Abstract
Immune hemolytic anemia is a rare adverse effect of ceftriaxone, a third-generation cephalosporin, which is a commonly used antibiotic. We describe a 60-years-old lady, a case of community-acquired pneumonia, who developed severe hemolysis after the first dose of ceftriaxone. Her hemoglobin dropped from 9.6 g /dl to 5.5 g /dl. However, she improved after discontinuation of the drug and blood transfusion. This report serves as a reminder to medical fraternity that life-threatening hemolysis can rarely follow administration of ceftriaxone.
KEY WORDS: ADRs, ceftriaxone, hemolysis
Introduction
About 30 years ago, methyldopa and penicillin were the two medications most commonly associated with drug-induced autoimmune hemolytic anemia (DIIHA). Methyldopa and intravenous penicillin accounted for 67% and 25%, of all drug-induced immune hemolytic anemia, respectively.[1] Currently, most cases of DIIHA are attributed to second- and third-generation cephalosporins, most commonly ceftriaxone, and this drug has become the most common cause of antibiotic-induced hemolysis.[2] We present a case of DIIHA following ceftriaxone use, managed successfully with withdrawal of the drug and supportive measures. Since this antibiotic is widely used by clinicians across all specialties, it is important to be aware of this possibility to enable us to make an early diagnosis.
Case Report
A 60-years-old lady, with no past history of any drug allergies, presented with fever and productive cough of five days duration. Clinically, she had fever, tachycardia, tachypnea with crackles in the right mammary area. X-ray chest confirmed pneumonia in the right middle zone [Figure 1]. On admission, her investigation were as in [Table 1]
Figure 1.
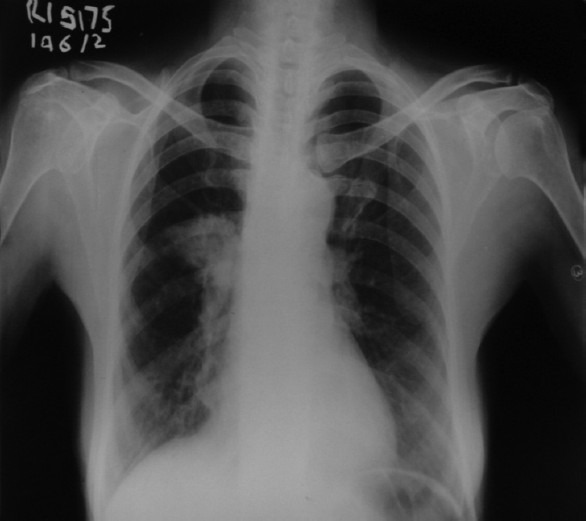
X-ray chest of the patient showing consolidation in right middle zone
Table 1.
Laboratory parameters of the patient suffering from ceftrixone-induced hemolysis
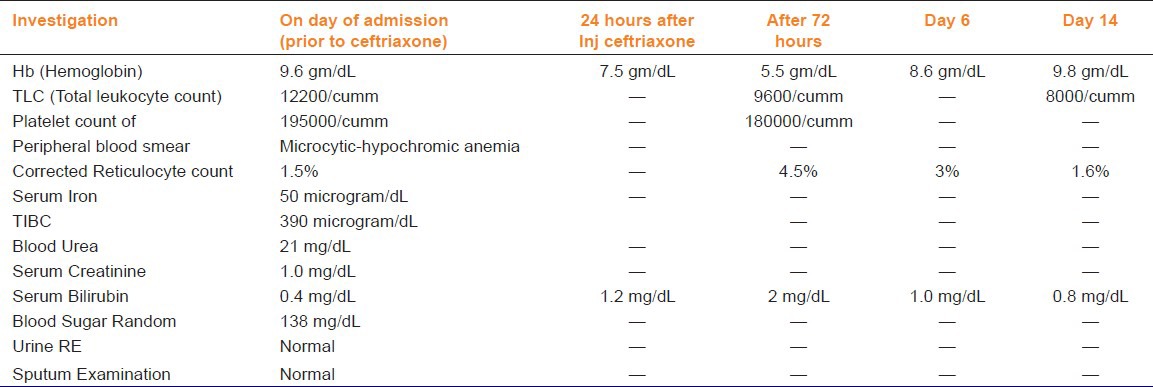
A diagnosis of community-acquired pneumonia was made, and patient was started on intravenous ceftriaxone one gram 12 hourly. Her hemoglobin dropped down to 7.5 g/dL within 24 hrs and further to 5.5 g/dL after 72 hrs, with peripheral blood smear showing marked polychromasia with three nucleated red cells per 100 white blood cells, schistocytes, a corrected reticulocyte count of 4.0% and LDH of 1221 units/L. Serum bilirubin rose to 2 mg/dl with indirect of 1.5 mg/dl. Direct Coombs test was positive. Urine for hemoglobin and hemosiderin was negative. A diagnosis of ceftriaxone-induced AIHA was made, and the drug was stopped immediately. Two units of packed red blood cells were transfused as she had symptomatic anemia, her hemoglobin increased to 8.6 g/dl and thereafter to 9.8 g/dl at discharge, after two weeks with no further deterioration. Bilirubin normalized on day six [Table 1].
Discussion
Ceftriaxone, a third-generation cephalosporin, is being commonly prescribed since 1984.[3] It is used as an antibiotic across almost all specialties for various conditions. Ceftriaxone-induced urticaria, rash, exanthem, and pruritus are the most common adverse effects and occur in about three percent of patients.[4] The first case of hemolysis induced by ceftriaxone was reported in 1991 by Garratty et al.[1] in a 52-year-old woman who was treated with ceftriaxone. This was the first case of immune hemolytic anemia associated with ceftriaxone, and also the first case of fatal cephalosporin-induced hemolytic anemia. Thereafter, in 1995, a case report of a 24-month-old boy with sickle cell disease who had cardiac arrest and died 36 hours later from multiple organ failure after starting ceftriaxone. Another case of a 16-year-old girl was reported in 1999 who had developed ceftriaxone-induced intravascular hemolysis leading to acute renal failure and death.
Drug-induced antibodies can be drug-dependent or drug-independent. Drug-dependent antibodies (antibodies react in vitro with RBC's, only in presence of drug) are produced by antibiotics like ceftriaxone and pipercillin. Antibody produced can be either IgG or IgM subtypes. These drugs attach to RBC, but do not bind covalently to RBC membrane proteins. Combination of drug and antibody creates an immunogen, which activates complement and results in acute intravascular hemolysis.[1] Drug-independent antibodies (drug not required to be present to detect antibodies in vitro) are produced by drugs as fludarabine, methyldopa, and penicillins. The antibody produced is mostly IgG.[5] These drugs combine covalently with RBC membrane proteins, and the antibody-coated RBC are taken up by macrophages.[3,4]
Ceftriaxone-induced AIHA, though rare, can be fatal if not thought of. It needs high index of clinical suspicion in patients treated with ceftriaxone who develop sudden drop in hemoglobin, hemoglobinuria, and evidence of hemolysis in peripheral blood smear. Beta lactam antibiotics should be used with caution in patients who have history of adverse effects to ceftriaxone because of cross reactivity.[6] According to the World Health Organization (WHO) system of causality definitions, the ADR (adverse drug reaction) in this reported case is categorized as probable with Naranjp algorithm score of six, as the patient developed hemolysis after intravenous administration of ceftriaxone. There was no other possible cause of hemolysis as no other drug was used with ceftriaxone. Rechallenge was not done due to inherent risk involved, but the patient showed improvement after stopping the drug.
Conclusion
Though drug-induced autoimmune hemolytic anemia is a rare adverse reaction, they may be fatal in some cases. Hence, the physicians should be vigilant while prescribing these drugs.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Garratty G, Postoway N, Schwellenbach J, McMahill PC. A fatal case of ceftriaxone (Rocephin)-induced hemolytic anaemia associated with intravascular immune hemolysis. Transfusion. 1991;31:176–9. doi: 10.1046/j.1537-2995.1991.31291142951.x. [DOI] [PubMed] [Google Scholar]
- 2.Arndt PA, Garratty G. The changing spectrum of drug-induced immune hemolytic anemia. Semin Hematol. 2005;42:137–44. doi: 10.1053/j.seminhematol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med. 2001;345:804–9. doi: 10.1056/NEJMra993637. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor FR, Dewdney JM, Weston RD, Wheeler AW. The immunogenicity of cephalosporin derivatives and their cross-reaction with penicillin. Immunology. 1966;10:21–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143–50. doi: 10.1016/j.blre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Pierce A, Nester T. Pathology consultation on drug-induced hemolytic anemia. Am J Clin Pathol. 2011;136:7–12. doi: 10.1309/AJCPBVLJZH6W6RQM. [DOI] [PubMed] [Google Scholar]


