Abstract
The metabolism of arachidonic acid (ARA) by cytochrome P450 ω/ω-1-hydroxylases leads to the formation of 20-hydroxyeicosatetraenoic acid (20-HETE), which is an important lipid-signaling molecule involved in regulation of vascular tone, angiogenesis, and inflammation. Development of a simple method to prepare 20-HETE would greatly facilitate the investigation of its biological activities. The nonpathogenic yeast Starmerella bombicola has been shown to convert exogenously added arachidonic acid to 20-HETE via the biosynthetic pathway of sophorolipids; however, the yield was low. Here we demonstrate that genetic knockout of multifunctional enzyme type 2 (MFE-2), which is involved in the β-oxidation of fatty acids, significantly increases the yield of ARA conversion to 20-HETE and allows practical preparation of 20-HETE.
Keywords: arachidonic acid, sophorolipids, 20-hydroxyeicosatetraenoic acid
The lipid mediators derived from the arachidonic acid (ARA) cascade are important because these signaling lipids regulate many critical biological processes from inflammation to tumorigenesis (1). Therefore, they are important therapeutic targets for many human diseases, and a significant proportion of drugs on the market targets lipid signaling of the ARA cascade. These lipid mediators are best known as prostaglandins and leukotrienes derived from the cyclooxygenase (COX) and lipoxygenase (LOX) pathways, respectively (1). Besides the intensively studied COX and LOX pathways, ARA is also a substrate of cytochrome P450 (CYP) ω/ω-1-hydroxylases (mainly CYP4A and CYP4F), which metabolize it to 19-hydroxyeicosatetraenoic acid (19-HETE) and 20-HETE (2). 20-HETE has been shown to have an array of largely detrimental effects, inducing hypertension, endothelial dysfunction, inflammation, cardiovascular diseases, angiogenesis, and tumor growth (3–8). Our recent research has shown that long-term administration of COX inhibitor rofecoxib caused a greater than 100-fold increase of circulating 20-HETE in a murine model, which suggests that the 20-HETE pathway contributes to the cardiovascular risks of COX inhibitors (9). More studies are needed to investigate the biological activities and mechanisms of 20-HETE.
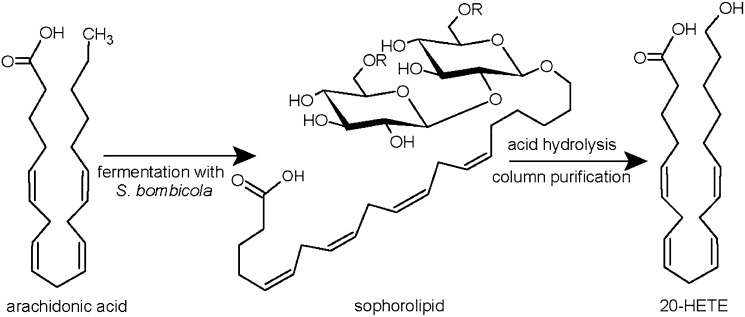
Development of a scalable method to prepare 20-HETE would greatly facilitate the evaluation of its biological activities and potential therapeutic applications, particularly in animal experiments that require relatively large amount of materials. 20-HETE is commercially available but prohibitively expensive ($4,000 per mg from Caymen Chemicals, Ann Arbor, MI). Chemical synthesis of 20-HETE has been reported (10); however, the multistep synthesis was challenging to carry out. Enzymatic conversion of ARA to 20-HETE using CYP enzyme(s) is attractive; however, the reaction is low-yield, is difficult to scale up, and requires the expensive cofactor NADPH or enzyme systems that generate NADPH. Biotransformation utilizing the nonpathogenic yeast Starmerella bombicola, which expresses CYP ω/ω-1-hydroxylases such as CYP52M1, is a promising alternative strategy (11). This yeast is known for its ability to produce sophorolipids, whose biosynthesis involves hydroxylation of fatty acids at the terminal or subterminal positions by the action of CYP ω/ω-1-hydroxylases in order to govern glycosidical coupling to the di-glucoside sophorose. The obtained sophorolipids can undergo further modification, such as acetylation and lactonization (supplementary Fig. I). The formed sophorolipids are secreted into the fermentation medium, making them easy to recover from the yeast culture with a yield up to 400 g/l (12). A recent study has shown that S. bombicola can convert the unnatural substrate ARA to 20-HETE (scheme shown in Fig. 1) (13); however, we found that the yield using the wild-type yeast was very low. Here we report that genetic knockout of multifunctional enzyme type 2 (MFE-2) gene in S. bombicola, which is involved in the β-oxidation of fatty acids, significantly increases the yield of ARA conversion to 19- and 20-HETE.
Fig. 1.
Schematic representation of the alternative production process for hydroxylated fatty acids. R = H or COCH3.
MATERIALS AND METHODS
Materials and chemicals
ARA was purchased from Nu-Chek Prep (Elysian, MN). Solvents used for flash chromatography and HPLC were purchased from Fisher Scientific (Pittsburgh, PA). Standards of 19-HETE, 20-HETE, 20-HETE-d6, and other lipid mediators were purchased from Cayman Chemical (Ann Arbor, MI).
CYP52M1 analysis
CYP52M1 (GenBank Accession EU552419) was expressed in Saccharomyces cerevisiae W(R) using pYeDP60 vector. Microsomes were isolated for the fatty acid hydroxylation assay by enzymatic digestion of the cell wall, followed by assisted hypotonic lysis and final CaCl2 precipitation as described (14). Hydroxylation assays were carried out by incubating 1 mg microsomal protein in 1 ml of 250 mM potassium phosphate buffer (pH 7.4) with 6.6 mM MgCl2, 6.6 mM glucose-6-phospate, 0.8 U glucose-6-phosphate dehydrogenase, 2.6 mM NADP+, and 0.2 mM fatty acid as substrate. The reaction was incubated at 30°C under shaking at 120 rpm for 3 h and quenched by adding 0.5 ml 0.8% (v/v) H2SO4. The reaction mixture was extracted with ethyl acetate, and the resulting extract was analyzed by gradient HPLC (Varian Prostar) and evaporative light scattering detection (ELSD; Alltech) and LC-MS (Waters) as described (11, 15).
Yeast fermentation
S. bombicola ATCC 22214 (wild-type) and the MFE-2-knockout M30 strain (patented by Ecover Belgium NV) (15, 16) were cultured with a glucose-rich medium as described by Prabhune et al. (13) or Lang et al. (17). An overnight preculture (5 ml) was used to inoculate 200 ml medium. After 48 h incubation at 30°C under shaking at 200 rpm, ARA dissolved in an equal volume of ethanol was added into the fermentation medium, and the incubation continued for another 5–12 days.
At the end of the fermentation, sophorolipids were extracted twice with an equal volume of ethyl acetate. The extracts were combined and evaporated to dryness using a rotary evaporator, and the dried extract was washed with hexane to remove any residual fatty acids, such as added ARA. The yield of the sophorolipids was expressed as grams of sophorolipids per volume of the fermentation (g/l). HPLC and LC-MS analysis of the sophorolipids was performed as described above.
Purification of 20-HETE
The recovered sophorolipids were hydrolyzed in 1M aqueous HCl under reflux in a N2 atmosphere for 2 h to liberate the hydroxyl fatty acids. The reaction product was extracted with dichloromethane (CH2Cl2), and then the CH2Cl2 extract was dried over anhydrous MgSO4, loaded onto a silica gel flash column, and eluted with 4% methanol in CH2Cl2. The fractions were pooled based on TLC (silica gel, 4% methanol in CH2Cl2, stained with KMnO4) and 1H NMR analysis. After the silica gel chromatography, fractions containing largely 19- and 20-HETE with minor impurities were obtained. The fractions containing 19- and 20-HETE were purified by HPLC using an Agilent 1100 HPLC system (Santa Clara, CA) equipped with a semipreparative silica gel HPLC column (Agilent Zorbax Sil, 250 × 9.4 mm, 5 µm, Agilent catalog no. 880952-201), which was eluted with a mobile phase of 2-propanol/hexane/acetic acid (8:92:0.1, v/v/v), detected at 210 nm with flow rate of 2–3 ml/min. The collected HPLC eluents were evaporated to dryness using a rotary evaporator, redissolved in CH2Cl2, washed with aqueous solution of NaHCO3 to remove residual acetic acid, dried over anhydrous MgSO4, evaporated to dryness, and stored at −80°C under a N2 atmosphere.
The structure of the purified 20-HETE was supported by NMR, as well as LC-MS/MS with coelution of standard 20-HETE and 20-HETE-d6. 1H NMR of 20-HETE (CDCl3, 400 MHz): δ 1.25–1.72 (m, 8H, H-3, 17, 18, and 19); 2.14 (m, 4H, H-4, and 16); 2.36 (tr, J = 6.4 Hz, 2H, H-2); 2.82 (m, 6H, H-7, 10, and 13); 3.67 (tr, J = 6.4 Hz, 2H, H-20); and 5.4 (m, 8H, H-5, 6, 8, 9, 11, 12, 14, and 15). The signal assignments were made by comparison with published NMR data (10).
LC-MS/MS analysis
The structure and purity of purified 20-HETE were further assessed by LC-MS/MS. The hydrolyzed sophorolipids or isolated 20-HETE was dissolved in methanol or acetonitrile to prepare a 1 mg/ml solution, then diluted 1,000–4,000 times for LC-MS/MS analysis. The solutions were injected into a LC-MS/MS system, including Agilent 1200SL (Santa Clara, CA) system coupled to AB Sciex 4000 QTrap system (Foster City, CA). The LC/MS/MS method was described earlier (18). The mass spectrometer was operated under negative electrospray mode. The MRM transition for 20-HETE was 319.2/275.2. other parameters for 20-HETE were DP-60 V, CE-26, and CXP-8. The identification of 20-HETE was implemented by comparing with 20-HETE and 20-HETE-d6 standard.
Endothelial cell proliferation assay
Human umbilical vein endothelial cells (HUVEC; Clonetics, Lonza, Walkersville, MD) were cultured in EBM-2 medium with supplements according to the manufacturer's instructions. Assays with HUVECs were conducted with cells from passage 2 to passage 6. HUVECs were plated into 24- or 96-well plates in complete endothelial medium and incubated overnight to allow cell attachment. The medium was changed to serum-free F-12K medium (ATCC, Manassas, VA) for 24 h, then treated with the prepared 20-HETE in F-12K medium for 18 h. Cell viability was assessed by a MTT (Sigma-Aldrich, Milwaukee, WI) or BrdU assay (Millipore, Billerica, MA).
Matrigel plug angiogenesis assay
All procedures and animal care were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis. A 0.5 ml growth-factor reduced Matrigel (BD Biosciences, San Jose, CA) premixed with 10 μg of the prepared 20-HETE (dissolved in 5 μl DMSO) and 20 U heparin (APP Pharmaceuticals, Schaumburg, IL) was subcutaneously injected into C57BL/6 mice at the abdominal area. After 4–7 days, the animals were euthanized to dissect the implanted Matrigel plugs. The gel plugs were weighted, homogenized in 1 ml PBS buffer, and centrifuged. Then the content of hemoglobin in the supernatant was analyzed by Drabkin's reagent (Sigma-Aldrich, St. Louis, MO) and normalized to the gel weights (19).
RESULTS AND DISCUSSION
Fatty acid hydroxylation by CYP52M1
Similar to other yeast species, S. bombicola expresses cytochrome P450 monooxygenases belonging to the CYP52 family. Members of this family show terminal or subterminal hydroxylating activity toward fatty acids or alkanes, and they are often involved in microbial metabolism of these substrates (20). CYP52 members display some similarity with other fatty acid ω/ω-1-hydroxylases of the CYP94 and CYP86 plant families, as well as with mammalian CYP4 members (21). To date, CYP52M1 of S. bombicola is the sole member of the CYP52M subfamily. This ω/ω-1-hydroxylase is uniquely involved in sophorolipid biosynthesis in which it governs the hydroxylation of a long-chain fatty acid required for coupling the carbohydrate moiety (22). The amino acid identity between CYP52M1 and the above-mentioned CYP families is 18–19% maximum; as an illustration, the alignment with CYP4A11 and CYP4F2 is depicted in supplementary Fig. II.
To investigate the potential of S. bombicola for the production of hydroxylated fatty acids, we studied the substrate selectivity of CYP52M1. The enzyme was heterologously expressed in S. cerevisiae W(R), and fatty acid hydroxylation assays were carried out using the isolated microsomes. Saturated fatty acids with a chain length shorter than 16 (such as C12:0, C14:0) or longer than 18 carbon atoms (C20:0, C22:0) were not hydroxylated at the ω/ω-1- positions, whereas the fatty acids (saturated, mono-, or polyunsaturated) with chain length of C16–C18 showed the highest reactivity (supplementary Table I). Indeed, the chain-length selectivity of C16–C18 is reflected in the natural sophorolipid fatty acid profile, in which only palmitic, palmitoleic, stearic, oleic, and linoleic acid are detected (23). Interestingly, unsaturated fatty acids with 20 carbon atoms (C20:1 and C20:4) were also hydroxylated by CYP52M1 (supplementary Table I). This could be explained the presence of cis double bond(s) in the unsaturated fatty acids, which may shorten the absolute length or change the conformation of the fatty acids. The activity of CYP52M1 toward unsaturated fatty acids with carbon chain lengths of 20 or higher suggests the feasibility to use S. bombicola for the production of hydroxylated long-chain fatty acids. It was possible to produce 20-HETE using the CYP52M1 overexpressing microsomes; however, the reaction was low-yield, and the expensive cofactor NADP(H) was required.
Strain and medium selection for ARA hydroxylation via the sophorolipid pathway
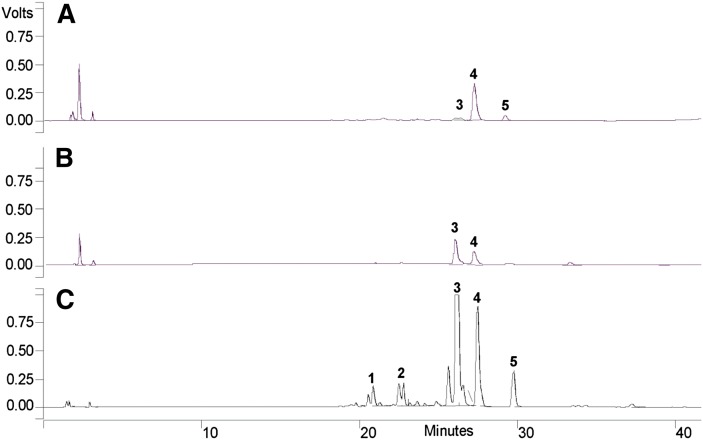
After confirmation of the enzymatic activity of CYP52M1 toward ARA, we studied whether S. bombicola could incorporate ARA into the sophorolipid biosynthetic pathway. First, we tried the wild-type strain of S. bombicola using two fermentation media: the first was described by Prahbune et al. (13) and was claimed to be suitable for efficient incorporation of ARA into the sophorolipids; the other one was described by Lang et al. (17) and is routinely used in our laboratory to produce native and new-to-nature sophorolipids or glycolipids. During the yeast cultivation, 0.25 g ARA (1.25 g/l) was added to the stationary cultures (2 days after initiation of fermentation), and sophorolipids were extracted after another 4-day cultivation. A yield of ∼0.3 g (1.5 g/l) sophorolipids was obtained using the two different media. However, the sophorolipids produced by the wild-type strain mainly contained de novo formed fatty acids but not exogenously added ARA (Fig. 2A). The low yield of ARA incorporation into the sophorolipids was supported by LC-MS/MS analysis of the HCl-hydrolyzed sophorolipids, which showed that the hydroxyarachidonates composed less than 0.01% of the hydrolyzed sophorolipids.
Fig. 2.
HPLC chromatograms of sophorolipids produced with ARA supplementation. (A) Wild-type strain with 1.25 g/l ARA. (B) M30 strain with 1.25 g/l ARA. (C) M30 strain with total addition of 5 g/l ARA. Identification of peaks upon LC-MS analysis: 1) acidic mono-acetylated sophorolipids with a C20:4 fatty acid tail (MW 686); 2) acidic di-acetylated sophorolipids with a C20:4 fatty acid tail (MW 728); 3) lactonic di-acetylated sophorolipids with a C20:4 fatty acid tail (MW 710); 4) de novo formed lactonic di-acetylated sophorolipids with a C18:1 fatty acid tail (MW 688); and 5) de novo formed lactonic di-acetylated sophorolipids with a C18:0 fatty acid tail (MW 690).
The low-yield incorporation of ARA was unexpected because, during the incubation, disappearance of the acid floating at the culture surface was observed and little residual ARA was retrieved in the hexane-washing step. These findings indicate that the ARA is preferentially degraded by β-oxidation instead of incorporated into the sophorolipid pathway. To test this hypothesis, we used a MFE-2-knockout strain of S. bombicola, which was disabled in the second (hydration) and third (second dehydrogenation) steps of the four-step β-oxidation pathway (24). In yeast, β-oxidation occurs only in the peroxisomes; this is in contrast to mammals in which the metabolism of fatty acids occurs both in peroxisomes and mitochondria (25). Moreover, yeasts only possess one MFE-2 isoform; this is in contrast to the pox and fox genes taking care of, respectively, the first and fourth reaction in β-oxidation. Hence, single knockout of this gene abolishes β-oxidation (26). Under the same culture conditions as reported above and by using the Lang medium, 0.9 g (4.5 g/l) sophorolipids were obtained. The yield could be further improved by adding 0.5 g (2.5 g/l) ARA instead of 0.25 g (1.25 g/l) in the stationary phase and with a second supplementation of 0.5 g (2.5 g/l) ARA 4 days later. Seven days after the initial addition, 3.8 g (19 g/l) sophorolipids were extracted.
HPLC and LC-MS analysis showed the mutant strain produced high levels of ARA-containing sophorolipids (Fig. 2B, C). Interestingly, the most dominant ARA sophorolipid was the di-acetylated lactonic form, indicating that the incorporation of this unnatural substrate does not hamper activity of the downstream acetyltransferase and lactonase, nor does it seems to affect the active transport required for secretion. LC-MS/MS analysis of the HCl-hydrolyzed sophorolipids (hydrolysis reaction yield of ∼30%) revealed that the hydroxyarachidonates compose ∼20% of the hydrolyzed sophorolipids (calculated yield of hydroxyarachidonates was 60 mg from 200 ml fermentation with addition of 0.25 g ARA). The high yields of ARA-containing sophorolipids in the mutant strain support our hypothesis that the exogenously added ARA in the wild-type strain is preferentially degraded by β-oxidation, genetic deletion of this process shunts the ARA into the sophorolipid biosynthetic pathway.
Purification of 19-HETE and 20-HETE from the ARA-containing sophorolipids
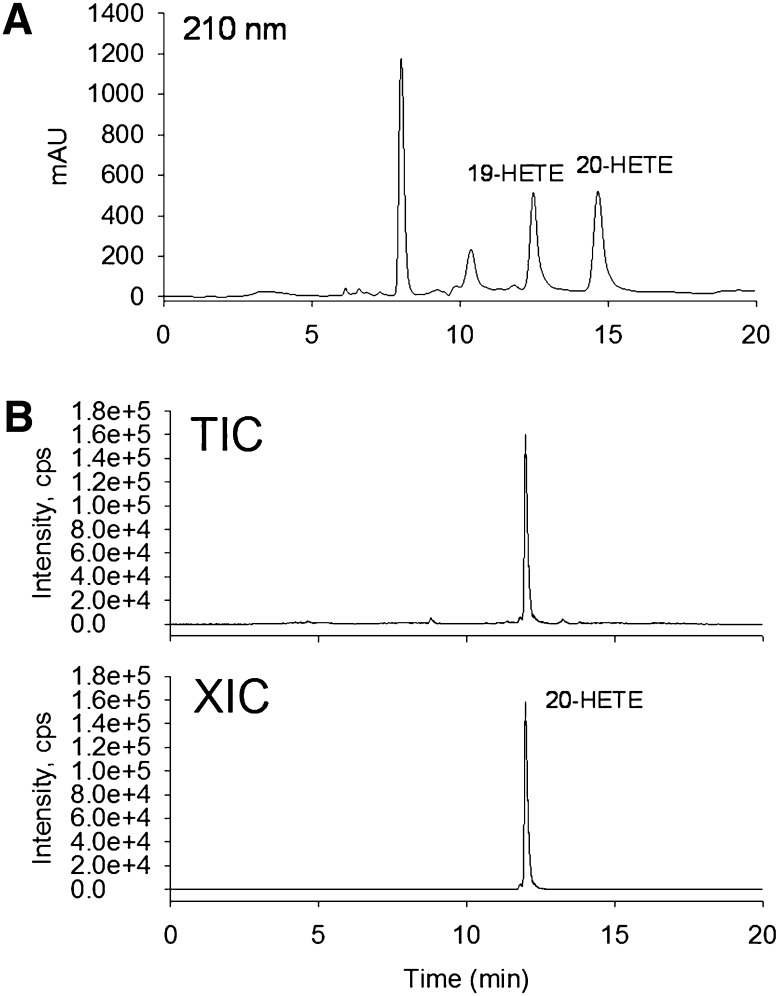
The hydroxyl fatty acids in the extracted sophorolipids are liberated by acid hydrolysis as described (13). The resulting reaction product is a complex mixture containing the hydroxylated forms of the de novo fatty acids from the yeast (C16:0, C18:0, C18:1, and C18:2 fatty acids), as well as hydroxyarachidonates (19- and 20-HETE). In our experiment, we found that simple flash column chromatography purification removed most impurities and generated a mixture containing mainly 19- and 20-HETE as assessed by 1H NMR and LC-MS/MS. The 19- and 20-HETE were separated by a semipreparative normal phase HPLC to obtain pure individual isomers (Fig. 3A). 19- and 20-HETE were not well resolved in reverse-phase HPLC (data not shown), which was consistent with a previous report (27).
Fig. 3.
(A) HPLC purification of 19- and 20-HETE, detection at 210 nm, flow rate = 2 ml/min. (B) LC-MS/MS analysis of isolated 20-HETE by HPLC. (Top) Total ion chromatogram (TIC). (Bottom) Extracted-ion chromatogram (XIC) using 319.2/275.1. The TIC shows that there is very little impurity in the purified 20-HETE. XIC of 20-HETE was used to support the elution retention time and amount of 20-HETE.
The structure and purity of isolated 19- and 20-HETE were supported by LC-MS/MS (Fig. 3B) and NMR analysis. The protons attached on the hydroxyl carbons of 19- or 20-HETE showed characteristic signals at δ 3.6–3.9 ppm, which are well separated from other 19- or 20-HETE NMR signals. H-19 of 19-HETE showed a signal at δ 3.83 (sex, J = 6.4 Hz, 1H, H-19), and H-20 of 20-HETE showed a signal at δ 3.67 (tr, J = 6.4 Hz, 2H, H-20). Before HPLC analysis, both signals were observed, consistent with a mixture of 19-HETE and 20-HETE. The isolated 20-HETE after HPLC purification only showed the signal at δ 3.67 (tr, J = 6.4 Hz), indicating 19- and 20-HETE were completely separated after HPLC purification (supplementary Fig. III).
20-HETE induces angiogenesis in vitro and in vivo
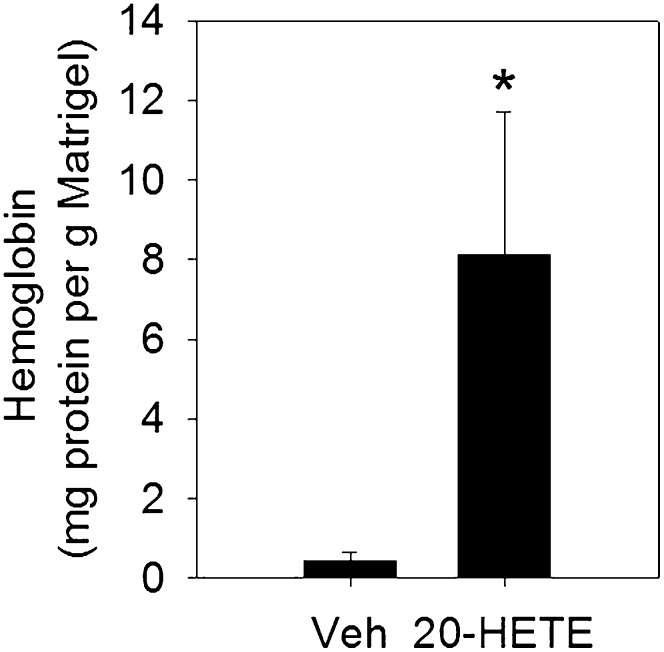
The development of a simple method to prepare 20-HETE could greatly facilitate the study of its biological activities, particularly in in vivo animal experiments. 20-HETE has been shown to induce angiogenesis (7); to test whether the prepared 20-HETE was functionally active, we tested the effect of the prepared 20-HETE on endothelial cell proliferation in HUVECs and angiogenesis in a Matrigel plug assay in mice. 20-HETE at 0.4–2.0 μM significantly increased cell proliferation after 18 h treatment in HUVECs (supplementary Fig. IV), which was consistent with a previous report for the proangiogenic effect of 20-HETE in vitro (7). Treatment with 10 μg 20-HETE in the implanted Matrigel plugs significantly induced angiogenesis in mice (Fig. 4), demonstrating its proangiogenic effect in vivo. Together, these results support that the prepared 20-HETE is biologically active.
Fig. 4.
20-HETE induces angiogenesis in a Matrigel plug assay in C57BL/6 mice (n = 4 mice per group). The results are expressed as mean ± SEM. *P < 0.05.
In conclusion, here we report an improved and scalable method to prepare 20-HETE using MFE-2-negative S. bombicola. This method will facilitate further studies to investigate the biological activities of 20-HETE. Moreover, preliminary results suggest that this method can be transferred to eicosapentaenoic (C20:5; EPA) and docosahexaenoic acid (C22:6; DHA), allowing synthesis of hitherto commercially unavailable hydroxylated polyunsaturated fatty acids.
Supplementary Material
Acknowledgments
The authors thank Lien Saey at Ghent University and Kyria Boundy-Mills and University of California-Davis for technical assistance.
Footnotes
Abbreviations:
- ARA
- arachidonic acid
- HETE
- hydroxyeicosatetraenoic acid
- MFE-2
- multifunctional enzyme type 2
This work was supported by National Institutes of Health Grants U24 DK097154, R01 ES-02710 (to B.D.H.), and P42 ES04699 (to B.D.H.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and four figures.
REFERENCES
- 1.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875 [DOI] [PubMed] [Google Scholar]
- 2.Kroetz D. L., Xu F. 2005. Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation. Annu. Rev. Pharmacol. Toxicol. 45: 413–438 [DOI] [PubMed] [Google Scholar]
- 3.Williams J. M., Murphy S., Burke M., Roman R. J. 2010. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J. Cardiovasc. Pharmacol. 56: 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng J., Ou J. S., Singh H., Falck J. R., Narsimhaswamy D., Pritchard K. A., Jr, Schwartzman M. L. 2008. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 294: H1018–H1026 [DOI] [PubMed] [Google Scholar]
- 5.Ishizuka T., Cheng J., Singh H., Vitto M. D., Manthati V. L., Falck J. R., Laniado-Schwartzman M. 2008. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J. Pharmacol. Exp. Ther. 324: 103–110 [DOI] [PubMed] [Google Scholar]
- 6.Miyata N., Roman R. J. 2005. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J. Smooth Muscle Res. 41: 175–193 [DOI] [PubMed] [Google Scholar]
- 7.Guo A. M., Arbab A. S., Falck J. R., Chen P., Edwards P. A., Roman R. J., Scicli A. G. 2007. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J. Pharmacol. Exp. Ther. 321: 18–27 [DOI] [PubMed] [Google Scholar]
- 8.Guo A. M., Sheng J., Scicli G. M., Arbab A. S., Lehman N. L., Edwards P. A., Falck J. R., Roman R. J., Scicli A. G. 2008. Expression of CYP4A1 in U251 human glioma cell induces hyperproliferative phenotype in vitro and rapidly growing tumors in vivo. J. Pharmacol. Exp. Ther. 327: 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J. Y., Li N., Yang J., Li N., Qiu H., Ai D., Chiamvimonvat N., Zhu Y., Hammock B. D. 2010. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc. Natl. Acad. Sci. USA. 107: 17017–17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manna S., Falck J. R., Chacos N., Capdevila J. 1983. Synthesis of arachidonic acid metabolites produced by purified kidney cortex microsomal cytochrome P-450. Tetrahedron Lett. 24: 33–36 [Google Scholar]
- 11.Van Bogaert I., Fleurackers S., Van Kerrebroeck S., Develter D., Soetaert W. 2011. Production of new-to-nature sophorolipids by cultivating the yeast Candida bombicola on unconventional hydrophobic substrates. Biotechnol. Bioeng. 108: 734–741 [DOI] [PubMed] [Google Scholar]
- 12.Van Bogaert I. N. A., Zhang J., Soetaert W. 2011. Microbial synthesis of sophorolipids. Process Biochem. 46: 821–833 [Google Scholar]
- 13.Prabhune A., Fox S. R., Ratledge C. 2002. Transformation of arachidonic acid to 19-hydroxy- and 20-hydroxy-eicosatetraenoic acids using Candida bombicola. Biotechnol. Lett. 24: 1041–1044 [Google Scholar]
- 14.Pompon D., Louerat B., Bronine A., Urban P. 1996. Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 272: 51–64 [DOI] [PubMed] [Google Scholar]
- 15.Van Bogaert I. N., Sabirova J., Develter D., Soetaert W., Vandamme E. J. 2009. Knocking out the MFE-2 gene of Candida bombicola leads to improved medium-chain sophorolipid production. FEMS Yeast Res. 9: 610–617 [DOI] [PubMed] [Google Scholar]
- 16.Van Bogaert I., Develter, and S. Fleurackers D. 2010 Method for the production of medium-chain sophorolipids. (Patent application PCT/EP2009/056190.) Publication WO 2009141407 A4. [Google Scholar]
- 17.Lang S., Brakemeier A., Heckmann R., Spockner S., Rau U. 2000. Production of native and modified sophorose lipids. Chim. Oggi. 10: 76–79 [Google Scholar]
- 18.Yang J., Schmelzer K., Georgi K., Hammock B. D. 2009. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Chem. 81: 8085–8093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adini A., Fainaru O., Udagawa T., Connor K. M., Folkman J., D'Amato R. J. 2009. Matrigel cytometry: a novel method for quantifying angiogenesis in vivo. J. Immunol. Methods. 342: 78–81 [DOI] [PubMed] [Google Scholar]
- 20.Van Bogaert I. N., De Mey M., Develter D., Soetaert W., Vandamme E. J. 2009. Importance of the cytochrome P450 monooxygenase CYP52 family for the sophorolipid-producing yeast Candida bombicola. FEMS Yeast Res. 9: 87–94 [DOI] [PubMed] [Google Scholar]
- 21.Nelson D. R. 2009. The cytochrome p450 homepage. Hum. Genomics. 4: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Bogaert I. N., Holvoet K., Roelants S. L., Li B., Lin Y. C., Van de Peer Y., Soetaert W. 2013. The biosynthetic gene cluster for sophorolipids: a biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol. Microbiol. 88: 501–509 [DOI] [PubMed] [Google Scholar]
- 23.Davila A-M., Marchal R., Vandecasteele J-P. 1994. Sophorose lipid production from lipidic precursors: predictive evaluation of industrial substrates. J. Ind. Microbiol. 13: 249–257 [Google Scholar]
- 24.Kunau W. H., Dommes V., Schulz H. 1995. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 34: 267–342 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka A., Osumi M., Fukui S. 1982. Peroxisomes of alkane-grown yeast: fundamental and practical aspects. Ann. N. Y. Acad. Sci. 386: 183–199 [DOI] [PubMed] [Google Scholar]
- 26.Hiltunen J. K., Wenzel B., Beyer A., Erdmann R., Fossa A., Kunau W. H. 1992. Peroxisomal multifunctional beta-oxidation protein of Saccharomyces cerevisiae. Molecular analysis of the fox2 gene and gene product. J. Biol. Chem. 267: 6646–6653 [PubMed] [Google Scholar]
- 27.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. 2010. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J. Biol. Chem. 285: 32720–32733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.