Abstract
Objective
To evaluate changes in iron, zinc and copper status of non-anemic Peruvian infants receiving daily supplements with 10 mg iron, 0.5 mg copper with or without 10 mg zinc from 6 to 18 months of age.
Methods
Overall, 251 infants were randomized to one of two daily supplements. Venous blood draws at 6, 12, and 18 months were taken to characterize hemoglobin, plasma ferritin, zinc and copper concentrations. Urinary excretion of zinc was also measured at each time point. Repeated measures ANOVA was used to evaluate changes over time and by supplement type.
Results
Both hemoglobin and copper concentrations increased significantly, while plasma ferritin decreased from 6 to 12 months of age (P < 0.05). Mean plasma zinc concentrations in the zinc treatment group were maintained over time, while that in the control group declined; differences by treatment were found at 12 and 18 months (P < 0.05). Urinary zinc concentration was increased in the zinc group at 12 months only. There was evidence that zinc treatment improved hemoglobin at 18 months of age (P = 0.09). Compliance with supplementation was high, with 81% of the intended dose consumed over the 12-month period.
Conclusions
Daily mineral supplementation over one year appears feasible and acceptable in this population, and a combined supplement can improve iron, zinc and copper status of infants at the same time.
Keywords: Zinc, iron, copper, supplements, infancy
INTRODUCTION
Studies indicate that infants in developing countries receive a diet low in minerals, including iron and zinc [1,2]. With respect to iron, many countries recommend that infants receive iron supplements (usually in the form of syrups) beginning at about 6 months of age to prevent and or treat anemia [3]. Despite dietary evidence of deficiency and documented potential benefits to health with zinc supplementation [4], zinc is not currently added to pediatric iron supplements in developing countries. Concerns have been raised about whether improvements in iron and zinc status can occur with a combined supplement [5,6], or that mineral supplements will negatively affect copper status [7,8] despite the lack in general of information on copper status of infants, particularly in developing countries. Further there is a paucity of information on adherence with daily mineral supplementation during infancy [9-11].
Between January 2006 and March 2007, we conducted a randomized controlled trial of daily zinc supplementation among Peruvian infants from 6 to 18 months of age. The goal of the study was to examine the effect of supplemental zinc on infant cognitive, behavioral and motor development. Non-anemic infants were randomized at 6 months of age to receive supplements containing 10 mg iron and 0.5 mg copper with or without 10 mg zinc. Here we present data examining the impact of these supplements on iron, zinc and copper status as well as the pattern of adherence with this regimen over the year-long period.
MATERIALS AND METHODS
The study was conducted in Villa Salvador, an urban settlement area in the greater metropolitan area of Lima, Peru. To identify eligible infants for the study, a geographic catchment area was selected near the primary care hospital “San Jose”. A household survey was conducted to identify infants 0-6 months of age as well as pregnant women. Field workers explained the study to mothers of infants 4-6 months of age, and if they were interested in potentially participating in the study, an appointment was made for them to visit the study clinic at the hospital to learn more about the study, provide signed consent and for final eligibility to be determined. Infants were eligible for the study if they had weighed more than 2500 grams at birth and had a gestational age > 37 completed weeks, were free of major malformations, genetic abnormalities, or health problems associated with developmental delays, had no known vision or hearing problems, and would remain in the hospital catchment area for the next 12 months. Following the approach of Lozoff et al [12], we considered infants with hemoglobin concentrations > 103 g/L as eligible for enrollment; those with values ≤ 103 g/L were treated with supplemental iron, monitored and excluded from the study. We chose this strategy because we were interested in testing the effects of preventing zinc deficiency during infancy on child development without confounding effects due to pre-existing anemia.
Infants were randomized to receive one of two liquid supplements to be taken daily until they reached 18 months of age. Randomization was in blocks of two stratified by infant gender. The liquid supplements contained 10 mg/d iron (as ferrous sulfate) and 0.5 mg/d copper (as copper oxide), and the liquid for the experimental group also contained 10 mg/d zinc (as zinc sulfate). The dosage of iron was set according to the Peruvian Ministry of Health (MOH) guidelines for preventing iron deficiency anemia in infancy. The dosage of zinc much higher than the current US R.D.A of 3 mg [13], but is the most common dose used in prior supplementation studies in this age group [4]. To ensure adequate copper status, given the dosages of iron and zinc, we chose a dosage of copper about twice the 2001 US R.D.A. for 12 to 18 month old infants [13].
The supplements were prepared in Lima, Peru by IQFARMA, a certified national laboratory, which follows Good Manufacturing Practices. The bottles containing the supplements as well as the appearance of the supplements were indistinguishable. The bottles had spoons metered to deliver 2.5 mL of supplement. A set of bottles were coded with each subject’s unique study identification number to facilitate distribution. The allocation list was held by the pharmaceutical company and the Director of the Instituto de Investigacion Nutricional (IIN) until the end of the study. The protocol was approved by the Institutional Review Boards at IIN, the University of Kansas, and The Johns Hopkins Bloomberg School of Public Health. It is a registered clinical trial (NCT00589264).
At enrollment, women were interviewed to gather background information on socioeconomic status, and family characteristics. All infants were evaluated by a physician and anthropometric measures of weight and length were taken following standard protocols [14]. Women were then provided an initial allocation of the supplement and were advised to give their infant one dose of the supplement daily. Monthly, infants were evaluated by the pediatrician. At that visit, the nurse received the used bottle and provided them with a new bottle of supplement. Each bottle was weighed prior to distribution, and the nurse weighed and then recorded the amount of liquid remaining in each returned bottle. On a weekly basis, fieldworkers visited the homes to inquire about illnesses since the last visit, as well as supplement administration. Specifically, they asked the mother to recall the days she had given the supplement to her infant since their last visit.
Measures of iron, copper and zinc status were assessed when infants were 6 (baseline), 12 and 18 months of age. A 5 mL blood sample was taken by venipuncture by a trained nurse at the clinic using trace mineral free tubes containing zinc-free heparin and disposable syringes. Urine samples were also collected in zinc-free containers at each time point. The samples were processed by the study nurse in the hospital laboratory, frozen at −20 °C, and, transported daily on ice to the laboratory at IIN. During the study, iron status was assessed using hemoglobin and hematocrit as indicators of anemia, and plasma ferritin as an indicator of iron stores. Hemoglobin and hematocrit were assessed at the clinic using standard methods by study nurse, and were included in the infant’s medical record. Plasma ferritin was determined at the IIN laboratory by ELISA using human antiferritin and antiferritin peroxidase antibodies (DAKO, Santa Barbara, CA USA). Ferritin standards were obtained from Diagnostic Products Corporation (Los Angeles, CA). Plasma copper and zinc and urinary zinc concentrations were determined at the IIN laboratory by atomic absorption spectrophotometry (AAS, Perkin Elmer 3100, Norwalk, CT).
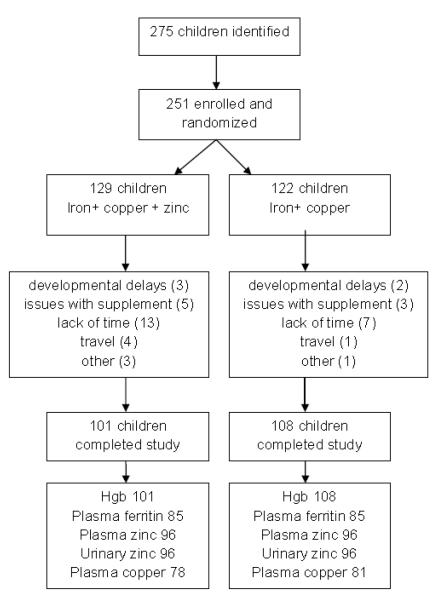
Originally the trial was designed to enroll 300 infants with an anticipated loss to follow up of 10%, and power calculations were made for the child development outcomes of the study. Due to funding constraints, we had to reduce the sample size. As shown in the study profile (Figure 1), 275 infants were evaluated for eligibility, and 251 were randomized, 122 to the iron + copper group, and 129 to the iron + copper + zinc group. As shown, there were 42 (13%) infants who left the study, leaving 108 in the iron + copper group and 101 in the iron + copper + zinc group. It was not possible to conduct full laboratory analyses for iron, copper and zinc status on all infants; decisions regarding sample for analysis were made based on funds, study completion and sample volume, with no consideration of treatment allocation or compliance. The numbers of infants contributing biochemical data varied by indicator and supplement type, and is most limiting for copper. Of interest is our ability to detect differences in response to supplementation by supplement type; given available sample sizes (with power = 0.80; alpha = 0.05), the minimal detectable differences by supplement type range from 0.040SD for hemoglobin to 0.45SD for plasma copper.
Figure 1.
Trial Profile
We utilized the adherence data to calculate the percent of the intended dose that was consumed by each child both overall and for each 6-month time period between blood draws and status determination. This was calculated by summing the weights of supplement delivered (weight of bottle given out – weight of the bottle returned) and dividing by the intended dose over the period to derive the percent of the intended dose consumed.
We applied t-tests to continuous variables and χ2 test to categorical variables to examine comparability in maternal and infant characteristics between supplement groups at enrollment. To determine relationships between biochemical parameters we used Pearson’s and Spearman’s correlations based on data distribution. Repeated measures analysis of variance (PROC MIXED) was performed to test changes in biochemical indicators across time, the effect of supplementation, and the interaction of supplement by time. We would expect to see an interaction of supplement by time for the zinc indicators, but because both supplements contained iron and copper we would not expect significant interaction terms, unless the presence of zinc differentially affected changes in these indicators over time. We included variables for infant sex, compliance and initial deficiency status, and tested for interactions between these variables and supplement type. Statistical analysis was accomplished using SAS version 9.3 (SAS Institute Inc., Cary, NC). The significance level was set at 0.05.
RESULTS
Shown in Table 1 are selected characteristics of the infants and their family at baseline; there were no differences in characteristics by supplement type. The average WAZ at enrollment was on the order of 0.5 SD for weight for age, but −0.5 SD for length for age.
Table 1.
Selected characteristics of Peruvian infants at enrollment by supplement type
| Characteristic | Iron + copper + zinc (N=101) |
Iron + copper (N=108) |
|---|---|---|
| Age (mo) | 6.3 ± 0.1 | 6.3 ± 0.1 |
| Girl (%) | 53.2 | 43.3 |
| Weight (kg) WAZ Length (cm) LAZ |
8.2 ± 1.0 0.5 ± 1.0 66.0 ± 2.1 −0.5 ± 0.9 |
8.1 ± 0.7 0.4 ± 0.7 65.8 ± 1.9 −0.6 ± 0.8 |
| Maternal age (y) Maternal schooling (y) Persons per bedroom‡ Dirt or temporary floor (%) |
27.4 ± 6.1 11.3 ± 2.2 2.0 (1.7, 2.7) 24.4 |
27.3 ± 5.6 11.2 ± 3.0 2.0 (1.5, 3.0) 30.8 |
Median (25th, 75th percentiles). No differences by supplement type were found, P < 0.05.
The enrollment characteristics of those who completed the study were compared with those lost to follow up. There were no differences in child, family characteristics, or initial hemoglobin concentrations, but those lost to follow up were significantly more likely to be girls and to be allocated to the supplemental zinc group. As shown in Figure 1, there were more children who left the study due to lack of time or because they disliked the supplement in the zinc group. The majority of these cases in the zinc group were girls.
We calculated the percent of the intended dose that children received. Over the 12 month period, children received 81% (median, 5th, 95th percentiles: 59%, 96%) of the intended amount to be supplemented. This did not differ by type of supplement consumed (Table 2).
Table 2.
Compliance (median (5th, 95th percentile)) with supplementation by supplement type
| Compliance | Iron + copper + zinc (N=101) |
Iron + copper (N=108) |
|---|---|---|
| Overall (% dose) 6 to 11 mo 12 to 17 mo |
81 (47, 96) 83 (44, 95) 82 (34, 96) |
82 (54, 94) 83 (51, 96) 83 (38, 98) |
No differences by supplement type were found, P < 0.05.
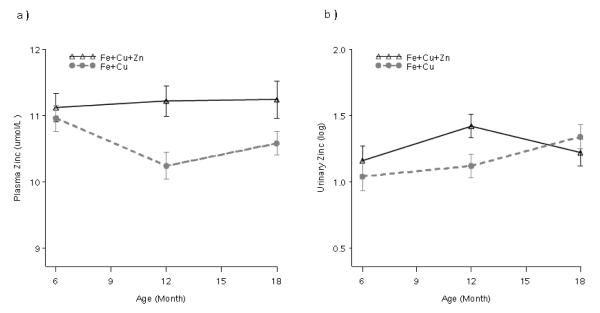
Concentrations of hemoglobin, ferritin, copper and zinc as well as urinary zinc by supplement type at each time point are presented in Table 3. Repeated measures modeling revealed treatment × time interactions for both plasma zinc and urinary zinc concentrations. No differences were found at baseline but at 12 and 18 months of age children receiving zinc had higher plasma zinc concentrations (1.00 ± 0.30; p < 0.01; 0.69 ± 0.32; p < 0.04). As shown in Figure 2(a), plasma zinc concentrations remained level over time in children receiving supplemental zinc, while they declined in those in the control group at 12 months and then rose slightly at 18 months. Urinary zinc concentrations were elevated in children receiving supplemental zinc at 12 months (0.30 ± 0.13; p < 0.03), but not at 18 months; this is consistent with the changes shown in Figure 2(b). Compliance with supplementation did not affect the differences observed over time, and no treatment × compliance interaction was detected.
Table 3.
Biochemical changes in iron, zinc and copper status of Peruvian infants 6, 12 and 18 months of age, by supplement type
| Age (mo) | |||
|---|---|---|---|
| 6 | 12 | 18 | |
| Hemoglobin (g/dL) Fe + Cu Fe + Cu + Zn |
112.6 ± 10.4 114.6 ± 9.7 |
116.4 ± 10.1b 117.6 ± 10.5b |
115.2 ± 9.1 117.3 ± 8.7c |
| Plasma Ferritin (umol/L)‡ Fe + Cu Fe + Cu + Zn |
27.5 (12.4, 60.7) 26.9 (12.0, 60.0) |
22.0 (11.6, 41.7)b 17.3 (9.4, 31.9)b |
26.4 (13.0, 53.7) 20.3 (11.2, 36.9) |
| Plasma Copper (umol/L) Fe + Cu Fe + Cu + Zn |
20.3 ± 4.0 21.0 ± 4.5 |
24.2 ± 3.9b 23.8 ± 4.3b |
24.0 ± 4.2 24.2 ± 3.5 |
| Plasma Zinc (umol/L) Fe + Cu Fe + Cu + Zn |
11.0 ± 2.0 11.1 ± 2.1 |
10.2 ± 1.9 11.2 ± 2.2a |
10.6 ± 1.7 11.2 ± 2.7a |
| Urinary Zinc (umol/L)‡ Fe + Cu Fe + Cu + Zn |
2.84 (0.97, 8.32) 3.21 (1.13, 9.09) |
3.08 (1.23, 7.73) 4.17 (1.78, 9.8)a |
3.79 (1.59, 9.03) 3.36 (1.30, 8.70) |
Geometric mean (−1 SD, + 1 SD). Repeated measures ANOVA were used to examine changes over time and by supplement type
supplement × time interaction, P < 0.05
change in concentration from baseline, P < 0.05
difference by supplement type, P < 0.05.
Figure 2.
Plasma (a) and urinary (b) zinc concentrations by type of supplement consumed by Peruvian infants at 6, 12 and 18 months. Significant differences (P < 0.05) by supplement type were found at 12 and 18 months (a) and 12 months (b).
As shown in Figure 3, plasma copper concentrations rose significantly from 6 to 12 months of age (3.27 ± 0.55; p < 0.001), but showed a non-significant increase from 12 to 18 months (0.42 ± 0.52; p = 0.41). No differences by supplement type were noted, nor were treatment × time interactions. Variation in compliance did not affect plasma copper concentrations overall or at any time point.
Figure 3.
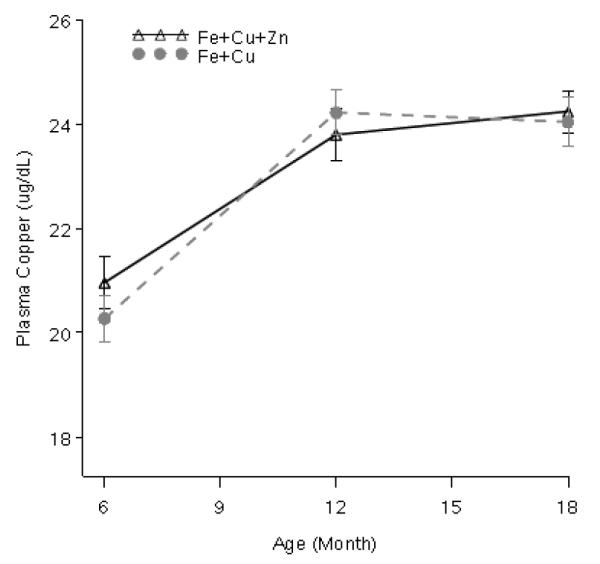
Plasma copper concentrations by supplement type consumed by Peruvian infants at 6, 12 and 18 months. No differences by supplement type were detected (P > 0.05). Concentrations increased from 6 to 12 months (P < 0.05).
To examine changes in hemoglobin and ferritin concentrations, we considered supplementation effects overall and stratified by whether or not the children had iron deficiency at baseline (Figure 4). Overall, we found that hemoglobin increased significantly from 6 to 12 months (0.27 ± 0.12; p < 0.03), but no further increase was detectable from 12 to 18 months. There were no differences by treatment at baseline or 12 months, but at 18 months, children who also received zinc had a trend toward higher hemoglobin concentration (0.21 ± 0.12; p = 0.09). When initial iron deficiency is considered (Figure 4), it became apparent that treatment differences were affected by initial iron deficiency status, and children who were iron deficient and did not receive zinc showed declining hemoglobin concentration over time, whereas those in the other 3 groups showed a similar increase in hemoglobin concentration over time. Overall, ferritin concentrations declined from 6 to 12 months (−0.27 ± 0.07; p < 0.001), but concentrations at 12 months were not statistically different from those at 18 months. There were no differences by supplement type and compliance did not affect observed changes.
Figure 4.
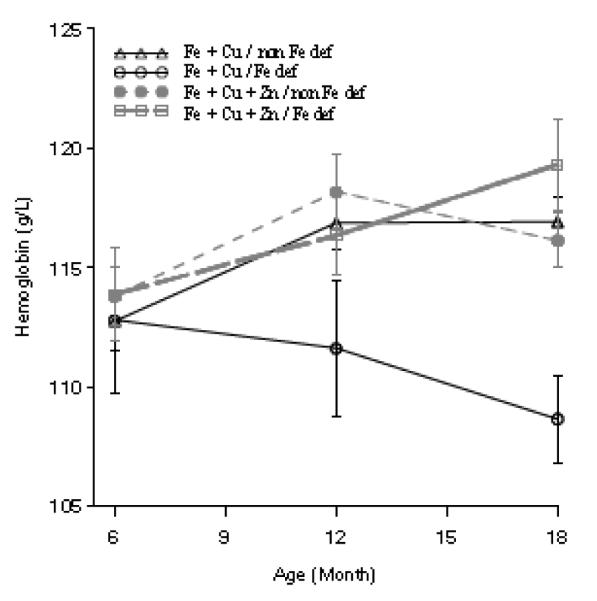
Hemoglobin concentrations by supplement type and iron deficiency at baseline in Peruvian infants at 6, 12 and 18 months. Differences in hemoglobin by treatment type at 18 months of age were noted (P=0.09). Hemoglobin increased from 6 to 12 months of age (P < 0.05).
DISCUSSION
Infants have high nutrient requirements to maintain their fast rate of growth. Breast milk satisfies their nutrient requirements for the first 6 months of life, but during the latter half of infancy the nutrient adequacy of complementary foods is insufficient to meet the iron and zinc needs of most infants in developing countries [15]. Thus, we expect to see some evidence of declining iron and zinc status of infants beginning around 6 months of life. Supplements are regarded as one means of maintaining nutrient status during this time. In this study, we provided daily supplements with the goal of maintaining the iron and copper status of these Peruvian infants while evaluating the effect of supplemental zinc on zinc status. Our results indicate that the combined supplement improved the iron, zinc and copper status of these infants. With supplemental zinc, plasma zinc concentrations were maintained over the 12 month study period, while declining concentrations were observed in the control group. Although not as clear cut, differences in urinary zinc excretion were observed at 12 months of age, consistent with better zinc status in the treatment group. The effect size for the differences in plasma zinc concentration at 12 months of 0.48 is lower than but consistent with the effect size of 0.60 (95% CI: 0.44, 0.77) derived by Brown et al [16] in a meta-analysis of 22 controlled supplementation trials. The effect size at 18 months is lower (0.27) and falls in the lower end of the estimated effect sizes from studies included in the meta-analysis.
Our results add to the literature addressing the concerns that because iron and zinc share some common pathways for absorption that their inclusion together in a supplement will not allow improvements in mineral status. Between 6 and 12 months of age hemoglobin concentration increased significantly overall, and as noted, there was a trend (P =0.09) toward greater hemoglobin concentration at 18 months in the infants receiving supplemental zinc. When stratified by initial iron deficiency, it became clear that among the iron deficient, supplemental zinc led to hemoglobin concentrations at 18 months similar to those achieved by infants who were non-iron deficient at baseline (whether or not they were supplemented with zinc). Some studies [17-19], including two from Peru, have reported improvements in hemglobin concentrations with supplemental zinc, but overall, Brown et al [16] concluded from a meta-analysis of 11 trials, no effect (either positive or negative) on hemoglobin concentration. We reported that plasma ferritin concentrations declined from 6 to 12 months in both groups despite supplementation, which is consistent with developmental changes; no further declines were observed from12 to 18 months, consistent with effects seen for hemoglobin and copper concentrations. Brown et al [16] concluded from the results of 10 trials that supplemental zinc had no effect on changes in plasma ferritin; there was some heterogeneity of effect, with the smallest impact found in studies also providing supplemental iron, such as the case we report here. Thus, our results confirm the findings of others that improvements in iron and zinc status in infants can occur with a combined supplement.
We added copper to the supplements to address concerns raised in the literature that supplemental zinc (and iron) would negatively affect copper status. We chose a dose that was considerably higher than the current US RDA but is still lower than the UL [13]. We utilized plasma copper concentration to evaluate copper status in these infants, primarily due to the ease of assay and the lower cost; although not the most sensitive indicator of copper status, it is still recommended for detecting population changes such as those studied here [20]. Study infants showed marked increases in plasma copper concentration between 6 and 12 months and then no further increases were noted. No differences were noted by treatment type, confirming the findings of the Brown et al [16] meta-analysis (based on 4 trials) of no adverse impact of supplemental zinc on plasma or serum copper concentrations.
There are several pieces of evidence to suggest that supplementation was more efficacious between 6 and 12 months than between 12 and 18 months. Declining efficacy is often thought to result from compliance fatigue – that caregivers or infants get tired of the daily supplements and begin to skip doses. Our results on compliance involving the weighing of the supplement bottle before and after use and comparing the amount dispensed with the potential dose provides strong evidence that this did not occur. The median compliance of 80% of dose delivered, suggests that infants received their supplements on 5 to 6 days out of 7. There are only a few studies documenting compliance with daily micronutrient supplements; however, most document levels of compliance equivalent to those we report here [9-11]. Thus, an important finding here is that the extended provision of daily supplements to improve micronutrient status appears feasible and acceptable in our and other studied populations in developing countries. It must also be mentioned that the addition of zinc to the syrup did not affect compliance.
There are limitations to our study that are worth mentioning. First, there is evidence of differential loss to follow up among those receiving supplemental zinc and among girls. The reasons for these findings are not clear, which should be investigated in further studies. Second, due to funding constraints we were not able to examine more indicators of mineral status, nor were we able to obtain findings on all infants who completed the study; additional indicators would have improved interpretation of the changes in copper status in particular. However, the indicators we chose were appropriate, especially for examining changes in status of groups, in this case, treatment groups. Third, a placebo group would also have allowed us to establish the changes in mineral status through the study period in the absence of supplementation. While desirable, this would not have been ethical in this care setting, with recommendations for the universal provision of supplemental iron to infants beginning at 6 months of age. There are numerous strengths to the study as well, including the use of multiple indicators of iron and zinc status, the use of the randomized design, two blood draws to examine changes over time and the attention to detail to document compliance over time.
CONCLUSIONS
Our results confirm high compliance with daily micronutrient supplementation among infants over a year-long period, and detectable improvements in iron and zinc status through a common delivery mechanism. Copper status was improved, but research is needed to interpret indicators of copper status in infants in developing countries.
ACKNOWLEDGEMENTS
We sincerely thank all the mothers and infants who participated in the study. We thank the health personnel of the Health Center “San José” in Villa El Salvador, the health authorities of the Ministry of Health, “DISA Lima Sur”, and MicroRed VES LPP for their collaboration. We thank Teresa Garcia for her help with data analysis.
Funded by a grant from the National Institutes of Child Health and Development (NICHD) HD045430.
Footnotes
The authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contribution: All authors conceived of the study design, contributed to the study data collection, and creation of the manuscript.
REFERENCES
- 1.Gibson RS, Ferguson EL, Lehrfeld J. Complementary foods for infant feeding in developing countries: their nutrient adequacy and improvement. Eur J Clin Nutr. 1998;52:764–770. doi: 10.1038/sj.ejcn.1600645. [DOI] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization) Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge. WHO; Geneva, Switzerland: 1998. [Google Scholar]
- 3.INACG/WHO/UNICEF. Stoltzfus RJ, Dreyfuss ML, International Nutritional Anemias Consultative Group (INACG) Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. International Life Sciences Institute (ILSI) Press; Washington DC: 1998. [Google Scholar]
- 4.Yacoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, Jhass A, Rudan I, Campbell H, Black RE, Bhutta ZA. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011 Apr 13;11(Suppl 3):S23. doi: 10.1186/1471-2458-11-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomons NW. Competitive interaction of iron and zinc in the diet: consequences for human nutrition. J Nutr. 1986;116(6):927–35. doi: 10.1093/jn/116.6.927. [DOI] [PubMed] [Google Scholar]
- 6.Olivares M, Pizarro F, Ruz M, de Romaña DL. Acute inhibition of iron bioavailability by zinc: studies in humans. Biometals. 2012;25(4):657–64. doi: 10.1007/s10534-012-9524-z. [DOI] [PubMed] [Google Scholar]
- 7.Botash AS, Nasca J, Dubowy R, Weinberger HL, Oliphant M. Zinc-induced copper deficiency in an infant. Am J Dis Child. 1992;146:709–711. doi: 10.1001/archpedi.1992.02160180069019. [DOI] [PubMed] [Google Scholar]
- 8.Turnlund JR. Copper. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. 9th ed Williams & Wilkins; Baltimore: 1999. pp. 241–252. [Google Scholar]
- 9.Bhutta Z, Klemm R, Shahid R, Rizvi A, Rah JH, Christian P. Treatment response to iron and folic acid alone is the same as with multivitamins and/or anthelminthics in severely anemic 6-24 month old children. J Nutr. 2009;139:1568–74. doi: 10.3945/jn.108.103507. [DOI] [PubMed] [Google Scholar]
- 10.Geltman PL, Hironaka LK, Mehta SD, Padilla P, Rodrigues P, Meyers AF, Bauchner H. Iron supplementation of low-income infants: a randomized clinical trial of adherence with ferrous fumarate sprinkles versus ferrous sulfate drops. Pediatr. 2009 May;154(5):738–43. doi: 10.1016/j.jpeds.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Azeredo CM, Cotta RM, Sant’Ana LF, Franceschini Sdo C, Ribeiro Rde C, Lamounier JA, Pedron FA. Greater effectiveness of daily iron supplementation scheme in infants. Rev Saude Publica. 2010;44(2):230–9. doi: 10.1590/s0034-89102010000200002. [DOI] [PubMed] [Google Scholar]
- 12.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–54. [PubMed] [Google Scholar]
- 13.Food and Nutrition Board. National Academy of Sciences –National Research Council . Dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 14.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 15.Krebs NF. Dietary zinc and iron sources, physical growth and cognitive development of breastfed infants. J.Nutr. 2000;130:358S–60S. doi: 10.1093/jn/130.2.358S. [DOI] [PubMed] [Google Scholar]
- 16.Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull. 2009;30(1 suppl):S12–S40. doi: 10.1177/15648265090301S103. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon K, Kolsteren PW, Prada AM, Chian AM, Velarde RE, Pecho IL, Hoeree TF. Effects of separate delivery of zinc or zinc and vitamin A on hemoglobin response, growth, and diarrhea in young Peruvian children receiving iron therapy for anemia. Am J Clin Nutr. 2004;80:1276–82. doi: 10.1093/ajcn/80.5.1276. [DOI] [PubMed] [Google Scholar]
- 18.Brown KH, Lopez de Romana D, Arsenault JE, Peerson JM, Penny ME. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity and plasma zinc concentrations of young Peruvian children. Am J Clin Nutr. 2007;85:538–47. doi: 10.1093/ajcn/85.2.538. [DOI] [PubMed] [Google Scholar]
- 19.Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Stoltzfus RJ. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5- to 11-mo old. J Nutr. 2006;136:427–34. doi: 10.1093/jn/136.9.2427. [DOI] [PubMed] [Google Scholar]
- 20.Harvey LJ, Ashton K, Hooper L, Casgrain A, Fairweather-Tait SJ. Methods of assessment of copper status in humans: a systematic review. Am J Clin Nutr. 2009;89(suppl):2009S–24S. doi: 10.3945/ajcn.2009.27230E. [DOI] [PubMed] [Google Scholar]