Abstract
BACKGROUND
Stored red blood cells (RBCs) undergo progressive deleterious functional, biochemical, and structural changes. The mechanisms responsible for the adverse effects of transfusing stored RBCs remain incompletely elucidated.
STUDY DESIGN AND METHODS
Awake wild-type (WT) mice, WT mice fed a high-fat diet (HFD-fed WT) for 4 to 6 weeks, and diabetic (db/db) mice were transfused with syngeneic leukoreduced RBCs or supernatant with or without oxidation (10% of total blood volume) after storage for not more than 24 hours (FRBCs) or 2 weeks (SRBCs). Inhaled nitric oxide (NO) at 80 parts per million was administered to a group of mice transfused with SRBCs. Blood and tissue samples were collected 2 hours after transfusion to measure iron and cytokine levels.
RESULTS
SRBCs had altered RBC morphology and a reduced P50. Transfusion of SRBCs into WT or HFD-fed WT mice did not produce systemic hemodynamic changes. In contrast, transfusion of SRBCs or supernatant from SRBCs into db/db mice induced systemic hypertension that was prevented by concurrent inhalation of NO. Infusion of washed SRBCs or oxidized SRBC supernatant into db/db mice did not induce hypertension. Two hours after SRBC transfusion, plasma hemoglobin (Hb), interleukin-6, and serum iron levels were increased.
CONCLUSION
Transfusion of syngeneic SRBCs or the supernatant from SRBCs produces systemic hypertension and vasoconstriction in db/db mice. It is likely that RBC storage, by causing in vitro hemolysis and posttransfusion hemoglobinemia, produces sustained NO scavenging and vasoconstriction in mice with endothelial dysfunction. Vasoconstriction is prevented by oxidizing the supernatant of SRBCs or breathing NO during SRBC transfusion.
Red blood cell (RBC) transfusion is a lifesaving therapy employed to treat patients with anemia or major blood loss. Transfused RBCs replace lost blood volume and transport oxygen to sustain tissues and organs. Every year, approximately 75 million units of blood are collected worldwide.1 In the United States, approximately 14 million units of RBCs are transfused annually, with an estimated mean duration of storage before transfusion of 16.4 days.2
During ex vivo storage, RBCs undergo numerous biochemical, structural, and functional alterations, which are collectively termed the “storage lesion.” RBC storage systems approved by the Food and Drug Administration (FDA) require that, after storage, the level of hemolysis in the storage bag remains below 1% and a minimum of 75% of the transfused RBCs persist in the recipient’s blood at 24 hours after transfusion.3 These two criteria may not ensure the safety and efficacy of stored RBC transfusions and only partially reflect the progressive alterations of RBCs during storage.4 Transfusion with blood stored for longer durations was associated with an increased rate of infection,5,6 mortality,7,8 and multiorgan failure,9,10 as well as longer hospitalizations.11–13 In contrast, other clinical studies of selected patient populations, including those undergoing cardiac surgical procedures, trauma victims, and critically ill patients, reported no association between the duration of RBC storage and adverse clinical outcomes.2,14–18
The precise mechanisms responsible for the deleterious effects on the recipient produced by stored RBC transfusion are not fully understood. Several possible mechanisms have been proposed. The “RBC deformability” theory suggests that tissue injury is attributable to impaired microcirculatory blood flow and oxygen delivery due to reduced RBC deformability.19,20 A reduction of oxygen delivery may cause ischemia by reducing oxygen delivery due to decreased 2,3-diphosphoglycerate (2,3-DPG) levels (and increased hemoglobin [Hb]-oxygen affinity) in stored RBCs.21 The “iron hypothesis” proposes that transfusing stored RBCs generates Hb degradation products (such as reactive iron) that can stimulate an inflammatory response by generating reactive oxygen species.22,23 Several groups have proposed a “nitric oxide (NO)-based” theory attributing some of the adverse events associated with transfusing stored RBCs to the inflammatory, thrombotic, and vasoactive effects of NO scavenging by excess plasma Hb levels.24,25 Recently, plasma Hb has been shown to exist in microparticles as well as free Hb.26 Others have attributed these NO depletion side effects to loss of S-nitroso-Hb during storage.27 We recently reported that extracellular Hb can induce hypertension in mice by scavenging NO produced by endothelial NO synthase.28 Moreover, we observed that mice with endothelial dysfunction due to high-fat-diet (HFD) feeding or diabetes mellitus are particularly sensitive to the vasoconstricting effects of extracellular Hb.29 Since storage of RBCs produces extracellular Hb (cell-free Hb and Hb-containing microparticles), it is conceivable that recipients with endothelial dysfunction are more vulnerable to the vasoconstrictor effects of stored RBCs.
Reproducible animal models of RBC storage can provide important insights into the effects of transfusing stored blood. Development of a murine model is especially valuable because it enables elucidation of the contribution of genetic factors to the responses associated with transfusing stored RBCs. Prior studies have documented the development of biochemical and functional alterations in stored murine30 and human RBCs.31,32 In our studies, we chose to utilize the leukoreduced murine RBC storage model described by Gilson and colleagues30 and Hod and colleagues.23 We studied the impact of transfusing fresh and stored syngeneic RBCs into C57BL/6 mice. The use of syngeneic blood transfusion allowed us to focus specifically on the effects of the storage lesion without the confounding effects of immune incompatibility
Our studies had three objectives. First, we characterized the storage lesion of murine RBCs stored for 2 weeks in several important respects (life span, hemolysis, 2,3-DPG depletion). Second, we compared the hematologic changes and inflammatory responses induced by transfusing fresh leukoreduced murine RBCs (FRBCs, stored ≤24 hr) and stored leukoreduced murine RBCs (SRBCs, stored for 2 weeks) into syngeneic recipients. Third, we investigated if the extracellular Hb released from SRBCs could be responsible for the deleterious effects of transfusing SRBCs in both awake and anesthetized wild-type (WT) mice, WT mice fed a HFD for 4 to 6 weeks, and mice with diabetes mellitus (db/db). We found that storage of murine RBCs for 2 weeks produced hemolysis levels in storage bags and a 24-hour survival rate after transfusion similar to those values observed in human RBCs stored for the FDA limit of 42 days.3 We report that murine RBCs stored for 2 weeks developed characteristics of the storage lesion. We observed that transfusion of SRBCs but not FRBCs markedly elevated serum iron levels and produced a systemic inflammatory response in WT mice fed a standard diet or a HFD and db/db mice. Transfusion of SRBCs into db/db mice induced systemic vasoconstriction and hypertension, which could be prevented by concurrent inhalation of 80 parts per million (ppm) NO. Infusion of supernatant from SRBCs also produced systemic vasoconstriction in db/db mice. In contrast, transfusion of either washed stored RBCs or oxidized supernatant from SRBCs into db/db mice did not induce systemic hypertension.
MATERIALS AND METHODS
Animals
All animal studies were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital (Boston, MA). We studied 8- to 10-week-old male C57BL/6J WT mice and B6.Cg-m+/+Leprdb/J (C57BL/6J background) db/db mice. Additional WT mice were fed a HFD (60 kcal% fat; Research Diets, Inc., New Brunswick, NJ) for 4 to 6 weeks. Eight- to 10-week-old, male green fluorescent protein (GFP) transgenic C57BL/6-Tg (UBC-GFP) 30Scha/J (C57BL/6J background) mice with GFP expressed on RBCs were used to measure RBC survival. All mice were obtained from The Jackson Laboratory (Bar Harbor, ME).
Murine blood collection and storage
Blood (approx. 1 mL/mouse) was withdrawn aseptically by open chest cardiac puncture from C57BL/6J WT mice into a syringe containing CPDA-1 (Sigma-Aldrich, St Louis, MO). The final concentration of CPDA-1 was 14%. After collection, blood was leukoreduced by passage through a neonatal leukoreduction filter (Pall Biomedical Products Co., East Hills, NY). The efficacy of leukoreduction was measured by a complete blood count (HemaVet veterinary analyzer, Heska, Loveland, CO). Leukoreduced blood was centrifuged for 15 minutes at 600 × g, adjusted to a hematocrit (Hct) of 70% to 75% by removing plasma, and stored in Eppendorf tubes at 4°C. The lipopolysac-charide (LPS) levels in samples of FRBCs or SRBCs were measured using a limulus amoebocyte lysate assay, as previously described.33
Preparation of stored RBC components
Supernatants were obtained after centrifuging FRBCs or SRBCs at 400 × g for 10 minutes at 4°C To minimize hemolysis induced by washing, FRBCs and SRBCs were gently washed with 10 vol of 1.5% sodium chloride and centrifuged at 400 × g for 10 minutes at 4°C Before transfusion, washed FRBCs or SRBCs were resuspended in fresh murine plasma to obtain a final Hct of 70% to 75%
To oxidize ferrous Hb in the supernatant of SRBCs, the supernatant was exposed to 800 ppm NO gas in nitrogen through an oxygenator (Living Systems Instrumentation, Inc., St Albans, VT) for 2 hours to produce methemoglobin (met-Hb).28 Supernatant (2 mL) was then dialyzed three times (Spectra/Por molecular porous membrane; MWCO, 12–14,000, Spectrum Laboratories, Inc., Rancho Dominguez, CA) against 3 L of 0.9% saline to reduce low-molecular-weight NO metabolites.
Biochemical, hematologic, and morphologic changes in SRBCs
Blood gas tensions (PCO2 and PO2), pH, and standard base excess (SBE) were measured in FRBCs and SRBCs (ABL800 FLEX, Radiometer, Westlake, OH). Electrolyte concentrations (sodium, potassium, calcium, chloride, HCO3−) and lactate were also determined in FRBCs and SRBCs (Critical Care Xpress, Nova Biomedical, Waltham, MA).
To determine Hb levels in the supernatant, FRBCs or SRBCs were centrifuged at 1700 × g for 8 minutes at 4°C, and supernatant Hb levels were measured with a Hb assay kit (QuantiChrom, BioAssaySystems, Hayward, CA). Hemolysis (%) was calculated using a standard formula:
Hemolysis (%) = (supernatant Hb × [1 - %Hct])/ total Hb × 100.34
To investigate the impact of storage on RBC morphology, FRBCs or SRBCs were smeared and stained with a manual staining system (Hema3, Fisher Diagnostics, Middletown, VA), and images were obtained using an upright microscope fitted with a dry objective (Nikon EDIPSE 80i and Plan Fluor 60×, respectively, Nikon Instruments, Inc., Melville, NY).
Oxygen dissociation curves (ODCs) of FRBCs and SRBCs (adding 20 µL of RBCs to 5 mL of Hemox solution containing 20 µL of additive-A and 10 µL of antifoaming agent) were determined with an analyzer (Hemox, TCS Scientific Corp., New Hope, PA). P50, defined as the partial pressure of oxygen at which Hb is 50% saturated, was calculated from the ODC. The levels of 2,3-DPG in FRBCs and SRBCs were measured using a 2,3-DPG kit (Roche Diagnostics, Mannheim, Germany).
Measuring murine RBC survival
To measure survival of transfused murine RBCs, we used the GFP-labeled RBC method reported by Gilson and coworkers.30 Briefly, blood was withdrawn from both UBC-GFP and WT mice by cardiac puncture into a 1-mL syringe containing CPDA-1, and blood mixtures were prepared containing 40% RBCs from UBC-GFP mice and 60% RBCs from WT mice, leukoreduced, centrifuged, and stored at 4°C for less than 24 hours (FRBCs) and 2 weeks (SRBCs). WT mice were transfused with 100 µL of FRBCs or SRBCs containing GFP-labeled RBCs via a tail vein. At 10 minutes, 30 minutes, 1 hour, 24 hours, and 8 days after transfusion, blood samples were obtained from the retroorbital space into heparinized glass microcapillary tubes. The percentage of GFP-positive RBCs was determined with a flow cytometer (BD Biosciences, San Jose, CA). The flow cytometer was calibrated to the same settings on different acquisition days by Sphero rainbow fluorescent particles (Spherotech, Inc., Lake Forest, IL). The RBC survival rate at 24 hours after transfusion (%) was calculated by dividing the percentage of GFP-positive RBCs measured 24 hours after transfusion by the percentage of GFP-positive RBCs at Time 0. Fluorescence at Time 0 was extrapolated by linear regression from the data at 10 minutes, 30 minutes, and 1 hour.
Biochemical assays 2 hours after transfusion
After transfusion of FRBCs or SRBCs, mice were killed at 2 hours with an intraperitoneal injection of pentobarbital (200 mg/kg). Approximately 1 mL of blood was withdrawn from each mouse by cardiac puncture into a heparinized syringe and centrifuged at 2700 × g, 4°C for 8 minutes. Heparinized plasma was obtained to measure blood chemistry, as well as interleukin-6 (IL-6), haptoglobin (Hp), and hemopexin (Hx) levels. Serum samples were obtained for the measurement of iron levels. Liver specimens were snap-frozen in liquid nitrogen for subsequent determination of heme oxygenase-1 (HO-1).
To compare the acute biochemical effects of transfusing FRBCs or SRBCs, we sacrificed 12 WT and 12 db/db mice at 10 minutes posttransfusion with an intraperito-neal injection of pentobarbital (200 mg/kg). Whole blood was obtained by cardiac puncture for blood chemistry measurements.
Measurement of serum iron levels
Serum iron levels were determined using an iron and unsaturated iron binding capacity assay (Thermo Fisher Scientific, Middletown, VA).
Measurement of plasma IL-6, Hp, and Hx levels
Plasma IL-6 levels were determined with a mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit (Duoset, R&D Systems, Minneapolis, MN). Plasma Hp and Hx levels were measured using mouse Hp and Hx ELISA kits, respectively (Life Diagnostics, Inc., West Chester, PA).
Quantification of tissue mRNA levels
Total mRNA was extracted from murine livers using reagent (Trizol, Invitrogen Life Technologies, Carlsbad, CA). cDNA was synthesized by the reverse transcriptase reaction (MMLV-RT, Invitrogen Life Technologies). Realtime amplification of transcripts was performed by the SYBR method using a thermal cycler (Mastercycler Realplex, Eppendorf, Hamburg, Germany). The relative expression of target transcripts was normalized to the levels of 18S rRNA and analyzed using the relative CT method. The sequences of primers used are listed in Table S1 (available as supporting information in the online version of this paper).
Noninvasive measurement of blood pressure in awake WT, HFD-fed WT, and db/db mice after transfusion of FRBCs or SBRCs
FRBCs, SRBCs, washed FRBCs and SRBCs, supernatants from FRBCs and SRBCs, and oxidized supernatant from SRBCs were transfused (total injectate volume was 10% of estimated blood volume) via a tail vein into WT, HFD-fed WT, or db/db mice. Systolic blood pressure (SBP) was measured with a noninvasive blood pressure system (XBP 1000, Kent Scientific, Torrington, CT).28,29 All tail vein injections were performed over 1 minute. The numbers of mice in each experimental group are reported in Table S2 (available as supporting information in the online version of this paper). One group of db/db mice received SRBCs while breathing 80 ppm NO in air (Medical-Technical Gases, Inc., Medford, MA) from 10 minutes before transfusion until 2 hours after transfusion using methods described previously.28
Invasive hemodynamic measurements in anesthetized mice
Invasive hemodynamic measurements were performed as described previously.29 Left ventricular pressure-volume loops and cardiac output, as well as mean arterial blood pressures, were measured at baseline and 5 minutes after transfusion with FRBCs, SRBCs, the supernatant from FRBCs, or the supernatant from SRBCs (10% of estimated blood volume, infused at a rate of 75 µL/min over 3 minutes through a separate catheter placed in the right jugular vein; please see Table S2 in the online supplement for the number of animals in each experimental group).
Statistical analysis
All values are expressed as mean ± SEM. Data were analyzed by a one-way analysis of variance (ANOVA) with Tukey adjustment (SigmaStat 3.0.1, Systat Software, Inc., San Jose, CA). SBP measurements in awake mice were analyzed by a repeated-measures two-way ANOVA with interaction. Probability values less than 0.05 were considered significant.
RESULTS
Characterization of stored murine blood
Since the storage of RBCs produces a host of biochemical changes, we examined these changes in the murine storage model. We compared FRBCs stored for not more than 24 hours with SRBCs stored for 2 weeks. In SRBCs, pH, PO2, HCO3−, and SBE were markedly decreased while PCO2 and lactate were greater than in FRBCs (Table 1). In addition, we observed that sodium levels were less and potassium levels were greater in SRBCs than in FRBCs. The level of hemolysis in SRBCs was markedly greater than that in FRBCs. Similarly, supernatant Hb levels were greater in SRBCs than in FRBCs. These results are consistent with the data of Makley and colleagues,31 who reported that, after prolonged storage (up to 35 days), murine RBCs exhibited a decreased pH and increased levels of potassium and supernatant Hb. Met-Hb levels measured in the supernatant of both FRBCs and SRBCs were less than 1% of the supernatant Hb (data not shown).
TABLE 1.
Comparison of blood chemistry in FRBCs (≤24 hr) and SRBCs (2 weeks)*
| Measure | FRBCs (n = 5) | SRBCs (n = 5) |
|---|---|---|
| pH | 7.06 ± 0.03 | 6.53 ± 0.03† |
| PCO2 (mmHg) | 16 ± 1 | 35 ± 4† |
| PO2 (mmHg) | 217 ± 9 | 140 ± 12† |
| HCO3− (mmol/L) | 4± 0 | 3 ± 0† |
| SBE (mmol/L) | −24 ± 0 | −30 ± 1† |
| Na+ (mmol/L) | 152 ± 0 | 137 ±1† |
| K+ (mmol/L) | 3.7 ± 0.4 | 34.4 ± 2.6† |
| Cl− (mmol/L) | 121 ± 1 | 120 ± 1 |
| Ca2+ (mmol/L) | 0.06 ± 0 | 0.05 ± 0 |
| Lactate (mmol/L) | 2.2 ± 0.3 | 18.3 ± 2.0† |
| Hb (g/dL) | 14.1 ± 1.6 | 12.9 ± 0.2 |
| Supernatant Hb (mg/dL) | 64 ± 6 | 571 ± 51† |
| Hemolysis (%) | 0.1 ± 0 | 0.8 ± 0† |
Values are mean ± SEM.
p < 0.01 differs versus FRBCs.
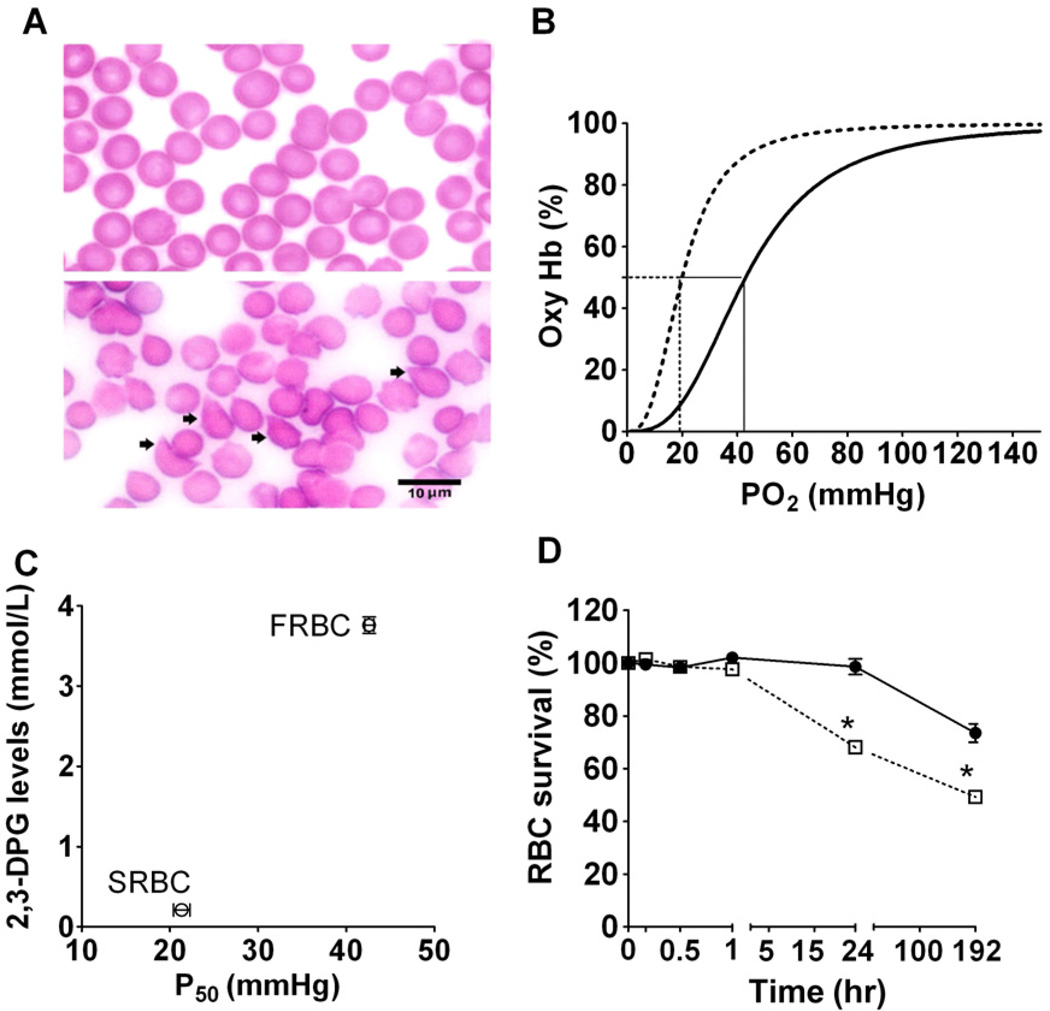
A previous study has shown that murine RBCs experience morphologic changes during storage;31 we therefore compared these changes in FRBCs with SRBCs. In FRBCs, cell morphology was dominated by discocytes (Fig. 1A, top panel). After storage for 2 weeks, the majority of RBCs were abnormally shaped (e.g., echinocytes or spheroechinocytes, as indicated by arrows in Fig. 1A, bottom panel). We observed storage-induced morphologic changes in murine RBCs that appear similar to those reported in stored human RBCs.31
Fig. 1.
Storage-induced morphologic, biochemical, and functional changes in murine RBCs in vitro. (A) Stained smears of FRBCs (top panel) and SRBCs (bottom panel); arrowheads in the bottom panel showing increased numbers of abnormally shaped RBCs after 2-week storage. (B) ODC of FRBCs (—) and SRBCs (- - -). P50, defined as the partial pressure of oxygen at which Hb is 50% saturated, was calculated from the ODC. The ODC of SRBCs was left-shifted with a P50 of 21 ± 1 mmHg; in contrast, the P50 of FRBCs was 43 ± 0 mmHg. (C) Comparison of changes in 2,3-DPG levels of FRBCs (n = 5) and SRBCs (n = 5) with P50 of FRBCs (n = 3) and SRBCs (n = 3). (D) FRBC (●; n = 6) or SRBC (□; n = 6) survival was determined by the percentage of GFP-labeled RBCs measured 24 hours after transfusion divided by the percentage of GFP-labeled RBCs at Time 0 in WT mice. *p < 0.01 differs versus FRBCs.
The primary function of the RBCs is to transport oxygen; thus, we measured the changes of Hb-oxygen affinity after RBCs storage. The ODC of SRBCs was markedly left-shifted, compared to FRBCs (21 ± 1 mmHg vs. 43 ± 0 mmHg, respectively; Fig. 1B, n = 5, p < 0.01). RBC 2,3-DPG levels decreased from 3.8 ±0.1 mmol/L in FRBCs to 0.2 ±0.1 mmol/L in SRBCs (Fig. 1C). Since oxygen affinity is modulated by the allosteric inhibitory binding effects of 2,3-DPG to Hb in mice, the reduction of 2,3-DPG with storage, similar to that observed in stored human RBCs, markedly augmented the oxygen affinity. Taken together, these results document that murine RBCs undergo biochemical, hematologic, and morphologic changes during the 2-week storage.
Impact of storage duration on RBCs survival after syngeneic transfusion
To track the survival of infused FRBCs and SRBCs, we studied the impact of storage on GFP-labeled RBCs. Twentyfour hours after transfusing GFP-labeled FRBCs, the fraction of GFP-labeled RBCs was 99 ± 3% of that calculated at Time 0 after infusion (Fig. 1D). Twentyfour hours after transfusing GFP-labeled SRBCs, the fraction of GFP-labeled RBCs was 68 ± 1% of that calculated at Time 0 after transfusion. Beginning 24 hours after transfusion, the rate of decrease in GFP-labeled RBCs was similar in FRBCs and SRBCs. These results are consistent with those reported by Gilson and colleagues30 and suggest that storage lesion seen in stored blood has a heterogeneous impact on RBCs.
Impact of FRBC and SRBC transfusion on Hct, Hb, and plasma Hb in WT, HFD-fed WT, and db/db mice
We assessed alterations of hematologic variables after transfusion, and we drew arterial blood samples from mice at 10 minutes (WT and db/db mice) and 2 hours (WT, HFD-fed WT, and db/db mice; Table S3, available as supporting information in the online version of this paper) after transfusion of FRBCs or SRBCs. Transfusion of either FRBCs or SRBCs increased Hct and total Hb levels at 10 minutes and 2 hours after transfusion in all studied mice. Transfusion with SRBCs, but not FRBCs, increased plasma Hb levels at 10 minutes and 2 hours after transfusion in all the groups of mice that we studied. These results indicate that increased plasma Hb levels after SRBCs transfusion is most likely due to the release of Hb during storage and after transfusion.
Serum iron levels after transfusion
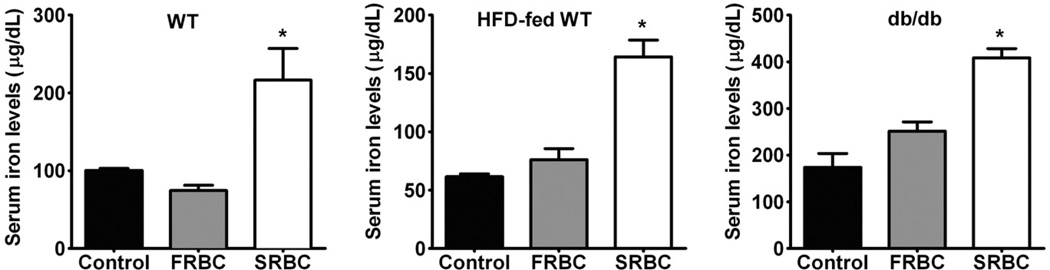
Since SRBCs have a shortened life span and are rapidly removed from the circulation, they could contribute iron from their heme to enhance the reactive oxygen species generation. In the current study, we measured serum iron levels in mice at 2 hours after transfusion of FRBCs or SRBCs (Fig. 2). Serum iron levels were greater at 2 hours after transfusion of SRBCs into WT, HFD-fed WT, and db/db mice, but not after transfusion of FRBCs. These results document that transfusion of SRBCs increases serum iron more than FRBC transfusion.
Fig. 2.
Serum iron levels 2 hours after transfusion with FRBCs or SRBCs in awake WT (A), HFD-fedWT (B), and db/db (C) mice. Control, no transfusion, n = 4/group; FRBCs, n = 6/group; SRBCs, n = 6/group. *p < 0.05 differs vs. control and FRBCs.
Inflammation after transfusion
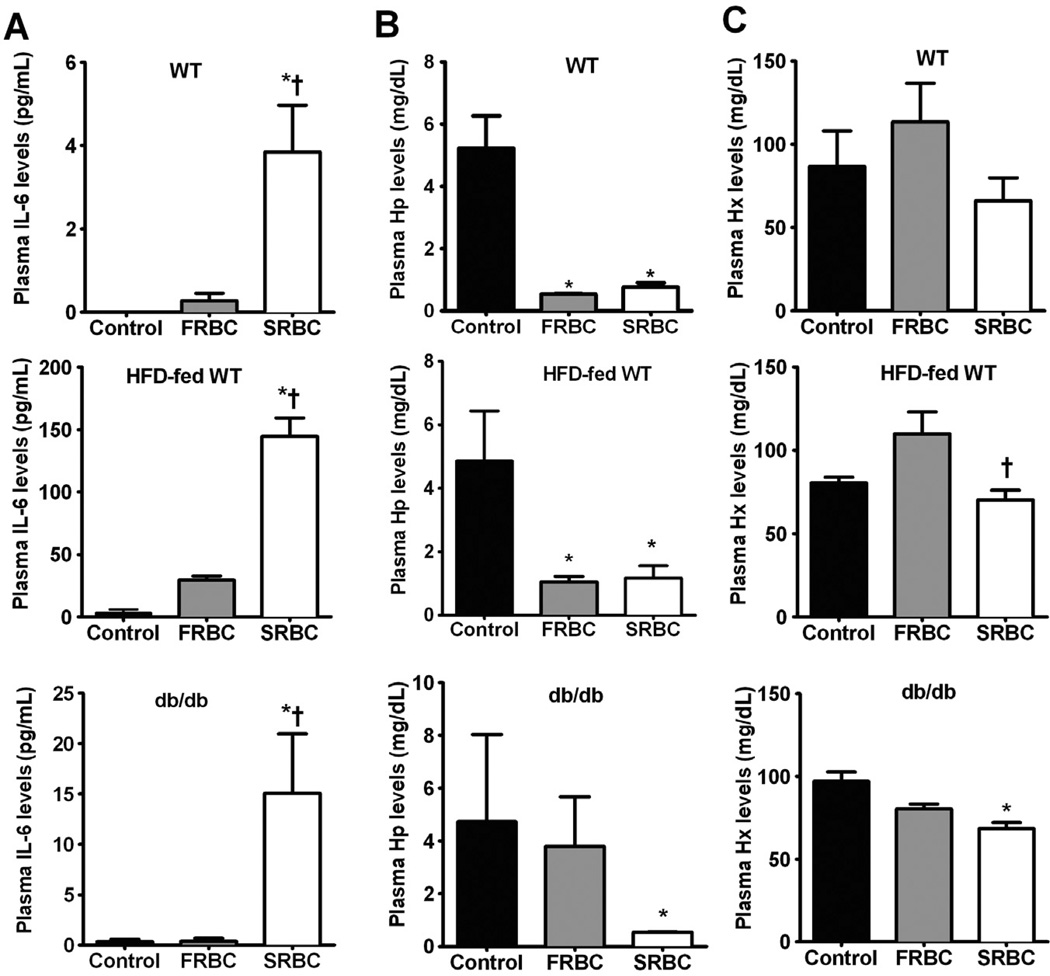
To learn whether transfusion of either FRBCs or SRBCs would induce a systemic inflammatory response in mice, IL-6 levels were measured in plasma samples collected from WT, HFD-fed WT, and db/db mice at 2 hours after transfusion (Fig. 3A). Transfusion of SRBCs, but not FRBCs, increased plasma IL-6 levels in WT, HFD-fed WT, and db/db mice (p < 0.05). Since approximately 97% of the white blood cells in stored cells were removed by leukoreduction and no endotoxin (e.g., LPS) was detected in the transfused samples, the increased IL-6 level is most likely a sequel of SRBC transfusion.
Fig. 3.
Plasma levels of IL-6 (A), Hp (B), and Hx (C) at 2 hours after transfusion of FRBCs or SRBCs in awake WT, HFD-fed WT, and db/db mice. Control, no transfusion, n = 4/group; FRBCs, n = 6/group; SRBCs, n = 6/group. *p < 0.05 differs versus control; †p < 0.01 differs versus FRBCs.
Plasma Hp, Hx, and hepatic HO-1 after transfusion
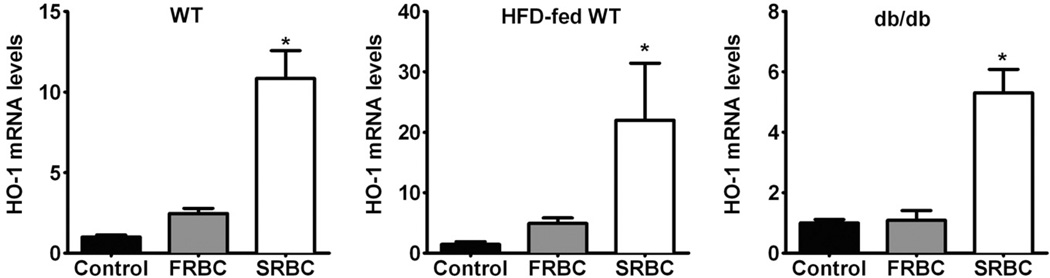
The primary protective mechanisms against extracellular Hb are often termed “antioxidant proteins,” such as Hp, Hx, and HO-1. Extracellular Hb binds to circulating Hp. Clearance of the Hb-Hp complex takes place within the circulation and the liver (macrophages), eventually leading to the breakdown of heme via HO-1.35 Hx serves as the primary carrier of plasma heme and participates in its clearance by transporting it to the liver.36 HO-1 is the inducible isoform of heme degradation and is protective against inflammatory injury.37 We measured increased levels of plasma Hb after SRBCs transfusion; therefore, we examined the impact of FRBC and SRBC transfusion on the mechanisms responsible for clearing plasma Hb. Plasma Hp levels decreased at 2 hours after either FRBC or SRBC transfusion (Fig. 3B). We noted that transfusion of SRBCs decreased plasma Hx levels at 2 hours in HFD-fed WT and db/db mice, but not in WT mice (Fig. 3C). Transfusion of FRBCs did not change Hx levels. At 2 hours after transfusion, we found that hepatic HO-1 mRNA levels were greater in WT, HFD-fed WT, and db/db mice after SRBC transfusion, but not after FRBC transfusion (Fig. 4, p < 0.05). These results suggest that since Hp levels at baseline are quite low in the mouse, the small amounts of Hb released in response to FRBC transfusion are sufficient to markedly reduce Hp levels, whereas the Hx levels are higher and were only modestly reduced at 2 hours after transfusion. Transfusion of SRBCs induced hepatic HO-1 mRNA levels due to increased heme released from SRBCs.
Fig. 4.
Changes of hepatic HO-1 mRNA levels at 2 hours after transfusion of FRBCs or SRBCs in awake WT (A), HFD-fed WT (B), and db/db (C) mice. Control, no transfusion, n = 4/group; FRBCs, n = 6/group; SRBCs, n = 6/group. *p < 0.05 differs versus control and FRBCs.
Hemodynamic sequelae of transfusing FRBCs and SRBCs
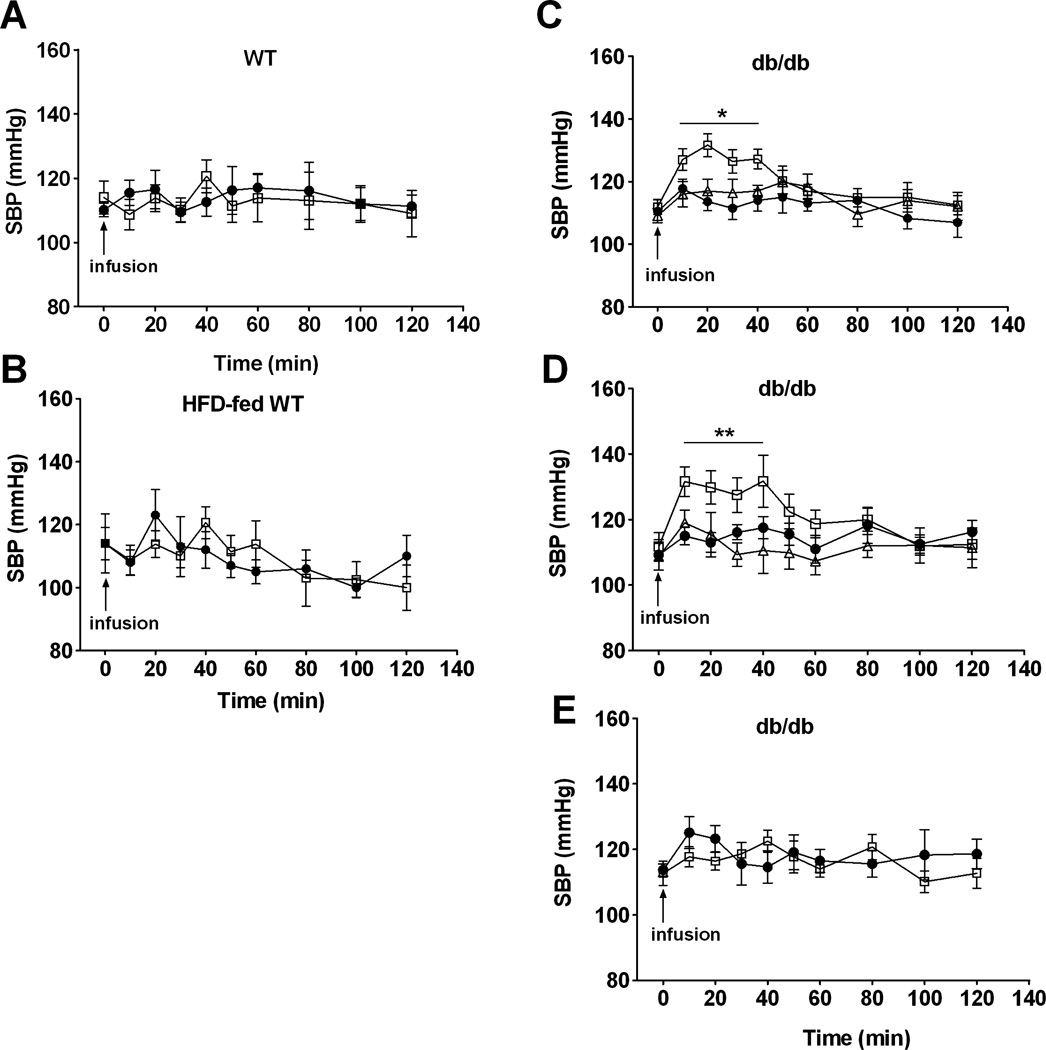
Our studies have shown that transfusing a blood substitute containing less than 1% of tetrameric Hb can produce systemic vasoconstrictor effects.29 To examine the effects of transfusing FRBCs and SRBCs on SBP, we administered FRBCs or SRBCs (as a 10% of blood volume injection) via a tail vein in awake mice. Transfusion of either FRBCs or SRBCs did not change SBP in awakeWT mice (Fig. 5A). In a recent study, we reported that mice with endothelial dysfunction (e.g., HFD-fed WT or db/db mice) are more sensitive to the vasoconstrictor effects of tetrameric Hb.29 In awake HFD-fed WT mice, transfusion of FRBCs or SRBCs did not change SBP (Fig. 5B). In contrast, transfusion of SRBCs, but not FRBCs, increased SBP in awake db/db mice (from 111 ± 2 mmHg at baseline to 127 ± 3 mmHg at 10 min, p <0.05, Fig. 5C). Since NO breathing prevented the systemic vasoconstriction induced by infusing tetrameric Hb in mice,28 we studied the ability of inhaled NO to prevent the vasoconstricting effects of transfusing db/db mice with SRBCs. We found that breathing 80 ppm NO beginning 10 minutes before transfusion and continuing for 2 hours thereafter completely prevented the systemic hypertension induced by the transfusion of SRBCs into db/db mice.
Fig. 5.
SBP (mmHg) after different types of transfusions (10% estimated blood volume) in awake WT, HFD-fed WT, or db/db mice. (A) Transfusion with FRBCs (●; n = 5) or SRBCs (□; n = 6) in awake WT mice. (B) Transfusion with FRBCs (●; n = 6) or SRBCs (□; n = 6) in awake HFD-fed WT mice. (C) Transfusion with FRBCs (●; n = 9) or SRBCs with (Δ; n = 12) or without (□; n = 9) breathing iNO (Δ; 80 ppm) in awake db/db mice. (D) Transfusion with supernatant from FRBCs (●; SFRBCs, n = 11), supernatant from SRBCs (□; SSRBCs, n = 7), or oxidized SSRBCs (Δ; n = 6) in awake db/db mice. (E) Transfusion with washed FRBCs (●; n = 9) or washed SRBCs (□; n = 6) in awake db/db mice. *p < 0.05 differs versus FRBCs and SRBCs plus iNO.
To investigate which component of SRBCs is responsible for its vasoconstricting effects, we transfused the supernatant obtained from SRBCs or washed SRBCs into awake db/db mice. Transfusing the supernatant of SRBCs (10% of estimated blood volume of db/db mouse38 or 250 µL, equivalent to four times the volume of supernatant given by transfusing 250 µL of SRBCs) increased systemic blood pressure (from 112 ± 4 to 132 ± 4 mmHg, p < 0.05), which lasted for 40 minutes (Figs. 5D and 5E).
To minimize hemolysis induced by washing, we found that suspension of SRBCs in 1.5 g% sodium chloride minimized acute hemolysis (Table S4, available as supporting information in the online version of this paper). The transfusion of washed SRBCs into db/db mice did not increase systemic blood pressure.
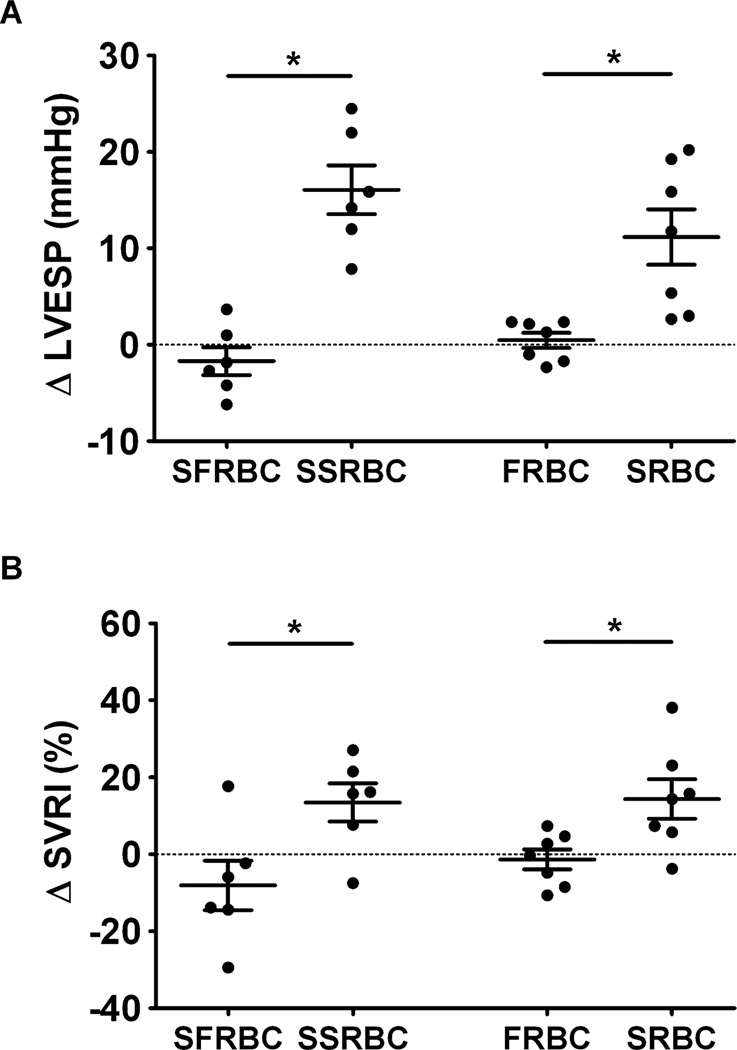
Invasive hemodynamic measurements obtained in anesthetized db/db mice confirmed our findings in awake db/db mice. Transfusion of SRBCs or supernatant from SRBCs increased left ventricular end-systolic pressure (LVESP; Fig. 6A) and systemic vascular resistance index (SVRI, Fig. 6B), whereas transfusion of FRBCs or supernatant from FRBCs did not alter these hemodynamic variables. After we oxidized the supernatant obtained from SRBCs by exposure to NO gas (thereby converting ferrous Hb to ferric Hb) with subsequent dialysis to remove low-molecular-weight components (e.g., nitrite and nitrate), transfusion into awake db/db mice did not increase their systemic blood pressure (Fig. 5D). These hemodynamic results suggest that infusion of the supernatant from SRBCs causes systemic vasoconstriction, which is most likely due to the infusion of supernatant Hb released during 2 weeks of storage.26,29
Fig. 6.
Comparison of changes in systemic hemodynamic measurements in anesthetized db/db mice before and after transfusion of supernatant of FRBCs (SFRBCs, n = 6), supernatant of SRBCs (SSRBCs, n = 6), FRBCs (n = 7) or SRBCs (n = 7). (A) Changes in LVESP (mmHg) before and 10 minutes after transfusion. (B) Changes in SVRI (%) before and 10 minutes after transfusion. *p < 0.05 differs versus either supernatant of FRBCs or the FRBC group
DISCUSSION
In this study, we report that in vitro storage of syngeneic murine RBCs for 2 weeks altered RBC morphology, increased levels of supernatant Hb (either free or in micro-particles), and decreased erythrocytic 2,3-DPG levels and P50. After transfusion, GFP-labeled SRBCs had a shorter life span than did GFP-labeled FRBCs. At 10 minutes and 2 hours after transfusion of SRBCs, plasma Hb levels were markedly greater than those after transfusion of FRBCs. Two hours after transfusion of SRBCs, both serum iron and plasma IL-6 levels increased. Plasma Hp levels decreased in response to both FRBC and SRBC transfusion (except in db/db mice) and Hx levels decreased only in HFD-fed WT and db/db mice transfused with SRBCs. SRBC transfusion increased systemic blood pressure and induced systemic vasoconstriction in db/db mice but not in WT or HFD-fed WT mice. Transfusing the supernatant from SRBCs, but not from FRBCs, induced systemic vasoconstriction in db/db mice. In contrast, transfusion of washed SRBCs or the oxidized supernatant obtained from SRBCs did not produce systemic vasoconstriction or increase blood pressure. Concomitant inhalation of 80 ppm NO prevented the systemic hypertension due to transfusion of SRBCs into db/db mice. Invasive hemodynamic measurements confirmed that transfusion of SRBCs into db/db mice increased LVESP and SVRI, but this did not occur with transfusion of either FRBCs or supernatant from FRBCs. Taken together, our findings suggest that ferrous Hb released into plasma scavenges NO and is the primary cause of the hypertension after transfusing SRBCs.
Gilson and colleagues30 as well as Hod and associates23 reported that after 14 days of storage in CPDA-1, murine RBCs had less than 1% in vitro hemolysis and an in vivo 24-hour RBC recovery of 64% to 65% measured with both GFP-labeled cells and 51Cr labeling. These values are similar to the data obtained from human blood stored for 42 days.3 In our study, we reproduced their RBC storage model, further characterized the biochemical and functional effects of storing murine RBCs, and investigated the hemodynamic and inflammatory sequelae of transfusing FRBCs and SRBCs in syngeneic mice.
It is worthwhile to note that we measured reduced levels of both 2,3-DPG and P50 in SRBCs. Winslow39 proposed an “autoregulation theory” suggesting that enhanced plasma O2 delivery by low-affinity cell-free Hb triggers arteriolar vasoconstriction. SRBCs and free Hb, both with a low P50 and high oxygen affinity, should produce less vasoconstriction than Hb in RBCs. However, our study provides evidence that the vasoconstriction induced by transfusing SRBCs is most likely due to NO dioxygenation by cell-free Hb and is not due to increased arteriolar oxygen delivery.
Oxidation of cell-free Hb can injure the host by exacerbating inflammation and by releasing cytotoxic free heme that can cause irreversible tissue damage and organ failure.35,40 In this study, an increase of both plasma Hb and serum iron levels was measured after SRBC transfusion. Hod and colleagues23 demonstrated a dramatic elevation of circulating proinflammatory cytokine levels after allogeneic transfusion of SRBCs (400 µL) or washed SRBCs into healthy WT mice and reported no increased inflammation after infusing either supernatant, stroma-free lysate, or ghosts of SRBCs.They also report the inflammatory response to infused allogeneic SRBCs was reduced by pretreatment with the iron chelator deferoxamine. They report similar results in WT mice after transfusing stored syngeneic RBCs (unpublished data). The more modest inflammation induced by transfusing SRBCs in our study may be attributable to a smaller transfusion volume or our syngeneic blood transfusion model. Moreover, we did not detect LPS in our FRBCs or SRBCs, suggesting that the inflammatory response induced by SRBC transfusion was not attributable to bacterial contamination of the blood during storage. The observation that SRBC transfusion induces an inflammatory response in the absence of immunologic differences between donor and recipient or LPS contamination suggests that the elevated plasma IL-6 levels measured after SRBC transfusion are due to transfusion of the stored RBCs and their supernatant.
Iron-derived reactive oxygen species can give rise to severe inflammation. Heme is an abundant source of reactive iron, and both heme and free iron can catalyze free radical reactions. Heme is hydrophobic and readily crosses cell membranes to increase oxidant damage to cells. It has been shown that prolonged storage (>17 days) of human RBCs produces significantly increased levels of nontransferrin bound iron in the supernatant.41 We measured an increase in serum iron levels 2 hours after SRBC transfusion, but not after FRBC transfusion.
Numerous cytoprotective mechanisms have evolved to protect against the toxicity produced by free Hb and free heme, including the Hb-binding protein, Hp; the heme-binding protein, Hx42–44; and the hememetabolizing enzyme, HO-1. Complexes of Hb-Hp or heme-Hx are transported to monocytes-macrophages and/or hepatic parenchymal cells, where the heme is metabolized by HO-1. In the mouse, protection against heme or Hb released during hemolysis is conferred by circulating Hx and Hp (although the level of Hp in mouse plasma is 20 times less than the human, the levels of Hx are nearly equal35). In addition, as a defense against oxidative stress, the vasculature and organs can up regulate the expression of HO-1.22 Catabolism of heme by HO-1 produces two anti-inflammatory products, carbon monoxide and biliverdin. In our study, a decrease of plasma Hp and Hx levels (except in WT mice), and an increase of HO-1 mRNA levels was measured 2 hours after SRBCs transfusion. Hp and Hx may partially prevent the toxicity of free Hb or heme by producing complexes of Hb-Hp or heme-Hx. The increase of hepatic HO-1 after SRBC transfusion is most likely due to its induction by heme released from SRBCs in hepatic macrophages.37
Our recent studies report that mice with endothelial dysfunction (e.g., WT mice fed a HFD or db/db mice) showed markedly enhanced vasoconstrictor responses to the NO-scavenging effects of infusing tetrameric Hb or Hb-based oxygen carriers.29 Similarly, in this study, we observed that SRBC transfusion–induced hypertension in db/db mice, but not in WT mice (with or without fat-feeding).We reasoned that mice with endothelial dysfunction are more vulnerable to the vasoconstrictor effects of extracellular Hb released from SRBCs. Because we observed that breathing NO prevented the vasoconstriction induced by SRBC transfusion, and that induced by transfusing supernatant from SRBCs, we proposed that SRBC-induced vasoconstriction was attributable to the cell-free Hb in the SRBC supernatant. Although we did not observe systemic hypertension in HFD-fed WT mice after SRBC transfusion, we speculate that db/db mice are more sensitive to the vasoconstricting effects of SRBC transfusion because they have more marked endothelial dysfunction than do HFD-fed WT mice. On the other hand, it remains possible that mechanisms other than NO scavenging by supernatant Hb may account for the ability of SRBCs to increase blood pressure in db/db mice.
We previously reported that inhaled NO, a safe method for administering NO, prevented the vasoconstriction caused by infusing tetrameric Hb.28 We therefore performed studies designed to test whether NO breathing could prevent the hypertension induced by transfusing SRBCs. An important finding of this study is that concurrently breathing 80 ppm NO prevented the systemic hypertension induced by transfusion of SRBCs into db/db mice. As others have shown in dogs,45 breathing NO at 80 ppm rapidly oxidizes plasma oxyhemoglobin (oxy-Hb) to met-Hb, which is unable to scavenge NO. In our prior studies, we also showed that pretreatment with inhaled NO prevented vasoconstriction induced by subsequent infusion of tetrameric Hb.28 Thus, we believe that breathing NO both oxidizes circulating oxy-Hb to met-Hb and loads the body’s stores with bioactive NO-containing molecules (e.g., nitrite and S-nitroso-Hb),46 thereby preventing the systemic vasoconstriction caused by SRBCs infusion in db/db mice. Although high doses (80 ppm) of inhaled NO oxidize and inactivate plasma Hb, the oxygen-carrying capacity of erythrocytic Hb is preserved by met-Hb reductase.
In this study, hemolysis occurred when murine RBCs were stored for 2 weeks with accumulation of extracellular Hb in supernatant to levels ninefold greater than that detected in the supernatant of blood stored for less than 24 hours. At both 10 minutes and 2 hours after transfusion of SRBCs, an increase in plasma Hb levels was detected. The increase in plasma Hb levels after SRBC transfusion may be attributable to both supernatant Hb released during storage as well as in vivo lysis of injured SRBCs after transfusion. One might expect with massive transfusion that persistent hemoglobinemia due to destruction of stored RBCs would produce sustained NO scavenging and vasoconstriction. It has been reported in a two-dimensional blood vessel model that a level of cell-free plasma Hb as lowas 1.61 mg/dL can limit NO bioavailability by scavenging NO.47 After transfusion of SRBCs in mice, the plasma Hb levels were well above this threshold. Most recently, Donadee and colleagues26 reported that after 42 days of storage of human RBCs, approximately 15% of supernatant Hb is contained in microparticles encapsulating Hb within a lipid cell membrane. Both free Hb and to a lesser extent encapsulated Hb can scavenge NO via the dioxygenation reaction and contribute to decreased NO bioavailability. When we oxidized the supernatant by NO exposure, it no longer produced vasoconstriction upon infusion into db/db mice.Thus, it is likely that transfusion of ferrous Hb in the supernatant of SRBCs caused systemic hypertension in highly sensitive db/db mice and that rapid destruction of transfused SRBCs contributed to the persistent elevation of plasma Hb at 2 hours.
If observations in mice can be extrapolated to human beings, caution should be taken when transfusing stored RBCs into patients with diabetes or other diseases inducing endothelial dysfunction (e.g., cardiovascular diseases such as atherosclerosis). Utilization of “younger” RBCs should be considered when patients with endothelial dysfunction require transfusion. The ability of supplementation of vascular NO levels with an NO donor compound or inhaled NO (to avoid systemic hypotension) to reduce the noxious effects of transfusing SRBCs in high-risk patients merits additional investigation.
In conclusion, we report that murine RBCs undergo significant biochemical, morphologic, and functional changes after 2 weeks of storage. Transfusion of SRBCs raised serum iron levels and induced a mild inflammatory response. Transfusion of SRBCs or supernatant from SRBCs caused systemic vasoconstriction and hypertension in db/db mice, which was prevented by breathing NO. Transfusing washed SRBCs or the oxidized supernatant obtained from SRBCs did not cause vasoconstriction in db/db mice. Thus, mice with endothelial dysfunction (db/db mice) are sensitized to the adverse hemodynamic effects of transfusing SRBCs. Transfused ferrous oxy-Hb (either free Hb or Hb in microparticles) released into the supernatant from RBCs during storage appears to be responsible for the vasoconstriction produced in db/db mice. Our current data support a possible link between the RBC storage lesion, Hb and heme release, and elevated iron levels in producing cardiovascular and inflammatory changes in highly susceptible recipients (e.g., db/db mice).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Ryan Carroll for his assistance in preparing stained murine RBC smears and Ms Fatima Y. Sammy for her assistance in measuring LPS levels in FRBCs and SRBCs. The authors thank Drs Rajeev Malhotra and Hui Zheng for statistical assistance.
This work was supported by Eleanor and Miles Shore 50th Anniversary Fellowship (217249) to BY at Harvard Medical School and by grants from the National Natural Science Foundation of China to CL (81000232) and from the NHLBI (R01 DK082971) to KDB.
ABBREVIATIONS
- db/db
diabetic (mice)
- FRBC(s)
fresh leukoreduced murine red blood cells (stored ≤24 hr)
- GFP
green fluorescent protein
- HFD
high-fat diet
- HO-1
heme oxygenase-1
- Hp
haptoglobin
- Hx
hemopexin
- LPS
lipopolysaccharide
- LVESP
left ventricular end-systolic pressure
- met-Hb
methemoglobin
- ODC(s)
oxygen dissociation curve(s)
- oxy-Hb
oxyhemoglobin
- SBE
standard base excess
- SBP
systolic blood pressure
- SRBC(s)
stored leukoreduced murine red blood cells (stored for 2 weeks)
- SVRI
systemic vascular resistance index
- WT
wild type
Footnotes
CONFLICT OF INTEREST
WMZ receives royalties from patents on inhaled nitric oxide licensed by Massachusetts General Hospital to Linde Corp., Munich, Germany, and Ikaria, Inc., Clinton, NJ. KDB has received grants from Ikaria, Inc., to study inhaled nitric oxide. WMZ and BY have applied for patents on inhaled nitric oxide and blood transfusion. The remaining authors report no conflicts of interest.
REFERENCES
- 1.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–426. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 2.Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci. 2010;43:95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–88. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 6.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. discussion 716-7. [DOI] [PubMed] [Google Scholar]
- 7.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg JA, McGwin G, Jr., Griffin RL, Huynh VQ, Cherry SA, 3rd., Marques MB, Reiff DA, Kerby JD, Rue LW., 3rd. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282-4. [DOI] [PubMed] [Google Scholar]
- 9.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 10.Basran S, Frumento RJ, Cohen A, Lee S, Du Y, Nishanian E, Kaplan HS, Stafford-Smith M, Bennett-Guerrero E. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103:15–20. doi: 10.1213/01.ane.0000221167.58135.3d. table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71:781–785. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- 12.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 13.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 14.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–1718. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 15.Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, Marin-Caballos A, Amaya-Villar R, Marin A, Puppo-Moreno A, Ferrandiz-Millon C, Flores-Cordero JM, Murillo-Cabezas F. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 17.Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, McClelland DB. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 18.Hebert PC, Chin-Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, Olberg B. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–1438. doi: 10.1213/01.ANE.0000148690.48803.27. table of contents. [DOI] [PubMed] [Google Scholar]
- 19.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Bommel J, de Korte D, Lind A, Siegemund M, Trouw-borst A, Verhoeven AJ, Ince C, Henny CP. The effect of the transfusion of stored RBCs on intestinal microvascular oxygenation in the rat. Transfusion. 2001;41:1515–1523. doi: 10.1046/j.1537-2995.2001.41121515.x. [DOI] [PubMed] [Google Scholar]
- 21.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 22.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 23.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16:515–523. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 25.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 26.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–594. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makley AT, Goodman MD, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Murine blood banking: characterization and comparisons to human blood. Shock. 2010;34:40–45. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novitsky TJ, Roslansky PF, Siber GR, Warren HS. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985;21:211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamel N, Goubran F, Ramsis N, Ahmed AS. Effects of storage time and leucocyte burden of packed and buffycoat depleted red blood cell units on red cell storage lesion. Blood Transfus. 2010;8:260–266. doi: 10.2450/2009.0131-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood aging, safety and transfusion: capturing the “Radical” menace. Antioxid Redox Signal. 2010;14:1713–1728. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 36.Gutteridge JM, Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988;256:861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immenschuh S, Baumgart-Vogt E, Mueller S. Heme oxygenase-1 and iron in liver inflammation: a complex alliance. Curr Drug Targets. 2010;11:1541–1550. doi: 10.2174/1389450111009011541. [DOI] [PubMed] [Google Scholar]
- 38.Yen TT, Stienmetz J, Simpson PJ. Blood volume of obese (ob-ob) and diabetic (db-db) mice. Proc Soc Exp Biol Med. 1970;133:307–308. [PubMed] [Google Scholar]
- 39.Winslow RM. Current status of blood substitute research: towards a new paradigm. J Intern Med. 2003;253:508–517. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 40.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001118. 51ra71. [DOI] [PubMed] [Google Scholar]
- 41.Marwah SS, Blann A, Harrison P, Lumley MA, Wright J, McDowell J, Phillips JD, Rea C, Bareford D. Increased non-transferrin bound iron in plasma-depleted SAG-M red blood cell units. Vox Sang. 2002;82:122–126. doi: 10.1046/j.1423-0410.2002.00153.x. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood. 2009;114:764–771. doi: 10.1182/blood-2009-01-198309. [DOI] [PubMed] [Google Scholar]
- 43.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Anti-oxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 44.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, Feelisch M, Zapol WM. Brief periods of nitric oxide inhalation protect against myocardial ischemia-reperfusion injury. Anesthesiology. 2008;109:675–682. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.