Abstract
There is a growing scientific agreement that the cellular redox regulators such as antioxidants, particularly the natural polyphenolic forms, may help lower the incidence of some pathologies, including metabolic diseases like diabetes and diabesity, cardiovascular and neurodegenerative abnormalities, and certain cancers or even have anti-aging properties. The recent researches indicate that the degree of metabolic modulation and adaptation response of cells to reductants as well as oxidants establish their survival and homeostasis, which is linked with very critical balance in imbalances in cellular redox capacity and signaling, and that might be an answer the questions why some antioxidants or phytochemicals potentially could do more harm than good, or why some proteins lose their function by increase interactions with glyco- and lipo-oxidation mediates in the cells (carbonyl stress). Nonetheless, pursue of healthy aging has led the use of antioxidants as a means to disrupt age-associated physiological dysfunctions, dysregulated metabolic processes or prevention of many age-related diseases. Although it is still early to define their exact clinical benefits for treating age-related disease, a diet rich in polyphenolic or other forms of antioxidants does seem to offer hope in delaying the onset of age-related disorders. It is now clear that any deficiency in antioxidant vitamins, inadequate enzymatic antioxidant defenses can distinctive for many age-related disease, and protein carbonylation can used as an indicator of oxidative stress associated diseases and aging status. This review examines antioxidant compounds and plant polyphenols as redox regulators in health, disease and aging processes with hope that a better understanding of the many mechanisms involved with these distinct compounds, which may lead to better health and novel treatment approaches for age-related diseases.
Keywords: Carbonyl stress, aging, phytochemicals, redox regulators, vitamin, diseases
Unmitigated stress as senescent factor
In living systems, the cells are exposed to environmental and endogenous stressors, which are initiated and promoted by physical, chemical, and biological stimuli triggering a series of events in order to counteract, adapt and survive. To maintain homeostasis, the cells are required to rapidly respond in a manner that will allow for redox balance, recycling of antioxidants, clearance of abnormal proteins and remodeling [1]. There are some main pathways of stress response intrinsic to cells including heat shock response, unfolded protein response, autophagy, antioxidant response, inflammatory response and DNA repair response [2]. Any deficit in the ability of the cell to perform these functions would have significant impact on the state of health of the tissues [3]. For instance, brain cells have a particularly high basal level of metabolic activity and use distinct oxidative damage repair mechanisms to remove oxidative damage from DNA, and accumulation of this damage in the background of a functional DNA repair response is associated with normal aging, but defective repair in brain cells can contribute to neurological dysfunction [4, 5]. On the other hand, dysfunctional ROS-induced DNA damage response is mediating to early asymptomatic stages of calcific aortic valve disease, and may be reversible by antioxidant enzymes delivery [6]. Similarly, chronic obstructive pulmonary disease reflects the exhausted response of respiratory tract to external oxidants like oxygen, pollutants, toxicants and cigarette smoke and characterized by chronic inflammation and airflow limitations due to the increased systemic oxidative stress [7].
Aging is a multifactorial process that depends on diverse molecular and cellular mechanisms, such as protein availability, genome maintenance and inflammation [8]. Proteins are among the main targets for oxidants due to their high rate constants for several reactions with reactive oxygen species (ROS) and their abundance in biological systems. The interactions of proteins with ROS may result in numerous post-translational, reversible or irreversible modifications, which may lead to a change in the structure and/or function of the oxidized protein. The level and the type of protein damage have an important for the maintenance of viability since most protein damage is non-repairable, and has deleterious consequences on protein structure and function. In addition, damaged and modified proteins can form cross-links and provide a basis for many senescence-associated alterations and may contribute to a range of human pathologies [9, 10]. Protein damage leading to the formation of carbonyl groups derives from direct oxidation of several amino acids side chains but can also derive through protein adducts formation with lipid peroxidation products and dicarbonyl glycating compounds. All these modifications have been implicated during oxidative stress, aging and age-related diseases [11]. The degrading systems as proteolytic systems and the lysosomal system provide a last line of antioxidative protection, removing irreversible damaged proteins and recycling amino acids for the continuous protein synthesis. It is now well known that during aging, both systems are affected and their proteolytic activity declines significantly [9, 12]. On the other hand, during aging, the continuous pressure on the immune system caused by repeated antigen stimulation, leads to an increase in activated cells and secretion of pro-inflammatory cytokines, such as TNFα, IL-6 [13]. These circulating pro-inflammatory factors may keep the immune system in a state of chronic low-level activation, a phenomenon described as “inflammaging” [14]. Eventually, this causes “immunosenescence,” that is, an age-related decline in the capacity of adaptive immunity, consisting of more specific responses carried out by B and T cells [15]. Shorter telomere length is also associated with advancing chronological age and also increased disease morbidity and mortality. Emerging studies suggest that redox stress accelerates the erosion of telomeres from very early in life and possibly even influences the initial (newborn) setting of telomere length [16]. Telomeres are shortened by each cell division until a critical length is reached and dysfunction ensues. DNA-repair pathways are then recruited and cells enter senescence, losing their capacity to proliferate. In addition to cell division, factors causing telomere shortening include DNA damage, inflammation, and oxidized protein accumulation [17, 18]
Nuclear enzyme poly(ADP-ribose) polymerase 1 (PARP1) represents an interesting player in several aging mechanisms and is discussed as a longevity assurance factor. There is a large body of evidence showing a positive correlation of poly(ADP-ribosyl)ation capacity and mammalian longevity. [12, 19]. In other side, the treatment with rapamycin, an inhibitor of mammalian target of rapamycin complex 1 (mTORC1) increases mammalian life span via increasing hepatic gluconeogenesis concomitant with increase insulin insensitivity [20], while PI3K-AKT-mTOR-ROS pathway is necessary for CKII inhibition-mediated cellular senescence in colon cancer [21].
Impaired redox homeostasis and diseases development
Aging has been defined as a progressive postmaturational decline in physiological capacity, accompanied by an increased susceptibility to disease and an increased mortality risk. A mounting body of evidence suggests that increased production of ROS-mediated damages is linked to aging processes and to the etiopathogenesis of aging-related diseases, such as cancer, diabetes, atherosclerosis and neurodegenerative diseases like Parkinson’s and Alzheimer’s.
The most of the research on aging focus on structural and functional alterations of the brain during aging, age-associated imbalances of defenses against oxidative stress and age-related alterations of the metabolism of extracellular amyloid beta plaques, because today, around 24 million people suffer from dementia and with aging of industrial populations this number will significantly increase throughout the next decades [22]. AGEs/ALEs modifications may explain many of the neuropathological and biochemical features of Alzheimer’s diseases such as extensive protein cross-linking, inflammation, oxidative stress and neuronal cell death [23]. In Parkinson’s disease, telomere is shorter in the presence of high oxidative stress as measured by carbonyl protein levels [24]. With age, senescent cells accumulate and express a senescence-associated secretory phenotype that is the robust secretion of many inflammatory cytokines, growth factors and proteases. On the basis of recently reported findings, authors propose that environmental stressors associated with Parkinson’s disease may act in part by eliciting senescence and the senescence-associated secretory phenotype within non neuronal glial cells in the aging brain, thus contributing to the characteristic decline in neuronal integrity that occurs in this disorder [25]. In prostate cancer, oxidative damage, an innate key event characterized by supraphysiological ROS concentrations, has been identified as one of the hallmarks of the aggressive disease phenotype [26]. Age-specific mortality rates from cardiovascular diseases and strokes increase with age throughout the later years of life [27, 28]. It was found that the content of lipofuscin (advanced cross-linked carbonyl protein adduct) is positively correlated with mitochondrial damage as well as with the production of ROS and associated with cardiovascular decline [29, 30]. Structural remodeling of dilated atria manifested as intracellular accumulation of fibrillar aggregates, lipofuscin, signs of myolysis and autophagy [31]. Large studies demonstrated that in the absence of other risk factors, aging per se causes the development of atherosclerosis [32]. Aging is also an independent factor associated with endothelial dysfunction even in the absence of other cardiovascular risk factors such as hypertension, diabetes mellitus, hypercholesterolemia, cigarette smoking or a sedentary lifestyle, as well as genetic factors [33, 34]. In addition, age-associated ocular morbidity, including macular degeneration, diabetic retinopathy and cataract has been linked with increased carbonyl stress [35, 36]. Skin aging is another complex biological process influenced by a combination of endogenous or intrinsic and exogenous or extrinsic factors, which trigger oxidative degeneration in dermal cell [37,38]. Many hormone levels such as dehydroepiandrosterone, insulin-like growth factor 1 fall with age in men and women, (reaching sometimes values as low as 10–20% of those encountered in young individuals), that is accompany to general failure [39, 40]. Carbonyl stress due to unmitigated hyperglycemia is important factor in complications development like cardiomyopathy, vasculopathy, neuropathy, nephropaty in diabetics captive with accelerated aging [41, 42, 43, 44]. Older adults with diabetes and altered glucose status likely represent a subset of the population at high risk for complications and adverse geriatric syndromes [45].
Cellular response to carbonyl stress in aging and age-related pathologies
Aging is a complex process which cannot be explained by a single pathway or even a set of closely related pathways. Redox biochemistry influences on most of cellular processes and has been shown to underpin aging and many human diseases. Integrating the complexity of redox signaling and regulation is perhaps one of the most challenging areas of biology [46]. Protein oxidative modifications, also known as protein oxidation, are a major class of protein posttranslational modifications. They are caused by reactions between protein amino acid residues and reactive oxygen species (ROS) or reactive nitrogen species (RNS) and can be classified into two categories: irreversible modifications and reversible modifications. Protein oxidation has been often associated with functional decline of the target proteins, which are thought to contribute to normal aging and age-related pathogenesis [9, 47]. It has now been recognized, however, that protein oxidation can also play a positive role in many cellular functions. This gradual realization of the beneficial roles of protein oxidation may be attributed to accumulating evidence that ROS and RNS are indispensible for cell survival and regeneration, and in many cases, they are required for recovery of cellular functions by creating positive stress conditions whereby cell survival mechanisms are reprogrammed to extend life span or to counteract severe, or lethal challenges[47, 48, 49]. Apparently, the adaptation to the increased oxidant or reductant status is important to cell survival and the cellular redox homeostasis is essential to maintain a normal long life.
Proteins can be modified by lipids as well as carbohydrates. Advanced glycation end products (AGE) formation has initially been described as a non-enzymatic reaction between proteins and glucose in the so-called Maillard reaction, but they are also more rapidly formed during oxidative stress and subsequent formation of reactive carbonyl compounds such as formaldehyde, acetaldehyde, acrolein, crotonaldehyde, glyoxal, methylglyoxal, glycolaldehyde, glycidaldehyde [50, 51]. Glyco-oxidation is a term used for glycation processes involving oxidation, and AGEs like pentosidine accumulate in tissue where they cross-link with proteins, e.g., collagen, inducing stiffness in arterioles and skin. They may also interact with receptor of AGE (RAGE) and other receptors, which lead to activation of intracellular transduction mechanisms resulting in cytokine release and further tissue damage in many diseases[11, 52–55]. During oxidative stress, reactive oxygen species also attack to polyunsaturated fatty acids (PUFAs) either in the cell membrane or circulating lipoprotein molecules. This oxidative decomposition of PUFAs initiates chain reactions that lead to the formation of a variety of reactive carbonyl species. Among them is 4-hydroxy-trans-2-nonenal (4-HNE), which is subsequently react by the so-called Michael addition mechanism with cysteine, histidine and lysine residues in proteins, generating relatively stable adducts known as advanced lipid peroxidation end products (advanced lipo-oxidation end products ALEs) [52, 53, 56]. Reactive carbonyl compounds (RCCs) may induce “carbonyl stress” characterized by the increased formation of adducts and cross-links on proteins. The growing number of evidence have demonstrated that carbonyl stress associates the promotion of cytotoxic events such as cell growth arrest, mitochondrial dysfunction, apoptosis, and necrosis by modifying cellular proteins and nucleic acids, contributing to the dysfunction and damages in tissues and to the progression of diseases [52, 53, 57, 58, 59, 60].
Serum AGEs (N(epsilon)-carboxymethyl-lysine [CML] or methylglyoxal [MG] derivatives) have been found higher in older compared to younger persons [61]. It has been reported that a certain subtype of schizophrenic patients exhibit idiopathic carbonyl stress with high plasma pentosidine levels and the depletion of vitamin B6, without underlying diabetes or chronic kidney disease, the two major causes of elevated AGEs [62]. In addition to brain [34, 63], there are many reports indicating increased advanced oxidative and glyco-oxidative protein damage markers in kidney [64, 65], heart [57, 34, 66], pancreas [67], aorta [68, 69],liver [70]and serum [71]by aging or accelerated aging models such as diabetes. Advanced oxidation protein products (AOPPs) represent dityrosine-containing cross-linked protein modifications formed mainly via myeloperoxidase reaction, has been demonstrate to accelerate the uremia-associated atherogenesis and renal fibrosis in children and adolescents [72]. Increased nitroxidative stress causes mitochondrial dysfunctions through oxidative modifications of mitochondrial DNA, lipids, and proteins, and the persistent mitochondrial dysfunction has been suggested to contribute to the development of alcoholic and nonalcoholic fatty liver disease [73]. It has been reported that the increased level of serum or tissue advanced glycation or glyco-oxidation end products (AGEs) is a risk factor for the production of colorectal cancer [74] and impairment of cognitive and memory functions [75, 76], associate with insulin resistance [77] and promote diabetic complications [78] such as diabetic peripheral sensory neuropathy [79].They also atherosclerotic risk markers in prediabetic and diabetic elderly subjects [71], markers for ocular complications in in vitro and in vivo models of human age-related eye diseases, such as cataracts [80]. Inhibition of ROS signaling pathways or blockade of sources of ROS in the pulmonary vasculature, targeting in particular Nox enzymes represent promising new therapeutic strategies in pulmonary hypertension [81]. AGEs and RAGE-mediated ROS production are involved in vascular aging. Proteins of the extracellular matrix and vascular basement membrane have longer half-lives and they thus accumulate AGEs to a greater extent [82]. Cross-linking of collagen and other proteins by AGEs/ALEs in the arteries increased the stiffness of the vasculature leading to changes in contractile mechanics of the vasculature [83].
In fact, the physiological levels of ROS are viewed as signaling molecules acting as redox signals, and their harmful effects are seen mostly as a result of shaded signaling, rather than due to direct damage to sensitive targets. According to this view, ROS such as hydrogen peroxide and superoxide are not just causative agents of aging but may also be agents that increase the life span by acting, for example, as prosurvival signals [2, 47, 84]. ROS have crucial roles in redox regulation of protein phosphorylation, ion channels, and transcription factors. ROS are also required for biosynthetic processes, including thyroid hormone production, cross-linking of extracellular matrix [85] and insulin release from pancreatic beta-cells [86, 87]. In pancreatic β-cells evidence is emerging that acute and transient glucose-dependent H2O2 contributes to normal glucose-stimulated insulin secretion. However, chronic and persistent elevation of H2O2, resulting from inflammation or excessive metabolic fuels such as glucose and fatty acids, may elevate antioxidant enzymes such that they blunt H2O2-inducedphysiologically redox signaling, thus impairing β-cell function [86, 87, 88, 89]. In association with this, the balance between the oxidized (NAD+) and the reduced (NADH) forms of nicotinamide adenine dinucleotidesis critical for the cell’s proper function and ultimately, for its survival. A decrease in the NAD+/NADH ratio causes these enzymes to decrease in activity (reductive stress), resulting in an altered metabolic situation that has been suggested recently be the first insult toward several pathologies, such as diabetes [90].
Vitamins as cellular redox regulators
Mammalian cells may induce several mechanisms to protect themselves against the detrimental effects of carbonyl stress. The effects of ROS are diminished by enzymatic antioxidants and by non-enzymatic dietary antioxidants (water- and lipid-soluble) that accumulate within the cells [91, 92, 93]. Enzymatic antioxidant defense systems include enzymes such as superoxide dismutase (SOD), catalase, glutathione dependent enzymatic system, existing within the cell to neutralize free radicals [94, 95]. It is believed that these enzymes are induced to protect cellular functions which continue in vivo homeostasis [96, 97]. On the other hand, nonenzymatic defenses contains water-soluble agents like glutathione, ascorbic acid, uric acid, plasma proteins and lipid-soluble agents like α-tocopherol, β-carotene, bilirubin, ubiquinone, isoflavones [92, 94, 98, 99]. Some of the dietary antioxidants like α-tocopherol, ascorbic acid and β-carotene neutralize radicals outside the cell [94]. Most carotenoids can be metabolized to vitamin A and have antioxidant activity especially against singlet oxygen. β-carotene is the most prominent member of the group of carotenoids, natural colorants that can be found in the human diet [100]. β-carotene supplementation can significantly reduce the rate of mitochondrial mutation in human dermal fibroblasts after UV irradiation [101]. A recent meta-analysis suggested that higher intake of carotenoids (beta-carotene, alpha- carotene, lycopene, beta-cryptoxanthin, lutein, and zeaxanthin) is associated with lower risk of esophageal cancer [102]. The results of another recent study showed that there is a significant inverse association between pancreatic cancer and nutrient/supplement groupings in a dose-dependent manner including magnesium, potassium, selenium, alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein and zeaxanthin, niacin, total alpha-tocopherol, total vitamin A activity, vitamin B6, and vitamin C [103].
Ubiquinone which is a small molecule (CoQ10) helps to generate energy for mitochondria by transporting electrons [104]. Coenzyme Q10 (CoQ10) is a fat-soluble, endogenous (synthesized by the body), vitamin-like substance that is mainly stored in the fat tissues of our body. Primary dietary sources of CoQ10 include oily fish (such as salmon and tuna), organ meats (such as liver), and whole grains. The amount of CoQ10 needed in human organism can be gained through a balanced diet; however in the market CoQ10 is available in several forms as a supplement, including soft gel capsules and tablets. As a fat-soluble substance it is better absorbed when taken with fat rich meals. The combined treatment of insulin with coenzyme Q10 has been shown to reduce lipid peroxidation, increase activity of antioxidant enzymes, total NO metabolites and bioavailability, decrease the level of atherogenic LDLP, normalize the elasticity of microvessels and modulate the elasticity of arterioles and capillaries in type 1 diabetic patients [105]. Q10 suppresses age-related inflammatory reactions and osteoclast differentiation by inhibiting oxidative stress in rats [106]. In another recent study indicates the protection of rat skeletal muscle fibers by either L-carnitine or coenzyme Q10 against statins toxicity mediated by mitochondrial reactive oxygen generation [107]. CoQ may improve spatial learning and attenuates oxidative damage when administered in relatively high doses and delayed until early senescence, after age-related declines have occurred. Thus, in individuals with age-associated symptoms of cognitive decline, high-CoQ intake has been suggested to be beneficial [108].
In this respect, Cod Liver Oil, is another studied antioxidant in our laboratory and mainly comprised of; omega-3 polyunsaturated fatty acids, eicosapentaenoic acid and docosahexaenoic acid as well as vitamin A, has been shown to improve redox homeostasis, endogenous antioxidant enzyme activities in the heart and blood vessels of diabetic models [109, 110]. Vitamin C, also named L-ascorbic acid, is water soluble, photosensitive and is the most important antioxidant in the hydrophilic phase. Vitamin C is not naturally synthesized by the human body and therefore adequate dietary intake of vitamin C is required and essential for a healthy human diet. Recent results indicate that ascorbic acid plays an important role in regulating the intracellular ROS levels in senescent cells and that the need for ascorbic acid is enhanced by cellular senescence [111]. The ascorbic acid synthesizing capacity declines over time to become a major factor in senescence-related diseases [112]. Alpha lipoic acid and vitamin E are other antioxidants in the focus of our laboratory. Alpha lipoic acid treatment of diabetic animals exerted low levels of superoxide and hydrogen peroxide, was able to reverse vessel antioxidative defense and improve hypertension [113, 114]. Alpha lipoic acid acts as a protective role on apoptotic cell death and suppress hyperglycaemia-induced apoptosis in rat cardiac tissue and down-regulates caspase-6, -8 and -9 activity, as well as expression levels of proapoptotic Bcl-2 proteins [115]. Dietary supplementation with N-acetyl cysteine, α-tocopherol and α-lipoic acid reduces the extent of oxidative stress and pro-inflammatory state in aged rat brain [116].The vitamin E complex is a group of 8 compounds called tocopherols. Tocopherol is a fat-soluble membrane bound and non-enzymatic antioxidant and consequently a free-radical scavenger especially of highly reactive singlet oxygen. Vitamin C and vitamin E act synergistically against UV-activated molecular oxidation. Protective role of vitamins E and C against oxidative stress caused by intermittent cold exposure has been reported in aging rat’s frontoparietal cortex [117]. Vitamin E administration produced significant improvement in red blood cell deformability and decrement in total oxidant status and oxidative stress index in aged rats with respect to young and aged control groups [118]. The average daily dietary and supplement intake of micronutrients (vitamin C, vitamin E, b-carotene, zinc, and folate) is associated with improved sperm DNA quality in older men [119]. In addition, there are a lot of data exist showing anti-cataract [120], anticancer [121] and anti-Alzheimer [122] effect of vitamin C or vitamin E which have proved that they are capable of preventing lipid peroxidation, thereby preventing the generation of free radicals. We showed that in diabetic animals, vitamin E prevents nerve conduction velocity impairment and increase the resistance to ischaemic conduction failure [123, 124], and also protect against retinal capillary basement membrane thickness and increment in vascular endothelial growth factor expression [125]. This vitamin was also able to reverse impaired sympathetic neurotransmission and abnormal vas deferens function [126], kidney dysfunction and abnormal microsomal Ca2+-ATPase activity [127] and leukocyte free radical generation in streptozotocin-diabetic rats [128].The functions of brain and aorta [129] as well as heart and liver [66, 130, 131] also protected by vitamin E through the inhibition of carbonyl stress-induced cellular destructions in diabetic rats.
Studies suggest that effects of UVA radiation, such as skin sagging or wrinkling can be prevented or at least minimized by topical or oral administration of astaxanthin, which is found in microalgae, yeast, salmon, trout, krill, shrimp, crayfish and crustacean [132]. Many other studies that tested oral vitamin D treatment showed the skin cancer prevention, which is linked to anti-aging effects [133]. In fact, laboratory investigations have now convincingly shown that vitamin D compounds protect the skin against the hazardous effects of various skin aging-inducing agents, including ultraviolet (UV) radiation [134]. Newly, the role of vitamin D has been revived regarding its potential advantage on delaying the aging process [135].
Phytochemicals in redox homeostasis and cell function
Undoubtedly, phytochemicals exhibit a wide array of physiological activities in humans. Ancient people had empirical knowledge that some plants and/or their extracts have great impact on health and disease regulation. For example, “Ayurveda” [136] and “Jamu” [137] recognized as a part of holistic, traditional medicine. In addition, the traditional uses many medicinal herbs including saffron and its pharmacological activities has been described Avicenna in Book II, Canon of Medicine (al-Qanun fi al-tib) and modern pharmacological findings on these compounds are compared with those mentioned in Avicenna’s monograph [138]. The evidence coming from the last 20 years research, showing that many dietary phytochemicals have exhibited pronounced bioactivities in a number of experimental models, increased attention to medicinal herbs again as phytotherapeutics [137]. In addition, a variety of epidemiological analysis have demonstrated that frequent ingestion of vegetables and fruits, which contain numerous phytochemicals, lowers the risk of onset of some diseases such as diabetes [89] and cardiovascular compliance [57, 109, 110]. However, the action mechanisms by which dietary phytochemicals show bioactivity still needs to be investigation and a fundamental question is why this class of chemicals has great potential for regulating health. As is well known that, the maintenance of redox signaling at physiological levels [88], protect and repair of biological proteins by molecular chaperones, such as heat shock proteins [57], and clearance of abnormal proteins by the ubiquitin-proteasome system [139] and autophagy [140] play central roles in health, some disease prevention, and longevity. Several recent studies have revealed that polyphenols, including curcumin (yellow pigment in turmeric), resveratrol (phytoalexin in grapes), oleuropein (olive leaf compound), quercetin (general flavonol in onions), isothiocyanates (present in cruciferous vegetables, such as broccoli and cabbage) and punicic acid (omega-5 fatty acids in pomegranate seed) are remarkable regulators of redox-dependent protein quality control systems, suggesting that their physiological and biological functions are exerted, at least in part, through activation of such unique mechanisms [88, 89, 140, 141].
Humans consume a wide range of foods, drugs, and nutraceuticals that are derived from plants and which modify the functioning of the nervous system, cardiovascular system and other organ functions and cellular signaling. Polyphenols are mostly found in fruits and plant-derived beverages such as fruit juices, tea, coffee, and red wine. Vegetables, cereals, chocolate and dry legumes are also sources for the total polyphenol intake [142]. Depending on the number of phenol rings and the way that these rings bind to one another, polyphenols can be classified into many different functional groups such as the phenolic acids, flavonoids, stilbenes, and lignans [143]. As cellular redox regulator, some phenolic compounds or polyphenolic-rich herbals have been shown to prevent formation or accumulation of AGEs in vivo [37, 144, 145, 146, 147]. Many studies showed that different polyphenols such as green and black tea polyphenols, grape seed proanthocyanidins, resveratrol, silymarin and genistein ameliorate adverse skin reactions following UV exposure [148, 149]. Polyphenols display a number of pharmacological properties in the gastrointestinal tract area, acting as antisecretory, cytoprotective, and antioxidant agents. Various polyphenolic compounds have been reported for their anti-ulcerogenic activity with a good level of gastric protection. Besides their action as gastroprotective, phenolic compounds namely, baicalein, cinnamic acid, oleuropein, rutin, quercetin, and tephrosin can be an alternative for the treatment of gastric ulcers [150]. Groving evidence demonstrating that prevention of formation or accumulation of AGEs by phenolic compounds or polyphenolic-rich herbals as cellular redox regulators is mediating their protective effect of tissues against oxidative damage in vivo [37, 144, 145, 146, 147].
There are variety of phenolics from simple low-molecular weight compounds, such as the simple phenylpropanoids, coumarins, and benzoic acid derivatives, to more complex structures such as flavanoids, stilbenes, and tannins. Flavanoids can be subdivided according to modifications of basic skeleton into chalcones, flavones, flavonols, flavanones, isoflavones, flavan-3-ols, and anthocyanins [151]. Resveratrol is an antioxidant, natural polyphenol, abundant in the skin of grapes (but not in the flesh) works both as a chelating agent and as a radical scavenger and in addition it takes part in inflammation by inhibiting the production of IL-8 by LPS-induced MAPK phosphorylation and a block of NFκB activation and IL-1β [152]. Resveratrol inhibits lipid peroxidase and protein carbonyl, protects spinal cord dorsal column from hypoxic injury by activating Nrf-2 [153]. Resveratrol possesses cancer chemopreventive activities [154], and has cardiovascular benefits via increased nitric oxide production and lowered levels of oxidized low-density lipoprotein [155]. Nuclear SIRT-1 enhanced by resveratrol was found to induce superoxide dismutase, an antioxidant enzyme which suppressed cell death in cardiomyocytes [156]. Resveratrol increases longevity through SirT1, which is activated with NAD+ supplied by an anti-aging enzyme PBEF. SirT1 interacts with an anti-aging transcription factor, FoxO1, which is negatively regulated by Akt [157]. There is a large body of evidence showing that curcumin has versatile biological and physiological activities, such as anti-inflammatory, anti-neurodegenerative, anti-Alzheimer’s disease, anti-obesity, anti-oxidative, anti-cancer, and anti-HIV activities [158]. At present, the binding proteins of curcumin are known to include cell survival proteins, protein kinases, protein reductases, histone acetyltransferase, histone deacetylase, glyoxalase I, xanthine oxidase, proteasome, HIV1 integrase, HIV1 protease, sarco (endo) plasmic reticulum Ca2+-ATPase, DNA methyltransferases 1, FtsZ protofilaments, carrier proteins, and others [159]. Furthermore, curcumin was found to protect from oxidative stress-induced damage in human endothelial cells via autophagy, which was executed by cytoplasmic localization and acetylation of FOXO1 for Atg7 activation [140, 160].
In fact the low levels of stress from physical, chemical and biological stressors often result in the functional improvement of cells, tissues, organs and organisms, a phenomenon termed physiological hormesis [161, 162].The seven major pathways of stress response like heat shock response, unfolded protein response, autophagy, antioxidant response, inflammatory response, DNA repair response and sirtuin response can be used as the screening platform for discovering, testing and monitoring the effects of novel hormetins [162]. As indicated largly by Rattan et al., such hormetins may be categorized as: (1) physical hormetins, such as exercise, hypergravity, heat and radiation; (2) biological and nutritional hormetins, such as infections, micronutrients, spices and other natural and synthetic compounds; and (3) psychological hormetins, such as mental challenge and focused attention or meditation [2, 161, 162]. According to this, several research articles have been published with respect to testing potential anti-aging herbalic hormetins, such curcumin, kinetin, rosmarinic acid, ferulic acid, hydroxytyrasol, slymarin and oleuropein [88, 89, 163].
A new approach that is feasible nowadays involves drug targeting for specific pathways that control oxidative, inflammatory and “proteotoxic stress”. In this new continuity, the transcription factor NF-E2-related factor-2 (Nrf2), master regulator of redox homeostasis, takes central role. Aged garlic extract containing S-allylcysteine (SAC) has been highlighting the ability to activate Nrf2 factor in cerebral cortex [164]. An herbal chemopreventive agent, oridonin, represents a novel class of Nrf2 activator, has enhancing effect on cellular redox capacity, reduces ROS, and improves survival of cells after arsenic exposure [165]. In glyco-oxidative conditions, high sulforaphane content broccoli, sprouts have been reported to activate the Nrf2-dependent antioxidant response-signaling pathway, attenuates oxidative stress, and inactivates inflammatory NF-κB [166].
A Chinese plant having iridoids, total glycosides such as morroniside and loganin and also a few polyphenols such as cornusiin A, B, and C, monomeric and trimeric hydrolysable tannins, were shownt to ameliorate hyperglycemia, proteinuria, renal AGE formation, and related protein expressions, i.e., receptor for AGEs, nuclear factor-kappaB, transforming growth factor-beta1, and Nepsilon-(carboxymethyl)lysine, in the same way as with aminoguanidine [167, 168].The anthraquinones, aurantio-obtusin, chryso-obtusin, obtusin, chryso-obtusin-2-O-beta-D-glucoside, physcion, emodin, chrysophanol, obtusifolin, and obtusifolin-2-O-beta-D-glucoside, isolated from an ethanolic extract of the seeds of Cassia tora, has been characterized as inhibitory against AGEs formation and rat lens aldose reductase and ocular dysfunction [169]. The phenolic compounds of pomegranate, Punica granatum L., which is our current research showed similar effects on aldose reductase and AGEs with wide-ranging health benefits [170].
On the other hand, phytochemicals have important signaling properties in the management of diabetes induction or the development of complications, and functional foods and their nutraceutical components are now considered as supplementary agents in type II diabetes. In this respect, a recent study showed that different fractions of Catharanthus roseus L. (Apocynaceae), Ocimum sanctum L. (Labiatae), Tinospora cordifolia Willd. (Menispermaceae), Aegle marmelos L. (Rutaceae), Ficus golmerata L. (Moraceae), Psoralea corlifolia L. (Fabaceae), Tribulus terrestris L. (Zygophyllaceae), and Morinda cetrifolia L. (Rubiaceae) have inhibitory activity on aldose reductase, a key enzyme implicated in cataractogenesis, and antioxidant agents [171,172]. The compounds with combined antioxidant and antiglycation properties seem to be more effective in treating diabetes mellitus or its complications [173]. Oral-treatment with anti-inflammatory and AGE-inhibitor, pyridoxamine inhibits AGE-induced ROS and inflammation and slow progression of degenerative spine changes in diabetes [174]. Almond skin is an abundant source of bioactive compounds and antioxidants, including polyphenolic flavonoids, catechin, epicatechin, contributing to rescued serum albumin from protein carbonylation induced by methylglyoxal, decreases gloxal induced hepatocyte cell death and ROS formation [175]. The effects of conventional reactive carbonyl species (RCS)-trapping agents including hydralazine, methoxylamine, aminoguanidine, pyridoxamine, carnosine, taurine and z-histidine hydrazide compared with herbal compounds in several experiments, and found that Cinnamon bark proanthocyanidins behave in a similar fashion as aminoguanidine, indicating great potential to be developed as agents to alleviate diabetic complications [176]. Natural Polyphenols including flavan-3-ols, thea flavins, cyanomaclurin, and dihydrochalcones or phenolic acids exert direct trapping agents of lipid peroxidation-derived adducts as acrolein and 4-hydroxy-trans-2-nonenal [177–179].
In our laboratory, we have testing antioxidant, redox regulator and anti-cytotoxic capacity of herbal compounds and natural products by using different in vitro cellular-based and biochemical assays and in vivo animal models. For example, we examined ethanolic seed and hull extracts of pomegranate with potential therapeutic use in prevention of diabetic complications [170]. We also found that a flavonol quercetin [141] and oleuropein [88, 89 180, 181] are able to reduce the risk for diabetes production or diabetic complications, age-related protein dysfunctions or protein adduct formations via affecting different cellular cascades related with carbonyl stress. Olive leaf polyphenols are the special interest of our laboratory, under the OLEA project we found that olive leaf poyphenolic mixture has cytoprotective and anti-inflamatory signaling effects tested in cardiomyocytes, insulin releasing β-cell or skin cell cultures stimulated by H2O2, 4-HNE or cytokine cocktail [88, 89, 180, 181]. In comparison with each phenolic component as oleuropein and hydroxytyrosol, the ethanolic olive leaf extract even in low concentrations was demonstrated more protective effectiveness in β-cell toxicity. Our studies using diabetic models showed improved insulin sensitivity, ameliorated antioxidant enzyme activity [34, 57, 64, 68, 70, 171], dysregulated glycated protein levels and lipid peroxidation products [34, 66, 70] by different antioxidant compounds.
In pancreatic β-cells, a hallmark of glucotoxicity is a profound oxidative stress, and the persistent oxidative stress is deleterious due to accumulation of oxidized proteins, lipids, and DNA. In the classic pathway of glucotoxicity, the spontaneous reactions of glucose and other sugars with amine residues of proteins, lipids, and nucleic acids are present [88, 89, 182–184]. In pancreatic β-cells an antioxidant defense can be considered as low [89] and polyol pathway is activated when excess glucose is converted to sorbitol in the presence of aldose reductase, consuming NADPH and thus contributing to pro-oxidation state [185]. Moreover, new discoveries have identified NADPH oxidases in β-cells as contributors to elevated cellular ROS leading to β-cell dysfunction and failure [186, 187]. B-cell function may be easily impaired under yet mild oxidative stress. Such stress imposes also activation of ROS-sensitive second messengers, such as p38 mitogen-activated protein kinase, p38MAPK [188], or c-Jun N-terminal kinase, JNK/SAPK [189] and then β-cell goes to apoptosis via caspases-dependent mechanisms without a significant increase in cytochrome c release [88, 89]. Activation of JNK pathway during oxidative stress results in decreased insulin gene expression by affecting the DNA binding activity of the epigenetic regulation of transcriptional factor pancreatic duodenal homeobox (PDX1) [190]. Thus, in turn, the beneficial effect of herbal antioxidants on diabetic patient could be explained besides the protection of oxidative destructions of macromolecules also by maintenance of PDX1. PDX1 plays pivotal role in proliferation, survival, and function of β-cells and activation of insulin gene expression [191, 192]. The cytokine-induced β-cell dysfunction and apoptosis is also based on ROS-induced intracellular signaling pathways [88, 193]. Cytokine-generated ROS induce expression of inducible nitric oxide synthase (iNOS) which results in NO• release and translocation of NFκB. In turn, NFκB induces NADPH oxidase as a major cytosolic ROS source [194]. Recently it has been demonstrated that quercetin, quercitrin are potential candidates to prevent β-cell death via the mitochondrial pathway and NF-κB signaling, and quercetin may be more efficacious than quercitrin as an anti-diabetic agent [195]. Similarly, myricetin protects against cytokine-induced cell death in RIN-m5f β-cells [196], and naringenin inhibit cytokine-induced toxicity in β-cells by enhancing cell survival through PI3-kinase pathway, independent of p-p38 MAPK or iNOS [197]. According to these, under the OLEA projects we found that olive leaf polyphenolic mixture or some olive leaf phenolic compounds,which are the special interest of our laboratory, carried out cytoprotective and anti-inflamatory effects tested insulin releasing β-cell by H2O2, 4-HNE or cytokine cocktail[88, 89, 180, 181] (Figure 1).
Figure 1.
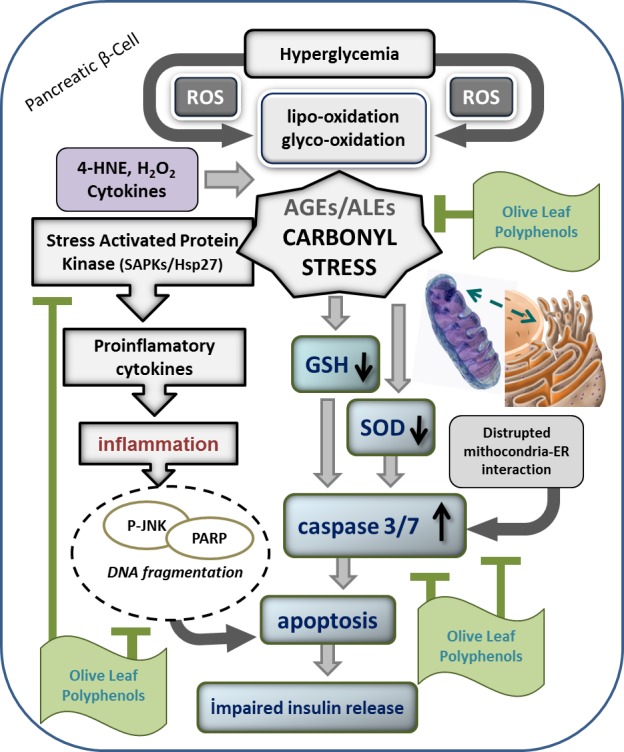
Inhibitory action mechanism of olive leaf polyphenolic mixture on down-stream apoptotic pathways triggering by carbonyl stress in pancreatic β-cells. ROS: Reactive oxygen species; SOD: Superoxide dismutase; GSH: Glutathione; AGEs: Advenced glyco-oxidation end products; ALEs: Advanced lipo-oxidation end products; PARP: Poly(ADP-ribose) polymerase; P-JNK: Phospho-c-Jun N-terminal kinase.
Cellular redox stress has been postulated as one major mechanism involved in the endothelial aging process and increased production of ROS leads to endothelial dysfunction in aging both in animals [57, 198] and in humans [32, 199]. There is a greater consensus over the reduced activity of the eNOS enzyme in aging [200]. Also, eNOS is regulated at post-transcriptional level involving PI3 kinase/Akt-dependent phosphorylation at Ser117, resulting in an increase in NO production from endothelial cells [32]. In aged animals, the PI3K/Akt pathway is diminished and eNOS gene expression and enzymatic activity levels decrease [201]. According to this, early experiments of our group showed that insulin-dependent and noninsulin-dependent diabetes lead to specific alterations in endothelium-dependent relaxation of rat aorta in basal and stimulated conditions [202–204]. This impaired relaxant feature of endothelium was accompanied to increased vasoconstrictor properties of diabetic resulting in high arterial blood pressure [203,204]. Our experiments also revealed that NIDDM causes a deficit in the vasorelaxation by endothelium, leading to an increase in contractility of human internal mammary artery and saphenous vein observed in patients under coronary artery bypass surgery [205]. In chronic state of disease, arterioles and arteries showed more sensitivity than non-diabetics to exogenously added ROS such as H2O2 [206, 207] and peroxynitrite [208] and excess superoxide was major mediator responsible for the enhanced transient nature of endothelium-dependent relaxation in aorta due to impaired NO bioavailability [209]. Furthermore, to the maintenance of physiological endothelium-mediated relaxation in chronically diabetic vessels, the involvement of H2O2 to acetylcholine-stimulated vasorelaxation was increased [209].
Under the Antioxidants in Diabetes-Induced Complications (ADIC) Study Group, we reported that vitamin E or lipoic acid treatment of STZ-diabetic rats can prevent abnormal contractility, structure and endothelial dysfunction in aorta, and the triglyceride-, lipid peroxidation-lowering and the endogen antioxidant enzyme activity modulating properties associate with the protective effect of these vitamins on the vasculature [110, 114, 130, 131, 210]. The elimination of superoxide/hydrogen peroxide by lipoic acid mediated the recovery of basal NO availability and the reversal of superoxide dismutase-induced relaxation in diabetic rat aorta [113]. On the other hand, using of vitamin A together with insulin provided a better metabolic control against cellular redox abnormalities and development of vascular complications in diabetes than using of insulin alone [210]. Vitamin E also ameliorated aldose reductase enzyme activity, protein adduct formation, retinal capillary basement membrane thickness and vascular endothelial growth factor expression in aged diabetic animals [125].
Additionally, some herbal polyphenols have ability to activate SIRT1 directly or indirectly judged in various animal models. Therefore, activation of SIRT1 by polyphenols may be essential for regulation of endothelial dysfunction as well as inflammation, senescence, and apoptosis. Molecular basis of SIRT1 in these processes is due to its ability to deacetylate miscellaneous proteins, such as NF-κB, p53, FOXO3, PPAR-γ, and eNOS [211, 212]. Several intervention studies have suggested that the consumption of flavonoid-, flavanone- or polyphenolic rich foods such as tea, grape polyphenols, cocoa, pomegranate juice and soya can improve endothelial function in patients with manifest cardiovascular and cerebrovascular disease [213–215]. Consumption of polyphenolic rich diet emphasized to be effective at reducing cardiovascular disease risk factors, particularly with respect to anti-hypertensive effects, inhibition of platelet aggregation and increasing endothelial-dependent vasodilation [216–218]. The studies performed with elderly people demonstrated that the consumption of a Mediterranean diet leads to an increase in NO bioavailability and vascular regeneration capacity [219]. Perez-Martinez et al., showed that the consumption of a Mediterranean diet reduced postprandial levels of cellular stress biomarkers such as lipid peroxide, protein carbonyl, SOD activity and plasma H2O2 compared to a saturated fat-rich diet in metabolic syndrome subjects [220]. Peña-Orihuela reported that monounsaturated fat consumption reduces oxidative stress as compared to saturated fat by inducing higher postprandial antioxidant response in adipose tissue, and thus, replacing saturated fatty acid for monounsaturated fatty acid may be an effective dietary strategy to reduce the oxidative stress in metabolic syndrome patients and its pathophysiological consequences [221]. Similarly, this diet significantly attenuated the postprandial inflammatory state, including NF-κB, metalloproteinase-9 and tumor necrosis factor-α [222, 223]. The results of the Yubero-Serrano’s study support the antioxidant effect of a Mediterenian diet and that exogenous CoQ supplementation has a protective effects against free radical over generation through the lowering of postprandial oxidative stress modifying the postprandial antioxidant protein levels and reducing the postprandial expression of antioxidant genes in peripheral blood mononuclear cells [224]. A recent study demonstrates for a first time that polyphenols, when used in conjunction with an intermittent feeding protocol, can enhance the longevity-promoting effects at least in mice [225].
Conclusion and Future Expectations
Aging is not controlled by a single mechanism and mainly depends on three major characteristics: stress response, the ability for damage prevention, repair and removal, and the ability for continuous remodeling and adaptation.
Accumulation of damaged macromolecules, including oxidatively damaged (carbonylated) proteins, is a hallmark of cellular and organismal aging. Thus understanding the molecular and cellular mechanisms that underlie the aging process would provide a good strategy to address the problems presented by the aging of the world’s population. Considering that micronutrients, phyto-chemicals are a very important source of redox regulators, in this review we analyzed the relationship between cellular redox stress, aging, and the mechanisms which may be involved in a higher survival rate and a lower incidence of the diseases associated with aging in populations which follow a healthy diet.
The large scale of findings show that cellular oxidative stress and also reductive stress (as indicated recently) are the major sources of damage in the dysregulation of biological systems and closely linked to the generation of impaired cellular stress response, physiological redox signaling and homeostasis, which contribute to the development of cellular senescence and accelerated aging of the organism. The contribution of carbonyl compounds and then protein adduct formations by advanced interaction of sugars/lipids with amino acids/peptides to the abnormal cellular redox metabolism is great interest of the recent research. The accumulation of adduct formation such as lipofuscin is implicated in normal aging and age-related diseases including Alzheimer’s disease, Parkinson’s disease, chronic obstructive pulmonary disease, myopathy, cardiovascular dysfunction and diabetes. Antioxidant plant extracts rich in polyphenols suggested to be increase lifespan. Polyphenols, natural cellular redox regulators or herbal compounds allowing physiological modulation of cells to redox stressors (when used in conjunction with an intermittent feeding) can enhance the longevity-promoting effects through reduction of cellular degenerative chaos and inflammation-related activity with a lower incidence of the diseases associated with aging. In the absence of large definitive clinical trials, present experimental findings at least in part have clear practical relevance for humans seeking to engage in health promoting behaviors.
Acknowledgments
Volkan Ergin is the Ph D student in department of Molecular Biology and Genetics in Faculty of Medicine, Gazi University, Ankara Turkey. Reza Ebrahimi Hariry is the Ph D student in department of Medical Pharmacology Faculty of Medicine, Gazi University, Ankara, Turkey. We wish to thanks to Gazi University Research Foundation No: 01/2010-126, 01/2011-09, 01/2012-70, EU-COST, Action B35 (Lipid Peroxidation Associated Disorders: LPO), EU-COST, Action BM1203 (EU-ROS), the Scientific and Technical Research Council of Turkey (TUBITAK) (Project no. 106S025, 108S239) and KOSGEB-2011-0850. We also thank Burcu Bali Ph D and “Farmasens” Co., Gazi University Techno Park, (www.farmasens.com) for their support the phytochemicals, Ankara, Turkey.
References
- [1].Rattan SI, Ali RE. Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann N Y Acad Sci. 2007;1100:424–30. doi: 10.1196/annals.1395.047. [DOI] [PubMed] [Google Scholar]
- [2].Rattan SI, Kryzch V, Schnebert S, Perrier E, Nizard C. Hormesis-based anti-aging products: a case study of a novel cosmetic. Dose Response. 2013;11:99–108. doi: 10.2203/dose-response.11-054.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Streit WJ, Xue QS. Alzheimer’s disease, neuroprotection, and CNS immunosenescence. Front Pharmacol. 2012;3:138. doi: 10.3389/fphar.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Canugovi C, Misiak M, Ferrarelli LK, Croteau DL, Bohr VA. The role of DNA repair in brain related disease pathology. DNA Repair (Amst) 2013;12:578–87. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sykora P, Wilson DM, 3rd, Bohr VA. Base excision repair in the mammalian brain: Implication for age related neurodegeneration. Mech Aging Dev. 2013 doi: 10.1016/j.mad.2013.04.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Neofytou E, Tzortzaki EG, Chatziantoniou A, Siafakas NM. DNA Damage Due to Oxidative Stress in Chronic Obstructive Pulmonary Disease (COPD) Int J Mol Sci. 2012;13:16853–64. doi: 10.3390/ijms131216853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mangerich A, Bürkle A. Pleiotropic cellular functions of PARP1 in longevity and aging: genome maintenance meets inflammation. Oxid Med Cell Longev. 2012;2012:321653. doi: 10.1155/2012/321653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Höhn A, König J, Grune T. Protein oxidation in aging and the removal of oxidized proteins. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.01.004. In Press. [DOI] [PubMed] [Google Scholar]
- [10].Butterfield DA, Dalle-Donne I. Redox proteomics. Antioxid Redox Signal. 2012;17:1487–9. doi: 10.1089/ars.2012.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Höhn A, Sittig A, Jung T, Grimm S, Grune T. Lipofuscinis formed independently of macroautophagy and lysosomal activity in stress-induced prematurely senescent human fibroblasts. Free Radic Biol Med. 2012;53:1760–9. doi: 10.1016/j.freeradbiomed.2012.08.591. [DOI] [PubMed] [Google Scholar]
- [13].de Gonzalo-Calvo D, Neitzert K, Fernández M, Vega-Naredo I, Caballero B, García-Macía M, Suárez FM, Rodríguez-Colunga MJ, Solano JJ, Coto-Montes A. Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic Biol Med. 2010;49:733–7. doi: 10.1016/j.freeradbiomed.2010.05.019. [DOI] [PubMed] [Google Scholar]
- [14].Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–46. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang J, Geiger H, Rudolph KL. Immunoaging induced by hematopoietic stem cell aging. Curr Opin Immunol. 2011;23:532–6. doi: 10.1016/j.coi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- [16].Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–8. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- [18].Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–83. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- [19].Behrens MI, Silva M, Salech F, Ponce DP, Merino D, Sinning M, Xiong C, Roe CM, Quest AF. Inverse susceptibility to oxidative death of lymphocytes obtained from Alzheimer’s patients and skin cancer survivors: increased apoptosis in Alzheimer’s and reduced necrosis in cancer. J Gerontol A Biol Sci Med Sci. 2013;67:1036–40. doi: 10.1093/gerona/glr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mirisola MG, Longo VD. A radical signal activates the epigenetic regulation of longevity. Cell Metab. 2013;17:812–3. doi: 10.1016/j.cmet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- [21].Park JH, Kim JJ, Bae YS. Involvement of PI3K-AKT-mTOR pathway in protein kinase CKII inhibition-mediated senescence in human colon cancer cells. Biochem Biophys Res Commun. 2013;433:420–5. doi: 10.1016/j.bbrc.2013.02.108. [DOI] [PubMed] [Google Scholar]
- [22].Kern A, Behl C. The unsolved relationship of brain aging and late-onset Alzheimer disease. Biochim Biophys Acta. 2009;1790:1124–32. doi: 10.1016/j.bbagen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- [23].Krautwald M, Münch G. Advanced glycation end products as biomarkers and gerontotoxins - A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease? Exp Gerontol. 2010;45:744–51. doi: 10.1016/j.exger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- [24].Watfa G, Dragonas C, Brosche T, Dittrich R, Sieber CC, Alecu C, Benetos A, Nzietchueng R. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J Nutr Health Aging. 2011;15:277–81. doi: 10.1007/s12603-010-0275-7. [DOI] [PubMed] [Google Scholar]
- [25].Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK. Environmental stress, aging and glial cell senescence: a novel mechanistic link to Parkinson’s disease? J Intern Med. 2013;273:429–36. doi: 10.1111/joim.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Paschos A, Pandya R, Duivenvoorden WC, Pinthus JH. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013 doi: 10.1038/pcan.2013.13. In Press. [DOI] [PubMed] [Google Scholar]
- [27].Cabrera MA, de Andrade SM, Mesas AE. A prospective study of risk factors for cardiovascular events among the elderly. Clin Interv Aging. 2012;7:463–8. doi: 10.2147/CIA.S37211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zamora PL, Villamena FA. Pharmacological approaches to the treatment of oxidative stress-induced cardiovascular dysfunctions. Future Med Chem. 2013;5:465–78. doi: 10.4155/fmc.13.15. [DOI] [PubMed] [Google Scholar]
- [29].Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann N Y Acad Sci. 2004;1019:70–7. doi: 10.1196/annals.1297.015. [DOI] [PubMed] [Google Scholar]
- [30].Tsyplenkova VG. Mechanisms of cardiac hystiocyte’s aging and death under noncoronary cardiac diseases. Arkh Patol. 2012;74:22–5. [PubMed] [Google Scholar]
- [31].Roosimaa M, Põdramägi T, Kadaja L, Ruusalepp A, Paju K, Puhke R, Eimre M, Orlova E, Piirsoo A, Peet N, Gellerich FN, Seppet E. Dilation of human atria: Increased diffusion restrictions for ADP, overexpression of hexokinase 2 and its coupling to oxidative phosphorylation in cardiomyocytes. Mitochondrion. 2012 doi: 10.1016/j.mito.2012.12.005. In Press. [DOI] [PubMed] [Google Scholar]
- [32].Marín C, Yubero-Serrano EM, López-Miranda J, Pérez-Jiménez F. Endothelial aging associated with oxidative stress can be modulated by a healthy Mediterranean diet. Int J Mol Sci. 2013;14:8869–89. doi: 10.3390/ijms14058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Barodka VM, Joshi BL, Berkowitz DE, Hogue CW, Jr, Nyhan D. Review article: implications of vascular aging. Anesth Analg. 2011;112:1048–60. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sakul A, Cumaoğlu A, Aydin E, Ari N, Dilsiz N, Karasu C. Age- and diabetes-induced regulation of oxidative protein modification in rat brain and peripheral tissues: consequences of treatment with antioxidant pyridoindole. Exp Gerontol. 2013;48:476–84. doi: 10.1016/j.exger.2013.02.028. [DOI] [PubMed] [Google Scholar]
- [35].Kawashima M, Ozawa Y, Shinmura K, Inaba T, Nakamura S, Kawakita T, Watanabe M, Tsubota K. Calorie restriction (CR) and CR mimetics for the prevention and treatment of age-related eye disorders. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.04.002. In Press. [DOI] [PubMed] [Google Scholar]
- [36].Lim XL, Teo BW, Tai BC, Wong TY, Ng DP. Pentosidine levels in nonproteinuric diabetes associated with both low estimated glomerular filtration rate and cataract. Diabetes Metab Syndr Obes. 2012;5:155–64. doi: 10.2147/DMSO.S32283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Danoux L, Mine S, Abdul-Malak N, Henry F, Jeanmaire C, Freis O, Pauly G, Cittadini L, André-Frei V, Rathjens A. How to help the skin cope with glycoxidation. Clin Chem Lab Med. 2013;2:1–8. doi: 10.1515/cclm-2012-0828. [DOI] [PubMed] [Google Scholar]
- [38].Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308–19. doi: 10.4161/derm.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Samaras N, Samaras D, Frangos Lordos E, Forster A, Philippe J. A review of age related dehydroepiandrosterone (DHEA) decline and its association with well-known geriatric syndromes Is treatment beneficial? Rejuvenation Res. 2013 doi: 10.1089/rej.2013.1425. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in aging and longevity. Nat Rev Endocrinol. 2013;9:366–76. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ceylan A, Karasu C, Aktan F, Güven C, Can B, Ozansoy G. Effects of simvastatin treatment on oxidant/antioxidant state and ultrastructure of diabetic rat myocardium. Gen Physiol Biophys. 2003;22:535–47. [PubMed] [Google Scholar]
- [42].Uluoglu C, Durakoglugil DB, Karasu C, Ozbey G, Gunes A, Zengil H. The effect of experimental diabetes on the twenty-four-hour pattern of the vasodilator responses to acetylcholine and isoprenaline in the rat aorta. Chronobiol Int. 2007;24:1081–94. doi: 10.1080/07420520701795332. [DOI] [PubMed] [Google Scholar]
- [43].Fan X, Sell DR, Zhang J, Nemet I, Theves M, Lu J, Strauch C, Halushka MK, Monnier VM. Anaerobic vs aerobic pathways of carbonyl and oxidant stress in human lens and skin during aging and in diabetes: A comparative analysis. Free Radic Biol Med. 2010;49:847–56. doi: 10.1016/j.freeradbiomed.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Heimfarth L, Loureiro SO, Pierozan P, de Lima BO, Reis KP, Torres EB, Pessoa-Pureur R. Methylglyoxal-induced cytotoxicity in neonatal rat brain: a role for oxidative stress and MAP kinases. Metab Brain Dis. 2013 doi: 10.1007/s11011-013-9379-1. In Press. [DOI] [PubMed] [Google Scholar]
- [45].Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42:333–47. doi: 10.1016/j.ecl.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murray DB, Haynes K, Tomita M. Redox regulation in respiring Saccharomyces cerevisiae. Biochim Biophys Acta. 2011;1810:945–58. doi: 10.1016/j.bbagen.2011.04.005. [DOI] [PubMed] [Google Scholar]
- [47].Cai Z, Yan LJ. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J Biochem Pharmacol Res. 2013;1:15–26. [PMC free article] [PubMed] [Google Scholar]
- [48].Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–8. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [49].Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–36. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- [50].Lozins’ka LM, Semchyshyn HM. Biological aspects of non-enzymatic glycosylation. Ukr Biokhim Zh. 2012;84:16–37. [PubMed] [Google Scholar]
- [51].Onyango AN. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem Phys Lipids. 2012;165:777–86. doi: 10.1016/j.chemphyslip.2012.09.004. [DOI] [PubMed] [Google Scholar]
- [52].Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42:1163–70. doi: 10.1007/s00726-010-0772-3. [DOI] [PubMed] [Google Scholar]
- [53].Neves D. Advanced glycation end-products: a common pathway in diabetes and age-related erectile dysfunction. Free Radic Res. 2013 doi: 10.3109/10715762.2013.821701. In Press. [DOI] [PubMed] [Google Scholar]
- [54].Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–821. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- [55].Arsov S, Graaff R, van Oe veren W, Stegmayr B, Sikole A, Rakhorst G, Smit AJ. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: a review. Clin Chem Lab Med. 2013;4:1–10. doi: 10.1515/cclm-2012-0832. [DOI] [PubMed] [Google Scholar]
- [56].Lo Pachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol. 2009;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Karasu C. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc Med J. 2010;4:240–256. doi: 10.2174/1874192401004010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Baraibar MA, Liu L, Ahmed EK, Friguet B. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid Med Cell Longev. 2012;2012:919832. doi: 10.1155/2012/919832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nakamura T, Cho DH, Lipton SA. Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp Neurol. 2012;238:12–21. doi: 10.1016/j.expneurol.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- [61].Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation end products: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–33. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Arai M, Miyashita M, Ichikawa T, Itokawa M. Carbonyl stress-related schizophrenia-perspective on future therapy and hypotheses regarding pathophysiology of schizophrenia. Seishin Shinkeigaku Zasshi. 2012;114:199–208. [PubMed] [Google Scholar]
- [63].Yanar K, Aydın S, Cakatay U, Mengi M, Buyukpınarbaşılı N, Atukeren P, Sitar ME, Sönmez A, Uslu E. Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic aging model. Basic Clin Pharmacol Toxicol. 2011;109:423–33. doi: 10.1111/j.1742-7843.2011.00756.x. [DOI] [PubMed] [Google Scholar]
- [64].Cumaoglu A, Stefek M, Bauer V, Ari N, Aricioglu A, Karasu C. Glycoxidative and nitrosative stress in kidney of experimental diabetic rats: effects of the prydoindole antioxidant stobadine. Neuro Endocrinol Lett. 2010;31:313–8. [PubMed] [Google Scholar]
- [65].Hamelin M, Borot-Laloi C, Friguet B, Bakala H. Increased level of glycoxidation product N(epsilon)-(carboxymethyl)lysine in rat serum and urine proteins with aging: link with glycoxidative damage accumulation in kidney. Arch Biochem Biophys. 2003;411:215–22. doi: 10.1016/s0003-9861(02)00735-x. [DOI] [PubMed] [Google Scholar]
- [66].Pekiner B, Ulusu NN, Das-Evcimen N, Sahilli M, Aktan F, Stefek M, Stolc S, Karasu C. Antioxidants in Diabetes-Induced Complications Study Group. In vivo treatment with stobadine prevents lipid peroxidation, protein glycation and calcium overload but does not ameliorate Ca2+-ATPase activity in heart and liver of streptozotocin-diabetic rats: comparison with vitamin E. Biochim Biophys Acta. 2002;1588:71–8. doi: 10.1016/s0925-4439(02)00141-2. [DOI] [PubMed] [Google Scholar]
- [67].Cumaoğlu A, Ozansoy G, Irat AM, Arıcıoğlu A, Karasu C, Arı N. Effect of long term, non-cholesterol lowering dose of fluvastatin treatment on oxidative stress in brain and peripheral tissues of streptozotocin-diabetic rats. Eur J Pharmacol. 2011;654:80–5. doi: 10.1016/j.ejphar.2010.11.035. [DOI] [PubMed] [Google Scholar]
- [68].Ulusu NN, Sahilli M, Avci A, Canbolat O, Ozansoy G, Ari N, Bali M, Stefek M, Stolc S, Gajdosik A, Karasu C. Pentose phosphate pathway, glutathione-dependent enzymes and antioxidant defense during oxidative stress in diabetic rodent brain and peripheral organs: effects of stobadine and vitamin E. Neurochem Res. 2003;28:815–23. doi: 10.1023/a:1023202805255. [DOI] [PubMed] [Google Scholar]
- [69].Muteliefu G, Shimizu H, Enomoto A, Nishijima F, Takahashi M, Niwa T. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelaminA through oxidative stress. Am J Physiol Cell Physiol. 2012;303:C126–34. doi: 10.1152/ajpcell.00329.2011. [DOI] [PubMed] [Google Scholar]
- [70].Cumaoglu A, Cevik C, Rackova L, Ari N, Karasu C. Effects of antioxidant stobadineon protein carbonylation, advanced oxidation protein products and reductive capacity of liver instreptozotocin-diabetic rats: role of oxidative/nitrosative stress. Biofactors. 2007;30:171–8. doi: 10.1002/biof.5520300304. [DOI] [PubMed] [Google Scholar]
- [71].Gradinaru D, Borsa C, Ionescu C, Margina D. Advanced oxidative and glycoxidative protein damage markers in the elderly with type 2 diabetes. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.03.034. In Press. [DOI] [PubMed] [Google Scholar]
- [72].Sebeková K, Klenovicsová K, Ferenczová J, Hedvig J, Podracká L, Heidland A. Advanced oxidation protein products and advanced glycation end products in children and adolescents with chronic renal insufficiency. J Ren Nutr. 2012;22:143–8. doi: 10.1053/j.jrn.2011.10.022. [DOI] [PubMed] [Google Scholar]
- [73].Song BJ, Abdelmegeed MA, Henderson LE, Yoo SH, Wan J, Purohit V, Hardwick JP, Moon KH. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic Fatty liver disease. Oxid Med Cell Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jiao L, Taylor PR, Weinstein SJ, Graubard BI, Virtamo J, Albanes D, Stolzenberg-Solomon RZ. Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1430–8. doi: 10.1158/1055-9965.EPI-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rahmadi A, Steiner N, Münch G. Advanced glycation end products as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer’s disease. Clin Chem Lab Med. 2011;49:385–91. doi: 10.1515/CCLM.2011.079. [DOI] [PubMed] [Google Scholar]
- [76].Fanelli F, Sepe S, D’Amelio M, Bernardi C, Cristiano L, Cimini A, Cecconi F, Ceru’ MP, Moreno S. Age-dependent roles of peroxisomes in the hippocampus of a transgenic mouse model of Alzheimer’s disease. Mol Neurodegener. 2013;8:8. doi: 10.1186/1750-1326-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tan KC, Shiu SW, Wong Y, Tam X. Serum advanced glycation end products (AGEs)are associated with insulin resistance. Diabetes Metab Res Rev. 2011;27:488–92. doi: 10.1002/dmrr.1188. [DOI] [PubMed] [Google Scholar]
- [78].Vlassara H, Striker G. Glycotoxins in the diet promote diabetes and diabetic complications. Curr Diab Rep. 2007;7:235–41. doi: 10.1007/s11892-007-0037-z. [DOI] [PubMed] [Google Scholar]
- [79].Jack M, Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl Res. 2012;159:355–65. doi: 10.1016/j.trsl.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Babizhayev MA, Yegorov YE. Therapeutic uses of drug-carrier systems for imidazole-containing dipeptide compounds that act as pharmacological chaperones and have significant impact on the treatment of chronic diseases associated with increased oxidative stress and the formation of advanced glycation end products. Crit Rev Ther Drug Carrier Syst. 2010;27:85–154. doi: 10.1615/critrevtherdrugcarriersyst.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- [81].Freund-Michel V, Guibert C, Dubois M, Courtois A, Marthan R, Savineau JP, Muller B. Reactive oxygen species as therapeutic targets in pulmonary hypertension. Ther Adv Respir Dis. 2013;7:175–200. doi: 10.1177/1753465812472940. [DOI] [PubMed] [Google Scholar]
- [82].Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology. 2012;58:227–37. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- [83].Ceylan A, Karasu C, Aktan F, Ozansoy G. Simvastatin treatment restores vasoconstriction and the inhibitory effect of LPC on endothelial relaxation via affecting oxidizing metabolism in diabetic rats. Diabetes Nutr Metab. 2004;17:203–10. [PubMed] [Google Scholar]
- [84].Bartosz G. Reactive oxygen species: destroyers or messengers? Biochem Pharmacol. 2009;77:1303–15. doi: 10.1016/j.bcp.2008.11.009. [DOI] [PubMed] [Google Scholar]
- [85].Brieger K, Schiavone S, Miller FJ, Jr, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- [86].Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic β-cell function. Diabetes Obes Metab. 2010;12(Suppl 2):141–8. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- [87].Račková L, Cumaoğlu A, Bağrıacık EU, Štefek M, Maechler P, Karasu C. Novel hexahydropyridoindole derivative as prospective agent against oxidative damage in pancreatic β cells. Med Chem. 2011;7:711–7. doi: 10.2174/157340611797928370. [DOI] [PubMed] [Google Scholar]
- [88].Cumaoğlu A, Ari N, Kartal M, Karasu C. Polyphenolic extracts from Olea europea L. protect against cytokine-induced β-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011;14:325–34. doi: 10.1089/rej.2010.1111. [DOI] [PubMed] [Google Scholar]
- [89].Cumaoğlu A, Rackova L, Stefek M, Kartal M, Maechler P, Karasu C. Effects of olive leaf polyphenols against H2O2 toxicity in insulin secreting β-cells. Acta Biochim Pol. 2011;58:45–50. [PubMed] [Google Scholar]
- [90].Teodoro JS, Rolo AP, Palmeira CM. The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol Mech Methods. 2013;23:297–302. doi: 10.3109/15376516.2012.759305. [DOI] [PubMed] [Google Scholar]
- [91].Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- [92].Lopaczynski W, Zeisel SH. Antioxidants, programmed cell death, and cancer. Nutrition Research. 2001;21:295–307. [Google Scholar]
- [93].Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–83. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- [94].Gaziano JM, Manson JE, Buring JE, Hennekens CH. Dietary antioxidants and cardiovascular disease. Ann N Y Acad Sci. 1992;669:249–58. doi: 10.1111/j.1749-6632.1992.tb17104.x. [DOI] [PubMed] [Google Scholar]
- [95].Grune T. Oxidative stress, aging and the proteasomal system. Biogerontology. 2000;1:31–40. doi: 10.1023/a:1010037908060. [DOI] [PubMed] [Google Scholar]
- [96].Zobali F, Avci A, Canbolat O, Karasu C. Effects of vitamin A and insulin on the antioxidative state of diabetic rat heart: a comparison study with combination treatment. Cell Biochem Funct. 2002;20:75–80. doi: 10.1002/cbf.957. [DOI] [PubMed] [Google Scholar]
- [97].Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromolecular Med. 2008;10:259–74. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–33. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- [99].Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3:4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–92S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Eicker J, Kürten V, Wild S, Riss G, Goralczyk R, Krutmann J, Berneburg M. Betacarotene supplementation protects from photoaging-associated mitochondrial DNA mutation. Photochem Photobiol Sci. 2003;2:655–9. doi: 10.1039/b300808h. [DOI] [PubMed] [Google Scholar]
- [102].Ge XX, Xing MY, Yu LF, Shen P. Carotenoid Intake and Esophageal Cancer Risk: a Meta-analysis. Asian Pac J Cancer Prev. 2013;14:1911–8. doi: 10.7314/apjcp.2013.14.3.1911. [DOI] [PubMed] [Google Scholar]
- [103].Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, Rabe KG, Anderson KE, Olson JE, Sinha R, Petersen GM. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Cancer. 2013;44:152–61. doi: 10.1007/s12029-012-9441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].[The influence of combined treatment on biochemical and functional characteristics in the patients with vascular complications of type 1 diabetes mellitus and different quality of compensation] Klin Med (Mosk) 2013;91:14–8. [PubMed] [Google Scholar]
- [106].Yoneda T, Tomofuji T, Ekuni D, Azuma T, Endo Y, Kasuyama K, Machida T, Morita M. Anti-aging Effects of Co-enzyme Q10 on Periodontal Tissues. J Dent Res. 2013;92:735–9. doi: 10.1177/0022034513490959. [DOI] [PubMed] [Google Scholar]
- [107].La Guardia PG, Alberici LC, Ravagnani FG, Catharino RR, Vercesi AE. Protection of rat skeletal muscle fibers by either L-carnitine or coenzyme Q10 against statins toxicity mediated by mitochondrial reactive oxygen generation. Front Physiol. 2013;4:103. doi: 10.3389/fphys.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shetty RA, Forster MJ, Sumien N. Coenzyme Q(10) supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age (Dordr) 2012 doi: 10.1007/s11357-012-9484-9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hünkar T, Aktan F, Ceylan A, Karasu C. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin diabetic rats. Cell Biochem Funct. 2002;20:297–302. doi: 10.1002/cbf.977. [DOI] [PubMed] [Google Scholar]
- [110].Ceylan-Isik A, Hünkar T, Aşan E, Kaymaz F, Ari N, Söylemezoğlu T, Renda N, Soncul H, Bali M, Karasu C. (Antioxidants in Diabetes-Induced Complications) The ADIC Study Group. Cod liver oil supplementation improves cardiovascular and metabolic abnormalities in streptozotocin diabetic rats. J Pharm Pharmacol. 2007;59:1629–41. doi: 10.1211/jpp.59.12.0004. [DOI] [PubMed] [Google Scholar]
- [111].Saitoh Y, Morishita A, Mito S, Tsujiya T, Miwa N. Senescence-induced increases in intracellular oxidative stress and enhancement of the need for ascorbic acid in human fibroblasts. Mol Cell Biochem. 2013;380:129–41. doi: 10.1007/s11010-013-1666-y. [DOI] [PubMed] [Google Scholar]
- [112].Iwama M, Amano A, Shimokado K, Maruyama N, Ishigami A. Ascorbic acid levels in various tissues, plasma and urine of mice during aging. J Nutr Sci Vitaminol (Tokyo) 2012;58(3):169–74. doi: 10.3177/jnsv.58.169. [DOI] [PubMed] [Google Scholar]
- [113].Koçak G, Karasu C. Elimination of superoxide/H2O2 by alpha-lipoic acid mediates the recovery of basal EDRF/NO availability and the reversal of superoxide dismutase-induced relaxation in diabetic rat aorta. Diabetes Obes Metab. 2002;4:69–74. doi: 10.1046/j.1463-1326.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- [114].Koçak G, Aktan F, Canbolat O, Ozoğul C, Elbeğ S, Yildizoglu-Ari N, Karasu C. ADIC Study Group. Antioxidants in Diabetes-Induced Complications Alpha-lipoic acid treatment ameliorates metabolic parameters, blood pressure, vascular reactivity and morphology of vessels already damaged by streptozotocin-diabetes. Diabetes Nutr Metab. 2000;13:308–18. [PubMed] [Google Scholar]
- [115].Bojunga J, Nowak D, Mitrou PS, Hoelzer D, Zeuzem S, Chow KU. Antioxidative treatment prevents activation of death-receptor- and mitochondrion-dependent apoptosis in the hearts of diabetic rats. Diabetologia. 2004;47:2072–80. doi: 10.1007/s00125-004-1572-7. [DOI] [PubMed] [Google Scholar]
- [116].Thakurta IG, Chattopadhyay M, Ghosh A, Chakrabarti S. Dietary supplementation with N-acetyl cysteine, α-tocopherol and α-lipoic acid reduces the extent of oxidative stress and proinflammatory state in aged rat brain. Biogerontology. 2012;13:479–88. doi: 10.1007/s10522-012-9392-5. [DOI] [PubMed] [Google Scholar]
- [117].Asha Devi S, Manjula KR, Subramanyam MV. Protective role of vitamins E and C against oxidative stress caused by intermittent cold exposure in aging rat’s frontoparietal cortex. Neurosci Lett. 2012;529:155–60. doi: 10.1016/j.neulet.2012.09.041. [DOI] [PubMed] [Google Scholar]
- [118].Kucukatay V, Bor-Kucukatay M, Gundogdu G, Erken G, Ozcan TO, Miloglu FD, Kadioglu Y. Vitamin E treatment enhances erythrocyte deformability in aged rats. Folia Biol (Praha) 2012;58:157–65. [PubMed] [Google Scholar]
- [119].Schmid TE, Eskenazi B, Marchetti F, Young S, Weldon RH, Baumgartner A, Anderson D, Wyrobek AJ. Micronutrients intake is associated with improved sperm DNA quality in older men. Fertil Steril. 2012;98:1130–7.e1. doi: 10.1016/j.fertnstert.2012.07.1126. [DOI] [PubMed] [Google Scholar]
- [120].Thiagarajan R, Manikandan R. Antioxidants and cataract. Free Radic Res. 2013;47:337–45. doi: 10.3109/10715762.2013.777155. [DOI] [PubMed] [Google Scholar]
- [121].Zarogoulidis P, Cheva A, Zarampouka K, Huang H, Li C, Huang Y, Katsikogiannis N, Zarogoulidis K. Tocopherols and tocotrienols as anticancer treatment for lung cancer: future nutrition. J Thorac Dis. 2013;5:349–52. doi: 10.3978/j.issn.2072-1439.2013.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Dysken MW, Guarino PD, Vertrees JE, Asthana S, Sano M, Llorente M, Pallaki M, Love S, Schellenberg GD, McCarten JR, Malphurs J, Prieto S, Chen P, Loreck DJ, Carney S, Trapp G, Bakshi RS, Mintzer JE, Heidebrink JL, Vidal-Cardona A, Arroyo LM, Cruz AR, Kowall NW, Chopra MP, Craft S, Thielke S, Turvey CL, Woodman C, Monnell KA, Gordon K, Tomaska J, Vatassery G. Vitamin E and memantine in Alzheimer’s disease: Clinical trial methods and baseline data. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.01.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Karasu C, Dewhurst M, Stevens EJ, Tomlinson DR. Effects of anti-oxidant treatment on sciatic nerve dysfunction in streptozotocin-diabetic rats; comparison with essential fatty acids. Diabetologia. 1995;38:129–34. doi: 10.1007/BF00400086. [DOI] [PubMed] [Google Scholar]
- [124].Skalska S, Kyselova Z, Gajdosikova A, Karasu C, Stefek M, Stolc S. Protective effect of stobadine on NCV in streptozotocin-diabetic rats: augmentation by vitamin E. Gen Physiol Biophys. 2008;27:106–14. [PubMed] [Google Scholar]
- [125].Yülek F, Or M, Ozoğul C, Isik AC, Ari N, Stefek M, Bauer V, Karasu C. Effects of stobadine and vitamin E in diabetes-induced retinal abnormalities: involvement of oxidative stress. Arch Med Res. 2007;38:503–11. doi: 10.1016/j.arcmed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [126].Güneş A, Ceylan A, Sarioglu Y, Stefek M, Bauer V, Karasu C. Antioxidants in Diabetes-induced Complications (ADIC) Study Group. Reactive oxygen species mediate abnormal contractile response to sympathetic nerve stimulation and noradrenaline in the vas deferens of chronically diabetic rats: effects of in vivo treatment with antioxidants. Fundam Clin Pharmacol. 2005;19:73–9. doi: 10.1111/j.1472-8206.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- [127].Dogru Pekiner B, Daş Evcimen N, Ulusu NN, Bali M, Karasu C. Effects of vitamin E on microsomal Ca2+-ATPase activity and calcium levels in streptozotocin-induced diabetic rat kidney. Cell Biochem Funct. 2003;21:177–82. doi: 10.1002/cbf.1016. [DOI] [PubMed] [Google Scholar]
- [128].Demiryürek AT, Karasu C, Stefek M, Stolc S. Effect of stobadine on leukocyte free radical generation in streptozotocin-diabetic rats: comparison with vitamin E. Pharmacology. 2004;70:1–4. doi: 10.1159/000074236. [DOI] [PubMed] [Google Scholar]
- [129].Das Evcimen N, Ulusu NN, Karasu C, Doğru B. Adenosine triphosphatase activity of streptozotocin-induced diabetic rat brain microsomes. Effect of vitamin E. Gen Physiol Biophys. 2004;23:347–55. [PubMed] [Google Scholar]
- [130].Karasu C, Ozansoy G, Bozkurt O, Erdoğan D, Omeroğlu S. Changes in isoprenaline-induced endothelium-dependent and -independent relaxations of aorta in long-term STZ-diabetic rats: reversal effect of dietary vitamin E. Gen Pharmacol. 1997;29:561–7. doi: 10.1016/s0306-3623(96)00577-0. [DOI] [PubMed] [Google Scholar]
- [131].Karasu C, Ozansoy G, Bozkurt O, Erdoğan D, Omeroğlu S. Antioxidant and triglyceride-lowering effects of vitamin E associated with the prevention of abnormalities in the reactivity and morphology of aorta from streptozotocin-diabetic rats. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Metabolism. 1997;46:872–9. doi: 10.1016/s0026-0495(97)90072-x. [DOI] [PubMed] [Google Scholar]
- [132].Suganuma K, Nakajima H, Ohtsuki M, Imokawa G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci. 2010;58:136–42. doi: 10.1016/j.jdermsci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- [133].Glossmann H. Vitamin D, UV, and skin cancer in the elderly: to expose or not to expose? Gerontology. 2011;57:350–3. doi: 10.1159/000322521. [DOI] [PubMed] [Google Scholar]
- [134].Reichrath J. Unravelling of hidden secrets: The role of vitamin D in skin aging. Dermatoendocrinol. 2012;4:241–4. doi: 10.4161/derm.21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Najmi Varzaneh F, Sharifi F, Hossein-Nezhad A, Mirarefin M, Maghbooli Z, Ghaderpanahi M, Larijani B, Fakhrzadeh H. Association of vitamin d receptor with longevity and healthy aging. Acta Med Iran. 2013;51:236–41. [PubMed] [Google Scholar]
- [136].Chopra A, Doiphode VV. Ayurvedic medicine. Core concept, therapeutic principles, and current relevance. Med Clin North Am. 2002;86:75–89. vii. doi: 10.1016/s0025-7125(03)00073-7. [DOI] [PubMed] [Google Scholar]
- [137].Stevensen C. JAMU: an Indonesian herbal tradition with a long past, a little known present and an uncertain future. Complement Ther Nurs Midwifery. 1999;5:1–3. doi: 10.1016/s1353-6117(99)80062-6. [DOI] [PubMed] [Google Scholar]
- [138].Hosseinzadeh H, Nassiri-Asl M. Avicenna’s (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27:475–83. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- [139].Rackova L, Snirc V, Jung T, Stefek M, Karasu C, Grune T. Metabolism-induced oxidative stress is a mediator of glucose toxicity in HT22 neuronal cells. Free Radic Res. 2009;43:876–86. doi: 10.1080/10715760903104374. [DOI] [PubMed] [Google Scholar]
- [140].Murakami A. Modulation of protein quality control systems by food phytochemicals. J Clin Biochem Nutr. 2013;52:215–27. doi: 10.3164/jcbn.12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Stefek M, Karasu C. Eye lens in aging and diabetes: effect of quercetin. Rejuvenation Res. 2011;14:525–34. doi: 10.1089/rej.2011.1170. [DOI] [PubMed] [Google Scholar]
- [142].Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81(1 Suppl):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- [143].Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- [144].Sajithlal GB, Chithra P, Chandrakasan G. Effect of curcuminon the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998;56:1607–14. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- [145].Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:180–5. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- [146].Kim HY, Kim K. Protein glycation inhibitory and antioxidative activities of some plant extracts in vitro. J Agric Food Chem. 2003;51:1586–91. doi: 10.1021/jf020850t. [DOI] [PubMed] [Google Scholar]
- [147].Tung YT, Wu JH, Kuo YH, Chang ST. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour Technol. 2007;98:1120–3. doi: 10.1016/j.biortech.2006.04.017. [DOI] [PubMed] [Google Scholar]
- [148].Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Korać RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. 2011;5:164–73. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–7. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- [152].Chang CC, Chang CY, Huang JP, Hung LM. “Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats”. Chin J Physiol. 2012;55:192–201. doi: 10.4077/CJP.2012.BAA012. [DOI] [PubMed] [Google Scholar]
- [153].Kesherwani V, Atif F, Yousuf S, Agrawal SK. Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf-2. Neuroscience. 2013;241:80–8. doi: 10.1016/j.neuroscience.2013.03.015. [DOI] [PubMed] [Google Scholar]
- [154].Díaz-Chávez J, Fonseca-Sánchez MA, Arechaga-Ocampo E, Flores-Pérez A, Palacios-Rodríguez Y, Domínguez-Gómez G, Marchat LA, Fuentes-Mera L, Mendoza-Hernández G, Gariglio P, López-Camarillo C. Proteomic Profiling Reveals That Resveratrol Inhibits HSP27 Expression and Sensitizes Breast Cancer Cells to Doxorubicin Therapy. PLoS One. 2013;8:e64378. doi: 10.1371/journal.pone.0064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Avila PR, Marques SO, Luciano TF, Vitto MF, Engelmann J, Souza DR, Pereira SV, Pinho RA, Lira FS, De Souza CT. Resveratrol and fish oil reduce catecholamine-induced mortality in obese rats: role of oxidative stress in the myocardium and aorta. Br J Nutr. 2013;4:1–11. doi: 10.1017/S0007114513000925. [DOI] [PubMed] [Google Scholar]
- [156].Mohar DS, Malik S. The Sirtuin System: The Holy Grail of Resveratrol? J Clin Exp Cardiolog. 2012;3(11):pii, 216. doi: 10.4172/2155-9880.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–8. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [158].Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- [159].Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28:1937–55. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, Pan Y, Li XJ. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–25. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- [161].Rattan SI. Hormesis in aging. Aging Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [162].Rattan SI, Fernandes RA, Demirovic D, Dymek B, Lima CF. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response. 2009;7:90–103. doi: 10.2203/dose-response.08-014.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Rattan SI. Rationale and methods of discovering hormetins as drugs for healthy aging. Expert Opin Drug Discov. 2012;7:439–48. doi: 10.1517/17460441.2012.677430. [DOI] [PubMed] [Google Scholar]
- [164].Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract-and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012;2012:907162. doi: 10.1155/2012/907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen W, Li J, Lou H, Wong PK, Zhang DD. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ Health Perspect. 2008;116:1154–61. doi: 10.1289/ehp.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Bahadoran Z, Mirmiran P, Azizi F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J Med Food. 2013;16:375–82. doi: 10.1089/jmf.2012.2559. [DOI] [PubMed] [Google Scholar]
- [167].Yamabe N, Kang KS, Goto E, Tanaka T, Yokozawa T. Beneficial effect of Corni Fructus, a constituent of Hachimi-jio-gan, on advanced glycation end-product-mediated renal injury in Streptozotocin-treated diabetic rats. Biol Pharm Bull. 2007;30:520–6. doi: 10.1248/bpb.30.520. [DOI] [PubMed] [Google Scholar]
- [168].Jang DS, Lee GY, Kim YS, Lee YM, Kim CS, Yoo JL, Kim JS. Anthraquinones from the seeds of Cassiatora with inhibitory activity on protein glycation and aldose reductase. Biol Pharm Bull. 2007;30:2207–10. doi: 10.1248/bpb.30.2207. [DOI] [PubMed] [Google Scholar]
- [169].Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–27. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- [170].Karasu C, Cumaoğlu A, Gürpinar AR, Kartal M, Kovacikova L, Milackova I, Stefek M. Aldose reductase inhibitory activity and antioxidant capacity of pomegranate extracts. Interdiscip Toxicol. 2012;5:15–20. doi: 10.2478/v10102-012-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Gacche RN, Dhole NA. Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: an attempt to standardize the botanicals for amelioration of diabetes complications. Food Chem Toxicol. 2011;49:1806–13. doi: 10.1016/j.fct.2011.04.032. [DOI] [PubMed] [Google Scholar]
- [172].Duraisamy Y, Gaffney J, Slevin M, Smith CA, Williamson K, Ahmed N. Aminosalicylic acid reduces the antiproliferative effect of hyperglycaemia, advanced glycation end products and glycated basic fibroblast growth factor in cultured bovine aortic endothelial cells: comparison with aminoguanidine. Mol Cell Biochem. 2003;246:143–53. [PubMed] [Google Scholar]
- [173].Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22:228–37. doi: 10.1002/ptr.2297. [DOI] [PubMed] [Google Scholar]
- [174].Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined Anti-Inflammatory and Anti-AGE Drug Treatments Have a Protective Effect on Intervertebral Discs in Mice with Diabetes. Plo S One. 2013;8:e64302. doi: 10.1371/journal.pone.0064302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Dong Q, Banaich MS, O’Brien PJ. Cytoprotection by almond skin extracts or catechins of hepatocyte cytotoxicity induced by hydroperoxide (oxidative stress model) versus glyoxal or methylglyoxal (carbonylationmodel) Chem Biol Interact. 2010;185:101–9. doi: 10.1016/j.cbi.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [176].Peng X, Cheng KW, Ma J, Chen B, Ho CT, Lo C, Chen F, Wang M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation end products. J Agric Food Chem. 2008;56:1907–11. doi: 10.1021/jf073065v. [DOI] [PubMed] [Google Scholar]
- [177].Zhu Q, Zheng ZP, Cheng KW, Wu JJ, Zhang S, Tang YS, Sze KH, Chen J, Chen F, Wang M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. ChemResToxicol. 2009;22:1721–7. doi: 10.1021/tx900221s. [DOI] [PubMed] [Google Scholar]
- [178].Lo CY, Hsiao WT, Chen XY. Efficiency of trapping methylglyoxal by phenols and phenolic acids. J Food Sci. 2011;76:H90–6. doi: 10.1111/j.1750-3841.2011.02067.x. [DOI] [PubMed] [Google Scholar]
- [179].Dogan I, Cumaoglu A, Aricioglu A, Ekmekci A. Inhibition of ErbB2 by herceptin reduces viability and survival, induces apoptosis and oxidative stress in Calu-3 cell line. Mol Cell Biochem. 2011;347:41–51. doi: 10.1007/s11010-010-0610-7. [DOI] [PubMed] [Google Scholar]
- [180].Rackova L, Cumaoglu M, Kartal M, Stefek M, Karasu C. Protection of insulin-producing INS-1 cells from H2O2-induced oxidative stress by phenolic extracts from olea europea L. Free Radic Res. 2008;42(Supplement 1):S98. [Google Scholar]
- [181].Cumaoglu A, Rackova L, Kartal M, Karasu C. Effects of olive extracts on free radical metabolism and glucose stimulated insulin release in INS-1E cell line exposed to H2O2. FEBS Journal. 2008;275(Supplement 1):377. [Google Scholar]
- [182].Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- [183].Lortz S, Tiedge M. Sequential inactivation of reactive oxygen species by combined overexpression of SOD isoforms and catalase in insulin-producing cells. Free Radical Biology and Medicine. 2003;34:683–688. doi: 10.1016/s0891-5849(02)01371-0. [DOI] [PubMed] [Google Scholar]
- [184].Hamaoka R, Fujii J, Miyagawa J, Takahashi M, Kishimoto M, Moriwaki M, Yamamoto K, Kajimoto Y, Yamasaki Y, Hanafusa T, Matsuzawa Y, Taniguchi N. Overexpression of the aldose reductase gene induces apoptosis in pancreatic beta-cells by causing a redox imbalance. J Biochem. 1999;126:41–7. doi: 10.1093/oxfordjournals.jbchem.a022434. [DOI] [PubMed] [Google Scholar]
- [185].Huang M, Joseph JW. Metabolomic analysis of pancreatic β-cell insulin release in response to glucose. Islets. 2012;4:210–22. doi: 10.4161/isl.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Taylor-Fishwick DA. NOX, NOX Who is There? The Contribution of NADPH Oxidase One to Beta Cell Dysfunction. Front Endocrinol (Lausanne) 2013;4:40. doi: 10.3389/fendo.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lu X, Liu J, Hou F, Liu Z, Cao X, Seo H, Gao B. Cholesterol induces pancreaticβ cell apoptosis through oxidative stress pathway. Cell Stress Chaperones. 2011;16:539–48. doi: 10.1007/s12192-011-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Chang KC, Hsu CC, Liu SH, Su CC, Yen CC, Lee MJ, Chen KL, Ho TJ, Hung DZ, Wu CC, Lu TH, Su YC, Chen YW, Huang CF. Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PloS One. 2013;8:e54374. doi: 10.1371/journal.pone.0054374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Subramanian SL, Hull RL, Zraika S, Aston-Mourney K, Udayasankar J, Kahn SE. cJun N-terminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2012;55:166–74. doi: 10.1007/s00125-011-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Lombardi A, Ulianich L, Treglia AS, Nigro C, Parrillo L, Lofrumento DD, Nicolardi G, Garbi C, Beguinot F, Miele C, DiJeso B. Increased hexosamine biosynthetic pathway flux dedifferentiates INS-1E cells and murine islets by anextra cellular signal-regulated kinase (ERK)1/2-mediated signal transmission pathway. Diabetologia. 2012;55:141–53. doi: 10.1007/s00125-011-2315-1. [DOI] [PubMed] [Google Scholar]
- [191].Ježek P, Dlasková A, Plecitá-Hlavatá L. Redox homeostasis in pancreatic β cells. Oxid Med Cell Longev. 2012;2012:932838. doi: 10.1155/2012/932838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Liu D, Pavlovic D, Chen MC, Flodström M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS-/-) Diabetes. 2000;49:1116–22. doi: 10.2337/diabetes.49.7.1116. [DOI] [PubMed] [Google Scholar]
- [193].Azevedo-Martins AK, Lortz S, Lenzen S, Curi R, Eizirik DL, Tiedge M. Improvement of the mitochondrial antioxidant defense status prevents cytokine-induced nuclear factor-kappa B activation in insulin-producing cells. Diabetes. 2003;52:93–101. doi: 10.2337/diabetes.52.1.93. [DOI] [PubMed] [Google Scholar]
- [194].Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013;1281:16–35. doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Dai X, Ding Y, Zhang Z, Cai X, Li Y. Quercetin and quercitrin protect against cytokine induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int J Mol Med. 2013;31:265–71. doi: 10.3892/ijmm.2012.1177. [DOI] [PubMed] [Google Scholar]
- [196].Ding Y, Zhang ZF, Dai XQ, Li Y. Myricetin protects against cytokine-induced cell death in RIN-m5f β cells. J Med Food. 2012;15:733–40. doi: 10.1089/jmf.2011.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lin CY, Ni CC, Yin MC, Lii CK. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine. 2012;59(1):65–71. doi: 10.1016/j.cyto.2012.04.011. [DOI] [PubMed] [Google Scholar]
- [198].Vander Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappa B. Circ Res. 2007;100:1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- [200].Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 2006;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- [201].LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008;295:H2280–8. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Altan VM, Karasu C, Ozüari A. The effects of type-1 and type-2 diabetes on endothelium-dependent relaxation in rat aorta. Pharmacol Biochem Behav. 1989;33:519–22. doi: 10.1016/0091-3057(89)90379-1. [DOI] [PubMed] [Google Scholar]
- [203].Karasu C, Altan VM. The role of endothelial cells on the alterations in vascular reactivity induced by insulin-dependent diabetes mellitus: effects of insulin treatment. Gen Pharmacol. 1993;24:743–55. doi: 10.1016/0306-3623(93)90241-o. [DOI] [PubMed] [Google Scholar]
- [204].Karasu C, Altan VM. The role of the endothelium on enhanced contractile response of non-insulin-dependent diabetic rat aortae: effects of insulin treatment. Gen Pharmacol. 1994;25:795–802. doi: 10.1016/0306-3623(94)90262-3. [DOI] [PubMed] [Google Scholar]
- [205].Karasu C, Soncul H, Altan VM. Effects of non-insulin dependent diabetes mellitus on the reactivity of human internal mammary artery and human saphenous vein. Life Sci. 1995;57:103–12. doi: 10.1016/0024-3205(95)00251-z. [DOI] [PubMed] [Google Scholar]
- [206].Karasu C. Increased activity of H2O2 in aorta isolated from chronically streptozotocin-diabetic rats: effects of antioxidant enzymes and enzymes inhibitors. Free Radic Biol Med. 1999;27:16–27. doi: 10.1016/s0891-5849(99)00028-3. [DOI] [PubMed] [Google Scholar]
- [207].Bauer V, Sotníková R, Drábiková K. Effects of reactive oxygen species and neutrophils on endothelium-dependent relaxation of rat thoracic aorta. Interdiscip Toxicol. 2011;4:191–7. doi: 10.2478/v10102-011-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zobali F, Cakici I, Karasu C. Effects of peroxynitrite on the reactivity of diabetic rat aorta. Pharmacology. 2001;63:58–64. doi: 10.1159/000056113. [DOI] [PubMed] [Google Scholar]
- [209].Karasu C. Time course of changes in endothelium-dependent and–independent relaxation of chronically diabetic aorta: role of reactive oxygen species. Eur J Pharmacol. 2000;392:163–73. doi: 10.1016/s0014-2999(00)00140-0. [DOI] [PubMed] [Google Scholar]
- [210].Zobali F, Besler T, Ari N, Karasu C. (Antioxidants in Diabetes-Induced Complications) TheADIC Study Group. Hydrogen peroxide-induced inhibition of vasomotor activity: evaluation of single and combined treatments with vitamin A and insulin in streptozotocin-diabetic rats. Int J Exp Diabetes Res. 2002;3:119–30. doi: 10.1080/15604280214484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–91. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Rodríguez-Mañas L, El-Assar M, Vallejo S, López-Dóriga P, Solís J, Petidier R, Montes M, Nevado J, Castro M, Gómez-Guerrero C, Peiró C, Sánchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–38. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- [214].Barona J, Aristizabal JC, Blesso CN, Volek JS, Fernandez ML. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J nutr. 142:1626–32. doi: 10.3945/jn.112.162743. [DOI] [PubMed] [Google Scholar]
- [215].Landberg R, Naidoo N, van Dam RM. Diet and endothelial function: from individual components to dietary patterns. Curr Opin Lipidol. 2012;23:147–55. doi: 10.1097/MOL.0b013e328351123a. [DOI] [PubMed] [Google Scholar]
- [216].Chong MF, Macdonald R, Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr. 2010;104(Supplement 3):S28–39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- [217].Ghosh D, Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res. 2009;53:322–31. doi: 10.1002/mnfr.200800182. [DOI] [PubMed] [Google Scholar]
- [218].Mulvihill EE, Huff MW. Antiatherogenic properties of flavonoids: implications for cardiovascular health. Can J Cardiol. 2010;26(Supplement A):17A–21A. doi: 10.1016/s0828-282x(10)71056-4. [DOI] [PubMed] [Google Scholar]
- [219].Yubero-Serrano EM, Garcia-Rios A, Delgado-Lista J, Delgado-Casado N, Perez-Martinez P, Rodriguez-Cantalejo F, Fuentes F, Cruz-Teno C, Tunez I, Tasset-Cuevas I, Tinahones FJ, Perez-Jimenez F, Lopez-Miranda J. Postprandial effects of the Mediterranean diet on oxidant and antioxidant status in elderly men and women. J Am Geriatr Soc. 2011;59:938–40. doi: 10.1111/j.1532-5415.2011.03381.x. [DOI] [PubMed] [Google Scholar]
- [220].Perez-Martinez P, Garcia-Quintana JM, Yubero-Serrano EM, Tasset-Cuevas I, Tunez I, Garcia-Rios A, Delgado-Lista J, Marin C, Perez-Jimenez F, Roche HM, Lopez-Miranda J. Postprandial oxidative stress is modified by dietary fat: evidence from a human intervention study. Clin Sci (Lond) 2010;119:251–61. doi: 10.1042/CS20100015. [DOI] [PubMed] [Google Scholar]
- [221].Peña-Orihuela P, Camargo A, Rangel-Zuñiga OA, Perez-Martinez P, Cruz-Teno C, Delgado-Lista J, Yubero-Serrano EM, Paniagua JA, Tinahones FJ, Malagon MM, Roche HM, Perez-Jimenez F, Lopez-Miranda J. Antioxidant system response is modified by dietary fat in adipose tissue of metabolic syndrome patients. J Nutr Biochem. 2013 doi: 10.1016/j.jnutbio.2013.02.012. In Press. [DOI] [PubMed] [Google Scholar]
- [222].Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, DeCaterina R, Carluccio MA. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–9. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- [223].Cruz-Teno C, Pérez-Martínez P, Delgado-Lista J, Yubero-Serrano EM, García-Ríos A, Marín C, Gómez P, Jiménez-Gómez Y, Camargo A, Rodríguez-Cantalejo F, Malagón MM, Pérez-Jiménez F, Roche HM, López-Miranda J. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: the LIPGENE study. Mol Nutr Food Res. 2012;56:854–65. doi: 10.1002/mnfr.201200096. [DOI] [PubMed] [Google Scholar]
- [224].Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Caballero J, Marin C, Gutierrez-Mariscal FM, Tinahones FJ, Villalba JM, Tunez I, Perez-Jimenez F, Lopez-Miranda J. Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q(10) in elderly men and women. Age (Dordr) 2013;35:159–70. doi: 10.1007/s11357-011-9331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Aires DJ, Rockwell G, Wang T, Frontera J, Wick J, Wang W, Tonkovic-Capin M, Lu J, E L, Zhu H, Swerdlow RH. Potentiation of dietary restriction-induced lifespan extension by polyphenols. Biochim Biophys Acta. 2012;1822:522–6. doi: 10.1016/j.bbadis.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


