Abstract
Neutralization capsid epitopes are important determinants for antibody-mediated immune protection against papillomavirus (PV) infection and induced disease. Chimeric L1 major capsid proteins of the human PV type 16 (HPV-16) and the bovine PV type 1 (BPV-1) with a foreign peptide incorporated into several capsid surface loops self-assembled into pentamers or virus-like particles (VLP). Binding patterns of neutralizing monoclonal antibodies (MAb) and immunization of mice confirmed (i) that regions around aa 282–286 and 351–355 contribute to neutralization epitopes and identified the latter region as an immunodominant site and (ii) that placing a foreign peptide in the context of an assembled structure markedly enhanced its immunogenicity. Pentamers disassembled from wild-type HPV-16 and BPV-1 VLPs displayed some of the neutralization epitopes that were detected on fully assembled VLPs, but were deficient for binding a subset of neutralizing MAb that inhibit cell attachment.
Papillomavirus (PV) infections are widespread in animals and humans, causing mostly benign epithelial proliferations, papillomas or warts, of the skin and mucous membranes (Howley, 1991; Lowy et al., 1994; zur Hausen, 1991). Infection with oncogenic types, most often human PV type 16 (HPV-16), has been aetiologically associated with the development of anogenital malignancies, in particular cervical cancer (zur Hausen, 1994). PVs are nonenveloped ~ 8 kb double-stranded DNA viruses with a T = 7 icosahedral symmetry and a diameter of approximately 55 nm (Baker et al., 1991). The capsid consists of 60 hexavalent and 12 pentavalent capsomers, with each capsomer being a pentamer composed of 5 L1 major capsid proteins. The second viral structural protein, the minor capsid protein L2, is required for genome encapsidation and the generation of infectious virions (Day et al., 1998; Roden et al., 1996a; Trus et al., 1997). Despite the development of in vitro and in vivo methods, there is still no efficient tissue culture system or laboratory animal to easily propagate HPV, which has hampered immunological and structural studies of the virion (Kreider et al., 1987; Meyers et al., 1997; Roden et al., 1996a).
In recent years progress has been achieved towards development of a prophylactic HPV vaccine to prevent genital HPV infection and associated benign and dysplastic lesions (Kirnbauer, 1996; Schiller & Okun, 1996). Capsid protein(s) L1 alone, or L1 plus L2, expressed in eukaryotic cells self-assembles into virus-like particles (VLP) which are morphologically and antigenically similar to native virions, but lack potentially oncogenic DNA. Immunization with L1 or L1/L2 VLP generated sera containing neutralizing antibodies that conferred type-specific and long-lasting protection in several animal models (Bell et al., 1994; Breitburd et al., 1995; Christensen et al., 1996; Kirnbauer et al., 1992, 1993, 1996; Rose et al., 1998; Suzich et al., 1995). Neutralizing antibodies are thus considered the major protective component in prophylactic PV vaccination.
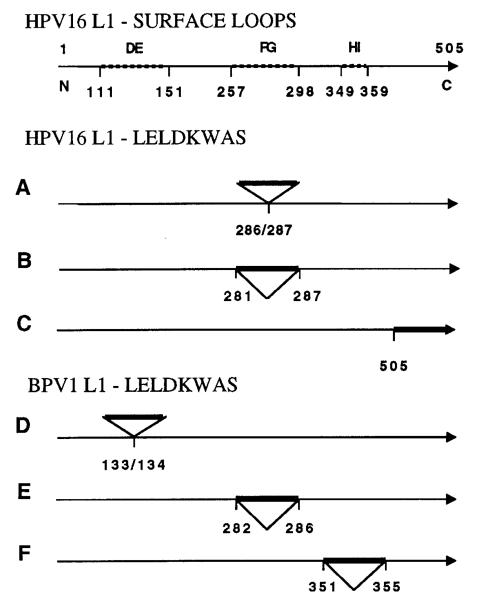
Because neutralization PV epitopes are non-linear and conformation-dependent, their amino acid composition and surface localization are incompletely characterized. Monoclonal antibody (MAb) binding and immunization studies have tentatively mapped neutralization epitopes of the low-risk HPV-11 along a 20 aa stretch of L1 (residues 120–140), immunodominant sites at aa 131–132 and 346–349 (Ludmerer et al., 1997, 2000), and have implicated L1 residues 50, 266 and 282 in HPV-16 neutralization epitopes (Roden et al., 1997; White et al., 1998). To further explore these epitopes and the potential of VLP as an immunogenic vaccine carrier, we engineered the 8 aa B-cell epitope LELDKWAS (Leu-Glu-Leu-Asp-Lys-Trp-Ala-Ser) from the human immunodeficiency virus type 1 (HIV-1) gp41 envelope glycoprotein into the L1 protein of HPV-16 and bovine PV type 1 (BPV-1) using inverse touchdown PCR (Ochman et al., 1988). Oligonucleotides encoding the epitope were cloned into several hypervariable regions of the L1 ORF of the HPV-16 baculovirus transfer vector 114K-L1-pSynwtVI- (Kirnbauer et al., 1993) and BPV-1 L1-pEVmod (Kirnbauer et al., 1992). These regions are likely to encode surface structures in the assembled capsid (Fig. 1) (Chen et al., 2000). For HPV-16 L1, recombinant baculovirus expression vectors were generated with the peptide inserted between amino acid residues 286/287, 281/287 (replacing aa 282–286) or fused to the C terminus as a control (Fig. 1A–C). We also designed BPV-1 L1 chimeras that incorporate the peptide in three different hypervariable regions, between amino acid residues 133/134, 282/286 (replacing aa 283–285) or 351/355 (replacing aa 352–354) (Fig. 1D–F). Recombinant proteins were immunoblotted using MAb Camvir-1 (Pharmingen) and AU-1 (BAbCO), directed against linear epitopes of HPV-16 L1 and BPV-1 L1, or MAb 2F5, which recognizes the LELDKWAS epitope (AIDS Research and Reference Reagent Program, NIAID, NIH, USA) (Muster et al., 1994) (data not shown). Following purification of high molecular mass complexes on density gradients, HPV-16 and BPV-1 L1 chimeric proteins were analysed by transmission electron microscopy (TEM). Micrographs demonstrated pentamers and full-size or smaller VLP, indicating that peptide insertion did not prevent L1 self-assembly into higher-ordered structures (data not shown). Further evidence for assembly of chimeric BPV-1 L1 proteins was obtained by analytical centrifugation on 20–40% sucrose density gradients demonstrating a migration pattern which corresponded to both wild-type (wt) L1 VLP (bottom of gradient) as well as disassembled wt L1 pentamers (top of gradient) that were run in parallel (data not shown). These results supported the presence of both VLP and pentamers in chimeric L1 preparations as observed by TEM.
Fig. 1.
Construction of chimeric HPV-16 (A–C) and BPV-1 (D–F) L1–LELDKWAS baculovirus expression vectors. HPV-16 capsid surface loops DE, FG, HI (dotted lines) and amino acid numbering are indicated (Chen et al., 2000). The LELDKWAS peptide (indicated by a bold line) was engineered into the L1 ORF between the indicated amino acid residues, encoding the following deduced chimeric L1 epitope sequences with the added amino acids in brackets: A, TANL(ELDKW)ASSN; B, KGSG(LELDKW)ASSN; C, KKRKL(ELDKWAS); D, DATL(ELDKWA)SVHF; E, RKVT(LELDKWAS)TQTT; and F, TPLT(LELDKWA)SSKF (oligonucleotide primer sequences are available on request). Loops and inserts are not drawn to scale. N, C, amino- and carboxy-terminus.
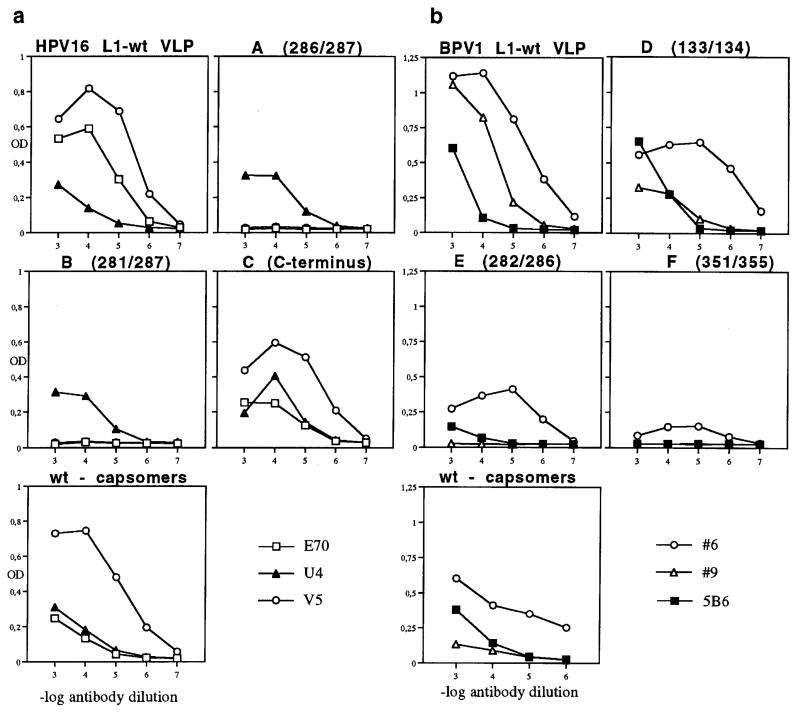
We next examined if amino acid deletion and/or peptide insertion at a hypervariable region of HPV-16 L1 would interrupt neutralizing MAb-binding to conformation-dependent epitopes by ELISA (Roden et al., 1997). Two classes of MAb that neutralize HPV-16 virions have been described, MAbs E70 and V5, which inhibit virus to cell binding (referred to here as class A), and U4, which does not inhibit binding (class B) (Roden et al., 1996b). By ELISA, both classes of MAb bound the HPV-16 C-terminal fusion protein similar to wt VLP (Fig. 2a). There was a complete loss of binding for class A antibodies E70 and V5 to chimeras A 286/287 and B 281/287. In contrast, binding of the class B MAb U4 to chimeras A 286/287 and B 281/287 was similar to that of wt VLP. These results confirm and extend our previous observation that amino acids around 282 are part of the E70 epitope and also contribute to the V5 epitope (Roden et al., 1997).
Fig. 2.
(a) Binding of HPV-16-neutralizing MAb to wt HPV-16 L1 VLP and chimeric HPV-16 L1 proteins A, B, C or disassembled wt capsomers by ELISA. MAbs E70 and V5 inhibit haemagglutination (class A); U4 does not inhibit haemagglutination (class B). (b) Binding of BPV-neutralizing MAb to wt BPV L1 VLP and chimeric BPV-1 L1 proteins D, E, F or disassembled wt capsomers by ELISA. Neutralizing MAbs #6 and #9 inhibit haemagglutination (class A); 5B6 does not inhibit haemagglutination (class B). OD, absorbance at 405 nm.
It has been shown that HPV-11 capsomers contain conformational epitopes found on intact VLP (Rose et al., 1998). To examine whether VLP are required for MAb binding or whether HPV-16 L1 pentamers are sufficient to display the epitopes, wt HPV-16 L1 capsids were disassembled into pentamers and the absence of VLP was verified by TEM and sucrose gradient (data not shown). By ELISA, MAbs V5 and U4 bound wt L1 pentamers with kinetics similar to wt VLP, whereas absorbance values for MAb E70 were significantly reduced. These results indicated that the E70 epitope is partially sensitive to VLP disassembly (Fig. 2a), and HPV-16 L1 pentamers appear sufficient for the display of the MAb V5 (class A) and U4 (class B) epitopes. As shown previously for low-risk HPV-11, we demonstrate for the first time that high-risk HPV-16 L1 capsomers display at least a subset of the neutralization epitopes found on full-size VLP.
As with HPV, two classes of MAb that neutralize BPV virions have been described, those represented by MAbs #6 and #9 (both of class A), and MAb 5B6 (class B) (Roden et al., 1996b). When BPV-1 chimeric L1 proteins or pentamers disassembled from wt L1 VLP under reducing conditions (3% 2-mercaptoethanol/150 mM NaCl) (McCarthy et al., 1998) were analysed by ELISA (Fig. 2b), binding of MAb #6 (class A) to wt L1 VLP, the chimeric BPV-1 L1 proteins D 133/134, E 282/286 and wt L1 pentamers was observed. Data for MAb #6 binding to chimera F 351/355 were inconclusive as absorbance values did not decline across 3 logs of antibody dilution. These results indicate that wt pentamers appear sufficient for the display of the #6 epitope and that the determinants were not contained within the targeted L1 regions of chimeras D 133/134 and E 282/286. Antibody #9 (class A) recognized VLP of wt L1 and chimera D 133/134, but no binding was seen with chimeras E 282/286 and F 351/355. This finding raises the possibility that peptide insertion at aa 282/286 and 351/355 had either directly targeted the respective BPV neutralization epitope or indirectly affected the quaternary structure of the particles. In addition, wt pentamers did not react, indicating that MAb #9 requires assembly into VLP for binding. The above findings add to published results indicating that residues 282–286 and 351–355 form part of the neutralization epitopes (Ludmerer et al., 1997, 2000; Roden et al., 1997; White et al., 1998). Reactivity of MAb 5B6 (class B) was specifically absent for construct F 351/355, indicating that peptide targeting to aa 351/355 interrupted the 5B6 epitope. This interpretation is consistent with the observation that aa 346 of the analogous region of HPV-11 L1 is critical for binding of a neutralizing MAb (Ludmerer et al., 1996), although an indirect effect on quaternary structure remains possible. Interestingly, in high resolution reconstructions, MAb 5B6 binds monovalently or divalently to (neighbouring) hexavalent capsomers just above intercapsomer cross-bridges (Trus et al., 1997). Taken together these data suggest that aa 351–355 contribute to the 5B6 epitope at a virion surface location close to the interpentamer connections. In addition, pentamers appear sufficient for the display of this neutralization epitope. Our results also identify the existence of two sub-classes of neutralizing antibodies within those antibodies that interfere with binding of particles to cell surface (class A). One sub-class (MAb #6) can bind both to intact VLPs and to pentamers/capsomers, while the other sub-class (MAb #9) requires the higher-ordered structure of VLP for binding. The BPV pentamer results are similar to those for the HPV-16 pentamers, which were deficient for E70 binding but bound V5 similarly to intact VLPs.
To examine the effect of peptide insertion on the immunogenicity of BPV-1 L1 proteins, three mice each were injected with native or denatured protein preparations and boosted twice in 2-week intervals. Two weeks after the last immunization, pooled sera were analysed for antibodies against the LELDKWAS peptide and for BPV-neutralizing activity using an in vitro focus-forming assay (Dvoretzky et al., 1980). Sera raised against native preparations of chimeras D 133/134, E 282/286 and wt VLP neutralized BPV virions with a titre of 1000, 100 and 10000 respectively. This indicated that these chimeric proteins retain PV-neutralization epitopes, although the potency to induce neutralization activity was lower than that of wt VLP. These results suggest the feasibility of inserting epitopes of choice into immunogenic regions of L1, e.g. to generate HPV vaccines with a broad-spectrum protection. In contrast, sera raised against chimera F 351/355 were non-neutralizing, suggesting that peptide insertion at residues 351/355 had disrupted a major immunogenic determinant(s).
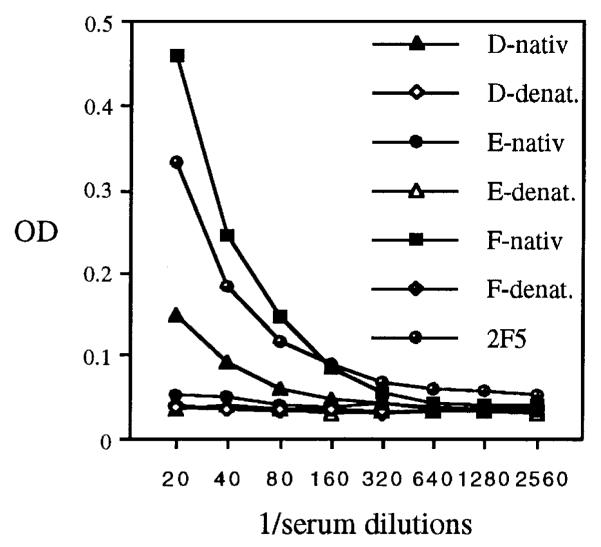
To evaluate the potential of assembled chimeric BPV-1 L1 proteins to induce antibodies against the inserted epitope, sera were analysed by ELISA using alkaline phosphatase (AP)–LELDKWAS as the antigen (Fig. 3) (Kerschbaumer et al., 1996). Antisera raised against chimera E 282/286 showed insignificant binding to the LELDKWAS peptide, whereas sera raised against chimera F 351/355 showed reactivity comparable to that obtained with MAb 2F5. Thus insertion of a foreign peptide at 351/355 (chimera F) generated a protein that efficiently induced anti-peptide antibodies, while binding of MAb 5B6 and the capacity to induce BPV-neutralizing antisera were completely abrogated. This change of function provides additional evidence that amino acids around 351/355 contribute to an immunodominant neutralization surface structure. Sera raised against chimera D 133/134 also demonstrated significant binding, implicating residues 133/134 as part of another immunogenic region. Remarkably, denatured proteins did not induce immunoreactivity against the peptide, although sera contained antibodies to linear L1 epitopes. This indicated that placing a foreign peptide in the context of an assembled structure markedly enhanced its immunogenicity, whereas the peptide appeared subdominant when presented as denatured (linear) chimeric L1–LELDKWAS protein.
Fig. 3.
Reactivity of immune sera in the LELDKWAS epitope ELISA. Three BALB/c mice each were injected subcutaneously with 50 μg (estimated by SDS–PAGE) of native or heat/SDS-denatured protein in complete Freund’s adjuvant followed by two boosts in incomplete Freund’s adjuvant at 2-week intervals. Two weeks after the last immunization, serum samples were collected, pooled and heat-inactivated. Twofold serial dilutions of immune sera raised against native or denatured chimeric L1 proteins D, E or F, or dilutions of MAb 2F5 (1 ng/ml) were reacted against the LELDKWAS peptide fused to the C terminus of AP, expressed in and affinity-purified from E. coli and attached to ELISA plates. OD, absorbance at 405 nm.
The sera neither reacted to AP–scF control protein nor neutralized macrophage (BAL) and T-cell tropic (IIIb) HIV-1 laboratory strains by in vitro assays, whereas MAb 2F5 directed against this epitope was neutralizing (data not shown). It is likely that in the context of chimeric VLP the epitope did not present in its native conformation as detected on infectious HIV virions. Alternatively, it is possible that the antibody concentration in the sera was too low to score in the assay.
This observation represents an important extension to previous results generating autoantibodies against a self-antigen (Chackerian et al., 1999), supporting the potential of a virus structure as a vaccine carrier (Zinkernagel & Hengartner, 1997).
Our results corroborate the functional relevance of the crystal structure of an HPV-16 L1 assembled into small T = 1 particles (Chen et al., 2000), demonstrating that highly variable positions exposed on the pentamer surface can tolerate insertion of heterologous amino acids without losing the salient structural characteristics of L1. By L1 sequence comparison, we had grafted the epitope into three distinct surface loops that connect strands of the β-jelly roll of the L1 monomer, i.e. the DE loop (comprised of HPV-16 aa 111–151), the FG loop (aa 257–298) and the HI loop (aa 349–359) (Fig. 1). Insertion resulted in either a loss or change of function for immunogenic capsid regions (Figs 2 and 3), supporting the role of surface loops as virion neutralization determinants.
The identification of neutralization, immunogenic and mutation-permissive capsid regions may be relevant for HPV vaccine design and allows a rational approach for the insertion of epitopes of choice. The display of foreign peptides in the context of an immunogenic self-assembled L1 oligomer can induce a vigorous humoral immune response and may thus become a more general procedure to overcome the low immunogenicity of free peptide antigens. This may become an attractive strategy to generate a cross-protective vaccine against multiple HPV types or other, e.g. sexually transmitted, diseases.
Acknowledgments
We thank Doug Lowy, John Schiller and Thomas Muster for critical reading of the manuscript. This work was supported by grants to R.K. from the Austrian Science Foundation (FWF P13365-MED), the Austrian National Bank (Jubiläumsfonds no. 7688) and the Kommission Onkologie of the University of Vienna Medical School.
References
- Baker TS, Newcomb WW, Olson NH, Cowsert LM, Olson C, Brown JC. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophysical Journal. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, Sunberg JP, Ghim SJ, Newsome J, Jenson AB, Schlegel R. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology. 1994;62:194–198. doi: 10.1159/000163910. [DOI] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. Journal of Virology. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proceedings of the National Academy of Sciences, USA. 1999;96:2373–2378. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Molecular Cell. 2000;5:557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- Christensen N, Reed C, Cladel N, Han R, Kreider J. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. Journal of Virology. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Roden RBS, Lowy DR, Schiller JT. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. Journal of Virology. 1998;72:142–150. doi: 10.1128/jvi.72.1.142-150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I, Shober R, Chattopadhyay SK, Lowy DR. Focus assay in mouse cells for bovine papillomavirus. Virology. 1980;103:369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Howley PM. Role of the human papillomaviruses in human cancer. Cancer Research. 1991;51(Suppl. 18):S5019–S5022. [PubMed] [Google Scholar]
- Kerschbaumer RJ, Hirschl S, Schwager C, Ibl M, Himmler G. pDAP2: a vector for construction of alkaline phosphatase fusion proteins. Immunotechnology. 1996;2:145–150. doi: 10.1016/1380-2933(96)00040-1. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R. Papillomavirus-like particles for serology and vaccine development. Intervirology. 1996;39:54–61. doi: 10.1159/000150475. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proceedings of the National Academy of Sciences, USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Taub J, Greenstone H, Roden R, Durst M, Gissmann L, Lowy DR, Schiller JT. Efficient self-assembly of human papillomavirus type 16 L1 and L1–L2 into virus-like particles. Journal of Virology. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Chandrachud L, O’Neil B, Wagner E, Grindlay G, Armstrong A, McGarvie G, Schiller J, Lowy D, Campo M. Virus-like particles of bovine papillomavirus type-4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- Kreider JW, Howett MK, Leure DA, Zaino RJ, Weber JA. Laboratory production in vivo of infectious human papillomavirus type 11. Journal of Virology. 1987;61:590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy DR, Kirnbauer R, Schiller JT. Genital human papillomavirus infection. Proceedings of the National Academy of Sciences, USA. 1994;91:2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmerer SW, Benincasa D, Mark GE. Two amino acid residues confer type specificity to a neutralizing, conformationally dependent epitope on human papillomavirus type 11. Journal of Virology. 1996;70:4791–4794. doi: 10.1128/jvi.70.7.4791-4794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmerer SW, Benincasa D, Mark GE, Christensen ND. A neutralizing epitope of human papillomavirus type 11 is principally described by a continuous set of residues which overlap a distinct linear, surface-exposed epitope. Journal of Virology. 1997;71:3834–3839. doi: 10.1128/jvi.71.5.3834-3839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmerer SW, McClements WL, Wang XM, Ling JC, Jansen KU, Christensen ND. HPV11 mutant virus-like particles elicit immune responses that neutralize virus and delineate a novel neutralizing domain. Virology. 2000;266:237–245. doi: 10.1006/viro.1999.0083. [DOI] [PubMed] [Google Scholar]
- McCarthy MP, White WI, Palmer Hill F, Koenig S, Suzich JA. Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro. Journal of Virology. 1998;72:32–41. doi: 10.1128/jvi.72.1.32-41.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C, Mayer TJ, Ozbun MA. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. Journal of Virology. 1997;71:7381–7386. doi: 10.1128/jvi.71.10.7381-7386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. Journal of Virology. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Gerber AS, Hartl DL. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden R, Greenstone H, Kirnbauer R, Booy F, Joel J, Lowy D, Schiller J. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. Journal of Virology. 1996a;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden R, Hubbert N, Kirnbauer R, Christensen N, Lowy D, Schiller J. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. Journal of Virology. 1996b;70:3298–3301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden RB, Armstrong A, Haderer P, Christensen ND, Hubbert NL, Lowy DR, Schiller JT, Kirnbauer R. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. Journal of Virology. 1997;71:6247–6252. doi: 10.1128/jvi.71.8.6247-6252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R, White W, Maolin L, Suzich J, Lane C, Garcea R. Human papillomavirus type 11 recombinant L1 capsomeres induce virusneutralizing antibodies. Journal of Virology. 1998;72:6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JT, Okun M. Papillomavirus vaccines: current status and future prospects. Advances in Dermatology. 1996;11:355–380. [PubMed] [Google Scholar]
- Suzich JA, Ghim S, Palmer-Hill FJ, White WI, Tamura JK, Bell J, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents thedevelopment of viral mucosal papillomas. Proceedings of the National Academy of Sciences, USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Roden RB, Greenstone HL, Vrhel M, Schiller JT, Booy FP. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nature Structural Biology. 1997;4:413–420. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- White WI, Wilson SD, Bonnez W, Rose RC, Koenig S, Suzich JA. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. Journal of Virology. 1998;72:959–964. doi: 10.1128/jvi.72.2.959-964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM, Hengartner H. Antiviral immunity. Immunology Today. 1997;18:258–260. doi: 10.1016/s0167-5699(97)80017-5. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Current Topics in Microbiology and Immunology. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]