Abstract
Liver disease continues to represent a critical mediator of morbidity and mortality in those with human immunodeficiency virus (HIV) infection. The frequent presence and overlap of concomitant injurious processes, including hepatitis C virus and hepatitis B virus infections, hepatoxicity associated with antiretroviral therapeutic agents, alcohol, and other toxins, in the setting of immunosuppression lead to rapid fibrotic progression and early development of end-stage liver disease. This conference summary describes the proceedings of a state-of-the-art gathering of international experts designed to highlight the status of current research in epidemiology, natural history, pathogenesis, and treatment of HIV and liver disease.
Epidemiologic and natural history studies have clearly demonstrated the significant role played by liver-associated morbidity and mortality among those infected with human immunodeficiency virus (HIV) in the post-HAART (highly active antiretroviral therapy) era. The reasons for this are complex, but include coinfections with hepatitis B virus (HBV), hepatitis C virus (HCV), drug-related hepatotoxicity, alcohol, and a modified cytokine milieu that contributes to increased rates of fibrotic progression and advanced liver disease. There is a mounting body of evidence that HIV directly infects, or through its protein products mediates, hepatic stellate cells (HSCs), hepatocytes, and hepatic-dwelling lymphocytes affecting collagen production, hepatocyte survival, and risk of hepatocellular carcinoma (HCC). Furthermore, there may be a complex interaction between gut wall permeability, which is mediated by HIV infection of the gut-associated lymphatic tissue, and hepatic clearance of endotoxin from the portal and systemic circulation. The hepatology community has an increasing responsibility in the care and management of patients living with HIV infection.
In September 2010, the 3rd International Forum on HIV and Liver Disease was convened in Jackson Hole, WY. This biennial event brings together national and international experts in the field of HIV and liver disease, drawing from the disciplines of hepatology, infectious diseases, epidemiology/public health, behavioral medicine, drug regulatory affairs, pharmaceutical development, and basic scientists in viral immunology, virology, and liver injury and repair. The meeting received National Institutes of Health support via a grant cofunded by the National Institute of Allergy and Infectious Diseases, National Institute of Diabetes and Kidney Diseases, and the National Institute on Alcohol Abuse and Alcoholism, as well as by unrestricted grant support from multiple pharmaceutical industry sponsors. The meeting sought to define the current status of knowledge in key areas related to epidemiology, pathogenesis, treatment, and management of liver disease in those with HIV infection. The proceedings of the prior two meetings were published in Hepatology, and this summary seeks to update the progress made in the previous 2 years and to redefine the cutting edge of research needs.
Epidemiology and Natural History
(Brooks, Brau, Garlassi, and Qazi)
Currently, approximately 1.1 million Americans are living with HIV. Those with cluster of differentiation (CD)4 counts less than 200 or experiencing an acquired immunodeficiency syndrome (AIDS)-defining opportunistic infection are considered to have AIDS. The incidence is 50,000–60,000 new cases of HIV infection per year. This is relatively stable and contributes to the increasing number of people living with HIV infection. Men are affected approximately 3 times more than women. The majority of infections in men (71%) occur in men who have sex with men (MSM). Approximately 10% are attributable to injection drug use (IDU). In contrast, 83% of new infections in women are associated with heterosexual contact. Data from New York shows an increasing attribution of infection to heterosexual sexual exposure. Black/African Americans are substantially more likely to have or acquire HIV infection, compared to other racial/ethnic groups. Hispanic/Latino men and women have greater risk than Caucasians but less than black/African-American individuals. Increased risk does not appear to be an artifact of increased testing. Among young MSM (age 13–24) there is a dramatic increase in HIV incidence. However, there is also a segmental increase in rates of new infection among those over 50 years of age in the United States and other countries. U.S. poverty areas (>20% residents with household income less than poverty level) have rates of HIV prevalence similar to that observed in Ethiopia, Haiti, Angola, and other developing countries. In these areas in the United States, household geography trumps race and ethnicity as a risk factor for HIV. The majority of new infections are in the southeast United States. Only New York City is not in the southeast among the top 10 metropolitan areas for incident HIV infection.
The introduction of HAART in 1996 led to a dramatic decrease in death and hospitalization related to HIV opportunistic infections. However, liver-related end organ disease, malignancy, metabolic disease, and neurocognitive dysfunction have dramatically increased among those living with HIV. The French mortality studies (2000 and 2005) describe increasing proportions of liver deaths (13.4%–15.4% over the 5-year interval), with a concomitant increase in deaths from HCC from 15% to 25% (P = 0.03).1 This observation highlights the need to focus on liver disease in HIV-infected patients. Studies have described increased rates of hepatic fibrosis among HIV-infected patients in cohorts where paired liver biopsies were evaluated. 2,3 Interestingly, CD4 lymphocyte count was not associated with risk of progression in several cohorts, but the presence of a detectable HIV viral load was.4 The progression from development of cirrhosis to liver failure also appears to be accelerated in HIV-positive individuals. Pineda et al. reported a 5-year incidence of hepatic decompensation among HIV positive cirrhotics, which is nearly twice the rate observed in those with HCV alone.5 There are no comparative incidence data regarding HCC development in cirrhotic patients with HIV. Data from several cross-sectional studies show increased rates of prevalence, but the denominator is not known, and this may represent increased rates of tumor reporting. Development of surveillance cohorts will be needed to clearly define HCC incidence. Data from one study suggested that HCC surveillance at precirrhotic stages of HCV/HIV infection may improve survival, but this will require study in larger cohorts. The survival of patients with HCC appears to be similar regardless of the presence of absence of HIV in the setting of HCV infection in some cohorts, but not others. The continuation of HAART after diagnosis of HCC was associated with improved survival in an Italian cohort.6
Acute HCV infection does appear to be more prevalent in HIV-positive MSM, with reports from many cities, including San Francisco, London, and Paris, documenting high rates of disease transmission associated with high-risk sexual practices. These include fisting and receptive and insertive anal sex when other sexually transmitted disease lesions are present. Recent phylogenetic analysis data suggest that there are parallel epidemics underway in Europe, the United States, and Australia, but there is little mixing between continents or even between major metropolitan areas in the United States. Some patients appear to have more significant liver disease on biopsy than might be suspected by duration of infection.7
HIV Treatment
(Kurtizkes and Feinberg)
When to Start
Antiretroviral therapy (ART) has saved millions of lives and is one of the most important breakthroughs in medicine. One of the big questions in HIV management is at what CD4 cell count should treatment be initiated. Historically, there was rapid appreciation for the efficacy of ART in preventing AIDS opportunistic infections, such as pneumocystis pneumonia. However, early ART combinations also caused substantial toxicities and were expensive. Because AIDS opportunistic infections chiefly occurred in persons with CD4 lymphocyte counts below 200/mm3, many guidelines recommended treatment only for persons in that low CD4 lymphocyte stratum. However, ART is now safer, and there are new data that suggest that ART should be administered at higher CD4 lymphocyte counts and, possibly, to all individuals with HIV infection. In fact, a recent Department of Health and Human Services (DHHS) panel recommended ART be given or at least considered for nearly all HIV-infected persons (http://aidsinfo.nih.gov/guidelines), echoing recommendations from the International Antiviral Society-USA (IAS-USA) and other groups.8 European guidelines are also under revision and are likely to reflect the view that earlier treatment of HIV should be considered, compared to current practices.
All guidelines now recommend ART treatment for persons with symptomatic HIV infection (e.g., an opportunistic infection) and at CD4 lymphocyte counts below 350/mm3. The pivotal study was done in Haiti. In a randomized, controlled study, persons who received ART at CD4 lymphocyte counts between 200 and 350/mm3 survived longer than those who started with CD4 lymphocyte counts below 200/ mm3.9 That study provides level 1A evidence for the guideline to start ART below 350/mm3. There are also data that suggest persons at even higher CD4 lymphocyte counts should be treated. The North American AIDS Cohort Collaboration on Research and Design is a large, observational, multisite cohort database that reported improved survival in patients who started ART at CD4 lymphocyte counts 350–500/mm3 and even over 500/mm3, compared to those not on ART, even after adjusting for differences, such as IDU, that might also affect ART and survival.10 Another study tested whether the toxicity (and expense) of therapy could be avoided by drug-free intervals. Patients were randomized to either start and continue ART or treat until CD4 lymphocyte count was sustained above 350/mm3, then stop and monitor until the count dropped back below that threshold and restart. This strategy was very popular with both patients and healthcare providers, because patients could have “drug holidays” and avoid some of the side effects of their antiretroviral regimens. Contrary to what was expected, those in the Strategies for Management of Anti Retroviral Therapy (SMART) study interrupted treatment group did worse and actually experienced more non-AIDS events.11 In contrast, another study showed an attenuation of treatment advantage in persons with CD4 lymphocyte counts near 400/mm3.12 Reflecting differences in the interpretation of these studies, European guidelines suggest ART should be deferred at CD4 lymphocyte counts above 500/mm3, whereas two U.S.-based guidelines recommend at least consideration of treatment at CD4 lymphocyte counts above 500/mm3 (www.eacs.eu/guidelines) (http://aidsinfo.-nih.gov/guidelines).13
What to Use
There is less controversy about what ART to use. The DHHS and IAS-USA guidelines provide a hierarchy of recommendations based on quality of evidence. There are three types of preferred regimens and four preferred regimens, as well as some alternatives that might be considered in certain circumstances (Fig. 1) (http://www.aidsinfo.nih.gov/guidelines).13
Fig. 1.

2010 current treatment guidelines for preferred initial antiretroviral regimens for HIV/HCV-coinfected patients. Rating of recommendation: A = strong; B = moderate; C = optional. Rating of evidence: I = data from randomized, controlled trials; II = data from well-designed nonrandomized trials or observational cohort studies with long-term clinical outcomes; III = expert opinion (www.aidsinfo.nih.-gov/guidelines; accessed on June 14, 2011).
HIV/HCV-Coinfected Persons
There is little strong evidence that ART decisions differ for HIV/HCV-coinfected persons. There are some data that suggest ART can attenuate the progression of liver fibrosis.14,15 However, other studies have not detected an effect of ART on fibrosis progression, and it is hard to discount factors, such as alcohol use, that might confound such analyses.16 In addition, because ART is recommended at higher and higher CD4 lymphocyte thresholds, the question of whether HCV infection provides an independent justification for ART refers to few individuals, perhaps only those with CD4 lymphocyte counts above 500/mm3, and available data provide very little insight into this particular subset. Likewise, there are few data to suggest that any particular ART is preferred in an HIV/HCV-coinfected person in relation to attenuation of liver disease progression. An indirect exception is tipranavir use, which is discouraged in patients with any form of advanced liver disease because of a ~3-fold increased risk of liver injury.17 There is concern regarding older regimens containing deoxynucleotide analogs because these drugs may increase the risk of hepatic steatosis. There are also concerns raised by some investigators about the development of hepatoportal sclerosis associated with previous use of didanosine (ddI).18,19
Pathogenesis
(Kleiner, Chang, Bansal, Sterling, and Szabo)
The mechanisms of liver injury in the HIV-infected patient reflect multiple derangements that attend HIV infection. A consideration of the pathogenesis of HIV itself is instructive. After acute HIV infection, there is a depletion of intestinal CD4 cells. HIV-mediated depletion of T cells in the gut promotes intestinal microbial translocation, which augments immune activation and bystander activation of T cells, ultimately hastening the loss of T cells.20 For pathogen-specific T cells, such as HCV-specific T cells, this leads to the loss or dysfunction of HCV-specific responses and loosened containment of infection. HIV-associated microbial translocation also stimulates hepatic fibrogenesis by virtue of the profibrotic effects of elevated circulating levels of lipopolysaccharide (LPS). LPS binds to Toll-like receptor 4 on HSCs and contributes to their release of extracellular matrix (ECM) by enhancing their sensitivity to the profibrogenic cytokine, transforming growth factor beta (TGF-β).21
HIV infection itself exerts direct and indirect effects on the liver, including induction of profibrogenic cytokine production by hepatocytes as well as nonparenchymal cells, including sinusoidal endothelial cells and Kupffer cells. There are limited reports of direct HIV infection of hepatocytes.22 More accepted is the role of gp120, the major HIV envelope glycoprotein, which has been shown to engage the chemokine receptors (and HIV coreceptors), CXCR4 and CCR5, on the hepatocyte and stellate cell surface.23,24 This engagement induces signal-transduction pathways that promote HCV replication, profibrogenic cytokine release (e.g., TGF-β), and oxidative stress.23,25,26
There is additional suggestive evidence from hepatic cell lines that HIV may infect HSCs, which results in the secretion of profibrogenic and -inflammatory cytokines, such as monocyte chemoattractant protein-1 and interleukin (IL)-6.27 Activated stellate cells are also triggered to release ECM, including collagen I, and tissue inhibitor of metalloproteinase 1, a key inhibitor of fibrolysis, in the presence of HIV. Additional data support that HCV also indirectly induces oxidative stress in HSCs; this may be a result of engagement by HCV of its coreceptor, CD81, on the surface of HSCs.28
There is evidence that HIV also promotes hepatic disease progression through independent effects on hepatocyte apoptosis. It is known that apoptosis is another important trigger for hepatic fibrogenesis, by virtue of the fact that phagocytosis of apoptotic hepatocytes by stellate cells may act as an important trigger for their activation.29 To this end, there are cell-line data to support the concept that rates of hepatocyte apoptosis are increased in the presence of HIV, HCV, or both infections.30–32
Taken together, the presence of HIV, whether through direct or indirect means, promotes hepatic fibrosis progression through several potential mechanisms. The finding that microbial translocation is associated with severity of liver disease in HIV/HCV-coinfected persons supports the hypothesis that strategies to minimize translocation could produce salutary effects.33 The observation of increased reactive oxygen species (ROS), TGF-β production, and apoptosis in hepatocytes and ROS and TGF-β production in HSCs exposed to HIV (with or without HCV) also suggest additional targeted therapeutic avenues. These data go a long way toward accounting for the observed accelerated disease progression of copathogens, such as HCV and HBV, in the HIV-coinfected host.
There are other important mediators of liver injury in the HIV-infected person. A major contributor is the development of hepatic steatosis. There appear to be multiple predisposing factors to steatosis in the HIV-infected person, including HIV infection itself and its treatment. Treatment with protease inhibitors can induce peripheral lipolysis, insulin resistance, and fatty liver. Peripheral insulin resistance can also be aggravated by proinflammatory mediators, such as tumor necrosis factor alpha (TNF-α), associated with the derangements of microbial translocation observed in the HIV-infected person. Additionally, nucleoside analogs used for years to treat HIV can themselves induce steatosis through the incorporation into mitochondrial DNA polymerases, with resultant mitochondrial dysfunction, including inhibition of beta-oxidation of fatty acids. Indeed, with advances in survival accompanying the use of HAART, metabolic derangements leading to cardiovascular and liver disease have emerged as the leading causes of morbidity and mortality in HIV-infected persons.34 Even with successful control of HCV and HBV disease, additional measures will likely be required to slow fatty liver disease progression in HIV-infected persons. The development of viable therapeutics in the management of nonalcoholic fatty liver disease (NAFLD) may be of great importance among HIV-infected persons, though it is unknown whether drugs effective for NAFLD would have any effect on steatosis/steatohepatitis resulting from antiretroviral agents.
Substance use and abuse are frequently found among HIV-infected persons. Indeed, heavy drinking among HIV+ persons occurs at rates double those seen in the general population. Heavy alcohol consumption has a direct impact on CD4 counts in persons not receiving ART, in addition to its effects on decreasing adherence and impairing virologic suppression among those receiving ART.35 Alcoholic liver injury shares many features with both HIV and HCV infection, as well as ART, including induction of oxidative stress, mitochondrial injury, and steatosis. Further, alcohol impairs both innate and adaptive immune responses to pathogens, already impaired in the context of HIV. For instance, both alcohol and HCV inhibit myeloid dendritic cell function.36 In addition, alcohol also causes alterations in gut barrier function and enhances permeability to microbial LPS that promotes the activation of HSCs, alterations already much in evidence in HIV infection. High circulating levels of TNF-α observed with alcohol only serve to exacerbate liver injury. Thus, alcohol only fans the flames of liver disease observed with HIV, its treatment, and its copathogens, further accelerating liver disease progression.
HCV Treatment
(Schooley, Chung, Bonacini, Fleischer, and Thio)
IL-28b as a Marker of Response to Pegylated Interferon and Ribavirin
The standard of care for treatment of HCV infection is pegylated interferon (PEG-IFN) and ribavirin (RBV), but may soon include HCV protease inhibitors. Phase III trials have been completed in HCV-monoinfected patients. Interestingly, the nucleotide sequence near the gene for IL-28b or lambda IFN 3 on chromosome 19 affects the likelihood of the spontaneous resolution of HCV infection and achieving a sustained virologic response (SVR) with PEG-IFN and RBV.37,38 At least seven single-nucleotide polymorphisms are highly predictive of these outcomes, including detection of the C or T allele at position rs12979860. The CC genotype is found more than twice as frequently in persons who have spontaneously cleared HCV infection than in those who had progressed to chronic HCV.38 Among persons with chronic genotype 1 infection who are treated with PEG-IFN/RBV, SVR is achieved in 69%, 33%, and 27% of Caucasians who have the CC, CT, and TT genotypes, respectively; among African Americans, SVR rates are 48%, 15%, and 13% for CC, CT, and TT genotypes, respectively. The predictive value of IL-28b genotype testing for SVR is also observed for HIV/HCV-coinfected individuals and is greater than that of the pretreatment HCV RNA level, age, and fibrosis stage.39 There is also evidence that HCV viral load may be influenced by the IL-28b polymorphism.
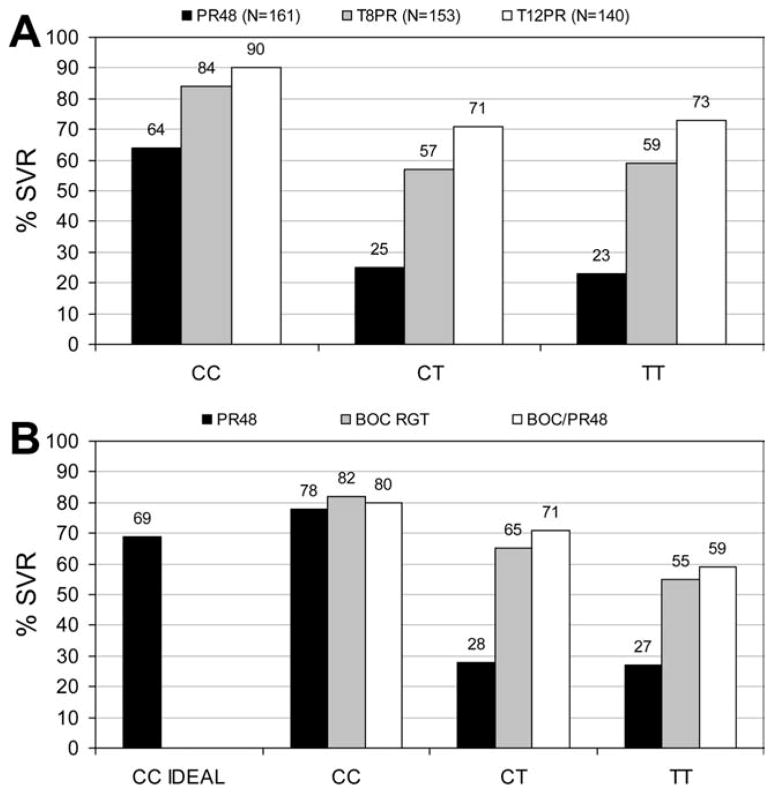
There is also a good deal of interest in the predictive value of IL-28b genotype in persons taking HCV protease inhibitors telaprevir (TVR) or boceprevir (BOC) along with PEG-IFN/RBV. Preliminary data have been reported since the Jackson Hole meeting showing that HCV-monoinfected Caucasian patients randomized to take TVR achieved SVR in 84%–90%, 57%–71%, and 59%–73% of patients with CC, CT, and TT genotypes, respectively40 (Fig. 2A). In Caucasian patients randomized to take BOC, SVR was achieved by 80%–82%, 65%–71%, and 55%–59% of patients with CC, CT, and TT genotypes, respectively41 (Fig. 2B). These data indicate that the IL-28b genotype is a powerful pretreatment predictor of response to PEG-IFN therapy and that its predictive value will be reduced when overall response rates increase with direct-acting HCV agents.
Fig. 2.
(A) Improvement in SVR in Caucasian naïve patients with TVR is greater with the IL-28b T allele. P, PEG-IFN; R, RBV; #, weeks of treatment; T, TVR. Figure created from data presented by Jacobson et al., EASL 2011. (B) Improvement in SVR in naïve patients with BOC is greater with the IL-28b T allele. P, PEG-IFN; R, RBV; #, weeks of treatment; RGT, response-guided therapy. Figure created from data presented by Poordad et al., at the 46th Annual Meeting of the International Liver Congress by the European Association for the Study of the Liver, March 30-April 3, 2011, Berlin, Germany.
Drug Development
HCV drug development is affected by the epidemiology and natural history of HIV/HCV-coinfected patients that are summarized above. HIV/HCV-coinfected patients are more likely to have substance abuse, social, and neuropsychiatric challenges than those with just HIV infection. In addition, there are also differences in HIV/HCV-coinfected persons, compared to those with just HCV, that reflect the biologic effects of HIV causing higher HCV RNA levels, more rapid progression of liver fibrosis, and lower SVR rates, compared to those without HIV (see below). Likewise, many HIV/HCV-coinfected patients are on ARTs that share metabolic pathways, such as cytochrome p450 (CYP) with HCV protease inhibitors, raising concerns about drug-drug interactions (see below). Collectively, these issues raise important questions of the timing, extent, and design of studies to test new HCV drugs in HIV/HCV-coinfected patients.
The most common cause of drug nonapproval is drug-induced liver injury (DILI), which leads to approximately 5% of liver transplants. Traditional teaching (Hy Zimmerman’s law) is that the most dangerous scenario is the combination of liver-related symptoms and jaundice. DILI can occur at any time, but generally 8–12 weeks after starting a medication. Overall, DILI occurs in approximately 5% of persons starting ART.42 Chronic HCV and HBV and recent start of nevirapine increase the risk.43 Especially in patients with ddI exposure, there are cases of noncirrhotic portal hypertension that present in HIV-infected persons without frank fibrosis. Histologic findings include nodular regenerative hyperplasia or hepatoportal sclerosis.19,44 However, the causal relationship to ddI remains unclear, and additional research in this area is clearly indicated. Characterization of toxicity related to long-term exposure to antiretroviral agents is poorly characterized, but may represent an important source of liver injury in this population. Additional studies are warranted.
Drug-Drug Studies
TVR is a cytochrome P450 3A4 (CYP3A4) substrate. Blood concentrations are reduced by ritonavir-boosted fosamprenavir, darunavir, lopinavir, and, to a lesser extent, atazanavir (ATV/r).45 Efavirenz (EFV) also reduces blood concentrations of TVR, an effect that can be offset, in part, by using a higher TVR dose (1,125 mg every 8 hours). TVR use significantly reduces the concentrations of darunavir and fosamprenavir. BOC is primarily metabolized by aldoketoreductases. BOC is an inhibitor of CYP3A4 and is not likely to be a CYP3A4 substrate. BOC trough concentrations are decreased 44% with EFV coadministrations. BOC concentrations are also decreased by 19% with low-dose ritonavir at steady state (100 mg twice-daily).46
HIV/HCV-coinfected patients are one of the special populations for which the U.S. Food and Drug Administration calls for HCV drug development studies that include data on drug-drug interactions and safety. For a drug with clear superiority in HCV-monoinfected persons (e.g., HCV protease inhibitors), an indication for HIV/HCV-coinfected patients could be achieved even with a single-arm study of approximately 300 patients that shows safety and SVR data that can be compared to historic PEG-IFN/RBV responses. Fewer patients (n = 100) may be required for approval of direct-acting antiviral use in Europe, according to draft guidelines provided by the European Medicines Agency (http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/02/WC500102109.pdf).
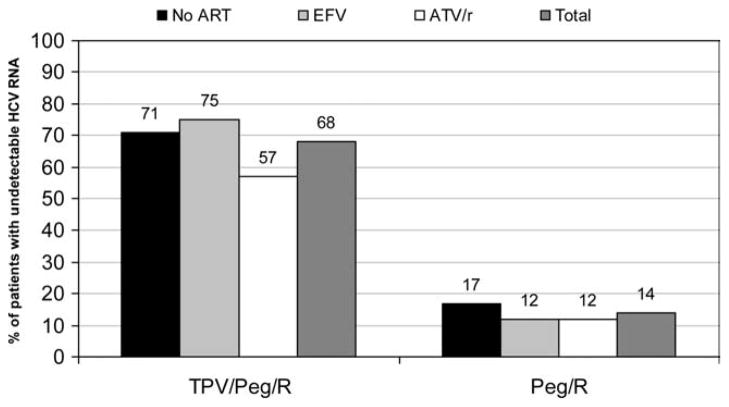
At the time of this meeting, studies were planned for genotype 1 HIV/HCV-coinfected patients for TVR and BOC, and preliminary data for TVR were subsequently reported at the Conference on Retroviruses and Opportunistic Infections in February 2011. For TVR, 60 HIV/HCV-coinfected patients were enrolled in two phases to receive TVR for 12 weeks with PEG-IFN 2a 180 μg/week and RBV 800 mg for 48 weeks. The study enrolled in two phases: part A included 20 patients with well-controlled HIV infection not on ART, whereas another 40 patients on ART, including emtricitabine/lamivudine and tenofovir along with either ATV/r or EFV, were enrolled in part B. The primary endpoint was the reduction in HCV RNA at day 29. Data on safety as well as 4- and 12-week HCV RNA reduction were reported for 13 subjects on no ART with well-controlled HIV infection (n = 13), 24 subjects taking EFV, and 22 subjects taking ATV/r.47 Reflecting the drug-drug interaction studies summarized above, TVR was given at 1,125 mg every 8 hours in patients taking EFV and the usual 750 mg every 8 hours in the other arms. The proportion of persons with undetectable HCV RNA at weeks 4 and 12 was substantially greater in the TVR arms (Fig. 3). There were no unexpected adverse events reported through week 12. The effect of using fixed-dose RBV versus weight-based dosing is unknown in this treatment group.
Fig. 3.
Higher week 12 response rates in HCV/HIV-coinfected patients in all groups taking TPV/Peg/R. Peg, PEG-IFN; R, RBV. Figure created from data presented by Sulkowski et al. at the 18th Conference on Retroviruses and Opportunistic Infections, February 27-March 2, 2011, Boston, MA.
The BOC trial underway planned to include 100 HIV/HCV-coinfected patients with a 4-week PEG-IFN/ RBV lead-in, followed by BOC. Subjects would be on ART and stratified on HCV RNA level and liver fibrosis stage. No results were available as of June 2011.
Resistance
Resistance is an important complication of any antiviral therapy. There are important similarities and differences between HIV, HCV, and HBV in the likelihood, pathogenesis, and clinical significance of antiviral resistance. HIV, HBV, and HCV copy their nucleic acid frequently using error-prone polymerases, creating a wide array of nucleotide variations. The HBV genome is approximately one-third as large as HIV or HCV and contains multiple overlapping reading frames, which limit the number of viable nucleotide variants. Thus, although HBV is capable of the highest replication of the three viruses, HBV has the most constrained genome and the least net viral diversity. Because both HIV and HBV nucleotides exist as DNA during at least part of the replication cycle, an archive of viruses can be established by the integration of viral and host DNA. No such mechanism has been demonstrated for HCV. Therefore, the long-term consequence of selecting for antiviral resistance may be lower than for HBV and HIV. Whereas direct-acting antiviral agents have been used for decades for HBV and HIV, they were just approved for HCV in spring 2011, and thus there is correspondingly less information on their clinical significance. Sequence analysis demonstrates that a small percentage of patients with HCV or HCV/HIV have baseline resistance mutations present upon population sequencing. However, there does not appear to be an increased risk in coinfected subjects.48 Available data indicate that resistant variants are detected in most instances in which there is a more than 1 log increase in HCV RNA level in someone taking a direct-acting anti-HCV drug. However, in many instances, those variants can no longer be detected 1–2 years after treatment is discontinued.49 Resistance appears to be more likely for HCV genotype 1a versus genotype 1b, and the rate of reversion to wild type is slower. More research is needed to determine whether individuals who have broken through a direct-acting HCV treatment have clinically meaningful reduced responses to future treatment regimens that include drugs that share a similar resistance mechanism.
End-Stage Liver Disease and Transplantation in HIV
(Stock and Brau)
Hepatic decompensation after development of advanced fibrosis/cirrhosis remains a serious endpoint in those with HIV and liver disease. Data regarding an accelerated course of disease are mixed. Many experts have shared experiences that suggest there is a rapid decline leading to hepatic decompensation, which occurs more often than in non-HIV infected populations. Increased risk of serious infection and sepsis was frequently cited. However, published reports provide mixed outcomes. Ragni et al. reported high rates of hepatic decompensation among hemophiliacs with end-stage liver disease.50,51 However, an analysis of United Network for Organ Sharing data did not support a finding of significant variance from the rate of survival predicted by the Model for End-Stage Liver Disease score.52 It is clear that additional work in this area is indicated to determine whether markers of rapid decompensation could be identified.
Liver transplantation outcomes have now been reported in two major cohort studies in the United States and Europe. Though patients with HBV/HIV-associated decompensated liver disease do very well, compared to those with HBV alone, patients with HCV infection have lower survival rates than matched controls. Interestingly, rates of acute cellular rejection are higher in both liver and kidney transplant recipients. The mechanism(s) underlying this observation remain unknown, but may relate to altered trough levels associated with variant dosing patterns of immunosuppressive agents that have pharmacokinetic interactions with antiretrovirals. Further research in this area is clearly indicated.
Liver disease continues to represent a major source of morbidity and mortality among those with HIV infection. There is continued evolution of antiretroviral drug treatment paradigms that reflect an increased interest in early intervention and amelioration of immune dysfunction, which may reduce rates of hepatic injury associated with immunosuppression and immune activation. However, long-term use of antiretroviral drugs may increase liver-associated metabolic derangements and, ultimately, result in more severe liver injury. A new era of HCV treatment holds great promise, but considerable research will be needed to mitigate potentially dangerous drug-drug interactions that could jeopardize both HCV and HIV treatment response rates. Research needs remain high in areas related to pathogenesis, natural history, changing disease epidemiology, and treatment intervention. Development and study of cohorts with advanced liver disease that may help characterize those at risk for decompensation and HCC are needed. Improvement in transplantation for patients with HCV/HIV coinfection remains a critical research agenda issue.
Acknowledgments
The project described was supported by Award Number R13 AI 071925 from the National Institute of Allergy and Infectious Diseases (NIAID) and was cofunded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NIDDK, NIAAA, or the National Institutes of Health.
Abbreviations
- AIDS
acquired immune deficiency syndrome
- ART
antiretroviral therapy
- ATV/r
ritonavir-boosted atazanavir
- BOC
boceprevir
- CD
cluster of differentiation
- CYP3A4
cytochrome P450 3A4
- ddI
didanosine
- DHHS
Department of Health and Human Services
- DILI
drug-induced liver injury
- ECM
extracellular matrix
- EFV
efavirenz
- HAART
highly active antiretroviral therapy
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HSCs
hepatic stellate cells
- IAS-USA
International Antiviral Society-USA
- IDU
injection drug use
- IL
interleukin
- LPS
lipopolysaccharide
- MSM
men who have sex with men
- NAFLD
nonalcoholic fatty liver disease
- PEG-IFN
pegylated interferon
- RBV
ribavirin
- ROS
reactive oxygen species
- SVR
sustained virologic response
- TGF-β
transforming growth factor beta; tumor necrosis factor alpha
- TVR
telaprevir
Footnotes
The meeting participants included the following individuals: The chairman was Kenneth E. Sherman, M.D., Ph.D., University of Cincinnati. The cochairs were Raymond T. Chung, M.D., Harvard Medical School; and David L. Thomas, M.D., M.P.H., Johns Hopkins University. The speakers (invited and selected) were Meena Bansal, M.D., Mt. Sinai School of Medicine; Pablo Barreiro, M.D., Ph.D., Hospital Carlos III, Madrid, Spain; Mauricio Bonacini, M.D., California Pacific Medical Center, University of California San Francisco; Norbert Brau, M.D., Bronx Veterans Affairs Medical Center; Susan W. Brobst, Ph.D., National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH); John T. Brooks, M.D., Ceners for Disease Control and Prevention; Kyong-Mi Chang, M.D., University of Pennsylvania; Harel Dahari, M.D., The University of Illinois; Mark Eckman, M.D., University of Cincinnati; Brian R. Edlin, M.D., M.P.H., SUNY Downstate College of Medicine; Russell Fleischer, PA-C, M.P.H., U.S. Food and Drug Administration; Judith Feinberg, M.D., University of Cincinnati; Lucia Gallego, M.D., Hospital Clinico San Carlos, Madrid, Spain; Elisa Garlassi, M.D., University of Modena and Reggio Emilia, Modena, Italy; Richard Green, M.D., Northwestern University; Maria Hernandez, M.D., University of Cincinnati; Jag Khalsa, Ph.D., National Institute on Drug Abuse, NIH; Luciana Kikuchi, M.D., University of Sao Paulo, Sao Paulo, Brazil; Nina Kim, M.D., University of Washington; David E. Kleiner, M.D., Ph.D., NIH; Dan Kuritzkes, M.D., Harvard Medical School; Christina Martin, Ph.D., University of Cincinnati; Anu Osinusi, M.D., M.P.H., NIH; Massimo Puoti, M.D., University of Brescia, Brescia, Italy; Nazia Qazi, M.D., Veterans Affairs Medical Center; Chip Schooley, M.D., University of California San Diego; Amy Shah, M.D., Virginia Commonwealth University; Gerald Sharp, M.D., NIAID, NIH; Richard Sterling, M.D., Virginia Commonwealth University; Peter G. Stock, M.D., Ph.D., University of California San Francisco; Robert Striker, M.D., University of Wisconsin School of Medicine and Public Health; Gyongi Szabo, M.D., University of Massachusetts; Chloe Thio, M.D., Johns Hopkins University; Glenn J. Treisman, M.D., Ph.D., Johns Hopkins University; Eugenia Vispo, M.D., Hospital Carlo III, Madrid, Spain; Joe Wang, Ph.D., National Institute on Alcohol Abuse and Alcoholism, NIH; Benjamin Westley, M.D., Brown University; and David Wyles, M.D., University of California San Diego.
View this article online at wileyonlinelibrary.com.
Potential conflict of interest: Unrestricted educational grants from pharmaceutical sponsors to support this meeting were provided by Genentech, Inc., Gilead Sciences, Inc., Merck & Co., Three Rivers Pharmaceuticals, LLC, and Vertex Pharmaceuticals, Inc. Continuing Medical Education credit and content oversight were provided by Medical Education Resources, Inc. Dr. Sherman advises and received grants from Merck, Vertex, SciClone, and Roche. He advises Bristol-Myers Squibb, GlaxoSmithKline, Baxter, and Regulus. He received grants from Gilead, Boehringer Ingelheim, Siemens, and Anadys. He holds other interests with Pfizer, Tibotec, and Medpace.
References
- 1.Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: the French national Mortalite 2005 study. J Hepatol. 2009;50:736–745. doi: 10.1016/j.jhep.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056–1063. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 3.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 4.Bräu N, Salvatore M, Ríos-Bedoya CF, Fernández-Carbia A, Paronetto F, Rodríguez-Orengo JF, et al. Slower fibrosis progression in HIV/ HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Pineda JA, Aguilar-Guisado M, Rivero A, Girón-González JA, Ruiz-Morales J, Merino D, et al. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49:1274–1282. doi: 10.1086/605676. [DOI] [PubMed] [Google Scholar]
- 6.Bräu N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S. -Canadian multicenter study. J Hepatol. 2007;47:527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Fierer DS. Epidemic of sexually transmitted hepatitis C virus infection among HIV-infected men. Curr Infect Dis Rep. 2010;12:118–125. doi: 10.1007/s11908-010-0088-1. [DOI] [PubMed] [Google Scholar]
- 8.Hicks CB. Guideline watch. Antiretroviral drug resistance testing—updated guidelines from the IAS-USA. AIDS Clin Care. 2008;20:64. [PubMed] [Google Scholar]
- 9.Severe P, Juste MA, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saaq MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 12.Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 14.Qurishi N, Kreuzberg C, Lüchters G, Effenberger W, Kupfer B, Sauerbruch T, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 15.Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, et al. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001;34:283–287. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SH, Thomas DL, Torbenson M, Brinkley S, Mirel L, Chaisson RE, et al. The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection. Hepatology. 2005;41:123–131. doi: 10.1002/hep.20541. [DOI] [PubMed] [Google Scholar]
- 17.Puoti M, Nasta P, Gatti F, Matti A, Prestini K, Biasi L, Carosi G. HIV-related liver disease: ARV drugs, coinfection, and other risk factors. J Int Assoc Physicians AIDS Care (Chic) 2009;8:30–42. doi: 10.1177/1545109708330906. [DOI] [PubMed] [Google Scholar]
- 18.Schiano TD, Uriel A, Dieterich DT, Fiel MI. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch. 2011;458:231–235. doi: 10.1007/s00428-010-1004-7. [DOI] [PubMed] [Google Scholar]
- 19.Vispo E, Moreno A, Maida I, Barreiro P, Cuevas A, Albertos S, Soriano V. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS. 2010;24:1171–1176. doi: 10.1097/QAD.0b013e3283389e26. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 21.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 22.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–330. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 25.Lin W, Tsai WL, Shao RX, Wu G, Peng LF, Barlow LL, et al. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology. 2010;138:2509–2518. 2518.e1. doi: 10.1053/j.gastro.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 27.Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–622. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J Biol Chem. 2011;286:2665–2674. doi: 10.1074/jbc.M110.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang JY, Shao RX, Lin W, Weinberg EM, Chung WJ, Tsai WL, et al. HIV infection increases HCV-induced hepatocyte apoptosis. J Hepatol. 2011;54:612–620. doi: 10.1016/j.jhep.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455–1460. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 32.Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV induces TRAIL sensitivity in hepatocytes. PLoS One. 2009;4:e4623. doi: 10.1371/journal.pone.0004623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 35.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–199. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo G, Dolganiuc A. Hepatitis C and innate immunity: recent advances. Clin Liver Dis. 2008;12:675–692. doi: 10.1016/j.cld.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rallón NI, Naggie S, Benito JM, Medrano J, Restrepo C, Goldstein D, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in HIV/ hepatitis C virus-coinfected patients. AIDS. 2010;24:F23–F29. doi: 10.1097/QAD.0b013e3283391d6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobson IM, Catlett I, Marcellin P, Bzowej NH, Muir AJ, Adda N, et al. Telaprevir substantially improved SVR rates across all IL28B genotypes in the ADVANCE trial. the 46th Annual Meeting of the International Liver Congress by the European Association for the Study of the Liver; March 30–April 3, 2011; Berlin, Germany. [Google Scholar]
- 41.Poordad F, Bronowicki J-P, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, et al. IL28B polymorphism predicts virologic response in patients with hepatitis C genotype 1 treated with boceprevir (BOC) combination therapy. the 46th Annual Meeting of the International Liver Congress by the European Association for the Study of the Liver; March 30–April 3, 2011; Berlin, Germany. [Google Scholar]
- 42.Soriano V, Puoti M, Garcia-Gascó P, Rockstroh JK, Benhamou Y, Barreiro P, et al. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 43.Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23–31. doi: 10.1086/341084. [DOI] [PubMed] [Google Scholar]
- 44.Kovari H, Ledergerber B, Peter U, Flepp M, Jost J, Schmid P, et al. Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis. 2009;49:626–35. doi: 10.1086/603559. [DOI] [PubMed] [Google Scholar]
- 45.van Heeswijk R, Vandevoorde A, Boogaerts G, Vangeneugden T, de Paepe E, Polo R, et al. Pharmacokinetic interactions between ARV agents and the investigational HCV protease inhibitor TVR in healthy volunteers. Conference on Retroviruses and Opportunistic Iinfections; February 27–March 2, 2011; Boston, MA. [Google Scholar]
- 46.Kasserra C, Hughes E, Treitel M, Gupta S, O’Mara E. Clinical pharmacology of BOC: metabolism, excretion, and drug-drug interactions. the 18th Conference on Retroviruses and Opportunistic Infections; February 27–March 2, 2011; Boston, MA. [Google Scholar]
- 47.Sulkowski MS, Dieterich D, Sherman K, Rockstroh J, Adda N, Mahnke L, et al. Interim analysis of a phase 2a double-blind study of TVR in combination with pegIFN-a2a and RBV in HIV/HCV co-infected patients. the 18th Conference on Retroviruses and Opportunistic Infections; February 27–March 2, 2011; Boston, MA. [Google Scholar]
- 48.Zamor PJ, Rouster S, Sherman KE. HCV NS3 mutations in HCV-HIV coinfected and HCV monoinfected subjects. the 61st Annual Meeting of the American Association for the Study of Liver Diseases; October 29–November 2, 2010; Boston, MA. [Google Scholar]
- 49.Zeuzem S, Sulkowski M, Zoulim F, et al. Long-term follow-up of patients with chronic hepatitis C treated with telaprevir in combination with peginterferon alfa-2a and ribavirin: interim analysis of the EXTEND study. Program and Abstracts of the 61st Annual Meeting of the American Association for the Study of Liver Diseases; October 29–November 2, 2010; Boston, MA. p. Abstract 227. [Google Scholar]
- 50.Ragni M, Belle S. Impact of human immunodeficiency virus infection on progress to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 51.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretransplant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver Transpl. 2005;11:1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 52.Subramanian A, Sulkowski M, Barin B, Stablein D, Curry M, Nissen N, et al. MELD score is an important predictor of pretransplantation mortality in HIV-infected liver transplant candidates. Gastroenterology. 2010;138:159–164. doi: 10.1053/j.gastro.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]