Abstract
Soluble guanylate cyclase (sGC) is a heterodimeric heme protein of ∼150 kDa and the primary nitric oxide receptor. Binding of NO stimulates cyclase activity, leading to regulation of cardiovascular physiology and providing attractive opportunities for drug discovery. How sGC is stimulated and where candidate drugs bind remains unknown. The α and β sGC chains are each composed of Heme-Nitric Oxide Oxygen (H-NOX), Per-ARNT-Sim (PAS), coiled-coil and cyclase domains. Here, we present the crystal structure of the α1 PAS domain to 1.8 Å resolution. The structure reveals the binding surfaces of importance to heterodimer function, particularly with respect to regulating NO binding to heme in the β1 H-NOX domain. It also reveals a small internal cavity that may serve to bind ligands or participate in signal transduction.
Keywords: nitric oxide, soluble guanylate cyclase, per-ARNT-sim domain, YC-1, X-ray crystallography, Manduca sexta
Introduction
Nitric oxide (NO) is produced in most mammalian cells and serves to regulate blood pressure, wound healing, memory formation, and numerous other physiological processes.1 The NO receptor is soluble guanylyl/guanylate cyclase (sGC), a large heterodimeric heme protein that is increasingly targeted for drug discovery in the treatment of cardiovascular disease.2 Two classes of compounds targeting sGC are now in clinical trial, one that stimulates the heme-containing protein (BAY 63-2521/riociguat),3,4 and another that functions to replace heme after loss due to oxidation (BAY 58-2667/cinaciguat and HMR1766/ataciguat).5,6 How NO or drug binding leads to cyclase stimulation and signal transduction in sGC is poorly understood.
sGC is composed of two homologous subunits, α and β. Multiple isoforms of each subunit have been identified; however, the most common isoform is the α1/β1 heterodimer.7 Each sGC subunit consists of four domains, an N-terminal Heme-Nitric Oxide Oxygen (H-NOX) domain8 (also called a SONO domain),9 a central Per-ARNT-Sim (PAS) domain,10 a coiled-coil domain and a C-terminal catalytic cyclase domain.11 NO binding to the heme in the β1-subunit leads to the formation of a pentacoordinated Fe–NO complex, stimulation of cyclase activity and production cGMP from GTP. Structural insight into the allostery underlying stimulation is lacking. Structures of individual sGC domains such as the β1 coiled-coil homodimer12 and the α1/β1 heterodimeric cyclase domain13 have recently been determined, as have bacterial homologues of the H-NOX and PAS domains.9,14–16 Yet an understanding of how these domains are arranged in the functional NO sensor remains unknown.
To fill this gap, we have developed sGC from Manduca sexta (tobacco hornworm) for biophysical and biochemical characterization.17–20 Manduca sexta sGC (Ms sGC) is highly homologous to its mammalian counterparts and responds well to YC-1, the parent compound for riociguat. Using homology modeling, small angle X-ray scattering (SAXS) and chemical cross-linking, we previously determined that Ms sGC lacking the cyclase domains (Ms sGC-NT) is an elongated molecule with a central parallel coiled-coil.20 In this model, the α1 subunit PAS domain directly contacts the heme-containing β1 subunit H-NOX domain20 and inhibits NO and CO binding.1 Here, we present the 1.8 Å crystal structure of the Ms sGC α1 PAS domain, which reveals the H-NOX binding surface and a small internal cavity.
Results
Crystal structure of the α1 PAS domain
Ms sGC α1 PAS protein was obtained from an Escherichia coli expression vector as a SUMO-tagged fusion protein. SUMO cleavage and purification yielded 2–3 mg of highly pure α1 PAS protein per liter of cell culture. Crystals of the wild-type α1 PAS domain (residues 279–404) were initially small and could not be improved, possibly due to a requirement for cysteine modification by the arsenic in the cacodylate-containing crystallization buffer.21–23 To overcome this, we made the triple cysteine mutant C285A, C352A, C374A. This protein crystallized under new conditions, yielding larger crystals with a rhombic dodecahedron morphology and diffraction to 1.8 Å resolution (Table I). Structure solution was by molecular replacement, using the Nostoc punctiforme signal transduction histidine kinase (Np STHK) PAS domain structure (PDB entry 2P04).15 Four nearly-identical copies of the α1 PAS domain were present in the asymmetric unit and were generally well ordered except for the loop between beta strands 4 and 5 (residues 357–361; also called Gβ and Hβ, Fig. 1) and the C-termini. All four C-termini were disordered and not included in the final refined models. In the final model, chains A and C included residues 279–391, chain B included residues 279–390 and chain D included residues 279–395.
Table I.
Crystallographic Data
| Data measurement | |
|---|---|
| PDB entry | 4GJ4 |
| Wavelength (Å) | 0.97950 |
| Space group | H32 |
| Unit cell parameters | a = b = 95.42 Å, c = 317.69 Å, α = β = 90°, γ = 120° |
| Resolution (Å)a | 23.7–1.8 (1.86–1.80) |
| Total reflections | 455433 (43841) |
| Unique reflections | 52047 (5122) |
| Completeness (%) | 100.0 (100.0) |
| Mean I/σI | 11.3 (1.9) |
| Redundancy | 8.75 (8.56) |
| Rmerge (%) | 5.5 (69.1) |
| Refinement | |
| Rwork (%) | 19.6 (39.8) |
| Rfree (%)b | 24.2 (42.1) |
| RMS deviation | |
| Bond lengths (Å) | 0.012 |
| Bond angles (°) | 1.55 |
| No. of solvent molecules | 176 |
| Ramachandran plot | |
| Most favored (%) | 89.9 |
| Allowed (%) | 10.1 |
Overall (outermost shell).
Five percent of data not used in refinement.
Figure 1.
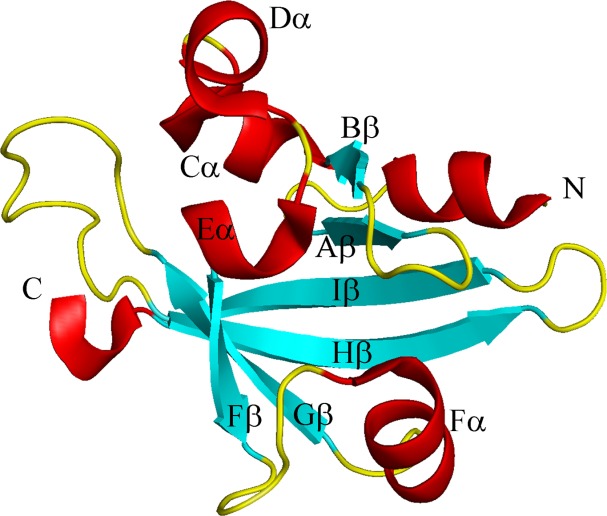
Ribbon diagram of Ms sGC α1 PAS highlighting secondary structure elements. Helix Fα, which splits into a β strand and α helix as compared with canonical PAS domains, is shown as Fβ and Fα.
The Ms sGC α1 PAS domain contains a typical PAS fold but one that is modified near the site where ligands often bind in PAS-containing proteins (Fig. 1).10 The core PAS fold consists of a five-stranded antiparallel beta-sheet with strands arranged in the sequence with order 2-1-5-4-3.10 The segment connecting strand 1 (also called Bβ, Fig. 1) to strand 5 (Gβ) is quite variable both in length and structure among PAS proteins and often provides a ligand-binding surface. Ligands commonly bind in a pocket formed between the beta 1–5 connecting strand and the interior face of the curved beta sheet. An N-terminal flanking helix is generally also present in PAS-containing proteins.
In α1 PAS, the beta 1–5 connecting strand displays a unique structure as compared with other PAS domains. In most PAS proteins, this segment includes four helices, generally referred to as Cα, Dα, Eα, and Fα. All four helices are present in Ms sGC α1 PAS; however, the residues that form the first half of Fα in a typical PAS domain are seen to form a new beta strand in α1 PAS (referred to as Fβ in Fig. 1), yielding an overall 6-stranded beta sheet with topology 2-1-6-5-4-3. The remaining portion of Fα is in a different orientation than typically observed in PAS domains and makes a critical contact with the β1 H-NOX domain, based on cross-linking studies.20 The helix includes residues Glu 340 and Lys 343, each of which can be cross-linked to β1 H-NOX residue Lys 170,20 indicating that the α1 PAS and β1 H-NOX domains are in direct contact in sGC.
Of additional interest is an internal cavity found directly behind the Fα helix in the α1 PAS structure (Fig. 2). This cavity is in a similar position to the ligand-binding site in other PAS domains, overlapping, for example, with the positions for heme in FixL24,25 and flavin in the FMN containing LOV domains.26,27 The cavity size is ∼36 Å,3 about two-thirds the size of a benzene ring. While it is tempting to suggest this cavity represents a ligand or protein binding site, a substantial rearrangement in the Fα helix would be required to accommodate anything larger than 3–4 atoms. Nonetheless, large rearrangements are known to occur in other PAS domains leading to ligand binding (see, for example, references28,29) and the cavity found in Ms sGC α1 PAS may yet have a ligand-binding function. YC-1 appears, however, not to bind to α1 PAS, but rather to bind to the β1 H-NOX domain (see footnote *).
Figure 2.
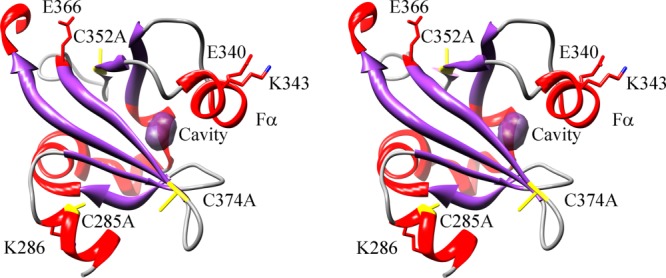
Ribbon drawing of Ms sGC α1 emphasizing the small internal pocket and inter-domain contact residues (cross-eyed stereo view). The small internal pocket found in the structure is highlighted in purple and the C285A, C352A, C374A mutations are shown in yellow. Also shown are residues Glu 340 and Lys 343, which can be cross-linked to the β1 H-NOX domain; residue Lys 286, which can be cross-linked to the β1 PAS domain; and residue Glu 366, which can be cross-linked to the β1 coiled-coil.
The four copies of α1 PAS in the asymmetric unit are quite similar, displaying similar internal cavity volumes and pairwise RMS deviations in Cα positions of 0.4–0.6 Å. Superpositioning of Ms sGC α1 PAS with Np STHK, which was used for molecular replacement, leads to an RMSD of 1.4 Å for 93 core residues (29% identity) when aligned using secondary structure matching (SSM).30 Superimposing Ms sGC α1 PAS with heme-containing FixL (PDB entry 1EW0)25 and FAD-containing PAS1 of NIFL (PDB entry 2GJ3)26 reveal RMSD values of 2.7 Å for 87 core residues (10% identity) and 2.7 Å for 86 core residues (11% identity), respectively. The key difference between the FixL and NIFL PAS1 structures is the position of the Fα helix.
Discussion
The α1 PAS crystal structure provides constraints for understanding domain arrangement in sGC. The overall fold is typical for PAS domains but displays a unique arrangement for the most variable region in the family, the segment connecting beta sheet strands 1 and 5. In this segment, the Fα helix is split into a sixth beta strand and a shorter helix with a new orientation with respect to most other PAS domains, but similar to that of Np STHK.15
In Ms sGC-NT, a parallel coiled-coil provides a platform on which the other domains assemble. Direct cross-links between the α1 PAS Fα helix and the β1 H-NOX domain near the heme pocket suggest the two domains are in direct contact, providing a means for allosteric regulation of the protein. Both domains also cross-link with the coiled-coil domain. Our working hypothesis is that YC-1 binding disrupts the α1 PAS/β1 H-NOX interaction, leading to a closed H-NOX domain and tighter CO and NO binding.
PAS domains often form homo- or hetero-oligomers as part of their function10 and the possibility that sGC forms an α1/β1 PAS dimer has been previously proposed. The most compelling data are based on the oligomer formed by Np STHK, which shares sequence homology with the sGC PAS domains.15 Np STHK forms a homodimer involving a hydrophobic patch near the N-terminus and a strand swap that allows Leu 8 from one chain to cover the hydrophobic patch of the other chain in the dimer. Several hydrogen bonds help stabilize the dimer while removal of the first seven residues in the protein abolishes the dimer. Intriguingly, the rat β1 PAS domain also dimerizes. In contrast, our construct for Ms α1 PAS runs as a monomer over a sizing column and appears to be monomeric in solution. The protein crystallizes as a rhombic dodecahedron, but this arrangement is likely an artifact of crystallization and of no physiological significance. The N-terminal hydrophobic patch at the heart of the Np STHK dimer interface is also found in Ms α1 PAS; however, it does not lead to a dimer interface. Our structure is six residues shorter at the N-terminus than that for the rat β1 PAS construct and, conceivably, this could alter dimer formation much as it did in Np STHK. Nonetheless, our cross-linking data for Ms sGC-NT include a link between α1 Lys 286 and β1 Glu 196, located at the N-termini of the two PAS domains, indicating the two PAS domains are in contact in the intact heterodimer.20
The Ms sGC α1 PAS structure also displays a small internal cavity behind the Fα helix (Fig. 2). Our binding data indicate YC-1 does not bind to this domain but do not rule out another role for this pocket in ligand binding. For ligand binding to occur, the beta 1–5 connecting segment, which includes the Fα helix, would need to rearrange, allowing the pocket to open up. There is precedent for such rearrangements in PAS domain proteins. For example, human PAS kinase has a dynamic Fα helix in the PAS A domain that allows for small molecule entry into the hydrophobic protein core, near to where the internal cavity lies in sGC α1 PAS.28 Importantly, the ligand-binding pocket is collapsed in the apo protein and only forms upon ligand binding. A second example is that of histidine kinase CitA, which uses a PAS domain for sensing citrate.29 In CitA, the beta 1–5 connecting segment is poorly ordered in the absence of citrate binding, but becomes well ordered in the complex with citrate bound in the protein interior. The loop connecting beta strands 4 and 5 also rearranges upon citrate binding, shifting inward. In Ms sGC α1 PAS, the beta 4–5 loop is poorly ordered and could serve a similar role in ligand binding or in signal transduction.
Materials and Methods
Ms sGC α1 PAS expression and purification
All chemicals were obtained from Sigma-Aldrich, restriction enzymes from New England Biolabs, and purification columns from GE Healthcare unless otherwise indicated. Ms sGC α1 PAS with a N-terminal His-tagged SUMO fusion was cloned into the pETHSUL vector,31 expressed in E. coli strain BL21 (DE3) pLysS, and purified after cleavage of the SUMO tag with SUMO hydrolase, as described elsewhere (see footnote *). The final material was concentrated to 10–15 mg/mL using a Vivaspin concentrator (Sartorius Stedim Biotech) and stored at –80°C. A final yield 2–3 mg of highly pure protein was obtained per liter of cell culture.
Crystallization
Initial crystallization conditions for Ms sGC α1 PAS were found using a PHOENIX protein crystallization robot (Art Robbins Instruments) and commercially available screens (Hampton Research and Qiagen). Crystals formed in a 96-well Intelli-Plate using sitting drop vapor diffusion at 4°C and precipitants of 1.4–1.6 M ammonium sulfate, 50 mM sodium cacodylate (pH 5.5–6.5) and 15 mM magnesium acetate tetrahydrate. Protein at 10–15 mg/mL was mixed with precipitant at ratios of 1:1 and 1:2. Cubic crystals appeared within 24–48 h after plate setup but failed to grow beyond 100 μm in size. Diffraction quality hexagonal crystals for Ms sGC P35α (cysteine triple mutant) were obtained by hanging drop vapor diffusion at 4°C using a precipitant solution of 1.5 M lithium sulfate, 0.1 M Hepes (pH 7.5). Small crystals were also observed from 4.3 M NaCl, 0.1 M Hepes (pH 7.5) and from 25% PEG 3350, 0.2 M NaCl, 0.1 M Hepes (pH 7.5). Total of 90% saturated lithium sulfate was used as the cryoprotectant and crystals were flash frozen in liquid nitrogen.
Data collection, structure solution, and refinement
X-ray diffraction data for Ms sGC α1 PAS (wild type) cubic crystals were measured remotely on SSRL beamline 9–2 (Stanford) using a MAR325 detector at T = 100 K and λ = 0.97950 Å. The data were processed in space group P213 to 3.7 Å resolution using CrystalClear.32 The unit cell parameters were a = b = c = 143.26 Å, and α = β = γ = 90°.
Diffraction data for hexagonal crystals of Ms sGC α1 PAS (triple mutant) were also measured remotely on SSRL beamline 7-1 (Stanford) using a MAR325 detector at T = 100 K, λ = 0.97950 Å and were processed to 1.8 Å with CrystalClear in hexagonal space group H32 (Table I). There were four molecules in the asymmetric unit. The structure was determined using molecular replacement as implemented in MrBUMP33,34 and search models generated from the structure of the Nostoc punctiforme signal transduction histidine kinase HNOXA domain (PDB entries 2P04 and 2P08),15 which yielded an ensemble model. Model building and refinement were performed using programs COOT and REFMAC5.35,36 Figures were prepared using PyMOL (W. L. DeLano, http://www.pymol.org) and UCSF Chimera.37 Model quality was evaluated with PROCHECK.38 Cavity volume was computed using CASTp.39
Atomic Coordinates
The atomic coordinates and structure factors have been deposited with the Protein Data Bank (PDB entry 4GJ4).
Acknowledgments
Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a national user facility operated by Stanford University on behalf of the USA Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311).
Glossary
- H-NOX domain
heme-nitric oxide/oxygen binding domain
- PAS domain
Per-ARNT-Sim domain
- SAXS
small angle X-ray scattering
- sGC
soluble guanylyl cyclase
- Ms sGC
Manduca sexta sGC
- Ms sGC-NT
Manduca sexta sGC lacking the catalytic domains.
Footnotes
Rahul Purohit, Bradley Fritz, Juliana The, Aaron Issaian, Andrzej Weichsel, Cynthia David, Eric Campbell, Andrew C. Hausrath, Leida Rassouli-Taylor, Elsa D. Garcin, Matthew J. Gage, and William R. Montfort, YC-1 Binding to the Beta Subunit of Soluble Guanylyl Cyclase Overcomes Allosteric Inhibition by the Alpha Subunit, in revision.
References
- 1.Ignarro LJ, editor. Nitric Oxide Biology and Pathobiology. Second ed. San Diego: Academic Press; 2010. [Google Scholar]
- 2.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belik J. Riociguat, an oral soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension. Curr Opin Investig Drugs. 2009;10:971–979. [PubMed] [Google Scholar]
- 4.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, Kern A, Knorr A, Lang D, Muenter K, Radtke M. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4:853–865. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt PM, Schramm M, Schroder H, Wunder F, Stasch JP. Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. J Biol Chem. 2004;279:3025–3032. doi: 10.1074/jbc.M310141200. [DOI] [PubMed] [Google Scholar]
- 6.Schindler U, Strobel H, Schonafinger K, Linz W, Lohn M, Martorana PA, Rutten H, Schindler PW, Busch AE, Sohn M, Topfer A. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol 69. 2006:1260–1268. doi: 10.1124/mol.105.018747. [DOI] [PubMed] [Google Scholar]
- 7.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 8.Cary SP, Winger JA, Derbyshire ER, Marletta MA. Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci. 2006;31:231–239. doi: 10.1016/j.tibs.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Nioche P, Berka V, Vipond J, Minton N, Tsai AL, Raman CS. Femtomolar sensitivity of a NO sensor from Clostridium botulinum. Science. 2004;306:1550–1553. doi: 10.1126/science.1103596. [DOI] [PubMed] [Google Scholar]
- 10.Moglich A, Ayers RA, Moffat K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure. 2009;17:1282–1294. doi: 10.1016/j.str.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Ruoho AE, Rao VD, Hurley JH. Catalytic mechanism of the adenylyl and guanylyl cyclases: modeling and mutational analysis. Proc Natl Acad Sci USA. 1997;94:13414–13419. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma X, Beuve A, van den Akker F. Crystal structure of the signaling helix coiled-coil domain of the beta1 subunit of the soluble guanylyl cyclase. BMC Struct Biol. 2010;10:2. doi: 10.1186/1472-6807-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allerston CK, von Delft F, Gileadi O. Crystal structures of the catalytic domain of human soluble guanylate cyclase. PLoS One. 2013;8:e57644. doi: 10.1371/journal.pone.0057644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellicena P, Karow DS, Boon EM, Marletta MA, Kuriyan J. Crystal structure of an oxygen-binding heme domain related to soluble guanylate cyclases. Proc Natl Acad Sci USA. 2004;101:12854–12859. doi: 10.1073/pnas.0405188101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Sayed N, Baskaran P, Beuve A, van den Akker F. PAS-mediated dimerization of soluble guanylyl cyclase revealed by signal transduction histidine kinase domain crystal structure. J Biol Chem. 2008;283:1167–1178. doi: 10.1074/jbc.M706218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X, Sayed N, Beuve A, van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. EMBO J. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Feng C, Hazzard JT, Tollin G, Montfort WR. Binding of YC-1 or BAY 41-2272 to soluble guanylyl cyclase induces a geminate phase in CO photolysis. J Am Chem Soc. 2008;130:15748–15749. doi: 10.1021/ja804103y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Murata LB, Weichsel A, Brailey JL, Roberts SA, Nighorn A, Montfort WR. Allostery in recombinant soluble guanylyl cyclase from Manduca sexta. J Biol Chem. 2008;283:20968–20977. doi: 10.1074/jbc.M801501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz BG, Hu X, Brailey JL, Berry RE, Walker FA, Montfort WR. Oxidation and loss of heme in soluble guanylyl cyclase from Manduca sexta. Biochemistry. 2011;50:5813–5815. doi: 10.1021/bi200794c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz BG, Roberts SA, Ahmed A, Breci L, Li W, Weichsel A, Brailey JL, Wysocki VH, Tama F, Montfort WR. Molecular model of a soluble guanylyl cyclase fragment determined by small-angle x-ray scattering and chemical cross-linking. Biochemistry. 2013;52:1568–1582. doi: 10.1021/bi301570m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson KB, Murphy JB, Das Sarma B. Reaction of cacodylic acid with organic thiols. FEBS Lett. 1972;22:80–82. doi: 10.1016/0014-5793(72)80224-2. [DOI] [PubMed] [Google Scholar]
- 22.Maignan S, Guilloteau JP, Zhou-Liu Q, Clement-Mella C, Mikol V. Crystal structures of the catalytic domain of HIV-1 integrase free and complexed with its metal cofactor: high level of similarity of the active site with other viral integrases. J Mol Biol. 1998;282:359–368. doi: 10.1006/jmbi.1998.2002. [DOI] [PubMed] [Google Scholar]
- 23.Winger JA, Derbyshire ER, Lamers MH, Marletta MA, Kuriyan J. The crystal structure of the catalytic domain of a eukaryotic guanylate cyclase. BMC Struct Biol. 2008;8:42. doi: 10.1186/1472-6807-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong W, Hao B, Mansy SS, Gonzalez G, Gilles-Gonzalez MA, Chan MK. Structure of a biological oxygen sensor: a new mechanism for heme- driven signal transduction. Proc Natl Acad Sci USA. 1998;95:15177–15182. doi: 10.1073/pnas.95.26.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyatake H, Mukai M, Park SY, Adachi S, Tamura K, Nakamura H, Nakamura K, Tsuchiya T, Iizuka T, Shiro Y. Sensory mechanism of oxygen sensor FixL from Rhizobium meliloti: crystallographic, mutagenesis and resonance Raman spectroscopic studies. J Mol Biol. 2000;301:415–431. doi: 10.1006/jmbi.2000.3954. [DOI] [PubMed] [Google Scholar]
- 26.Key J, Hefti M, Purcell EB, Moffat K. Structure of the redox sensor domain of Azotobacter vinelandii NifL at atomic resolution: signaling, dimerization, and mechanism. Biochemistry. 2007;46:3614–3623. doi: 10.1021/bi0620407. [DOI] [PubMed] [Google Scholar]
- 27.Crosson S, Moffat K. Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc Natl Acad Sci USA. 2001;98:2995–3000. doi: 10.1073/pnas.051520298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amezcua CA, Harper SM, Rutter J, Gardner KH. Structure and interactions of PAS kinase N-terminal PAS domain: model for intramolecular kinase regulation. Structure. 2002;10:1349–1361. doi: 10.1016/s0969-2126(02)00857-2. [DOI] [PubMed] [Google Scholar]
- 29.Sevvana M, Vijayan V, Zweckstetter M, Reinelt S, Madden DR, Herbst-Irmer R, Sheldrick GM, Bott M, Griesinger C, Becker S. A ligand-induced switch in the periplasmic domain of sensor histidine kinase CitA. J Mol Biol. 2008;377:512–523. doi: 10.1016/j.jmb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 31.Weeks SD, Drinker M, Loll PJ. Ligation independent cloning vectors for expression of SUMO fusions. Protein Expr Purif. 2007;53:40–50. doi: 10.1016/j.pep.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pflugrath JW. The finer things in X-ray diffraction data collection. Acta Crystallogr D. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 33.Keegan RM, Long F, Fazio VJ, Winn MD, Murshudov GN, Vagin AA. Evaluating the solution from MrBUMP and BALBES. Acta Crystallogr D Biol Crystallogr. 2011;67:313–323. doi: 10.1107/S0907444911007530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keegan RM, Winn MD. MrBUMP: an automated pipeline for molecular replacement. Acta Crystallogr D Biol Crystallogr. 2008;64:119–124. doi: 10.1107/S0907444907037195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera – a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 38.Laskowski RA, MacArthur MW, Moss DS, Thorton JM. PROCHECK: A program to check the stereochemical quality of protein structures. J Appl Crystal. 1993;26:283–291. [Google Scholar]
- 39.Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]


