Abstract
The organism’s ability to adapt to the changing sensory environment is due in part to the ability of the nervous system to change with experience. Input and synapse specific Hebbian plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), are critical for sculpting the nervous system to wire its circuit in tune with the environment and for storing memories. However, these synaptic plasticity mechanisms are innately unstable and require another mode of plasticity that maintains homeostasis to allow neurons to function within a desired dynamic range. Several modes of homeostatic adaptation are known, some of which work at the synaptic level. This review will focus on the known mechanisms of experience-induced homeostatic synaptic plasticity in the neocortex and their potential function in sensory cortex plasticity.
Introduction
Hebbian plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), is essential to strengthen or weaken specific connections within neuronal circuits to store information as relative differences in the gain between competing inputs. However, for proper functioning of the nervous system neuronal firing must be maintained within a desired “target range” of activity but Hebbian plasticity alone is insufficient to provide such stability. To the contrary, Hebbian plasticity has an innate positive feedback, which destabilizes neural firing. For example, LTP of inputs would increase the firing of the postsynaptic neuron, which could further potentiate other inputs to the cell by increasing the probability of pre- and postsynaptic spike correlation. Therefore, there has to be additional mechanism(s) in place that can provide stability to neuronal firing. This ensures that neurons remain flexible and plastic to changing inputs, but also maintain a physiologically relevant range of firing to avoid excitotoxicity caused by hyperexcitability and to prevent the loss of valuable information after a sustained period of quiescence. The term “homeostatic plasticity” is used to describe changes that allow neurons to adjust their activity and compensate for prolonged periods of increased or decreased input activity. There are several ways in which cortical neurons can stabilize their own activity in response to prolonged changes in incoming signals, including altering their intrinsic excitability or changing the relative strength of excitatory and inhibitory inputs [reviewed in (Turrigiano and Nelson, 2004)] as well as adapting their plasticity mechanisms in accordance to the “sliding threshold” model (Bear, 1995; Bear et al., 1987; Bienenstock et al., 1982). Homeostatic plasticity allows for the adjustment of overall neuronal activity while preserving the relative strength of individual synapses, and therefore is particularly important to maintain physiological functions in situations of chronic alterations in neuronal drive, as would happen with the loss of a sensory modality or when changes in network activity are triggered by various neurological conditions. In this review, we will focus on experience-driven homeostatic changes that occur in sensory cortical areas. It is especially critical for the sensory cortices to adequately adapt to prolonged periods of sensory deprivation or overstimulation because it impacts the organism’s ability to survive in a changing environment.
1. Experience-dependent homeostatic regulation of excitatory synapses
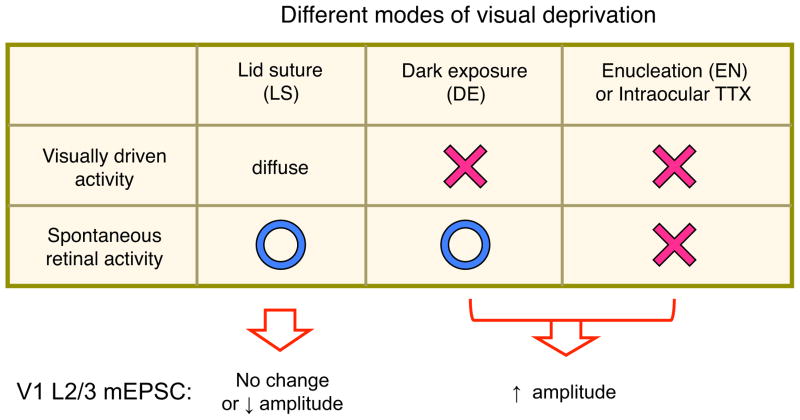
Homeostatic plasticity was initially demonstrated in vitro as a scaling of quantal amplitude of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated synaptic responses to alterations in activity of cultured neurons, such that chronic inactivity produces larger miniature excitatory postsynaptic currents (mEPSCs) while a prolonged increase in activity decreases the amplitude of mEPSCs (O’Brien et al., 1998; Turrigiano et al., 1998). Since this initial proposal of a mechanism by which neurons homeostatically regulate activity, numerous studies have followed up to examine the molecular mechanisms as well as in vivo counterparts [reviewed in (Lee, 2012; Turrigiano, 2008)]. One of the initial models used to demonstrate homeostatic synaptic plasticity in vivo is the visual cortex, which has long been used as a model for studying various forms of experience-dependent plasticity. To manipulate neural activity in vivo that can result in homeostatic adaptation of visual cortical neurons, various visual deprivation paradigms have been used including dark rearing (DR), dark exposure (DE), monocular or binocular lid suture, binocular enucleation, and monocular tetrodotoxin (TTX) injections. While all of these manipulations should alter incoming sensory information to visual cortex (V1) to a varying degree, their effects on cortical neurons vary (Figure 1). For example, several days of intraocular TTX injections, DR from birth, several days of DE, or binocular enucleation all homeostatically scale up AMPAR-mEPSC amplitudes in V1 layer 2/3 (L2/3) pyramidal neurons (Desai et al., 2002; Gao et al., 2010; Goel et al., 2006; Goel and Lee, 2007; Goel et al., 2011; He et al., 2012), while lid suture either decreases (Maffei and Turrigiano, 2008) or does not change the average mEPSC amplitude (He et al., 2012) in the same neurons. These discrepancies probably result from the different sensory deprivation paradigms used. It is known that diffuse light penetrating through the eyelids produces some degree of cortical activation (Blais et al., 2008), which may prevent homeostatic plasticity or even produce LTD (Rittenhouse et al., 1999). Collectively the visual deprivation experiments suggest that a complete lack of visually driven cortical activity is needed to elicit homeostatic synaptic plasticity in L2/3 of V1 (Figure 1).
Figure 1. Homeostatic regulation of excitatory synapses in L2/3 of V1 depends on the mode of visual deprivation.
Manipulations that trigger homeostatic scaling up of mEPSC amplitude are the ones that prevent visually driven activity (DE or EN). The residual visual activity through the eye lids prevent homeostatic synaptic plasticity, but instead either result in no change in the average mEPSC or produce synaptic depression, likely via a LTD-type of mechanism.
It is pertinent to mention that monocular deprivation (MD) paradigms have traditionally been used in the context of studying Hebbian synaptic changes related to ocular dominance plasticity (ODP) (Frenkel and Bear, 2004; Gordon and Stryker, 1996; Hubel and Wiesel, 1970; Sawtell et al., 2003). Specifically, MD using monocular lid suture initially decreases the strength of the closed eye inputs and later strengthens the open eye inputs to V1 neurons, phenomena which respectively mimic LTD and LTP (Frenkel and Bear, 2004; Rittenhouse et al., 1999; Sawtell et al., 2003; Yoon et al., 2009). Though the initial weakening of the deprived eye inputs may seem to contradict studies reporting homeostatic scaling up of mEPSCs following monocular deprivation paradigms, there are some key differences in experimental design when considering these findings. Studies reporting homeostatic scaling up of mEPSCs following MD were observed in the contralateral monocular zone of V1, which receives only the deprived eye inputs (Desai et al., 2002; Maffei et al., 2004; Maffei and Turrigiano, 2008). Furthermore, these studies utilize intraocular TTX injection, which completely silences all retinal activity. Therefore, in these studies the postsynaptic neuron experiences a total reduction or absence of visually driven activity. It is also known that MD-induced weakening of the deprived eye inputs are far less effective with intraocular TTX-injection method of monocular inactivation than monocular lid suture or monocular blurring (Frenkel and Bear, 2004; Rittenhouse et al., 1999; Rittenhouse et al., 2006). It is of interest to note that while intraocular TTX injection can abolish all retinal activity (both visually-driven and spontaneous), it paradoxically produces rhythmic oscillatory firing of thalamic neurons in the lateral geniculate nucleus (LGN) (Linden et al., 2009). Furthermore, intraocular TTX injection increases, while monocular lid suture decreases, the correlative firing between two LGN neurons when compared to recordings from normal controls (Linden et al., 2009). Therefore, the quality and pattern of neural activity arriving to V1 likely varies across different modes of visual deprivation.
While the exact alterations in neural activity arriving at V1 remain unclear for each visual deprivation paradigm, the consensus is that a prolonged absence of visually driven activity (as opposed to spontaneous activity) produces homeostatic scaling up of mEPSC amplitudes, while uncorrelated visually driven activity triggers LTD-type of synaptic weakening (Figure 1). In support of the idea that lacking visually driven activity produces homeostatic synaptic changes, specifically removing visually driven activity, while leaving spontaneous retinal activity intact with DE, globally scales up synapses in L2/3 of V1 (Gao et al., 2010; Goel et al., 2006; Goel and Lee, 2007; Goel et al., 2011). DE-induced homeostatic changes are reversible, because re-exposing DE animals to light reduces the amplitude of mEPSCs in L2/3 neurons of V1 to match that of normal animals (Gao et al., 2010; Goel et al., 2006; Goel and Lee, 2007; Goel et al., 2011). In contrast to intraocular TTX injection or DE paradigms, MD via lid-suture initially weakens the deprived eye inputs to the binocular zone of V1 contralateral to the deprived eye (Frenkel and Bear, 2004; Rittenhouse et al., 1999; Sawtell et al., 2003; Yoon et al., 2009). The binocular zone receives input from both eyes, hence there is visually driven activity from the open eye, albeit this is quite weak in rodents due to a large contralateral bias. In addition, as mentioned above, there is also visually driven activity arising from diffuse light across the closed lid of the dominant contralateral eye. Therefore, the lid-suture paradigm does not eliminate all visually driven activity. The uncorrelated visually driven activity arising from the closed eye then triggers LTD-type of Hebbian synaptic weakening (Frenkel and Bear, 2004; Rittenhouse et al., 1999; Rittenhouse et al., 2006). It is thought that the overall reduction in neural activity caused by the initial depression of the deprived eye inputs then either slides down the synaptic modification threshold to promote LTP at the open eye inputs (Frenkel and Bear, 2004; Ranson et al., 2012) or initiates a global scaling up of excitatory inputs (Mrsic-Flogel et al., 2007) to manifest the delayed open eye input potentiation (see Section 4 for more details).
Laminar specificity of homeostatic changes
It is clear that there are laminar differences in homeostatic synaptic plasticity in V1 following visual deprivation (Figure 2). For instance, Desai et al. (2002) reported that L2/3 and layer 4 (L4) neurons of the mouse visual cortex have distinct windows of “critical period”, during which modulation of visual activity can affect synaptic gain. For example, L4 has an early and narrow critical period for homeostatic synaptic plasticity (Desai 2002), which opens at postnatal day 16 (P16) and closes by P21. In L2/3, homeostatic plasticity is elicited at a later age (by P21) (Desai et al., 2002; Goel and Lee, 2007) and persists at least through P90 (Goel and Lee 2007). Layer 6 (L6) appears to have a unique response to visual deprivation in that there is an age-dependent reversal in the polarity of synaptic change between P16 and P21 (Petrus et al., 2011). In L6 pyramidal neurons, DE initiated at P16 increases, while DE initiated at P21 decreases, the average amplitude of mEPSCs. This suggests that either there is a developmental change in the cortical activity in L6 circuit after DE, or that there is a change in the mechanism by which L6 neurons adapt to DE with synaptic scaling-like mechanism at younger ages and LTD-type of plasticity later on (Petrus et al., 2011). In either case, the results suggest that experience alters sensory processing differently based on the developmental stage of the cortex. The developmental switch in the polarity of excitatory gain change in L6 is interesting considering a recent finding that L6 neurons act to bidirectionally modulate the gain of visually evoked activity in other cortical layers independent of its projection back to the LGN (Olsen et al., 2012). While homeostatic synaptic plasticity has not been studied in layer 5 (L5) neurons directly, visual deprivation between P19-P21 is reported to reduce the intrinsic excitability of L5 pyramidal neurons (Nataraj et al., 2010). Therefore, it is clear that cortical neurons adapt to losing visual experience in a laminar-specific manner.
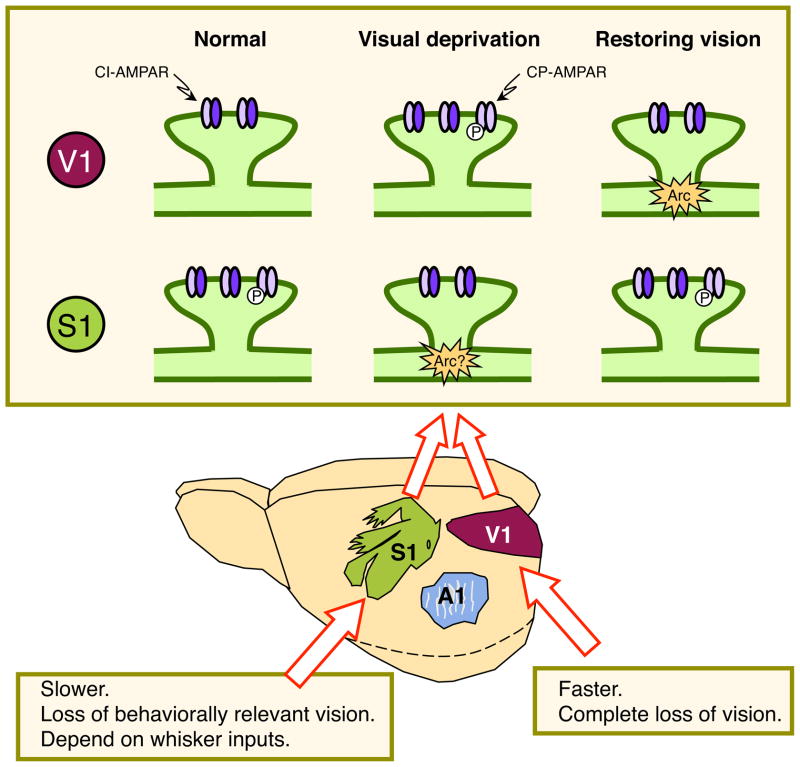
Figure 2. Lamina-specific homeostatic adaptation to visual deprivation in V1.
Principal neurons of different lamina are shown, and their known modes of homeostatic adaptation at different ages are noted. Results for visual deprivation leading to complete loss of visually driven activity (see Figure 1) is shown, except for L5 neurons where lid suture was used. Note that there is a switch in the polarity of synaptic changes in L4 and L6 during the course of development, such that homeostatic adaption occurs only early in development. L2/3 changes are initiated later and persist through adulthood. E/I: excitation/inhibitory balance. IE: intrinsic excitability.
Adding to the complexity, each lamina of cortex has unique inputs and outputs (Binzegger et al., 2004) hence there is a possibility that specific inputs may respond differentially to the same change in visual experience. Multiplicative synaptic scaling was observed initially in cortical cultured neurons when activity was deprived pharmacologically with TTX (Turrigiano et al., 1998). Based on this experimental data, it was proposed that multiplicative synaptic scaling reflects a global adaptation of excitatory synaptic gain. This mode of homeostatic adaptation is thought to be critical for preserving information storage at individual synapses while allowing homeostatic adaptation of neuronal firing (Lee, 2012; Turrigiano, 2008; Turrigiano and Nelson, 2004). However, in vivo homeostatic adaptation of L2/3 neurons only follows the rules of multiplicative synaptic scaling in early development (Gao et al., 2010; Goel and Lee, 2007; Goel et al., 2011; He et al., 2012), but not when visual deprivation occurs later in life (Goel and Lee, 2007). Furthermore, homeostatic synaptic changes observed during development in L6 are also not multiplicative at any age (Petrus et al., 2011). The non-multiplicative homeostatic synaptic scaling likely reflects changes at a subset of synapses. At this point, it is not known whether the non-multiplicative synaptic scaling of cortical neurons in vivo is due to changes in activity of specific inputs, but there is evidence of input-specific synaptic scaling from in vitro studies (Beique et al., 2011; Kim and Tsien, 2008; Lee et al., 2013). In particular, inactivating a specific presynaptic axon by expressing inward-rectifying potassium channel (Kir1.4) specifically scales up AMPA receptor function in the apposing dendritic spine without altering the strength of neighboring spines (Beique et al., 2011). Moreover, there is recent evidence that input-specific homeostatic plasticity operates in vivo. In the optic tectum of Xenopus laevis tadpoles, two days of DE produces homeostatic strengthening of visual inputs without changes in the strength of mechanosensory inputs, which converge onto the same tectal neuron (Deeg and Aizenman, 2011). Such synapse-specific scaling may be especially critical in a complex neural network, which receives diverse inputs with different activity levels as is in the sensory cortices. Thalamorecipient layers of primary sensory cortices (i.e. L4 and L6) receive two main distinct types of inputs: thalamocortical (TC) and intracortical (IC). Although less numerous, TC synapses from the LGN to L4 principal neurons tend to have more release sites and higher probability of release than IC synapses (Gil et al., 1999; Stratford et al., 1996), which is why TC synapses are thought to be the primary driver of these neurons. On the other hand, IC inputs reflect projections from other layers or other cortical areas. Hence the information content and the activity levels arising from TC and IC inputs are likely to be very different. Thus, input-specific homeostatic synaptic changes may be more beneficial to complex cortical circuits with multiple inputs, in terms of proper adaptation to incoming activity. Whether this is the case will require further studies.
Molecular mechanisms of experience-dependent homeostatic synaptic plasticity
Homeostatic synaptic plasticity is mediated at least in part by modulation of the number, subunit composition, and conductance of AMPARs [for a recent review see (Lee, 2012)]. AMPARs exist as tetramers made up of subunits including GluA1, 2, 3, and 4 (or GluR1-4), with GluA1 and GluA2 being more prevalent in most cortical areas [reviewed in (Traynelis et al., 2010)]. Due to RNA editing, GluA2 subunits contain a positively charged arginine residue, which is bulkier than the genetically encoded glutamine, at the pore loop (Burnashev et al., 1992; Sommer et al., 1991). This confers GluA2-containing AMPARs with their hallmark electrophysiological properties. These include impermeability to Ca2+, a linear current-voltage (I-V) relationship, and insensitivity to polyamines [reviewed in (Liu and Zukin, 2007; Traynelis et al., 2010)]. In contrast, AMPARs lacking GluA2 are Ca2+ permeable, display inward rectification of current, have larger conductance, and are blocked by polyamines, especially at positive potentials. Though initially described in a subset of interneurons (Bochet et al., 1994; Isa et al., 1996; McBain, 1998; Otis et al., 1995; Washburn et al., 1997), recent studies indicate that Ca2+-permeable AMPARs (CP-AMPARs) are present at pyramidal synapses under certain conditions [reviewed in (Isaac et al., 2007; Liu and Zukin, 2007)], including homeostatic adaptation to inactivity [reviewed in (Lee, 2012)].
Sensory experience-dependent homeostatic synaptic plasticity can alter receptor composition, phosphorylation, and conductance of AMPARs at cortical synapses. For example, visual deprivation in the form of DE scales up mEPSCs of L2/3 neurons in V1 and increases the content of GluA1, but not GluA2, at the postsynaptic density (PSD) of V1 (Goel et al., 2006). These changes correlated with the appearance of functional CP-AMPARs, which are likely GluA1 homomers, at synapses (Goel et al., 2006; Goel et al., 2011). The plasma membrane targeting of GluA1 in V1 requires phosphorylation of GluA1 on the serine 845 (S845) residue (Goel et al., 2011) similar to what has been reported in other brain areas (Esteban et al., 2003; Oh et al., 2006; Sun et al., 2005). GluA1-S845 is phosphorylated by cAMP-dependent protein kinase (PKA) (Roche et al., 1996), and targeted by various neuromodulators linked to the cAMP signaling (Chao et al., 2002; Hu et al., 2007; Qian et al., 2012; Seol et al., 2007). While increasing GluA1-S845 phosphorylation via pharmacological activation of beta-adrenergic receptors is able to increase the amplitude of mEPSCs in L2/3 of V1, the increase did not occur via multiplicative scaling as seen with DE (Goel et al., 2011). This suggests that there are likely other mechanism(s) responsible for providing multiplicative scaling, and that phosphorylation of GluA1-S845 itself is likely a targeting signal for increasing cell surface GluA1 levels. One peculiar aspect of L2/3 synapses in V1 is that both pharmacological phosphorylation of GluA1-S845 and mutation of GluA1-S845 to an alanine residue to prevent phosphorylation increase the amplitude of mEPSCs and synaptic expression of CP-AMPARs (Goel et al., 2011). This is quite distinct from CA1 synapses, where mEPSC amplitude is not affected by these two manipulations (He et al., 2011). This led to a speculation that synaptic expression of CP-AMPARs may be more permissive at cortical synapses compared to CA1 (He et al., 2011; Lee and Kirkwood, 2011). There is additional evidence that CP-AMPARs expression is relatively tightly controlled at CA1 synapses. For example, CP-AMPARs are located predominantly at perisynaptic locations, and only express at synapses in small quantities following mGluR activation (He et al., 2009) or only transiently after LTP under limited conditions (Guire et al., 2008; Plant et al., 2006)[but see (Adesnik and Nicoll, 2007)]. This contrasts the relatively robust recruitment of synaptic CP-AMPARs following visual deprivation in V1 (Goel et al., 2006; Goel et al., 2011), as well as with single whisker experience in rodent barrel cortex (Clem and Barth, 2006).
In contrast to the proposed role of GluA1 in homeostatic adaptation to visual deprivation (Goel et al., 2006; Goel et al., 2011; He et al., 2012), Gainey et al. (2009) reported that GluA2 rather than GluA1 is critical for scaling synapses in V1. The apparent contradiction may be due to several factors. One difference is that the mode of visual deprivation is different: the studies showing GluA1 dependence were done with DE (Goel et al., 2006; Goel et al., 2011; He et al., 2012), while GluA2 dependence was demonstrated using monocular TTX injection and recording from the monocular zone of V1 (Gainey et al., 2009). As discussed above, these two modes of visual deprivation may produce different degrees of activity changes in vivo in V1. Another possibility is that the GluA1 changes may happen in conjunction with GluA2-dependent mechanisms. In a recent study, we showed that DE increases the conductance of synaptic AMPAR without changes in the number of open channels at peak current (He et al., 2012). This idea is consistent with an interpretation that synaptic expression of GluA1 CP-AMPARs may be replacing existing synaptic GluA2 to mediate up-scaling.
Experience-dependent homeostatic plasticity in other cortical areas
Although it is tempting to make a generalization that changes in activity produce uniform results across all brain areas, not all cortices respond in the same manner and their basal synaptic transmission may differ as well. There are many similarities and differences between visual (V1), auditory (A1) and somatosensory (S1) cortical plasticity. Some of the differences may be due to the distinct qualities of various sensory inputs and processing required for proper sensory perception, but some may be apparent differences arising from studying distinct cell types or laminae. As mentioned previously, distinct layers in V1 respond differentially to sensory deprivation (Figure 2), and also the mode of visual deprivation is an important determinant (Figure 1).
Sensory neural hearing loss (SNHL) produced by bilateral cochlear ablation increases the strength of excitatory synapses in L2/3 of A1 (Kotak et al., 2005). SNHL is similar to binocular enucleation, in that it removes both sensory evoked activity as well as spontaneous activity arising in the sensory organ. As mentioned above, binocular enucleation scaled up L2/3 synapses in V1 (He et al., 2012), which highlights the similarity of L2/3 neurons in A1 and V1 when responding to sensory organ damage. However, there are qualitative differences in that mEPSC frequency is reduced in A1 L2/3 neurons following SNHL (Kotak et al., 2005), but unaltered in V1 L2/3 with enucleation (He et al., 2012).
In rodent barrel cortex (S1BF), whisker deprivation studies have revealed dramatic changes in both synaptic function and connectivity [reviewed in (Feldman and Brecht, 2005)]. However, it is pertinent to note that the majority of whisker deprivation studies are done under conditions that promote competition of different whisker inputs, such as depriving a single row of whiskers or a checkerboard deprivation paradigm, which elicit Hebbian plasticity. In contrast, uniform deprivation of all whiskers produces minimal change in synaptic function of S1BF neurons (Finnerty and Connors, 2000; He et al., 2012), unless such deprivation is done from birth (Popescu and Ebner, 2010). In particular, a week of bilateral whisker deprivation initiated later in development does not alter the average amplitude or frequency of mEPSC in L2/3 S1BF neurons (He et al., 2012). The apparent lack of homeostatic synaptic changes in L2/3 S1BF following bilateral whisker deprivation was suggested to be due to its similarity to bilateral lid suture manipulation, which does not elicit global homeostatic synaptic changes in L2/3 of V1 (He et al., 2012). A recent study reported that unilateral infraorbital nerve resection, which is expected to abolish all tactile driven activity, potentiates thalamocortical inputs to S1BF (Yu et al., 2012). These results further support the idea that a complete loss of sensory driven activity is needed to trigger homeostatic synaptic plasticity in primary sensory cortices.
2. Experience-dependent homeostatic adaptation of inhibitory synapses
There are several aspects of inhibitory transmission in V1 that are affected by periods of visual deprivation, including reduced expression of GABA and the GABA synthesizing enzyme glutamic acid decarboxylase (GAD) (Benevento et al., 1995; Hendry and Jones, 1986; Huang et al., 1999; Kreczko et al., 2009) and alterations in the overall maturation of inhibitory circuits (Huang et al., 1999). While there is a relative wealth of knowledge regarding experience-dependent homeostatic changes in excitatory drive in the cortex, there is much less known about homeostatic adaptation of the inhibitory circuit after altered sensory input. Inhibitory tone in the cortex is a function of several different aspects of inhibitory transmission, including diverse inhibitory cell types, excitatory drive to these inhibitory neurons, and, of course, the way in which the inhibitory neurons affect each other and excitatory neurons in the circuit. It is perhaps not surprising then, that experience does not alter all classes of inhibitory cells in all layers in the same manner. While maintaining excitatory-to-inhibitory (E/I) balance is thought critical for normal cortical functions, in some cases experience driven homeostatic adaptations in cortical inhibitory transmission occur inversely with excitatory changes, yielding a net shift in the E/I balance (discussed below). Presumably, the shift in E/I balance is an adaptive mechanism to maintain cortical activity within a desired functional range.
Excitatory drive onto inhibitory neurons
The first reports of homeostatic plasticity in culture revealed that excitatory and inhibitory circuits change differently after prolonged changes in activity. Specifically, blocking neuronal activity with TTX causes excitatory synapses onto pyramidal neurons to be scaled up, without changes in excitatory drive onto interneurons (Turrigiano et al., 1998). To the contrary, pharmacologically enhancing activity in neuronal cultures via brain-derived neurotrophic factor (BDNF) (Rutherford et al., 1998) or through addition of GABAA receptor antagonist bicuculline (Chang et al., 2010) increases the average amplitude of mEPSCs onto GABAergic neurons, suggesting a net increase in inhibition. These data suggest that the excitatory synapses onto inhibitory neurons only adapt to increased activity. Consistent with this idea, sensory experience (which is expected to increase patterned activity in the cortex) is critical for the developmental increase in the strength of excitatory inputs to GABAergic neurons in S1BF. Excitatory thalamic input to feed-forward inhibitory interneurons in L4 of S1BF shows a developmental strengthening, which is attenuated in whisker-trimmed animals (Chittajallu and Isaac, 2010). This study was performed during the second postnatal week, an age at which thalamocortical inputs to L4 stellate cells do not respond to sensory deprivation (Feldman and Brecht, 2005). Thus, the same whisker deprivation does not alter the strength of thalamic input to L4 stellate cells nor the synaptic strength of unitary inhibitory inputs from L4 interneuron to stellate cells (Chittajallu and Isaac, 2010). This suggests that the modification of excitatory inputs to L4 interneurons is the main locus of adaptation in L4 S1BF in response to whisker deprivation after the critical period of plasticity for L4 stellate neurons has closed. The net outcome of these changes conforms to the idea that these are homeostatic adaptations, because it is predicted to increase the E/I balance in response to sensory deprivation. Whether these types of adaptation exist beyond the initial developmental period needs to be tested.
Homeostatic adaptation of inhibitory synapses
Early studies that directly measured inhibitory currents in cultured neocortical neurons revealed that after two days of TTX-induced activity blockade, miniature inhibitory postsynaptic currents (mIPSCs) in pyramidal neurons scale down due to a loss of GABAARs clustered at the synapse and an overall loss of functional inhibitory synapses (Kilman et al., 2002). In contrast, prolonged increase in activity triggers accumulation of postsynaptic GABAARs (Rannals and Kapur, 2011). Both of these studies also report corresponding alterations in presynaptic GABA synthesizing enzyme levels, as well as changes in mIPSC frequency, suggesting that these homeostatic changes involve both pre- and post-synaptic mechanisms. Reduced inhibitory transmission after periods of neuronal inactivity would result in an overall increase in excitability of pyramidal neurons, which would complement homeostatic increase in excitatory transmission to increase the E/I balance in the network.
As discussed above, many sensory deprivation paradigms have been developed over the years to identify experience-dependent alterations in network activity in vivo, and different layers of the cortex have distinct critical periods during which experience can shape excitatory synapses. In L4 of V1, 2 days of monocular deprivation from postnatal day 14 to 17 (P14–17) causes a decrease in the amplitude of unitary IPSCs (uIPSCs) from fast-spiking interneurons to principal neurons in the contralateral monocular zone (Maffei et al., 2004). This was accompanied by an increase in the strength of excitatory inputs between principal neurons of L4, which indicates an increase in E/I balance (Maffei et al., 2004). In line with the idea that this is a homeostatic adaptation to inactivity, visual deprivation at this early age increases the spontaneous firing of L4 principal neurons (Maffei et al., 2004). However, this type of homeostatic adaptation in L4 only occurs in early development, and the same visual deprivation paradigm initiated at P18 does not alter the recurrent excitatory connections of the principal neurons in L4, but increases the uIPSC amplitude (Maffei et al., 2006). These alterations would decrease the E/I balance, and are consistent with the finding that spontaneous activity in L4 principal neurons are decreased with visual deprivation at this later age (Maffei et al., 2006). These results illustrate that the mechanisms by which the cortical circuit adapts to changes in sensory inputs may be distinct depending on the developmental age. The opposite regulation of inhibitory inputs to L4 principal neurons by sensory deprivation during different phases of development is reminiscent of the opposite changes in the strength of excitatory inputs to L6 principal neurons (Petrus et al., 2011). These changes would act to increase E/I balance to the principal neurons during early development, but decrease this at a later age. L4 and L6 are the major thalamorecipient layers, and whether the developmental change in how they adapt to sensory deprivation reflects any specific nature of thalamocortical inputs is unclear at this point. It is interesting to note that the developmental switch seems to coincide with the transition from pre-critical to critical period for cortical plasticity (Feller and Scanziani, 2005).
In L2/3 of V1 changes in the E/I balance seem dependent on the mode of visual deprivation. For instance, 2 days of monocular TTX injection leads to an increase, while the same duration of monocular lid suture decreases, the E/I ratio of L2/3 pyramidal neurons in the monocular zone of V1 (Maffei and Turrigiano, 2008). Using a minimal stimulation paradigm, it was shown that the changes in E/I ratio with intraocular TTX injection are due to an increase in L4 to L2/3 excitatory inputs and a concomitant decrease in the inhibitory inputs, but monocular lid suture only decreased the amplitude of excitatory inputs (Maffei and Turrigiano, 2008). This conforms to the idea that V1 L2/3 neurons differentially adapt their inhibitory network to distinct visual deprivation paradigms. This parallels the effect of various modes of binocular deprivation on homeostatic regulation of mEPSCs in L2/3 V1 neurons (Figure 1). Distinct from monocular deprivation paradigms and in vitro studies, a week of DE does not alter the amplitude, but decreases the frequency, of mIPSCs recorded from L2/3 V1 neurons (Gao et al., 2011). These changes were not accompanied by alterations in presynaptic parameters of evoked IPSCs (eIPSCs) or the density of inhibitory synapses as measured by GAD-65 puncta density (Gao et al., 2011). These data suggest that experience driven homeostatic plasticity can selectively modify spontaneous inhibitory transmission while leaving evoked inhibitory transmission unaltered, perhaps allowing experience to set the overall inhibitory “tone” or “background noise” in sensory systems while preserving the temporal response properties of cortical neurons which critically depend on action potential evoked inhibition (Zhang et al., 2011).
3. Potential relationship between synaptic scaling and sliding threshold
As mentioned in the introduction, synaptic scaling is not the only mechanism used to maintain homeostasis in cortical circuits. In particular, the sliding threshold is another homeostatic mechanism that can provide stability to a network. The sliding threshold model differs from synaptic scaling in that it maintains neuronal homeostasis by altering the induction threshold of LTP and LTD (Bear, 1995, 1996; Bear et al., 1987; Bienenstock et al., 1982). For example, in the sliding threshold model a neuron would adapt to a prolonged increase in activity by increasing the threshold for LTP induction, while chronic inactivity would decrease the LTP induction threshold. In essence, enhanced neuronal activity promotes LTD at synapses, which would act to dampen excitatory inputs and reduce excitability. On the other hand, chronic inactivity facilitates LTP induction at synapses, which would increase the impact of excitatory inputs in driving neuronal firing. Thus, the regulation of the synaptic modification threshold in effect provides a negative feedback to stabilize neuronal responses. In order for sliding threshold to properly maintain neuronal firing, the synaptic modification threshold needs to “slide” with a longer time scale (i.e. hours to days) compared to LTP/LTD which occur within seconds to minutes (Bear, 1995, 1996; Bear et al., 1987; Bienenstock et al., 1982). It is pertinent to note that a key difference between synaptic scaling and sliding threshold is that the latter can only be input-specific and activity-dependent, because it relies on Hebbian modification of active inputs. Although there is evidence that one prevalent mechanism for sliding the synaptic modification threshold is through changes in NMDA receptor (NMDAR) function (Philpot et al., 2007; Philpot et al., 2003; Philpot et al., 2001; Quinlan et al., 1999a; Quinlan et al., 1999b), in principle any manipulation that alters the postsynaptic Ca2+ signal could alter the threshold as well (Cummings et al., 1996). While synaptic scaling and sliding threshold are largely considered two independent mechanisms to maintain homeostasis, there are several indications that they may interact at various levels. Indeed, recent studies showed that chronic inactivity or sensory deprivation paradigms, which scale up excitatory synapses, promote LTP induction (Arendt et al., 2013; Guo et al., 2012).
The initial studies of synaptic scaling, which were done in vitro neuronal cultures, demonstrated that this mode of homeostatic synaptic plasticity is independent of NMDARs (O’Brien et al., 1998; Turrigiano et al., 1998). The NMDAR independence of synaptic scaling distinguishes this form of plasticity from sliding threshold, which is known to depend on changes in NMDAR function (Philpot et al., 2007; Philpot et al., 2003; Philpot et al., 2001; Quinlan et al., 1999a; Quinlan et al., 1999b). However, there is evidence that blocking NMDAR activity can accelerate synaptic scaling (Sutton et al., 2006) and knocking out the obligatory GluN1 (or NR1) subunit of NMDAR increases the amplitude of mEPSCs (Adesnik et al., 2008). These results suggest that alterations in NMDAR activity may also influence synaptic scaling mechanisms in addition to sliding the LTP/LTD threshold. The effect of NMDAR function on synaptic scaling may involve regulation of retinoic acid (RA) signaling with subsequent local translation and synaptic expression of GluA1-containing AMPARs (Aoto et al., 2008). Experimental evidence suggests that inhibiting NMDAR in conjunction with TTX, but not TTX alone, up-regulates RA synthesis (Aoto et al., 2008). RA then binds to retinoic acid receptor alpha (RARα), which relieves the brake on local translation of dendritic mRNAs, including that of the GluA1 subunit (Maghsoodi et al., 2008; Poon and Chen, 2008). This mechanism can serve to increase the synaptic content of CP-AMPARs, which are GluA1-homomers, following prolonged inactivity (Aoto et al., 2008; Beique et al., 2011; Ju et al., 2004; Sutton et al., 2006; Thiagarajan et al., 2005). Whether similar mechanisms act in vivo is unclear, but may underlie synaptic recruitment of CP-AMPARs at L2/3 synapses in V1 after DE (Goel et al., 2006; Goel et al., 2011). It would also be of interest to know whether sensory experience induced homeostatic synaptic plasticity is NMDAR dependent. Considering a relatively large contribution of NMDAR responses in sensory evoked potentials (Fox et al., 1989; Lavzin et al., 2012; Miller et al., 1989), alterations in sensory inputs may also have a direct impact on NMDAR signaling.
Another mechanism through which synaptic scaling and sliding threshold may interact is via synaptic recruitment of CP-AMPARs by inactivity, which could alter postsynaptic calcium transients to influence the LTP/LTD threshold. Because of differences in their physiological properties, synapses containing CP-AMPARs integrate neuronal activity differently than those without CP-AMPARs. Specifically, CP-AMPARs exhibit faster decay kinetics than Ca2+-impermeable AMPARs (Hollmann et al., 1991), which predict diminished summation at low input frequencies. Conversely, the activity-dependent relief from polyamine block in CP-AMPARs predicts enhancement of the summation of synaptic responses following periods of high input activity (Rozov and Burnashev, 1999; Rozov et al., 1998). As a result, synaptic expression of CP-AMPARs following a period of inactivity may serve to slide the induction threshold for LTP/LTD by altering the temporal summation properties of inputs. In addition, because of their Ca2+ permeability, it is likely that CP-AMPARs may enhance the activation of signaling cascades dependent on Ca2+. CP-AMPAR mediated Ca2+ influx may summate with NMDAR mediated Ca2+ signaling, which is predicted to enhance LTP at the expense of LTD. There is evidence that Ca2+ through CP-AMPARs can support LTP. For example, GluA2 knock-out mice, which express CP-AMPARs at synapses, produce LTP (Jia et al., 1996; Meng et al., 2003; Wiltgen et al., 2010) exclusively mediated by Ca2+ influx through CP-AMPARs (Asrar et al., 2009) even at hyperpolarizing membrane potentials (Wiltgen et al., 2010). These studies suggest that the experience-dependent homeostatic changes, which recruit CP-AMPARs to synapses, may in fact be sufficient to alter the induction threshold for LTP/LTD in neurons. In line with this idea, a recent study showed that 2 days of DE, which is known to recruit CP-AMPAR to L2/3 synapses in V1 (Goel et al., 2011), slides the threshold to promote LTP and suppress LTD at L4 to L2/3 synapses (Guo et al., 2012).
A recent study adds another mechanism of interaction between synaptic scaling and sliding threshold. Arendt et al. (2013) demonstrated that chronic inactivity not only scales up the strength of existing synapses, but also promotes the formation of silent synapses. Silent synapses are excitatory synapses that contain NMDA receptors but lack functional AMPA receptors and hence are “silent”‘ under resting membrane potentials (Isaac et al., 1995; Liao et al., 1995). A key feature of silent synapses is that they can be “unsilenced” by LTP via recruitment of AMPA receptors (Isaac et al., 1995; Liao et al., 1995; Liao et al., 1999). Ardent et al. (2013) demonstrated that neurons chronically treated with TTX had additional silent synapses, which were then “unsilenced” to enhance the magnitude of LTP. Thus, chronic inactivity may promote LTP by creating more synapses.
4. In vivo functions of experience-dependent homeostatic synaptic plasticity
There is emerging evidence that ocular dominance plasticity (ODP) is orchestrated by the coordinated recruitment of Hebbian and homeostatic synaptic plasticity. It is well documented that a brief MD functionally disconnects the deprived eye inputs to V1 (Hubel and Wiesel, 1970; Wiesel and Hubel, 1965a, b). The basic cellular mechanisms underlying ODP have been extensively studied in rodents. The main idea emerging from many studies is that there are two phases to ODP: an initial depression of the closed eye inputs followed by a delay potentiation of the open eye inputs. Evidence suggests that the former is mediated by LTD-like Hebbian plasticity, while the latter is mediated by homeostatic synaptic plasticity such as sliding threshold or synaptic plasticity. After a brief period of MD, V1 neurons decrease responsiveness to the closed eye, which is then followed by an increase in response to the open eye (Frenkel and Bear, 2004) resulting in a preferential responsiveness to the remaining open eye (Gordon and Stryker, 1996). There is clear evidence that the initial weakening of the closed eye inputs is due to LTD of excitatory synapses (Heynen et al., 2003; Rittenhouse et al., 1999), which is mainly due to the degradation of the quality of the visual input rather than a general reduction in retinal illumination (Rittenhouse et al., 2006). The delayed potentiation of the open eye inputs was initially proposed to be due to the sliding down of LTP induction threshold by the loss of the closed eye inputs, which promotes potentiation of synapses serving the open eye inputs (Frenkel and Bear, 2004). Later this idea was challenged and it was suggested that the open eye potentiation is due to homeostatic synaptic scaling triggered by losing the closed eye inputs. This proposal was based on the observation that both the open eye and closed eye inputs display a delayed potentiation (Mrsic-Flogel et al., 2007). The latter idea was further supported by the demonstration that the delayed potentiation of the open eye inputs following MD does not occur in the TNFalpha knockout mouse (Kaneko et al., 2008), which specifically lack up-scaling of excitatory synapses by inactivity (Stellwagen and Malenka, 2006). However, a recent study showed that synaptic scaling based potentiation of the open eye inputs only occur in young mice, but not in adults (Ranson et al., 2012). TNFα knockouts lack the delayed potentiation of the open eye inputs during the critical period (Kaneko et al., 2008), but display normal delayed potentiation in adults (Ranson et al., 2012). On the other hand, mice specifically lacking LTP (i.e. CaMKIIα-T286A mutant) display normal open eye potentiation when young, but lack this in adults (Ranson et al., 2012). These results indicate that the mechanisms of the delayed open eye potentiation seen following MD changes with age, and fits nicely with data showing that V1 L2/3 neurons exhibit multiplicative synaptic scaling only during early development, but not in adults (Goel and Lee, 2007). In adults, the non-multiplicative increase in mEPSC amplitude suggests that only a subset of synapses remain plastic (Goel and Lee, 2007) consistent with recruitment of input-specific plasticity mechanisms such as LTP. It is possible that MD in adults slides down the LTP threshold such that spontaneous cortical activity may be sufficient to produce LTP at a subset of active synapses, which would result in the delayed potentiation of the open eye inputs following MD (Ranson et al., 2012; Sawtell et al., 2003).
In addition to unimodal homeostatic synaptic plasticity, there are also cross-modal homeostatic changes apparent in vivo. It is well documented that losing a sense produces compensation of the remaining senses in a phenomenon coined as “cross-modal plasticity” [reviewed in (Bavelier and Neville, 2002)]. Studies aimed at understanding human sensory compensation are widespread, but experiments addressing the synaptic basis for this phenomenon are just beginning to surface. Cross-modal changes in excitatory synaptic strength have been observed in L2/3 of S1 and A1 of visually deprived mice (Goel et al., 2006; He et al., 2012) (Figure 3). Depriving vision via a week of DE decreases the average amplitude of mEPSCs, which is reversed by subsequent visual experience. The cross-modal synaptic changes follow the rules of multiplicative synaptic scaling, at least in juveniles (Goel et al., 2006; He et al., 2012), which suggests global adaptation in synaptic strength. Interestingly, cross-modal synaptic changes require a longer duration of DE than unimodal changes (He et al., 2012). Furthermore, while a milder form of vision loss (i.e. lid suture) can trigger a cross-modal decrease in mEPSC amplitudes in S1BF, the unimodal increase in mEPSCs requires a complete loss of visually driven activity (He et al., 2012). Despite the difference in the sensory requirement, at the molecular level cross-modal homeostatic synaptic adaptation employs the same mechanisms as unimodal changes observed in V1 (Figure 3). For instance, in S1 the cross-modal scaling down of mEPSCs by visual deprivation is accompanied by removal of synaptic AMPARs (He et al., 2012), including CP-AMPARs (Goel et al., 2006), while scaling up of mEPSCs by re-exposure to light recruits CP-AMPARs to synapses (Goel et al., 2006). In addition to homeostatic synaptic plasticity, cross-modal plasticity can also occur via Hebbian mechanisms. It was demonstrated that 3 days of bilateral lid suture potentiates L4 inputs to L2/3 neurons in S1BF by transiently driving GluA1 containing AMPARs to synapses (Jitsuki et al., 2011). This LTP-like cross-modal change was accompanied by sharpening of whisker maps, measured as a decrease in the response to surround whiskers in the visually deprived mice (Jitsuki et al., 2011). This study attributed the sharpened whisker maps to LTP of the L4 inputs to L2/3 neurons, which is expected to increase the impact of feedforward sensory information arising from the principal whisker. However, a global scaling down of mEPSCs in L2/3 of S1BF following visual deprivation (Goel et al., 2006) could also explain the sharper whisker map by reducing the strength of previously weak inputs (such as those from surround whiskers) below the action potential threshold. This in essence would sharpen the receptive field (RF) of L2/3 neurons in S1BF. There is evidence that global scaling down of cortical excitatory synapses may sharpen neuronal RFs. For example, orientation selectivity is broadened in Arc knockout mice (Wang et al., 2006), which lack down scaling of mEPSCs by visual experience (Gao et al., 2010).
Figure 3. Visual deprivation induced unimodal and cross-modal homeostatic synaptic plasticity.
Unimodal changes in V1 and cross-modal changes in S1 require distinct sensory requirements. At the cellular level, unimodal and cross-modal changes occur in opposite directions at excitatory synapses and follows the rules of multiplicative synaptic scaling. The underlying molecular mechanisms are conserved, such that scaling up of synapses in both cortical areas involve synaptic expression of CP-AMPARs, and scaling down depend on immediate early gene Arc and remove of synaptic AMPARs.
Homeostatic mechanisms of maintaining optimal synaptic performance need not be limited to sensory experience. Sleep is hypothesized to maintain the integrity of neuronal circuits via homeostatic mechanisms [reviewed in (Tononi and Cirelli, 2003)]. During wakefulness synapses are actively processing information, including but not limited to sensory experience, which may increase overall excitability in the system. Sleep has been hypothesized to homeostatically down-regulate cortical excitability in order to normalize the synaptic strength, which then facilitates memory consolidation and enhance performance on a variety of motor and procedural tasks [reviewed in (Stickgold et al., 2001)]. In support of this hypothesis, molecular markers of LTP increase during the wake cycle, while markers of LTD increase during sleep (Vyazovskiy et al., 2008). However, whether sleep associated synaptic depression is truly mediated by synaptic scaling type of global homeostatic synaptic plasticity is unclear at this point. At least in V1, sleep induced consolidation of ODP is mediated in part by facilitation of LTP-like mechanisms that enhance the open eye inputs (Aton et al., 2009).
It is pertinent to point out that deciphering whether synaptic change is due to synaptic scaling or Hebbian LTP/LTD-type of mechanism is often difficult. For example, neuromodulators linked to cAMP signaling, which promote LTP (Seol et al., 2007), produce apparent global scaling up of mEPSCs in L2/3 of V1 (Huang et al., 2012). On the other hand, neuromodulators linked to phospholipase C (PLC) enhance LTD globally to scale down synapses (Huang et al., 2012). A surprising aspect of this particular study is that the same visual experience either triggers global LTP or global LTD depending on the neuromodulator present (Huang et al., 2012). This study underscores the difficulty in distinguishing synaptic scaling from Hebbian plasticity in vivo, where the exact nature of change in activity following sensory manipulation or the neuromodulatory tone is often unknown.
Concluding remarks
Sensory cortices process and store information about the world from a multitude of incoming signals, integrate them, and produce appropriate responses for the organism. Hebbian-type of plasticity is considered critical for developmental fine tuning and rewiring of the cortical circuits in tune with the environment, as well as for memory storage. However, such changes at synapses and circuits require another level of plasticity that can provide homeostasis, such that neurons can function within its dynamic range and prevent over-excitation or insufficient activation. Cortical circuits are endowed with multiple homeostatic plasticity mechanisms, many of which target the regulation of both excitatory and inhibitory synaptic transmission. These homeostatic synaptic plasticity mechanisms are required to work in coordination to keep balance in neuronal function despite the changing sensory environment or processing requirements.
Highlights.
Sensory cortices require homeostasis.
Both excitatory and inhibitory synapses adapt to sensory deprivation.
Mechanisms of homeostatic synaptic changes are laminar and age specific.
Homeostatic synaptic plasticity is integral to normal cortical function.
Acknowledgments
This work was supported by grants from the National Institute of Health (NIH) (R01-EY014882 to H.-K.L and F31-NS079058 to E.P.). The authors would like to thank Dr. Alfredo Kirkwood for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci U S A. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Sarti F, Chen L. Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J Neurosci. 2013;33:2087–2096. doi: 10.1523/JNEUROSCI.3880-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrar S, Zhou Z, Ren W, Jia Z. Ca(2+) permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PLoS ONE. 2009;4:e4339. doi: 10.1371/journal.pone.0004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15:1–4. doi: 10.1016/0896-6273(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci U S A. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS. gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais BS, Frenkel MY, Kuindersma SR, Muhammad R, Shouval HZ, Cooper LN, Bear MF. Recovery from monocular deprivation using binocular deprivation. J Neurophysiol. 2008;100:2217–2224. doi: 10.1152/jn.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Isaac JT. Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nature Neurosci. 2010;13:1240–1248. doi: 10.1038/nn.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- Deeg KE, Aizenman CD. Sensory modality-specific homeostatic plasticity in the developing optic tectum. Nature Neurosci. 2011;14:548–550. doi: 10.1038/nn.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Curr Opin Neurobiol. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Connors BW. Sensory deprivation without competition yields modest alterations of short-term synaptic dynamics. Proc Natl Acad of Sci U S A. 2000;97:12864–12868. doi: 10.1073/pnas.230175697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Sato H, Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989;9:2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Huang S, Jacobs C, Song L, Kirkwood A, Lee H-K. Experience-dependent regulation of inhibitory synapses in the superficial layers of mouse visual cortex. Society for Neuroscience Abstract 798.18 2011 [Google Scholar]
- Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci. 2010;30:7168–7178. doi: 10.1523/JNEUROSCI.1067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron. 1999;23:385–397. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, Megill A, Takamiya K, Huganir RL, Lee HK. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoS ONE. 2011;6:e18264. doi: 10.1371/journal.pone.0018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Huang S, de Pasquale R, McGehrin K, Lee HK, Zhao K, Kirkwood A. Dark exposure extends the integration window for spike-timing-dependent plasticity. J Neurosci. 2012;32:15027–15035. doi: 10.1523/JNEUROSCI.2545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Goel A, Ciarkowski CE, Song L, Lee HK. Brain area specific regulation of synaptic AMPA receptors by phosphorylation. Communicative & Integrative Biology. 2011;4:569–572. doi: 10.4161/cib.4.5.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Petrus E, Gammon N, Lee HK. Distinct sensory requirements for unimodal and cross-modal homeostatic synaptic plasticity. J Neurosci. 2012;32:8469–8474. doi: 10.1523/JNEUROSCI.1424-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986;320:750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang S, Trevino M, He K, Ardiles A, Pasquale R, Guo Y, Palacios A, Huganir R, Kirkwood A. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Itazawa S, Iino M, Tsuzuki K, Ozawa S. Distribution of neurones expressing inwardly rectifying and Ca(2+)-permeable AMPA receptors in rat hippocampal slices. J Physiol. 1996;491 ( Pt 3):719–733. doi: 10.1113/jphysiol.1996.sp021252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Tsien RW. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron. 2008;58:925–937. doi: 10.1016/j.neuron.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczko A, Goel A, Song L, Lee HK. Visual deprivation decreases somatic GAD65 puncta number on layer 2/3 pyramidal neurons in mouse visual cortex. Neural Plast. 2009;2009:415135. doi: 10.1155/2009/415135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature. 2012;490:397–401. doi: 10.1038/nature11451. [DOI] [PubMed] [Google Scholar]
- Lee HK. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Frontiers Mol Neurosci. 2012;5:17. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Kirkwood A. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Seminars in Cell & Dev Biol. 2011;22:514–520. doi: 10.1016/j.semcdb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Queenan BN, Rozeboom AM, Bellmore R, Lim ST, Vicini S, Pak DT. Mossy Fiber-CA3 Synapses Mediate Homeostatic Plasticity in Mature Hippocampal Neurons. Neuron. 2013;77:99–114. doi: 10.1016/j.neuron.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O’Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nature Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Linden ML, Heynen AJ, Haslinger RH, Bear MF. Thalamic activity that drives visual cortical plasticity. Nat Neurosci. 2009;12:390–392. doi: 10.1038/nn.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghsoodi B, Poon MM, Nam CI, Aoto J, Ting P, Chen L. Retinoic acid regulates RARalpha-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc Natl Acad Sci U S A. 2008;105:16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ. A short-term mechanism of plasticity for interneurones? J Physiol. 1998;511 (Pt 2):331. doi: 10.1111/j.1469-7793.1998.331bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Miller KD, Chapman B, Stryker MP. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1989;86:5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nataraj K, Le Roux N, Nahmani M, Lefort S, Turrigiano G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron. 2010;68:750–762. doi: 10.1016/j.neuron.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Raman IM, Trussell LO. AMPA receptors with high Ca2+ permeability mediate synaptic transmission in the avian auditory pathway. J Physiol. 1995;482 ( Pt 2):309–315. doi: 10.1113/jphysiol.1995.sp020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus E, Anguh TT, Pho H, Lee A, Gammon N, Lee HK. Developmental switch in the polarity of experience-dependent synaptic changes in layer 6 of mouse visual cortex. J Neurophysiol. 2011;106:2499–2505. doi: 10.1152/jn.00111.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory Role of NR2A for Metaplasticity in Visual Cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARalpha. Proc Natl Acad Sci U S A. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Ebner FF. Neonatal sensory deprivation and the development of cortical function: unilateral and bilateral sensory deprivation result in different functional outcomes. J Neurophysiol. 2010;104:98–107. doi: 10.1152/jn.00120.2009. [DOI] [PubMed] [Google Scholar]
- Qian H, Matt L, Zhang M, Nguyen M, Patriarchi T, Koval OM, Anderson ME, He K, Lee HK, Hell JW. beta2-Adrenergic receptor supports prolonged theta tetanus-induced LTP. J Neurophysiol. 2012;107:2703–2712. doi: 10.1152/jn.00374.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rannals MD, Kapur J. Homeostatic Strengthening of Inhibitory Synapses Is Mediated by the Accumulation of GABAA Receptors. J Neurosci. 2011;31:17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson A, Cheetham CE, Fox K, Sengpiel F. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc Natl Acad Sci U S A. 2012;109:1311–1316. doi: 10.1073/pnas.1112204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Siegler BA, Voelker CC, Shouval HZ, Paradiso MA, Bear MF. Stimulus for rapid ocular dominance plasticity in visual cortex. J Neurophysiol. 2006;95:2947–2950. doi: 10.1152/jn.01328.2005. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol. 1998;511 ( Pt 2):361–377. doi: 10.1111/j.1469-7793.1998.361bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: offline memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, Sibley D. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965a;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965b;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, Saab F, Tonegawa S, Heinemann SF, O’Dell TJ, Fanselow MS, Vissel B. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BJ, Smith GB, Heynen AJ, Neve RL, Bear MF. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc Natl Acad Sci U S A. 2009;106:9860–9865. doi: 10.1073/pnas.0901305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Thalamocortical inputs show post-critical-period plasticity. Neuron. 2012;74:731–742. doi: 10.1016/j.neuron.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Zhou Y, Tao HW. Perspectives on: information and coding in mammalian sensory physiology: inhibitory synaptic mechanisms underlying functional diversity in auditory cortex. J Gen Physiol. 2011;138:311–320. doi: 10.1085/jgp.201110650. [DOI] [PMC free article] [PubMed] [Google Scholar]