Abstract
A valid method of studying age related degenerative pathologies is to study human genetic diseases that appear to accelerate many, though not necessarily all, features of the aging process. Such diseases are described as progeroid syndromes because of their possible relevance to many aspects of aging and age related disease. This article describes the recent progress made at the cellular and molecular levels in understanding the pathogenesis of one of the best characterised of these disorders, Werner's syndrome. These observations are related to some of the less well characterised progeroid syndromes within the context of the cell senescence hypothesis of aging, a theory formulated to explain the aging of regenerative tissue in normal individuals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp R. C., Chang E., Kashefi-Aazam M., Rogaev E. I., Piatyszek M. A., Shay J. W., Harley C. B. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995 Sep;220(1):194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Harley C. B. Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res. 1995 Jul;219(1):130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermbach G., Mayer U., Naumann G. O. Human lens epithelial cells in tissue culture. Exp Eye Res. 1991 Feb;52(2):113–119. doi: 10.1016/0014-4835(91)90251-9. [DOI] [PubMed] [Google Scholar]
- Blake D. A., Yu H., Young D. L., Caldwell D. R. Matrix stimulates the proliferation of human corneal endothelial cells in culture. Invest Ophthalmol Vis Sci. 1997 May;38(6):1119–1129. [PubMed] [Google Scholar]
- Brown W. T., Zebrower M., Kieras F. J. Progeria, a model disease for the study of accelerated aging. Basic Life Sci. 1985;35:375–396. doi: 10.1007/978-1-4899-2218-2_24. [DOI] [PubMed] [Google Scholar]
- Campisi J. The biology of replicative senescence. Eur J Cancer. 1997 Apr;33(5):703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Sharf B. B. Cellular senescence and DNA synthesis. Thymidine incorporation as a measure of population age in human diploid cells. Exp Cell Res. 1973 Feb;76(2):419–427. doi: 10.1016/0014-4827(73)90394-7. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Motulsky A. G. Werner syndrome: entering the helicase era. Bioessays. 1996 Dec;18(12):1025–1027. doi: 10.1002/bies.950181214. [DOI] [PubMed] [Google Scholar]
- Faragher R. G., Hardy S. P., Davis T., Dropcova S., Allen M. C. Cycling Werner's syndrome fibroblasts display calcium-dependent potassium currents. Exp Cell Res. 1997 Feb 25;231(1):119–122. doi: 10.1006/excr.1996.3437. [DOI] [PubMed] [Google Scholar]
- Faragher R. G., Kill I. R., Hunter J. A., Pope F. M., Tannock C., Shall S. The gene responsible for Werner syndrome may be a cell division "counting" gene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12030–12034. doi: 10.1073/pnas.90.24.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber A., Chang C., Sell C., Ptasznik A., Cristofalo V. J., Hubbard K., Ozer H. L., Adamo M., Roberts C. T., Jr, LeRoith D. Failure of senescent human fibroblasts to express the insulin-like growth factor-1 gene. J Biol Chem. 1993 Aug 25;268(24):17883–17888. [PubMed] [Google Scholar]
- Fukuchi K., Martin G. M., Monnat R. J., Jr Mutator phenotype of Werner syndrome is characterized by extensive deletions. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5893–5897. doi: 10.1073/pnas.86.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Tanaka K., Kumahara Y., Marumo K., Pride M. B., Martin G. M., Monnat R. J., Jr Increased frequency of 6-thioguanine-resistant peripheral blood lymphocytes in Werner syndrome patients. Hum Genet. 1990 Feb;84(3):249–252. doi: 10.1007/BF00200569. [DOI] [PubMed] [Google Scholar]
- Gao C. Y., Zelenka P. S. Cyclins, cyclin-dependent kinases and differentiation. Bioessays. 1997 Apr;19(4):307–315. doi: 10.1002/bies.950190408. [DOI] [PubMed] [Google Scholar]
- Garvik B., Carson M., Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995 Nov;15(11):6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart E., Bauer R., Raub U., Schinzel M., Ruprecht K. W., Jonas J. B. Spontaneous and induced chromosomal instability in Werner syndrome. Hum Genet. 1988 Oct;80(2):135–139. doi: 10.1007/BF00702855. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. In vitro assessment of keratinocyte aging. J Invest Dermatol. 1983 Jul;81(1 Suppl):184s–189s. doi: 10.1111/1523-1747.ep12541084. [DOI] [PubMed] [Google Scholar]
- Goto M., Rubenstein M., Weber J., Woods K., Drayna D. Genetic linkage of Werner's syndrome to five markers on chromosome 8. Nature. 1992 Feb 20;355(6362):735–738. doi: 10.1038/355735a0. [DOI] [PubMed] [Google Scholar]
- Grandin N., Reed S. I., Charbonneau M. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 1997 Feb 15;11(4):512–527. doi: 10.1101/gad.11.4.512. [DOI] [PubMed] [Google Scholar]
- Greenhaw G. A., Hebert A., Duke-Woodside M. E., Butler I. J., Hecht J. T., Cleaver J. E., Thomas G. H., Horton W. A. Xeroderma pigmentosum and Cockayne syndrome: overlapping clinical and biochemical phenotypes. Am J Hum Genet. 1992 Apr;50(4):677–689. [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Villeponteau B. Telomeres and telomerase in aging and cancer. Curr Opin Genet Dev. 1995 Apr;5(2):249–255. doi: 10.1016/0959-437x(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Setlow R. B. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The cell biology of aging. J Invest Dermatol. 1979 Jul;73(1):8–14. doi: 10.1111/1523-1747.ep12532752. [DOI] [PubMed] [Google Scholar]
- Henderson S., Allsopp R., Spector D., Wang S. S., Harley C. In situ analysis of changes in telomere size during replicative aging and cell transformation. J Cell Biol. 1996 Jul;134(1):1–12. doi: 10.1083/jcb.134.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashikawa T., Fujiwara Y. Normal level of unscheduled DNA synthesis in Werner's syndrome fibroblasts in culture. Exp Cell Res. 1978 May;113(2):438–442. doi: 10.1016/0014-4827(78)90386-5. [DOI] [PubMed] [Google Scholar]
- Hoppenreijs V. P., Pels E., Vrensen G. F., Treffers W. F. Effects of platelet-derived growth factor on endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):150–161. [PubMed] [Google Scholar]
- Kill I. R., Faragher R. G., Lawrence K., Shall S. The expression of proliferation-dependent antigens during the lifespan of normal and progeroid human fibroblasts in culture. J Cell Sci. 1994 Feb;107(Pt 2):571–579. doi: 10.1242/jcs.107.2.571. [DOI] [PubMed] [Google Scholar]
- Kill I. R., Shall S. Senescent human diploid fibroblasts are able to support DNA synthesis and to express markers associated with proliferation. J Cell Sci. 1990 Nov;97(Pt 3):473–478. doi: 10.1242/jcs.97.3.473. [DOI] [PubMed] [Google Scholar]
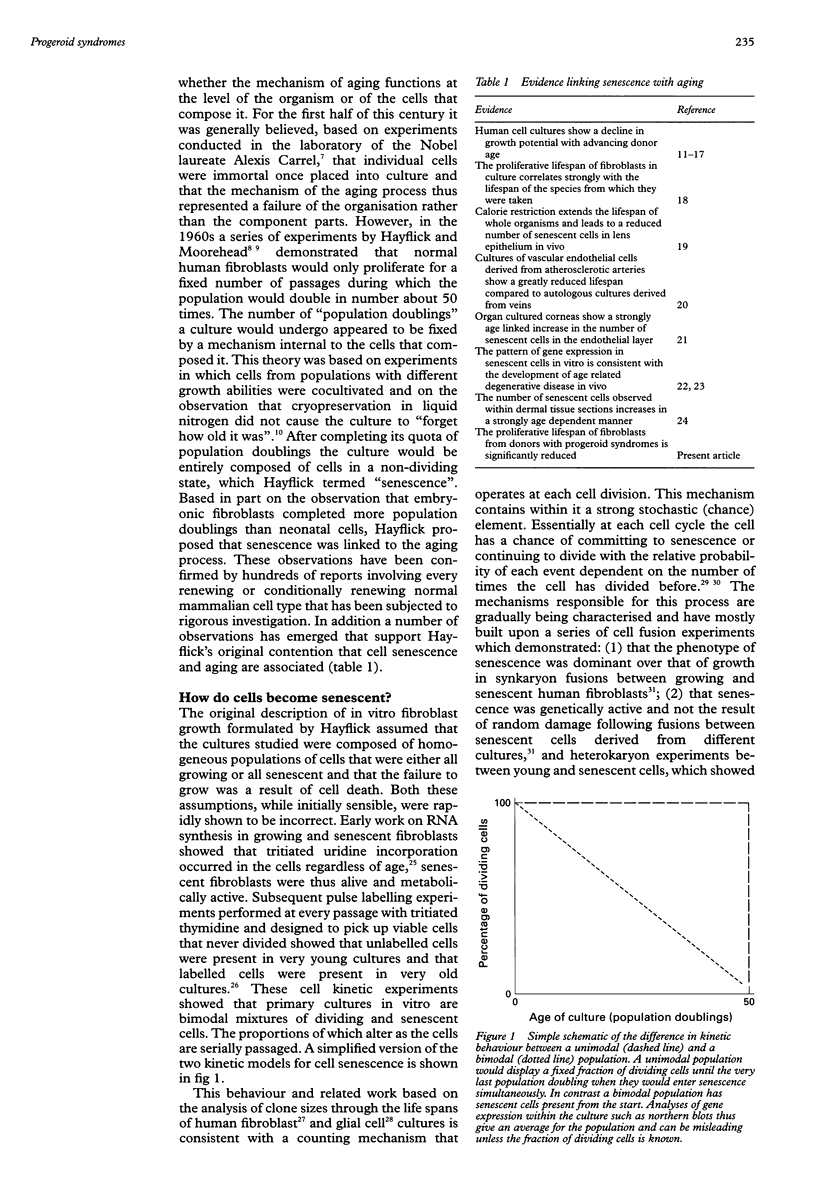
- Kirkwood T. B., Holliday R. The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci. 1979 Sep 21;205(1161):531–546. doi: 10.1098/rspb.1979.0083. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. B. Human senescence. Bioessays. 1996 Dec;18(12):1009–1016. doi: 10.1002/bies.950181211. [DOI] [PubMed] [Google Scholar]
- Kodama S., Yamada H., Annab L., Barrett J. C. Elevated expression of mitochondrial cytochrome b and NADH dehydrogenase subunit 4/4L genes in senescent human cells. Exp Cell Res. 1995 Jul;219(1):82–86. doi: 10.1006/excr.1995.1207. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B., Moerman E. J., Jones R. A., Goldstein S. Identification of gene sequences overexpressed in senescent and Werner syndrome human fibroblasts. Exp Gerontol. 1996 Jan-Apr;31(1-2):159–174. doi: 10.1016/0531-5565(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Leckie B. J. High blood pressure: hunting the genes. Bioessays. 1992 Jan;14(1):37–41. doi: 10.1002/bies.950140108. [DOI] [PubMed] [Google Scholar]
- Li Y., Yan Q., Wolf N. S. Long-term caloric restriction delays age-related decline in proliferation capacity of murine lens epithelial cells in vitro and in vivo. Invest Ophthalmol Vis Sci. 1997 Jan;38(1):100–107. [PubMed] [Google Scholar]
- Lin J. J., Zakian V. A. The Saccharomyces CDC13 protein is a single-strand TG1-3 telomeric DNA-binding protein in vitro that affects telomere behavior in vivo. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13760–13765. doi: 10.1073/pnas.93.24.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens M. H., Feng J., Andrews W. H., Enlow B. E., Saati S. M., Tonkin L. A., Funk W. D., Villeponteau B. Cataloging altered gene expression in young and senescent cells using enhanced differential display. Nucleic Acids Res. 1995 Aug 25;23(16):3244–3251. doi: 10.1093/nar/23.16.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. D., Taylor A. The in vitro replicative potential and cellular morphology of human lens epithelial cells derived from different aged donors. Curr Eye Res. 1987 Dec;6(12):1453–1457. doi: 10.3109/02713688709044509. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. Attempted hybridizations with senescent human fibroblasts. J Cell Physiol. 1973 Aug;82(1):129–132. doi: 10.1002/jcp.1040820115. [DOI] [PubMed] [Google Scholar]
- Liu S., Thweatt R., Lumpkin C. K., Jr, Goldstein S. Suppression of calcium-dependent membrane currents in human fibroblasts by replicative senescence and forced expression of a gene sequence encoding a putative calcium-binding protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2186–2190. doi: 10.1073/pnas.91.6.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llerena J. C., Jr, Murer-Orlando M. Bloom syndrome and ataxia telangiectasia. Semin Hematol. 1991 Apr;28(2):95–103. [PubMed] [Google Scholar]
- Lydall D., Weinert T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science. 1995 Dec 1;270(5241):1488–1491. doi: 10.1126/science.270.5241.1488. [DOI] [PubMed] [Google Scholar]
- Macieira-Coelho A., Pontén J., Philipson L. The division cycle and RNA-synthesis in diploid human cells at different passage levels in vitro. Exp Cell Res. 1966 Jun;42(3):673–684. doi: 10.1016/0014-4827(66)90280-1. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Hirose Y., Langmore J. P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997 Mar 7;88(5):657–666. doi: 10.1016/s0092-8674(00)81908-x. [DOI] [PubMed] [Google Scholar]
- Martin G. M. Genetics and aging; the Werner syndrome as a segmental progeroid syndrome. Adv Exp Med Biol. 1985;190:161–170. doi: 10.1007/978-1-4684-7853-2_5. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- Metcalfe J. A., Parkhill J., Campbell L., Stacey M., Biggs P., Byrd P. J., Taylor A. M. Accelerated telomere shortening in ataxia telangiectasia. Nat Genet. 1996 Jul;13(3):350–353. doi: 10.1038/ng0796-350. [DOI] [PubMed] [Google Scholar]
- Millis A. J., Sottile J., Hoyle M., Mann D. M., Diemer V. Collagenase production by early and late passage cultures of human fibroblasts. Exp Gerontol. 1989;24(5-6):559–575. doi: 10.1016/0531-5565(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Murano S., Thweatt R., Shmookler Reis R. J., Jones R. A., Moerman E. J., Goldstein S. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991 Aug;11(8):3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Norris P. G., Limb G. A., Hamblin A. S., Lehmann A. R., Arlett C. F., Cole J., Waugh A. P., Hawk J. L. Immune function, mutant frequency, and cancer risk in the DNA repair defective genodermatoses xeroderma pigmentosum, Cockayne's syndrome, and trichothiodystrophy. J Invest Dermatol. 1990 Jan;94(1):94–100. doi: 10.1111/1523-1747.ep12873952. [DOI] [PubMed] [Google Scholar]
- Nugent C. I., Hughes T. R., Lue N. F., Lundblad V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996 Oct 11;274(5285):249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- Ogryzko V. V., Hirai T. H., Russanova V. R., Barbie D. A., Howard B. H. Human fibroblast commitment to a senescence-like state in response to histone deacetylase inhibitors is cell cycle dependent. Mol Cell Biol. 1996 Sep;16(9):5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima J., Campisi J., Tannock T. C., Martin G. M. Regulation of c-fos expression in senescing Werner syndrome fibroblasts differs from that observed in senescing fibroblasts from normal donors. J Cell Physiol. 1995 Feb;162(2):277–283. doi: 10.1002/jcp.1041620213. [DOI] [PubMed] [Google Scholar]
- Pawelec G., Sansom D., Rehbein A., Adibzadeh M., Beckman I. Decreased proliferative capacity and increased susceptibility to activation-induced cell death in late-passage human CD4+ TCR2+ cultured T cell clones. Exp Gerontol. 1996 Nov-Dec;31(6):655–668. doi: 10.1016/s0531-5565(96)00097-6. [DOI] [PubMed] [Google Scholar]
- Perillo N. L., Naeim F., Walford R. L., Effros R. B. In vitro cellular aging in T-lymphocyte cultures: analysis of DNA content and cell size. Exp Cell Res. 1993 Jul;207(1):131–135. doi: 10.1006/excr.1993.1171. [DOI] [PubMed] [Google Scholar]
- Perillo N. L., Walford R. L., Newman M. A., Effros R. B. Human T lymphocytes possess a limited in vitro life span. Exp Gerontol. 1989;24(3):177–187. doi: 10.1016/0531-5565(89)90009-0. [DOI] [PubMed] [Google Scholar]
- Pontén J., Stein W. D., Shall S. A quantitative analysis of the aging of human glial cells in culture. J Cell Physiol. 1983 Dec;117(3):342–352. doi: 10.1002/jcp.1041170309. [DOI] [PubMed] [Google Scholar]
- Prothero J., Gallant J. A. A model of clonal attenuation. Proc Natl Acad Sci U S A. 1981 Jan;78(1):333–337. doi: 10.1073/pnas.78.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhme D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk D., Bryant E., Au K., Hoehn H., Martin G. M. Systematic growth studies, cocultivation, and cell hybridization studies of Werner syndrome cultured skin fibroblasts. Hum Genet. 1981;58(3):310–316. doi: 10.1007/BF00294930. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz V. P., Zakian V. A., Ogburn C. E., McKay J., Jarzebowicz A. A., Edland S. D., Martin G. M. Accelerated loss of telomeric repeats may not explain accelerated replicative decline of Werner syndrome cells. Hum Genet. 1996 Jun;97(6):750–754. doi: 10.1007/BF02346184. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997 Mar 7;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Shall S., Stein W. D. A mortalization theory for the control of the cell proliferation and for the origin of immortal cell lines. J Theor Biol. 1979 Jan 21;76(2):219–231. doi: 10.1016/0022-5193(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Sikora E., Kamińska B., Radziszewska E., Kaczmarek L. Loss of transcription factor AP-1 DNA binding activity during lymphocyte aging in vivo. FEBS Lett. 1992 Nov 9;312(2-3):179–182. doi: 10.1016/0014-5793(92)80930-f. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Pereira-Smith O. M. Genetic and molecular studies of cellular immortalization. Adv Cancer Res. 1990;54:63–77. doi: 10.1016/s0065-230x(08)60808-8. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Pereira-Smith O. M. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996 Jul 5;273(5271):63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Whitney R. G. Intraclonal variation in proliferative potential of human diploid fibroblasts: stochastic mechanism for cellular aging. Science. 1980 Jan 4;207(4426):82–84. doi: 10.1126/science.7350644. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Dulić V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995 Jun;17(6):537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- Thweatt R., Goldstein S. Werner syndrome and biological ageing: a molecular genetic hypothesis. Bioessays. 1993 Jun;15(6):421–426. doi: 10.1002/bies.950150609. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W. Arteriosclerosis. Impairment of cellular interactions in the arterial wall. Ann N Y Acad Sci. 1992 Dec 26;673:321–330. doi: 10.1111/j.1749-6632.1992.tb27467.x. [DOI] [PubMed] [Google Scholar]
- Venable M. E., Lee J. Y., Smyth M. J., Bielawska A., Obeid L. M. Role of ceramide in cellular senescence. J Biol Chem. 1995 Dec 22;270(51):30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- Webb D. K., Evans M. K., Bohr V. A. DNA repair fine structure in Werner's syndrome cell lines. Exp Cell Res. 1996 May 1;224(2):272–278. doi: 10.1006/excr.1996.0137. [DOI] [PubMed] [Google Scholar]
- Weirich-Schwaiger H., Weirich H. G., Gruber B., Schweiger M., Hirsch-Kauffmann M. Correlation between senescence and DNA repair in cells from young and old individuals and in premature aging syndromes. Mutat Res. 1994 Feb;316(1):37–48. doi: 10.1016/0921-8734(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Wellinger R. J., Ethier K., Labrecque P., Zakian V. A. Evidence for a new step in telomere maintenance. Cell. 1996 May 3;85(3):423–433. doi: 10.1016/s0092-8674(00)81120-4. [DOI] [PubMed] [Google Scholar]
- West M. D., Shay J. W., Wright W. E., Linskens M. H. Altered expression of plasminogen activator and plasminogen activator inhibitor during cellular senescence. Exp Gerontol. 1996 Jan-Apr;31(1-2):175–193. doi: 10.1016/0531-5565(95)02013-6. [DOI] [PubMed] [Google Scholar]
- Wistrom C., Villeponteau B. Cloning and expression of SAG: a novel marker of cellular senescence. Exp Cell Res. 1992 Apr;199(2):355–362. doi: 10.1016/0014-4827(92)90445-e. [DOI] [PubMed] [Google Scholar]
- Yu C. E., Oshima J., Fu Y. H., Wijsman E. M., Hisama F., Alisch R., Matthews S., Nakura J., Miki T., Ouais S. Positional cloning of the Werner's syndrome gene. Science. 1996 Apr 12;272(5259):258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- Yu C. E., Oshima J., Wijsman E. M., Nakura J., Miki T., Piussan C., Matthews S., Fu Y. H., Mulligan J., Martin G. M. Mutations in the consensus helicase domains of the Werner syndrome gene. Werner's Syndrome Collaborative Group. Am J Hum Genet. 1997 Feb;60(2):330–341. [PMC free article] [PubMed] [Google Scholar]
- Zeng G., Millis A. J. Differential regulation of collagenase and stromelysin mRNA in late passage cultures of human fibroblasts. Exp Cell Res. 1996 Jan 10;222(1):150–156. doi: 10.1006/excr.1996.0019. [DOI] [PubMed] [Google Scholar]
- Zeng G., Millis A. J. Expression of 72-kDa gelatinase and TIMP-2 in early and late passage human fibroblasts. Exp Cell Res. 1994 Jul;213(1):148–155. doi: 10.1006/excr.1994.1184. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T., Saretzki G., Döcke W., Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995 Sep;220(1):186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]