Abstract
Insect pollination benefits over three quarters of the world's major crops. There is growing concern that observed declines in pollinators may impact on production and revenues from animal pollinated crops. Knowing the distribution of pollinators is therefore crucial for estimating their availability to pollinate crops; however, in general, we have an incomplete knowledge of where these pollinators occur. We propose a method to predict geographical patterns of pollination service to crops, novel in two elements: the use of pollinator records rather than expert knowledge to predict pollinator occurrence, and the inclusion of the managed pollinator supply. We integrated a maximum entropy species distribution model (SDM) with an existing pollination service model (PSM) to derive the availability of pollinators for crop pollination. We used nation-wide records of wild and managed pollinators (honey bees) as well as agricultural data from Great Britain. We first calibrated the SDM on a representative sample of bee and hoverfly crop pollinator species, evaluating the effects of different settings on model performance and on its capacity to identify the most important predictors. The importance of the different predictors was better resolved by SDM derived from simpler functions, with consistent results for bees and hoverflies. We then used the species distributions from the calibrated model to predict pollination service of wild and managed pollinators, using field beans as a test case. The PSM allowed us to spatially characterize the contribution of wild and managed pollinators and also identify areas potentially vulnerable to low pollination service provision, which can help direct local scale interventions. This approach can be extended to investigate geographical mismatches between crop pollination demand and the availability of pollinators, resulting from environmental change or policy scenarios.
Introduction
The importance of ecosystems to human well-being was documented by the Millennium Ecosystem Assessment, which also recognised that the majority of pollinators are in decline or threatened [1]. Crop pollination is a key ecosystem service vital to the maintenance of both wild plant communities and agricultural productivity. Over three quarters of the world's major crops benefit from insect pollination, with an economic value estimated to be around € 153 billion globally in 2005 and approximately € 500 million in the United Kingdom [2]–[4]. Pollination services are mainly provided by wild pollinators (bees, hoverflies, flies, moths, beetles) and domesticated bees (primarily honey bee Apis mellifera). The recent declines observed in pollinators, mainly bees [5], [6], may therefore impact on the production of and profits from pollinator-dependent crops. For instance, long-term trends of global crop production suggest that to compensate for a 3–8% yield reduction expected in absence of animal pollination, the expansion of agricultural land would be much greater (ca. 25%, and proportionally much greater in the developing world), which in turn could accelerate habitat destruction and contribute to further pollination loss [7].
Knowing spatial patterns of managed and wild pollinators is therefore crucial to estimate their availability to crops and to inform management strategies. In general, however, we have incomplete knowledge of where wild pollinators occur. To overcome this, a recent approach proposed by Lonsdorf et al. [8] derives the probability of occurrence of wild bees from a relative availability (from 0 to 1) of nesting sites and floral resources within a landscape, assessed for a few large guilds of species. This probability is then used to derive the relative pollinator service available to a particular crop, taking into account crop location, its potential pollinators and their foraging distance.
Here we propose an approach that combines the Lonsdorf model to derive pollinator services, with predicted pollinator occurrences from species records rather than from landscape suitability. One of the preferred tools to predict species spatial patterns from geographically and temporally sparse biodiversity data are species distribution models (SDMs), which now offer a wide range of approaches due to enhanced computational resources, increasing availability of spatially explicit environmental information and accessibility of species occurrence databases [9], [10]. SDMs mainly differ in the requirements of the species records (e.g. presence and true absence, presence and background, presence only) and in the algorithms used to define the species niche as a function of the predictors (e.g. regression methods, machine learning techniques, Bayesian statistics) [11]. While it is unlikely that a single modelling approach will outperform all others in any situation, comparative work [10], [12] helps to identify the main elements affecting model performance, and thus represents a valuable resource to orient the end user in the choice of the modelling approach. In this study we use the maximum entropy method implemented within the freely available software MaxEnt [13], to derive species distributions from sparse pollinator records. MaxEnt is a general purpose machine-learning technique that estimates the potential distribution of the species by estimating the probability distribution of maximum entropy (i.e. that is most spread out), subject to the constraints derived from the available occurrence data [13]. MaxEnt has received increasing attention within the field of SDM (File S1: Fig. S1–1), both in single species applications [14], [15] and in comparisons of algorithms [16], [17]. Extensive experimental work has allowed guidance for several settings of MaxEnt modelling [18], as well as drawing attention to the main elements affecting its performance [19]–[21].
First, we describe the main steps and tests carried out to calibrate the MaxEnt model; we then show the predictions of the calibrated model for a representative sample of crop-pollinators within Great Britain; finally, we use the predicted species distributions to derive the potential pollination service of wild pollinators and managed honey bees, using the annual legume field bean Vicia faba as a test case. In conclusion, we discuss some of the methodological advantages of our approach, the remaining challenges and how it can be further applied to other ecological questions.
Materials and Methods
Datasets
Wild pollinator data
We used presence-only records of wild bees and hoverflies collected within the period 2000–2010 (“Bees, Wasps and Ants Recording Society”, BWARS [22]; “Hoverfly Recording Scheme”, HRS [23]). The spatial accuracy of the data varied between 10 m and 10 km; we chose 1 km2 as a suitable resolution to balance the aim to derive patterns at a national extent as well as to inform decisions at the local scale. We registered all records with accuracy finer than or equal to 1 km to a grid of 1 km2 cells, removing duplicates so that within each cell there was only one record of the same species. We use the term “records” to mean the number of original records for each species, with accuracy finer or equal to 1 km, whilst we use “occurrences” to denote the number of 1 km2 grid cells occupied by a species.
To calibrate the model, we selected a subset of pollinator species representing a range of geographic distributions. To follow a repeatable and objective procedure, we used hierarchical cluster analysis to group the species based on the number of occurrences, minimum and maximum latitude and longitude (expressed as northing and easting on the British National Grid), and spatial distribution. Spatial distribution was measured as the longest distance within the third quartile of the pairwise distances between the occurrences of each species; we preferred the third quartile over the fourth quartile, to avoid potential outliers and thus obtain a better characterisation of the species' distribution extent [24]. We selected about one third of the species from within each resulting cluster, ensuring representation across genera. Finally, we used visual inspection of the species occurrence maps to confirm they represented contrasting geographic distributions (e.g. ranging from few to many occurrences, and from narrow to wide range). The selection was carried out separately for bees and hoverflies.
Six hoverfly species and 22 bee species were selected (File S2: Table S2–1). The number of occurrences ranged from 232 to 4048 for hoverflies and from 12 to 4144 for bees, and longest third quartile distance ranged from 215.4 km to 312.6 km for hoverflies and from 63.8 km to 763.9 km for bees.
Managed pollinator data
The distribution of the managed honey bees was derived from the optional beekeeping register BeeBase, held by the National Bee Unit at the Food and Environment Research Agency [25]. The number of bee foragers was modelled at the 4 km2 resolution from the size and location of apiaries in England and Wales using the estimated average foraging distance [26]–[28]. At the time of this study, a sufficient coverage of apiaries from which to model forager numbers was not available for Scotland. To match the grain to the other pollinator data, the number of foragers was divided by four, assuming a uniform distribution across the four 1 km2 grid cells. The data were then linearly rescaled between 0 and 1, to provide a relative score of honey bee foragers per km2.
Environmental predictors
We use four types of environmental predictors (Table 1):
Table 1. Environmental predictors used to derive species distribution models.
| Variable theme | Variable name | Variable definition |
| Topography | *AspNS | Aspect = sin (rad (aspect)) |
| †AspEW | Aspect = cos (rad (aspect)) | |
| Climate | ‡Isoth | Isothermality % |
| TAR | Temperature Annual Range | |
| MTDQ | Mean Temperature of Driest Quarter | |
| MTCQ | Mean Temperature of Coldest Quarter | |
| RainSeasCV | Precipitation Seasonality (Coefficient of Variation) | |
| RainCQ | Precipitation Coldest Quarter (mm) | |
| Land-cover | BLW | Broadleaf woodland |
| ConW | Coniferous woodland | |
| AR | Arable | |
| GrassImp | Improved grassland | |
| GrassSN | Semi-natural grassland | |
| MHB | Mountain, heath, bog | |
| SW | Saltwater | |
| FW | Freshwater | |
| Coast | Coastal | |
| UrbGar | Built-up areas and gardens | |
| Pesticides | Pest | Average number of risk hectares |
AspNS = sine (radiant [aspect angle in degree]); †AspEW = cosine (radiant [aspect angle in degree]); ‡Isothermality % = Mean Diurnal Range (MDR)/Temperature Annual Range (TAR); where MDR = Mean of monthly (max temp – min temp)); TAR = Max Temperature of Warmest Month – Min Temperature of Coldest Month. Isothermality is a quantification of how large the day-to-night temperature oscillation is in comparison to the summer-to-winter oscillation. A value of 100 would represent a site where the diurnal temperature range is equal to the annual temperature range. A value of 50 would indicate a location where the diurnal temperature range is half of the annual temperature range.
Land cover classes: 10 continuous variables representing cover percent for Great Britain, from CEH Land Cover Map 2007 [29].
Bio-climatic data [30]: six variables derived from 25 km2 gridded monthly averages of minimum temperature, maximum temperature and precipitation for the 1991–2000 period (to provide an average climate characterizing the decade preceding the oldest species records), obtained from UKCP09 [31] and resampled to 1 km2 grain. Data were computed within R software environment 2.13.0 [32].
Topography: two indices describing aspect (i.e. slope orientation), derived from a 10 m horizontal interval digital elevation model [33] resampled to 1 km2.
Pesticides: treated hectares that pose a potential risk to bees per hectare of crop grown, derived from the Pesticides Usage Survey [34] and linked to cropping data from the Defra June Agricultural Survey [35]. The impact was assessed for honey bees, due to data availability for this species [36], [37].
Bio-climatic and topographic variables were selected using Jolliffe's Principal Component Analysis [38] to minimize multicollinearity [39]. Details of this procedure and the Pearson's correlation between the chosen variables are in File S2: Table S2–2.
Species distribution models
Choice of the background data
Species distribution models were carried out within MaxEnt 3.3.3 k [13], [40]. One of the advantages of MaxEnt is that presence-only data can be used. In this case the MaxEnt probability is defined over a sample of points taken from the study region (“background points”) which may, or may not, contain the species presence records [13].
To derive the MaxEnt probability, in addition to the environmental conditions at localities where a species is found, the model requires a sample from the background. This assumes a uniform survey effort over the entire study area, but if this assumption is violated the background information should reflect the sample bias. A possible correction is to restrict the selection of the background points to a region where a target group of species has been observed by similar methods [19]. We tested for violation of this assumption by comparing the AUC (Area Under the Curve of the Receiver Operating Characteristic) of models based on all known records of crop pollinators in Great Britain (i.e. the target group background, TGB), against the AUC of models based on n-time sampling an equal number of points from the entire study area (referred as null models) [41]. We found that the average AUC of 10 sets of 5000 points drawn from the TGB was significantly greater than the average AUC of 100 null models from the entire study area (0.771±0.002 and 0.543±0.005 respectively). The background localities for the individual SDMs were therefore drawn from within the TGB.
Model calibration
During the model calibration we evaluated the single and combined effects of changing two main MaxEnt settings:
Choice of the feature classes (the functions) used to fit the data: default settings currently allow for six feature classes (Linear, Quadratic, Product, Threshold, Hinge and Categorical), provided sufficient samples are available [42]. For each species, we compared models with default settings against models built with Hinge features alone, which are base functions for piecewise linear splines [18]. When using Hinge alone, we modified the threshold for minimum sample size to 12 (rather than 15, the default) to allow its application to the full set of calibration species (which included Andrena niveata with 12 records and Lasioglossum semilucens with 13).
Default prevalence: prevalence is defined as the probability of presence at ordinary occurrence points and, when absence data are not available, MaxEnt assigns it 0.5 [18]. It is defined over specific spatial and temporal scales, which should be taken into account particularly when working with pools of species differing in their rarity [42]. For each species, we compared models with default prevalence 0.5 to models where this value was modified to reflect the species commonality relative to the rest of the species within the pollinator set. To our knowledge there are no theoretically based rules to adjust this value; we therefore empirically rescaled it, considering the number of available records, the number of occurrences and the number of years with non-zero observations over the temporal scale (2000–2010). Each species was then assigned a new prevalence, from 0.1 to 0.5 (File S3: Table S3–1, Fig. S3–1, Figs S3–2.1 and S3–2.2).
Model performance
We evaluated the effects of changing default settings on model calibration with two metrics:
The model testing AUC and its standard deviation (AUCSD), using mixed effect models with species as random factor. We tested whether changing default parameters significantly affected the AUC and its variability between different models.
The standard deviation (SD) of the Permutation importance (%) between predictors and background, using generalized linear models. The Permutation importance is derived by randomly permuting the values of each predictor between presence and background in turn; the model is re-evaluated on the permuted values and the resulting drop in training AUC is then normalized to percentages. We expected that a model with good discriminatory power would result in a greater spread between the significance of the different predictors.
All models were carried out through k-fold cross-validation, where data are divided into k mutually exclusive subsets: for each run, k–1 of them are combined into a set for training, and one is used for the prediction (i.e. model testing). The number of mutually exclusive subsets was 10 for all species. Evaluation was performed on the average of the cross-validation runs (for AUC, AUCSD, Permutation importance and its SD).
After completing the model calibration, we used null models to test whether the resulting SDMs provided a significantly better fit than expected by chance alone. With presence-only data the maximum achievable AUC is <1 [43]: namely, it is 1-a/2, with a being the true fraction of the study area occupied by a species, typically unknown when absence data are not available [13]. To assess SDM accuracy, therefore, we compared the average AUC value of each species SDM (AUCSDM) with the average AUC value of a set of null models (AUCNM) where species records were replaced by randomly chosen locations [41]. We expected AUCSDM > AUCNM.
Following the assessment of model performance, we tested whether the predictors that were most important for fitting the training data were also the most important for predicting species distribution. Single-predictor models are built within MaxEnt for the training and testing phases: we ranked them according to their gain (a measure of model fit), assigning one the model with the lowest increase in gain. We then computed the Spearman's rank correlation between training and testing models for each predictor, using Mean and Mode. Their observed correlations were tested against the frequency of randomly generated correlations, using 999 bootstrap replicates [44]. Lastly, we also tested whether the Mean of each predictor was correlated to its Mode, for the pooled set of training and testing models.
Application to crop pollinators
Pollinator distribution models
The settings chosen from the model calibration were used to derive SDMs for the wild pollinators of field bean. We used expert knowledge from our team and existing literature [45] to select species known to pollinate field bean. For each species, we used “10th percentile training presence” as threshold to derive a binary map (1 = presence, 0 = absence) from the predicted continuous probability of each of the cross-validation runs. We summed together the 10 binary maps and we took the areas where the sum equalled 10 (i.e. areas where all 10 runs had predicted presence) as the presence area for that species. This strict criterion implies that the sites where all 10 runs have predicted presence identify conditions of greatest suitability for the species. The effects of this choice compared to a less conservative criterion are presented in the results. We then assigned to each presence area the average probability of presence derived from the 10 model runs, this became the predicted likelihood of occurrence for that particular species. This map was used as pollinator source to derive the potential pollinator service.
For consistency with the modelled distributions of wild pollinators, we applied a threshold to the probability of occurrence of managed honey bees to distinguish absence from presence. We used the fifth percentile as a cut-off, corresponding to a 0.001 probability of occurrence, and we assigned “absence” to areas with probability below this threshold. This threshold is less conservative than the one used for wild pollinators, to reflect the fact that the data on managed pollinators are based on information updated annually and on dispersal functions empirically derived.
Crop distribution
We used distributional records of field bean from the Defra 2010 June Agricultural Survey and mapped to an original grain of 4 km2, which we resampled to 1 km2 to match the grain of the SDMs.
Pollinator service
We adapted the model by Lonsdorf et al. [8], which focuses on wild bees. The model maps an index of potential pollinator abundance (“pollinator source map”), based on the relative availability of nesting sites and floral resources across the landscape as provided by expert knowledge and/or field observations. The source map is used to estimate the potential pollinator service Pos [8]:
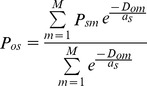 |
(1) |
Where: Psm = relative index for pollinator species s on map unit m, based on the pollinator source map; Dom = (Euclidean) distance between map unit m and crop cell o; as = average foraging distance of species s. Equation 1 is the distance-weighted proportion of M cells occupied by foraging pollinators [46]. The score Pos therefore represents the relative abundance (from 0 to 1) of the pollinator species s visiting each crop cell, i.e. the pollination service from species s.
The main difference between Lonsdorf's model and ours is the input used to generate the potential pollinator source (Psm): in our case, it is not derived from landscape suitability scores for nesting sites and floral resources, but from SDMs based on actual species records. We discuss the implications later in the text.
The total service Po of S pollinator species visiting cell o is [8]:
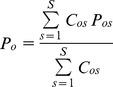 |
(2) |
Where Cos is 1 if the crop on farm o requires pollinator s, and 0 otherwise.
The model was carried out in NetLogo 5.0.1 [47]; the outputs were exported to ArcGIS 10.0 [48] for visualization.
Wild pollinator foraging distances were estimated from expert knowledge within our team and existing literature [49], [50]: we used 1 km for Andrena labialis, A. wilkella, Bombus hortorum, B. lucorum, B. muscorum and Osmia rufa; we doubled this distance forB. lapidarius, B. pascuorum and B. terrestris. We used the estimated foragers' occurrence on the crop parcels as a proxy for the service provision by managed pollinators, as this dataset already accounted for their typical foraging distance.
Results
Model calibration
Modifying default settings for feature class and prevalence did not significantly affect model performance (AUC) (P>0.5 for all, File S4: Table S4–1); variability between cross-validation runs (AUCSD) was also not affected, with the exception of modifying prevalence for features class All in hoverflies, which increased AUCSD (File S4: Table S4–2). In contrast, the ability to discriminate the importance of the different predictors, measured by the SD of the Permutation importance (%) was greater in models built using Hinge feature class alone (P≤0.001 in bees and hoverflies, File S4: Table S4–3); within bees this effect was even stronger when Hinge was used in combination with modified prevalence. In addition, the more complex response curves allowed by the default settings All suggested in some cases a possible overfit (a representative subset of these curves is shown in File S4: from Fig. S4–1.1 to Fig. S4–1.4).
Based on these patterns we chose Hinge feature class alone (with modified prevalence) to derive SDMs for the set of pollinators relevant to British crops.
Model performance
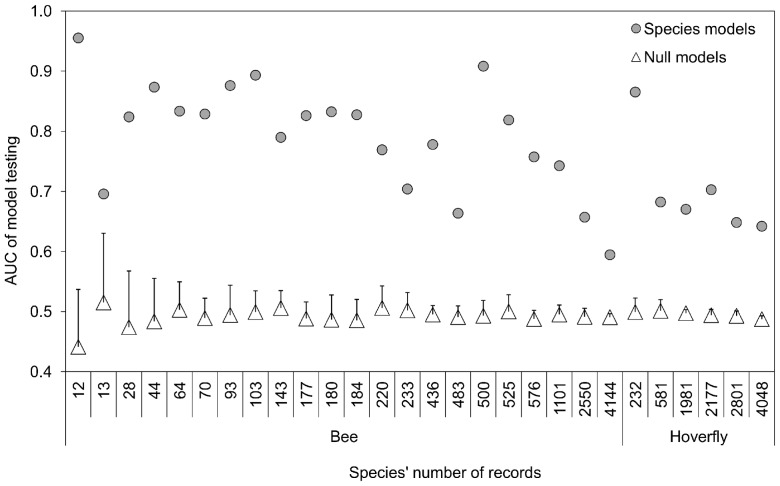
SDMs provided a significantly better fit than expected by chance alone for all the species (Fig. 1 shows the results for the AUC of the testing phase; a similar pattern was observed for the AUC of the training phase).
Figure 1. Performance of the calibrated SDMs against performance of the null models.
Model performance is measured as the AUC of model testing. Error bars show the SD of the null models (10 sets for each species, each modelled with 10-fold cross-validation). The number of available records is used to plot different species along the x-axis.
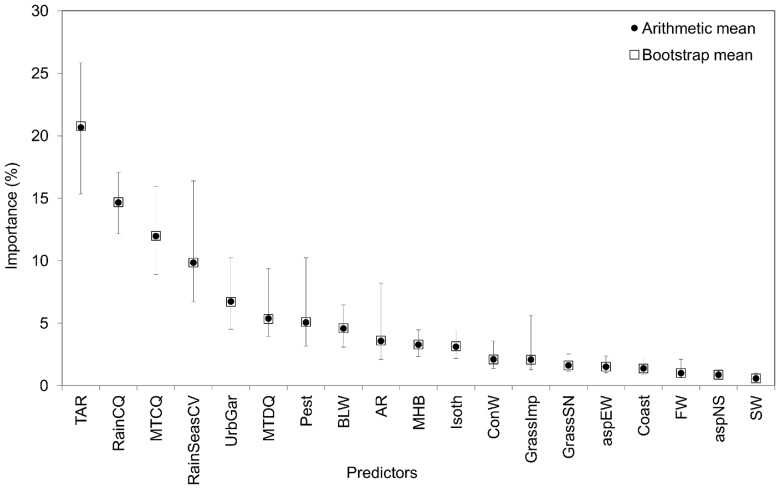
Of the predictors tested (Table 1), climatic variables generally ranked higher than the others, although with variation between species. In particular, Temperature Annual Range (TAR), Precipitation of the Coldest Quarter (RainCQ), Mean Temperature of the Coldest Quarter (MTCQ) and Precipitation Seasonality (RainSeasCV) were the predictors with the greatest importance (Fig. 2 and File S5: from Fig. S5–1.1 to Fig. S5–1.4).
Figure 2. Importance of different predictors.
Arithmetic and bootstrap mean and 95% confidence interval of each predictor's importance, pooled across species. Confidence interval shows the 95% biased-corrected accelerated percentile, based on 999 replicates. Predictors are defined in Table 1.
The Mean and Mode of the predictors' importance were significantly correlated between training and testing phase (ρMean = 0.974; ρMode = 0.944; File S5: Figs S5–2 and S5–3). The correlation between Mean and Mode of the pooled set of training and test models across species was also significant (ρ = 0.940; File S5: Figs S5–3 and S5–4).
Pollinator distribution models
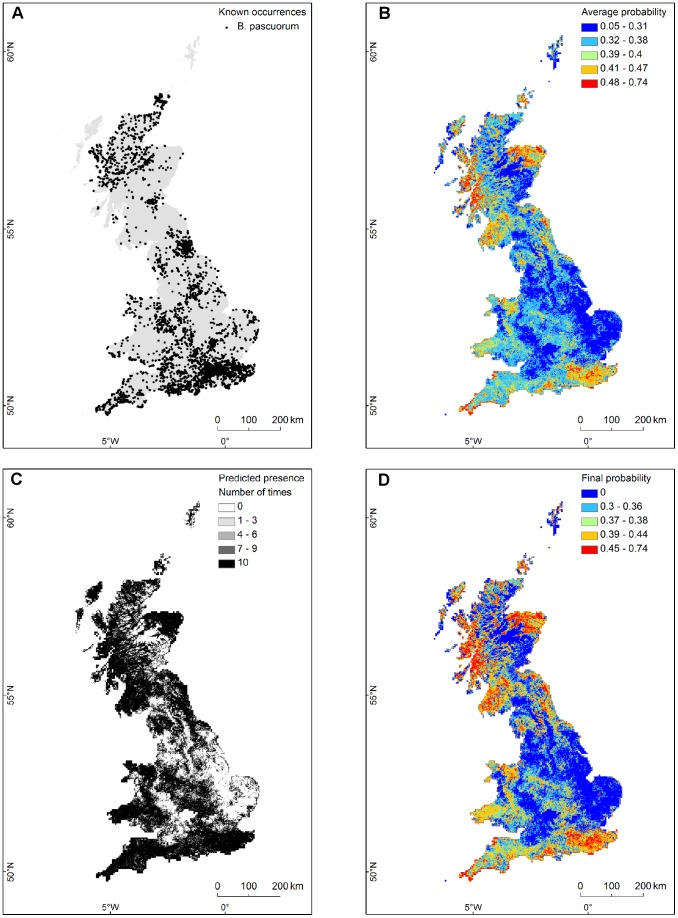
Figure 3 shows an example of outputs for Bombus pascuorum, one of the pollinator species of field beans. The average probability of presence from the 10 cross-validation models ranged from 0.05 to 0.74. The fraction of the 4144 occurrences available for B. pascuorum predicted as presence after converting each model prediction into a binary map (using 10th percentile training presence as threshold) was 0.90±0.003 (mean ± SD). This fraction decreased to 0.86 when 0 was assigned to any area predicted absence by at least one binary map, while retaining the average probability only in areas identified as “presence” by all 10 binary maps.
Figure 3. SDM outputs for Bombus pascuorum.
Outputs from the SDM for B. pascuorum: (A): known occurrences; (B): predicted MaxEnt average probability from the 10-fold cross-validation models, using geometric interval classes from blue to red; (C): summed presence from the 10 binary maps (10 indicates areas where all 10 models predicted presence and 0 areas where all models predicted absence); (D): final predicted probability for B. pascuorum used as input for the pollinator service, derived from assigning the average probability values in (C) only to the areas where all models predicted presence, and 0 to any area predicted “absence” by at least one binary map. Map projection: British National Grid (BNG).
Across species, the average fraction of observed occurrences captured within each species' final area of presence was 0.84±0.030 (mean ± SD). This fraction was positively but non-significantly correlated with the number of available occurrences (Spearman ρ = 0.64, significance assessed with 1000 permutations of samples without replacement, yielding a frequency ∼0.06). Across species, the average final area of presence was 16%±9% smaller than the average from the 10 runs, and negatively correlated with the number of species occurrences (Spearman ρ = −0.85, observed with a frequency ∼0.005 from 1000 permutations). Had we derived the final area of presence from sites predicted by at least nine runs rather than by all 10 runs, the fraction of captured occurrences would be on average 3% greater (± 2%) than the one obtained with the stricter criterion, and negatively correlated with the number of species occurrences (Spearman ρ = −0.86, observed with a frequency ∼0.005 from 1000 permutations).
Pollinator service
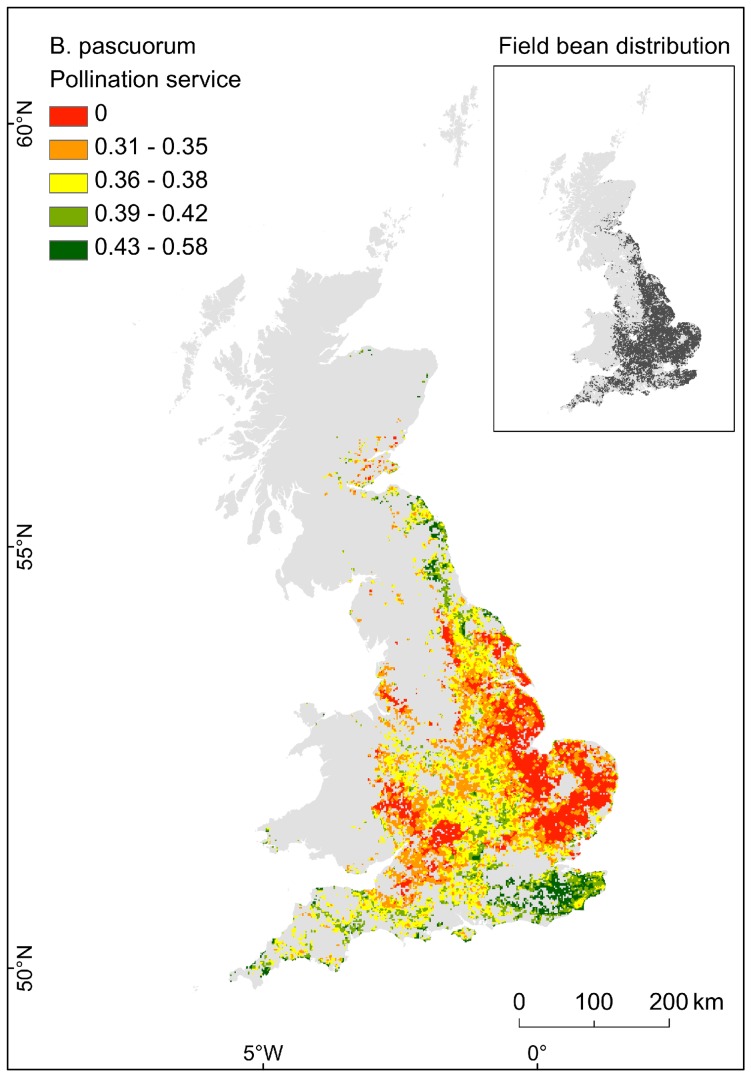
Figure 4 shows an example of potential pollinator service to field bean for Bombus pascuorum, as relative scores from 0 to 1. Predictions ranged from 0 to 0.58 and areas evaluated as zeroes indicate crop fields outside the typical foraging distance of B. pascuorum (i.e. no pollination service). Results for the remaining wild pollinators of field bean are in File 6: from Fig. S6–1 to Fig. S6–8.
Figure 4. Pollination service to field beans, from Bombus pascuorum.
The potential pollination service is represented using geometric intervals, with the exclusion of the zero class which was manually defined. Areas evaluated as 0 indicate crop fields outside the foraging distance of B. pascuorum (i.e. no pollination service). Map projection: BNG.
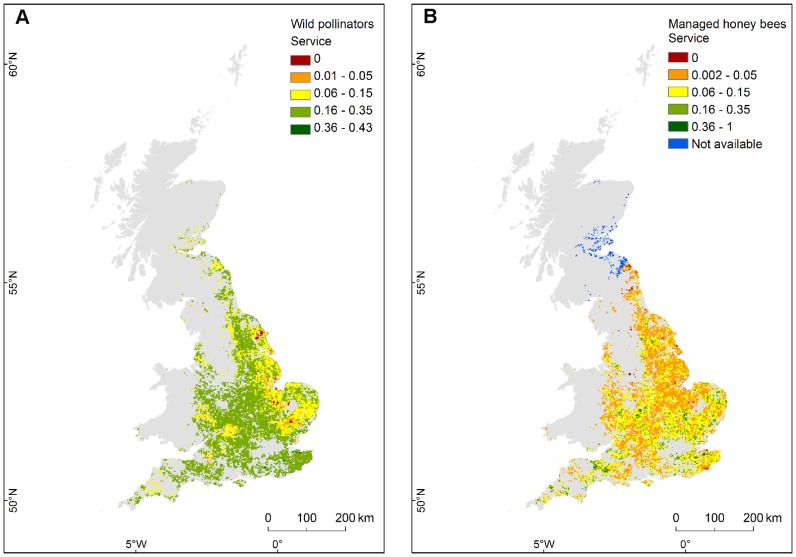
The summed outputs across the nine wild pollinator species, used as a proxy for the total potential pollinator service for field bean, ranged from 0 to 0.43, with a minimum service of 0.01 (Fig. 5(A)): regions close to zero indicate areas where pollinator service is predicted to be low. The predicted pollination service from managed honey bees ranged from 0 (i.e. field bean cells without service from honey bees) to 1, with minimum service of 0.002 (Fig. 5(B)). We also identified areas where pollinator service to field bean cannot be estimated due lack of information on the distribution of managed honey bees (blue regions in Fig. 5(B); File S7: Fig. S7–1 shows the underlying probability of honey bee occurrence).
Figure 5. Pollination service to field beans, from wild and managed pollinators.
Maps show the potential pollination service to field beans, provided by nine wild pollinator species (A) and by managed honey bees (B). Zero indicates areas lacking pollinator service (minimum service is 0.01 from wild pollinators, 0.002 from managed honey bees). Interval classes are manually defined to the same scale. Blue colour in (B) indicates areas where pollination service cannot be estimated due to missing information on honey bees' presence. Map projection: BNG.
Taken together, maps in Fig. 5 may be used to qualitatively compare the predicted spatial patterns of potential pollinator service to field bean, based on the current likelihood of occurrence of wild and managed pollinator species in Great Britain. We did not combine the two maps or make quantitative comparisons of patterns across the two groups, due to the different methods used to generate their underlying likelihood of species occurrence. The honey bee index was derived from the estimated maximum forager density based on reported hive location, apiary type and typical foraging distance; the wild pollinator index, instead, was based on a probability of occurrence, which did not take into account number of individuals per species.
Discussion
In this study we have predicted the current potential distribution of the main crop pollinators of field beans in Great Britain, to derive the potential service provision. Pollinator availability for crop pollination was based on SDMs from species occurrences, rather than on landscape suitability scores from expert knowledge. Potential service provision was assessed for wild and managed pollinators, which to our knowledge has never been done at this scale.
The calibration of the SDMs played an important part in this process making best use of the large species dataset and warranting use of the model outputs as inputs in the pollination service model.
For crops benefiting from insect pollination, we assumed that likelihood of species occurrence can be used as a proxy for potential pollinator service provision, thus implying two main premises: the first one is that the two variables scale proportionally; the second one is that a unit difference in the likelihood of occurrence in one species means the same change in service provision as in a different species.
Species distribution models
Prior to the modelling work, we tested for sample selection bias within the pollinator records to define the appropriate background: opportunistic records are in fact a great resource to predict species distribution, but they rarely provide a representative sample of the study area. The effects of the choice of background on model predictions are widely documented [19], [41], [51] and therefore it was important that this step was carried out at the start.
In the absence of an independent dataset covering the extent of our study region and the entire spectrum of species, each SDM was built using replication through cross-validation, so that after splitting the occurrence data into groups, models were built and tested using all the groups in turn. An advantage of this method, over using a single partitioning for training and testing, is that it uses all the data for validation, thus making better use of small datasets and minimising the impact of possible outliers.
During model calibration, we used AUC as a threshold-independent measure of model performance. Sole reliance on this method has been criticised [52], [53] as AUC depends on predictive success and not on explanatory value and it is affected by the geographical extent of the model; the latter point is particularly important if AUC is used to compare modelling performance between different species or between models built with different base datasets. In our study, however, we used AUC to compare models based on the same datasets and within species.
The similar AUC between models derived with default settings for feature class and models derived with Hinge alone has been observed in at least one other study [42]. In addition, the similar variation in model performance between the 10-fold cross-validation runs, independent of the feature class, indicated comparable stability in their predictions.
Our results on the importance of different predictors indicated a superior discriminatory power within models built with Hinge alone, probably due to the greater flexibility of fitted functions when All feature classes are allowed. It also became apparent that some of the response curves derived from single-predictor models were too narrowly fitted to the training data when allowing for All feature classes (see also [54]), which further supported the choice of using the Hinge alone.
The effect of changing the default prevalence to reflect the relative rarity of each species was significant (and positive) only within the bee group, possibly due to their greater variation in number of records. Modifying prevalence has implications for the maximum value predicted by the MaxEnt logistic output [18], noticeable when comparing response curves generated with default and modified prevalence. Since logistic outputs should be interpreted in relation to a temporal and spatial scale appropriate for each species [42], modifying the prevalence allowed us to make the outputs of the SDMs more comparable across species, and to account for their relative differences when evaluating crop pollination service.
The results on predictors' importance highlight within and across species properties (training vs. testing and mean vs. mode respectively). The within species agreement on the predictors' importance between training and testing data suggests that the models are transferable. With climatic predictors being in general the most important ones, this also indicates the possibility of investigating the effects of projected climate changes on the future distribution of wild pollinators. This aspect is of particular interest given the projected shifts in suitable environmental conditions predicted for many taxa including pollinators [55], and the potential phenological mismatch within mutualistic relations, such as plants and pollinators [56], [57]. The significant correlation between the Mean and the Mode used to rank predictors' importance can be interpreted as a general agreement on their relative importance across species.
Applications to crop pollinators
We adapted Lonsdorf's [8] model to derive pollinator service, using the SDMs derived for the field bean pollinators as inputs. Our choice was motivated by three main reasons: firstly, for the extent of our study area, it would be difficult to rely on expert knowledge to provide landscape suitability scores for pollinators and expert opinion may not be available for poorly known species. Secondly, regularly maintained databases with nation-wide pollinator records offered us the opportunity to rely on actual, albeit opportunistic, sightings. These data have already proven instrumental in detecting changes in species richness across temporal and spatial scales [6], [58]. Thirdly, our approach also accounted for the contribution of managed pollinators, providing the opportunity to compare patterns of pollination service between wild and domesticated pollinators. This is particularly important, given the potentially changing contribution made by both types of crop pollinators in the UK [59]. There is increasing evidence highlighting the importance of wild pollinators to crop production worldwide [60]. However, agricultural intensification and alteration of natural habitats, have shown negative effects on wild pollinator communities [61], [62] and for appropriate mitigation measures to be designed [63], [64], it is crucial to understand how different pollinator species are distributed in space and how this is determined by relationships with their abiotic environment. We believe that the work described here can be used to this end.
Our study has provided predicted PSM for a specific crop, field bean, as a case study to demonstrate how the general approach can be applied to other crops. For application of this method elsewhere we highlight several advantages and further challenges. For instance, since the results are spatially explicit, they can be used to simultaneously investigate the predicted pollinator supply and the underlying extent of crop parcels. This information can help quantify relevant risk factors such as the fraction of crop vulnerable to low pollinator supply. As previously illustrated by the recent work of Lautenbach et al. [65] in their map of global pollination benefits, spatially explicit information of this kind can provide a first instrument to prioritize areas where policies aiming at preserving pollination services and mitigating potential pollinator deficits for agricultural crops can be effectively targeted.
Whilst the cross-validation approach used during the SDM allowed us to use the available species occurrences to train and validate the models, testing for significant correlation between the PSM predictions and the pollination service actually provided, would require additional data, which are currently unavailable. In particular, we would need empirical information on pollinator density, flower visitation rate and fruit set for a representative set of crop parcels. Given the extent of the study region, parcels would need to be selected along the gradient of the environmental variables captured by the model, and power analysis would be needed to determine how may parcel replicates would be necessary to achieve the desired level of confidence. An additional difficulty relates to the scale (resolution) of the current model, which is suitable for country-wide and local scale patterns, but may be too coarse to draw correlations with what is observed at the crop parcel scale. We recommend that future applications of our method consider building models with species and environmental layers matching the spatial scale of the field work, thereby allowing direct testing of predictions. The empirical information being collected in different agricultural systems worldwide has already proven instrumental for drawing general patterns, such as the relative importance of wild pollinators vs. managed pollinators for enhancing fruit set (e.g. [60] and references therein). A number of studies funded under the UK Insect Pollinator Initiative, may provide the information needed towards a first validation step over the next few years.
Conclusion and Next Steps
The primary interest of our study was to show how the Lonsdorf et al. [8] pollination service model can be integrated with the MaxEnt species distribution model [13] to predict geographical patterns of pollination service to crops. We chose these two models since they both have peer-reviewed track records of successful applications in their respective fields but, to our knowledge, they have never been used in combination. The two main elements of novelty in our study are the use of pollinator records rather than expert knowledge to predict wild pollinator occurrence, and the inclusion of managed pollinator data. This approach allowed us to map the relative contribution of each pollinator group, and also identify areas potentially vulnerable to low service provision. Thus the outputs can help direct local scale mitigation measures, such as agri-environment scheme options. Despite the difficulties common to proxy-based approaches [66] the method we have proposed is sufficiently flexible to incorporate different environmental variables of biological relevance, which may be available for other geographic regions, useful to refine predictions, or relevant when the models are applied to smaller spatial extents. The last point should be of particular interest to studies at the field parcel scale, where detailed information of landscape elements may be collected and used to build the models. The possibility to correlate relative scores and proxies to empirical data is likely to provide relevant information for both the SDM and the PSM: for instance, using information on farm management and landscape composition and configuration, Kennedy et al. [67] have assessed the strength of the correlation between different predictors of bee abundance and richness and empirical data collected in 39 crop systems across the globe.
Looking ahead, the inclusion of local pollinator abundance and of the pollination effectiveness of different pollinators are arguably the most urgent next challenges we need to face to help to translate the relative suitability scores into units of crop pollination service and ultimately yield. Service provision, in fact, results from species' efficiency and local abundance.
We have used field beans as test case, but the method we have illustrated can be applied to other crops, provided that their distribution and main pollinators are known. In addition, this approach can be extended to investigate the projected effects of climate change on pollination services. To do that, it would require predictive SDMs for both the crop of interest and its pollinators, to reveal any compositional change in the pollinator community, as well as any potential geographical mismatch between crop and pollinators.
Supporting Information
Figure S1–1: Number of records from Web of Knowledge for applications of MaxEnt in species distribution models. Search criteria: Topic = “Maxent” AND “Species distribution”; Years = from 2006 to 2012; access date: 28/08/2012.
(PDF)
Table S2–1: Species selected for model calibration. Sample size equals to the number of occupied 1 km2 grid cells, which becomes the area occupied by a species solely based on existing records; quartile distance is the longest distance between all pairwise records for a particular species within its 3rd quartile. Table S2–2: Pearson's correlation between selected topographic and bio-climatic variables. Predictors are defined in the main text.
(PDF)
Figure S3–1: Number of species within each class of modified prevalence (τ), for bees (grey) and hoverflies (black). Table S3–1: Revised values of τ for species used during model calibration. Figure 3 –2.1 Single response curves for Andrena niveata with default and modified prevalence. Response of A. niveata to mean temperature of the driest quarter, with default (0.5, panel A) and modified prevalence (0.1, panel B). Modifying the prevalence changes the maximum probability of presence from ∼0.65 to ∼0.17. The response curves are based on a (MaxEnt) model created using only the focal predictor. The curves show the mean response of the 10 runs (red) and the mean +/− one standard deviation (blue). Figure 3 –2.2: Single response curves for Rhingia rostrata with default and modified prevalence. Response of R. rostrata to percentage of arable land, with default (0.5, panel A) and modified (0.3, panel B) prevalence. The maximum predicted probability of presence changes from ∼0.55 to ∼0.35. The response curves are based on a (MaxEnt) model created using only the focal predictor. The curves show the mean response of the 10 runs (red) and the mean +/− one standard deviation (blue).
(PDF)
Table S4–1: Results of the mixed model evaluating the influence of different model settings on the model performance. Model performance from the AUC of test data, for Bee and Hoverfly. Fixed effects only are shown here. A star (*) indicates that modified values of prevalence were used. Table S4–2: Results of the mixed model evaluating the influence of different model settings on the variability of the model performance. Model performance from the Standard Deviation of the AUC of test data (from the 10 cross-validations), for Bee and Hoverfly. Fixed effects only are shown here. A star (*) indicates that modified values of prevalence were used. Table S4–3: Results on the discriminatory ability of models built with different feature classes and prevalence, from generalized linear models. The importance of different predictors was better discriminated in models built using Hinge feature class alone. All* = All features classes allowed, with modified prevalence. Hinge = only Hinge feature class, with default prevalence. Hinge* = only Hinge feature class, with modified prevalence. Figure S4–1.1: Single response curves for Andrena barbilabris with default feature class and hinge only. Response of A. barbilabris (prevalence = 0.5) to the mean temperature of driest quarter as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations. Figure S4–1.2: Single response curves for Syrphus ribesii with default feature class and hinge only. Response of S. ribesii (prevalence = 0.4) to the temperature annual range as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations. Figure S4–1.3: Single response curves for Bombus muscorum with default feature class and hinge only. Response of B. muscorum (prevalence = 0.3) to the coefficient of variation of precipitation seasonality, as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations. Figure S4–1.4: Single response curves for Osmia rufa with default feature class and hinge only. Response of Osmia rufa (prevalence = 0.2) to the mean temperature of coldest quarter, as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations.
(PDF)
Figure S5–1.1: Predicted probability of occurrence of Andrena labiata along the temperature annual range. Figure S5–1.2: Predicted probability of occurrence of Andrena minutuloides along the precipitation seasonality. Figure S5–1.3: Predicted probability of occurrence of Halictus rubicundus along the precipitation of the coldest quarter. Figure S5–1.4: Predicted probability of occurrence of Megachile maritima along the mean temperature of the coldest quarter. Figure S5–2: Rank correlation within training and testing data, for predictors Mean and Mode. Spearman's rank correlations: Mean (open squares and dashed line): ρ = 0.974; Mode (filled circles and solid line): ρ = 0.944. Both correlations are significant, based on 999 bootstrap replicates (Fig. S5–4 panel A and B). Figure S5–3: Rank correlation between Mean and Mode, for the pooled set of training and test models across species. Spearman's rank correlation: ρ = 0.940, significant based on 999 bootstrap replicates (Fig. S5–4 panel C). Figure S5–4: Distributions of bootstrap and observed Spearman's rank correlations. A: correlation of predictors' Mean between training and testing phase; B: correlation of predictors' Mode between training and testing phase; C: correlation between Mean and Mode for the pooled set of training and testing data. In all three cases the observed correlation are significantly greater than those generated from 999 bootstrap replicates.
(PDF)
Figure S6–1: Probability of occurrence (A) and potential pollinator service (B) for A. labialis . Figure S6–2: Probability of occurrence (A) and potential pollinator service (B) for A. wilkella . Figure S6–3: Probability of occurrence (A) and potential pollinator service (B) for B. hortorum . Figure S6–4: Probability of occurrence (A) and potential pollinator service (B) for B. lapidarius . Figure S6–5: Probability of occurrence (A) and potential pollinator service (B) for B. lucorum . Figure S6–6: Probability of occurrence (A) and potential pollinator service (B) for B. muscorum . Figure S6–7: Probability of occurrence (A) and potential pollinator service (B) for B. terrestris . Figure S6–8: Probability of occurrence (A) and potential pollinator service (B) for O. rufa .
(PDF)
Figure S7–1: Probability of occurrence of managed honey bees. The original density of foragers was linearly rescaled to 0–1 and the 0–1 and the 5th percentile threshold was adopted to distinguish absence from presence (corresponding to a 0.001 probability of occurrence). Map projection: British National Grid.
(PDF)
Acknowledgments
CP acknowledges D. Allon and L.G. Carvalheiro for their help in accessing some of the datasets. The authors acknowledge the reviewers for their constructive comments to this manuscript. Authors from JA-G to KES are listed in alphabetical order.
Funding Statement
This study was carried out within the project “Sustainable pollination services for UK crops” (http://www.reading.ac.uk/caer/Project_IPI_Crops/project_ipi_crops_index.html), funded jointly by BBSRC, Defra, NERC, the Scottish Government, the Wellcome Trust and the LWEC, under the Insect Pollinators Initiative (https://wiki.ceh.ac.uk/display/ukipi/Home). JA-G received funding from the Mexican National Council for Science and Technology (CONACyT), reference 214731/310005. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hassan R, Scholes R, Ash N (2005) Ecosystem and Human Well-being: Current State & Trends. Findings of the Condition and Trends Working Group: Island Press. 47 p. [Google Scholar]
- 2. Gallai N, Salles J-M, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68: 810–821. [Google Scholar]
- 3. Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, et al. (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc Biol Sci Ser B 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK National Ecosystem Assessment (2011) The UK National Ecosystem Assessment Technical Report. Cambridge.
- 5. Potts SG, Roberts SPM, Dean R, Marris G, Brown MA, et al. (2010) Declines of managed honey bees and beekeepers in Europe. J Apic Res 49: 15–22. [Google Scholar]
- 6. Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, et al. (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands Science. 313: 351–354. [DOI] [PubMed] [Google Scholar]
- 7. Aizen MA, Garibaldi LA, Cunningham SA, Klein AM (2009) How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann Bot 103: 1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lonsdorf E, Kremen C, Ricketts T, Winfree R, Williams N, et al. (2009) Modelling pollination services across agricultural landscapes. Ann Bot 103: 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Modell 135: 147–186. [Google Scholar]
- 10. Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, et al. (2006) Novel methods improve prediction of species' distributions from occurrence data. Ecography 29: 129–151. [Google Scholar]
- 11.Peterson AT, Soberon J, Pearson RG, Anderson RP, Martinez-Meyer E, et al.. (2011) Ecological niches and geographic distributions: Princeton University Press.
- 12. Tsoar A, Allouche O, Steinitz O, Rotem D, Kadmon R (2007) A comparative evaluation of presence-only methods for modelling species distribution. Divers Distrib 13: 397–405. [Google Scholar]
- 13. Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190: 231–259. [Google Scholar]
- 14. Blach-Overgaard A, Svenning J-C, Dransfield J, Greve M, Balslev H (2010) Determinants of palm species distributions across Africa: the relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography 33: 380–391. [Google Scholar]
- 15. Anderson RP, Raza A (2010) The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J Biogeogr 37: 1378–1393. [Google Scholar]
- 16. Tognelli MF, Roig-Junent SA, Marvaldi AE, Flores GE, Lobo JM (2009) An evaluation of methods for modelling distribution of Patagonian insects. Rev Chil Hist Nat 82: 347–360. [Google Scholar]
- 17. Hernandez PA, Graham CH, Master LL, Albert DL (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29: 773–785. [Google Scholar]
- 18. Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31: 161–175. [Google Scholar]
- 19. Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, et al. (2009) Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl 19: 181–197. [DOI] [PubMed] [Google Scholar]
- 20. Royle JA, Chandler RB, Yackulic C, Nichols JD (2012) Likelihood analysis of species occurrence probability from presence-only data for modelling species distributions. Methods in Ecology and Evolution 3: 545–554. [Google Scholar]
- 21. Anderson RP, Gonzalez I Jr (2011) Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with Maxent. Ecol Modell 222: 2796–2811. [Google Scholar]
- 22.Bees, Wasps and Ants Recording Society website. Available: http://www.bwars.com/. Accessed: June 2011.
- 23.Hoverfly Recording Scheme website. Available: http://www.hoverfly.org.uk/. Accessed: June 2011.
- 24. Aguirre-Gutiérrez J, Carvalheiro LG, Polce C, van Loon EE, Raes N, et al. (2013) Fit-for-purpose: Species distribution model performance depends on evaluation criteria –Dutch hoverflies as a case study. PLoS ONE 8: e63708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BeeBase website. Available: https://secure.fera.defra.gov.uk/beebase/index.cfm. Accessed: July 2012.
- 26. Beekman M, Ratnieks FLW (2000) Long-range foraging by the honey-bee, Apis mellifera L. Funct Ecol. 14: 490–496. [Google Scholar]
- 27. Waddington KD, Visscher PK, Herbert TJ, Richter MR (1994) Comparisons of forager distributions from matched honey-bee colonies in suburban environments. Behav Ecol Sociobiol 35: 423–429. [Google Scholar]
- 28. Visscher PK, Seeley TD (1982) Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 63: 1790–1801. [Google Scholar]
- 29.Morton D, Rowland C, Wood C, Meek L, Marston C, et al.. (2011) Final Report for LCM2007– the new UK land cover map.
- 30. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- 31.UKCP09: Gridded observation data sets wesbite. Available: http://www.metoffice.gov.uk/climatechange/science/monitoring/ukcp09/. Accessed: July 2011.
- 32.R Development Core Team (2011) R: A Language and Environment for Statistical Computing. 2.13.0 ed. Vienna, Austria: R Foundation for Statistical Computing.
- 33.Edina website. Available: http://edina.ac.uk/digimap/description/products/. Accessed: June 2011.
- 34.Pesticide Usage Survey wesbite. Available: http://www.fera.defra.gov.uk/scienceResearch/scienceCapabilities/landUseSustainability/surveys/index.cfm. Accessed: March 2012.
- 35.DEFRA June Agricultural Survey website. Available: http://www.defra.gov.uk/statistics/foodfarm/landuselivestock/junesurvey/junesurveyresults/. Accessed: August 2011.
- 36. Mineau P, Harding KM, Whiteside M, Fletcher MR, Garthwaite D, et al. (2008) Using reports of bee mortality in the field to calibrate laboratory derived pesticide risk indices. Environ Entomol 37: 546–554. [DOI] [PubMed] [Google Scholar]
- 37. EPPO (2010) Environmental risk assessment scheme for plant protection products. Chapter 10: Honeybees. EPPO Bulletin 40: 323–331. [Google Scholar]
- 38. Jolliffe IT (1973) Discarding Variables in a Principal Component Analysis, II: Real Data. Applied Statistics 22: 21–31. [Google Scholar]
- 39. Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8: 993–1009. [DOI] [PubMed] [Google Scholar]
- 40.Maximum Entropy Modeling of Species Geographic Distributions website. Version 3.3.3k available: http://www.cs.princeton.edu/~schapire/maxent/ Accessed: November 2011.
- 41. Raes N, ter Steege H (2007) A null-model for significance testing of presence-only species distribution models. Ecography 30: 727–736. [Google Scholar]
- 42. Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, et al. (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17: 43–57. [Google Scholar]
- 43. Wiley E, McNyset K, Peterson AT, Robins C, Stewart AM (2003) Niche modeling and geographic range predictions in the marine environment using a machine-learning algorithm. Oceanography 16: 120–127. [Google Scholar]
- 44.Crawley MJ (2007) The R book. Chichester: John Wiley & Sons Ltd.
- 45.Free JB (1993) Insect Pollination of Crops. London: Academic Press Limited.
- 46. Winfree R, Dushoff J, Crone EE, Schultz CB, Budny RV, et al. (2005) Testing Simple Indices of Habitat Proximity. The American Naturalist 165: 707–717. [DOI] [PubMed] [Google Scholar]
- 47.Wilensky U (1999) NetLogo. Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL. Available: http://ccl.northwestern.edu/netlogo/. Accessed: 16 May 2012.
- 48.ESRI (2009) ArcGIS Desktop 10. 10.0 ed.
- 49. Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153: 589–596. [DOI] [PubMed] [Google Scholar]
- 50. Hagen M, Wikelski M, Kissling WD (2011) Space Use of Bumblebees (Bombus spp.) Revealed by Radio-Tracking. PLoS ONE 6: e19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution 3: 327–338. [Google Scholar]
- 52. Termansen M, McClean CJ, Preston CD (2006) The use of genetic algorithms and Bayesian classification to model species distributions. Ecol Modell 192: 410–424. [Google Scholar]
- 53. Austin M (2007) Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol Modell 200: 1–19. [Google Scholar]
- 54. Syfert MM, Smith MJ, Coomes DA (2013) The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 8: e55158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giannini TC, Acosta AL, Garófalo CA, Saraiva AM, Alves-dos-Santos I, et al. (2012) Pollination services at risk: Bee habitats will decrease owing to climate change in Brazil. Ecol Modell 244: 127–131. [Google Scholar]
- 56. Gordo O, Sanz JJ (2005) Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia 146: 484–495. [DOI] [PubMed] [Google Scholar]
- 57. Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant-pollinator interactions. Ecol Lett 10: 710–717. [DOI] [PubMed] [Google Scholar]
- 58. Keil P, Biesmeijer JC, Barendregt A, Reemer M, Kunin WE (2011) Biodiversity change is scale-dependent: an example from Dutch and UK hoverflies (Diptera, Syrphidae). Ecography 34: 392–401. [Google Scholar]
- 59. Breeze TD, Bailey AP, Balcombe KG, Potts SG (2011) Pollination services in the UK: How important are honeybees? Agric, Ecosyst Environ 142: 137–143. [Google Scholar]
- 60. Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, et al. (2013) Wild Pollinators Enhance Fruit Set of Crops Regardless of Honey Bee Abundance. Science 339: 1608–1611. [DOI] [PubMed] [Google Scholar]
- 61. Kremen C, Williams NM, Thorp RW (2002) Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci U S A 99: 16812–16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carvalheiro LG, Seymour CL, Veldtman R, Nicolson SW (2010) Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J Appl Ecol 47: 810–820. [Google Scholar]
- 63. Klein A-M, Brittain C, Hendrix SD, Thorp R, Williams N, et al. (2012) Wild pollination services to California almond rely on semi-natural habitat. J Appl Ecol 49: 723–732. [Google Scholar]
- 64. Carvalheiro LG, Seymour CL, Nicolson SW, Veldtman R (2012) Creating patches of native flowers facilitates crop pollination in large agricultural fields: mango as a case study. J Appl Ecol 49: 1373–1383. [Google Scholar]
- 65. Lautenbach S, Seppelt R, Liebscher J, Dormann CF (2012) Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 7: e35954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lautenbach S, Kugel C, Lausch A, Seppelt R (2011) Analysis of historic changes in regional ecosystem service provisioning using land use data. Ecol Indic 11: 676–687. [Google Scholar]
- 67. Kennedy CM, Lonsdorf E, Neel MC, Williams NM, Ricketts TH, et al. (2013) A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol Lett 16: 584–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–1: Number of records from Web of Knowledge for applications of MaxEnt in species distribution models. Search criteria: Topic = “Maxent” AND “Species distribution”; Years = from 2006 to 2012; access date: 28/08/2012.
(PDF)
Table S2–1: Species selected for model calibration. Sample size equals to the number of occupied 1 km2 grid cells, which becomes the area occupied by a species solely based on existing records; quartile distance is the longest distance between all pairwise records for a particular species within its 3rd quartile. Table S2–2: Pearson's correlation between selected topographic and bio-climatic variables. Predictors are defined in the main text.
(PDF)
Figure S3–1: Number of species within each class of modified prevalence (τ), for bees (grey) and hoverflies (black). Table S3–1: Revised values of τ for species used during model calibration. Figure 3 –2.1 Single response curves for Andrena niveata with default and modified prevalence. Response of A. niveata to mean temperature of the driest quarter, with default (0.5, panel A) and modified prevalence (0.1, panel B). Modifying the prevalence changes the maximum probability of presence from ∼0.65 to ∼0.17. The response curves are based on a (MaxEnt) model created using only the focal predictor. The curves show the mean response of the 10 runs (red) and the mean +/− one standard deviation (blue). Figure 3 –2.2: Single response curves for Rhingia rostrata with default and modified prevalence. Response of R. rostrata to percentage of arable land, with default (0.5, panel A) and modified (0.3, panel B) prevalence. The maximum predicted probability of presence changes from ∼0.55 to ∼0.35. The response curves are based on a (MaxEnt) model created using only the focal predictor. The curves show the mean response of the 10 runs (red) and the mean +/− one standard deviation (blue).
(PDF)
Table S4–1: Results of the mixed model evaluating the influence of different model settings on the model performance. Model performance from the AUC of test data, for Bee and Hoverfly. Fixed effects only are shown here. A star (*) indicates that modified values of prevalence were used. Table S4–2: Results of the mixed model evaluating the influence of different model settings on the variability of the model performance. Model performance from the Standard Deviation of the AUC of test data (from the 10 cross-validations), for Bee and Hoverfly. Fixed effects only are shown here. A star (*) indicates that modified values of prevalence were used. Table S4–3: Results on the discriminatory ability of models built with different feature classes and prevalence, from generalized linear models. The importance of different predictors was better discriminated in models built using Hinge feature class alone. All* = All features classes allowed, with modified prevalence. Hinge = only Hinge feature class, with default prevalence. Hinge* = only Hinge feature class, with modified prevalence. Figure S4–1.1: Single response curves for Andrena barbilabris with default feature class and hinge only. Response of A. barbilabris (prevalence = 0.5) to the mean temperature of driest quarter as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations. Figure S4–1.2: Single response curves for Syrphus ribesii with default feature class and hinge only. Response of S. ribesii (prevalence = 0.4) to the temperature annual range as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations. Figure S4–1.3: Single response curves for Bombus muscorum with default feature class and hinge only. Response of B. muscorum (prevalence = 0.3) to the coefficient of variation of precipitation seasonality, as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations. Figure S4–1.4: Single response curves for Osmia rufa with default feature class and hinge only. Response of Osmia rufa (prevalence = 0.2) to the mean temperature of coldest quarter, as modelled by default settings for feature class (A) and hinge only (B). See main text for explanations.
(PDF)
Figure S5–1.1: Predicted probability of occurrence of Andrena labiata along the temperature annual range. Figure S5–1.2: Predicted probability of occurrence of Andrena minutuloides along the precipitation seasonality. Figure S5–1.3: Predicted probability of occurrence of Halictus rubicundus along the precipitation of the coldest quarter. Figure S5–1.4: Predicted probability of occurrence of Megachile maritima along the mean temperature of the coldest quarter. Figure S5–2: Rank correlation within training and testing data, for predictors Mean and Mode. Spearman's rank correlations: Mean (open squares and dashed line): ρ = 0.974; Mode (filled circles and solid line): ρ = 0.944. Both correlations are significant, based on 999 bootstrap replicates (Fig. S5–4 panel A and B). Figure S5–3: Rank correlation between Mean and Mode, for the pooled set of training and test models across species. Spearman's rank correlation: ρ = 0.940, significant based on 999 bootstrap replicates (Fig. S5–4 panel C). Figure S5–4: Distributions of bootstrap and observed Spearman's rank correlations. A: correlation of predictors' Mean between training and testing phase; B: correlation of predictors' Mode between training and testing phase; C: correlation between Mean and Mode for the pooled set of training and testing data. In all three cases the observed correlation are significantly greater than those generated from 999 bootstrap replicates.
(PDF)
Figure S6–1: Probability of occurrence (A) and potential pollinator service (B) for A. labialis . Figure S6–2: Probability of occurrence (A) and potential pollinator service (B) for A. wilkella . Figure S6–3: Probability of occurrence (A) and potential pollinator service (B) for B. hortorum . Figure S6–4: Probability of occurrence (A) and potential pollinator service (B) for B. lapidarius . Figure S6–5: Probability of occurrence (A) and potential pollinator service (B) for B. lucorum . Figure S6–6: Probability of occurrence (A) and potential pollinator service (B) for B. muscorum . Figure S6–7: Probability of occurrence (A) and potential pollinator service (B) for B. terrestris . Figure S6–8: Probability of occurrence (A) and potential pollinator service (B) for O. rufa .
(PDF)
Figure S7–1: Probability of occurrence of managed honey bees. The original density of foragers was linearly rescaled to 0–1 and the 0–1 and the 5th percentile threshold was adopted to distinguish absence from presence (corresponding to a 0.001 probability of occurrence). Map projection: British National Grid.
(PDF)