Abstract
Arterioles, capillaries and venules all actively change their cellular functions and phenotypes during inflammation in ways that are essential for maintenance of homeostasis and self-defense, and are also associated with many inflammatory disorders. Endothelial cells (ECs), together with pericytes and extracellular matrix proteins, can regulate blood flow, the coagulation cascade, fluid and solute exchange, and leukocyte trafficking. While capillary and venular functions in inflammation are well characterized, the arteriolar contribution to inflammation has only recently come into focus. Arterioles differ from venules in structure, EC morphology, shear environment, expression and distribution of surface ligands, hence regulation and function of arteriolar wall cells during inflammation may also be distinct from venules. Recent work indicates that in response to pro-inflammatory stimuli, arterioles alter barrier function, and support leukocyte and platelet interactions through upregulation of adhesion molecules. This suggests that in addition to their role in blood flow regulation, arterioles may also participate in inflammatory responses. In this review we will discuss mechanisms that characterize arteriolar responses to proinflammatory stimuli. We will detail how distinct arteriolar features contribute to regulation of barrier function and leukocyte-EC interactions in inflammation, and further highlight the potential priming effects of arteriolar responses on venular function and progression of inflammatory responses.
Keywords: endothelial cells, morphology, permeability, leukocytes, neutrophils, adhesion molecules, shear stress, inflammatory responses
Endothelial cells form a semi-permeable barrier that is a primary player in regulating both the exchange of fluid and solutes and leukocyte trafficking in both healthy tissue and disease. This barrier is essential for proper tissue function and homeostasis. A very widely accepted paradigm is that small venules and capillaries are the main site for solute and fluid exchange, while leukocyte extravasation occurs mainly in post capillary venules. However, emerging evidence (34–36, 96, 98) suggests that arterioles, while playing a key role in regulation of blood pressure and flow, can also alter their barrier function in response to stress or pro-inflammatory stimuli, thus contributing to fluid and solute exchange. Furthermore, activated arterioles can support leukocyte interactions (8, 37, 50, 100), which may have important implications given recent findings suggesting that in microvessels, leukocyte engagement of surface endothelial receptors contributes significantly to the regulation of barrier function (5, 98). Not surprisingly, disregulation of arteriolar EC function results in significant changes, including thickening of the vessel wall and impaired metabolic and inflammatory responses; these changes are critically associated with multiple disorders, including thrombosis, atherosclerosis, hypertension, heart disease and stroke.
Endothelial cells and barrier function
In an integrated microvascular network, small arterioles diverge into capillaries, which in turn converge into post capillary venules. Vascular ECs lining the lumen of these vessels serve as a semi-permeable barrier that separates blood contents from the interstitial compartments (64). Small molecular weight substances including gases, ions and small solutes can cross the endothelium by passive diffusion or via active carriers, driven ultimately by their concentration gradient. However, both the transport of larger solutes such as albumin, as well as diapedesis of immune cells that reside in the blood, are restricted, and regulation of these processes is substantially, although of course not exclusively, a function of the endothelial monolayer. Gradients of macromolecules such as albumin contribute to the net flux of water (73, 108), as well transport of nutrients, lipophilic fatty acids, hormones, and other metabolic by-products across the vessel wall (109). Thus maintaining proper barrier function, with tight regulation of solute and water exchange between the blood and the extravascular space, is essential for tissue homeostasis under both normal and pathological conditions.
Importantly, in addition to regulating solute and fluid exchange, ECs play an important role in regulating leukocyte trafficking, both for surveillance purposes in normal tissue (10) and leukocyte recruitment during inflammation (18, 62, 66). In response to inflammatory stimuli, the barrier becomes more permissive to water and macromolecules as well as to extravasating leukocytes. Unless the inflammation is resolved and the inflammatory cues subside, these alterations in the endothelial barrier will ultimately be manifest as tissue edema and/or inflammatory diseases including, for example, acute respiratory distress syndrome, rheumatoid arthritis, inflammatory bowel disease and atherosclerosis.
Both fluid and solute exchange and leukocyte TEM are generally assumed to occur exclusively in capillaries and postcapillary venules, thus the regulation of capillary and venular permeability in normal and inflamed tissue has been extensively studied (reviewed in (64, 65)). Similarly, leukocyte interactions with venular ECs have been well characterized (reviewed in (62, 67, 83)). Arterioles on the other hand, have been somewhat neglected, and their barrier function properties are still poorly understood.
Why should we care about arterioles?
The immediate answer is because arteriolar EC function changes in response to inflammatory cues. As arterioles do not exist in isolation, but are a part of an integrated vascular network, responses upstream in the arteriolar components of the network are likely to affect cellular functions downstream (in capillaries and the venular microcirculation), thus contributing to the global outcome.
Arterioles are small resistance vessels and unlike venules are wrapped circumferentially by one or more layers of smooth muscle cells (71, 106). This morphology, together with their ability to locally vasodilate or vasoconstrict in response to a wide variety of chemical and electrical messages, defines arterioles as modifiers of resistance to flow and regulators of blood pressure. However, in addition to these fundamental homeostatic functions, (which in turn have the capacity to increase convective flux of material across the arteriolar wall), there is emerging evidence (presented in this review) that arterioles also contribute directly to solute exchange via changes in permeability, and furthermore, play a role in regulating leukocyte trafficking. Although the combined surface area of capillaries and venules is usually estimated as being at least five times that of arterioles, arterioles nevertheless offer a considerable surface area that can support leukocyte-EC interactions and the exchange of solutes and fluid, thus their contribution to tissue homeostasis has the capacity to be significant. There is therefore good reason to seek to understand mechanisms regulating the permeability of arterioles to solutes and fluid on the one hand, and to leukocytes on the other, and to determine whether they differ from or are similar to these processes as observed in venules.
Arteriolar responses to inflammatory cues
It is well established that in response to inflammatory stimuli post capillary venular permeability to both water and solutes dramatically increases. It is also established that venules support leukocyte interactions, and during inflammation, through upregulation of a variety of surface adhesion molecules, these vessels permit rapid mobilization of leukocytes from the circulation into the interstitium. Importantly, similarly to venules, arteriolar responses to inflammatory stimuli are also characterized by increased expression of EC adhesion molecules, enhanced solute permeability, and initiation of leukocyte-EC interactions, as we discuss below.
Changes in adhesion molecule expression and distribution
EC adhesion molecules act as both adhesive ligands for leukocytes and as signaling receptors for ECs, thus expression and distribution of adhesion molecules is essential for leukocyte recruitment and EC function. In venules, members of the selectin family, viz P-, E- and L-selectin (54), as well members of the immunoglobulin superfamily, such as ICAM-1 (57, 115), VCAM-1 (15, 105), and PECAM-1 (68), are essential for leukocyte recruitment. Similarly, VE-Cadherin expressed at the interendothelial junctions is essential for the integrity of the vessel wall and, along with VE-cadherin-associated junctional accessory molecules, can play an important role in leukocyte TEM (6, 92, 99). We have shown that under normal (unstimulated) conditions arteriolar ECs, similarly to venular ECs, express low but detectable levels of P- and E-selectin, as well as ICAM-1 and VCAM-1 (100, 101). Importantly, numerous studies have shown that the expression of adhesion molecules in arterioles in various tissue beds is significantly increased during inflammation. For example, four hours activation with TNFα results in a dramatic increase in the expression of P- and E-selectin, as well as ICAM-1 and VCAM-1 in cremaster muscle arterioles (100). Similarly, treatment with exogenous Ang II, or treatment with L-NAME to block eNOS, (which produces elevated levels of endogenous Ang II) results in upregulation of these same four adhesion molecules in arterioles (69, 78). Increased ICAM-1 expression has also been observed in arterioles in transplanted rat lungs post reperfusion (20), and after PAF stimulation of cultured coronary microvascular ECs (33). Similarly, VCAM-1 expression is increased in kidney arterioles in renal disease (13).
Leukocyte interactions with ECs are dependent on, and are defined by, the expression of surface adhesion molecules, thus increased expression of adhesion molecules in arteriolar ECs is likely one of the main reasons for the induction of leukocyte-EC interactions in arterioles. Additionally, some adhesion molecules appear to serve as signaling receptors regulating EC solute permeability. Specifically, ICAM-1 mediated signaling has been directly implicated in increased albumin permeability in arterioles (96, 98).
Induction of leukocyte-EC interactions
While leukocyte rolling interactions are observed in control venules (43, 49, 100), and leukocyte adhesion and TEM are observed after activation (54), in arterioles leukocyte-EC interactions are induced only under certain inflammatory conditions. For example, leukocyte rolling is observed in cremaster muscle arterioles after treatment with the pro-inflammatory cytokines IL-1 and TNFα (50, 96, 101, 104). Leukocyte adhesion in arterioles is stimulated by ischemia and reperfusion (40, 56), and in the presence of physiologically relevant doses of Ang II, whether through exogenous administration (8), or release of endogenous Ang II after blockade of NOS (69).
Changes in macromolecular permeability
It is well recognized that both capillary and venular permeability are increased during inflammation. Interestingly, there is evidence that coronary arteriolar permeability is also regulated in a dynamic way, as coronary arterioles increase their permeability to proteins as an adaptation to exercise training (34), or, acutely, during exposure to adenosine (35). These findings provided some of the first direct evidence for regulated solute exchange in arterioles. Although capillaries provide the vast majority of surface area for exchange, the finding that arteriolar permeability is also regulated is of great significance as metabolically active tissue also, of course, lies in proximity to arterioles, which in cardiac tissue, appears, in focal areas, to be devoid of capillaries (35). Recently we extended these findings to demonstrate, in a skeletal muscle model, that arterioles not only support solute exchange, but that this exchange is tightly regulated in arterioles just as it is in venules under both normal non-stimulated conditions and during inflammation (96, 98). Using intravital confocal microscopy we showed that arteriolar permeability to albumin under non-inflamed conditions was controlled through PKC-mediated signaling. However upon activation with TNFα this was rapidly altered to become independent of PKC but dependent on Src activation (98). These data imply that different mechanisms are key for regulation of permeability in unstimulated vs activated conditions, an idea that has yet to be tested directly but which has important implications for how we view this regulation as part of homeostatic compared to inflammatory mechanisms. Increased arteriolar permeability has also been observed in diabetic or VEGF activated rat retinas (11). Furthermore in this work the authors found that the observed changes in vascular permeability were initiated in arterioles and later observed in post-capillary venules, suggesting that indeed, in intact vascular networks, upstream responses (in arterioles) can affect the function of downstream vessels (venules).
Distinct features of arterioles as compared to venules and consequent effects on permeability and leukocyte-EC interactions
Arterioles differ from venules in their structure, EC morphology, and shear environment, as well as in expression and distribution of surface ligands, suggesting that the regulation of endothelial barrier function in arterioles may also be distinct from venules. In the next paragraphs we discuss the possible implications of these distinct features of arterioles on regulation of solute permeability and leukocyte-EC interactions.
Structure
In venules, macromolecules and leukocytes crossing the vascular wall sequentially encounter the glycocalyx, endothelial cells, an extensive although discontinuous pericyte layer, and the extracellular matrix (ECM) (53). Arterioles are distinct from venules in that arteriolar ECs are also circumferentially wrapped by one or more layers of SMC on the ablumenal side of the EC basal lamina. Thus, in contrast to the venular wall that is relatively thin, the arteriolar wall is a thick, multilayered structure, as illustrated in the electron micrographs (Fig 1). This wall structure supports rapid communication between ECs and SMCs (27, 55, 113), and is pivotal for regulation of vascular resistance and hence is essential for regulation of blood flow (110). Interestingly, whether this wall structure impedes or promotes solute exchange and recruitment of immune cells is still not clear. One possibility is that the additional SMC layer in arterioles might pose an obstacle for macromolecular flux across arteriolar wall and potentially affect leukocyte TEM. Indeed, to date leukocyte TEM has not been observed in arterioles. A tight barrier is presumably beneficial for healthy tissue where efflux of luminal antigens, blood proteins and immune cells must be contained. On the other hand, bi-directional communication between ECs and SMCs might contribute to regulation of responses that facilitate solute exchange. Indeed, there is clear evidence that macromolecules can cross both the EC and SMC layers as they traverse the arterial wall. Early work using electron microscopic observations in normal blood vessels showed that large molecules such as horse radish peroxidase (~ 40 KD) can cross both EC and SMC layers within 15 min of intravenous injection in both rat aortas and mouse carotid arteries (23). Moreover, under pathological conditions such as formation of atherosclerotic plaques or thrombosis, the SMC layer undergoes drastic reorganization leading to a compromised vessel wall barrier, thus allowing enhanced leakage of luminal contents (41).
Figure 1. Morphology of microvessel wall barrier differs in arterioles and venules.
Scanning electron micrographs show a cross sectional view of a mouse cremaster muscle arteriole (left panel) and venule (right panel). Red blood cells can be seen in the arteriole, and leukocytes interacting with the vessel wall can be seen in the venule. Arrows indicate ECM surrounding each vessel; asterisks indicate skeletal muscle myocytes. Scale bar: 10 µm.
In capillaries and post-capillary venules, ECM components and the pericyte sheath appear to play an important role in maintaining blood vessel integrity and regulation of both vascular permeability and leukocyte extravasation. For example, expression of the ECM component hyaluronan has been directly linked to the endothelial barrier, with higher hyaluronan expression being associated with lower permeability (82). EC attachment to the ECM (through integrin receptors) can stabilize EC junctions, thus limiting vascular leakage (64). Not surprisingly, patients with collagen vascular diseases show increased capillary permeability (60). Furthermore, dysregulation of matrix metalloproteases impairs matrix integrity hence EC function and survival (85). Moreover ECM-EC interactions have the ability to induce signaling events in ECs, thus regulating EC barrier function. For example, ECM interactions with αvβ3 integrin result in activation of VEGFR-2 (94), ligation of which can increase endothelial permeability (76).
Arteriolar ECs are also known to assemble ECM, and to recruit pericytes (9). However, to date the contribution of the pericyte sheath and the ECM to regulation of barrier function and leukocyte-EC interactions in arterioles has not been explored.
Interestingly it has been noted that matrix protein composition is different between microvessel and large vessel endothelia (16). Since different ECM proteins interact with different endothelial receptors, these findings suggest that the distribution of ECM proteins and subsequent specific ECM-EC interactions could help determine the barrier properties of different types of microvessels. This also raises the possibility that matrix composition surrounding venules might be different from that surrounding arterioles; an idea that should be explored in future work.
Contractile pericytes that are wrapped around capillaries in retina and cerebral circulation have been implicated in regulation of blood flow by controlling capillary diameter (79). Pericytes that are intimately associated with venular ECs within the region of the venular basement membrane have been implicated in regulation of leukocyte TEM, with migrating leukocytes appearing to traverse gaps in the pericyte sheath (81). Similarly, variations in the composition and density of the venular basement membrane are implicated in regulation of leukocyte TEM (107). Furthermore, leukocyte binding to ECM components during TEM can affect leukocyte function. For example, neutrophil β2-integrin interactions with the ECM protein fibrinogen regulate neutrophil apoptosis, hence contributing to neutrophil clearance during inflammation (114). Since the cellular morphology of the arteriolar wall with respect to the arrangement and density of SMCs, pericytes, and ECM, differs substantially from that in venules (71), it is not unreasonable to infer that regulatory processes for permeability of the arteriolar wall to macromolecules and to leukocytes will also likely differ from mechanisms defined in the venular microcirculation.
Intriguingly, we and others have shown that leukocyte TEM in venules is not random, but is localized to specific regions, which we have termed “portals”. The majority of these portals are tri-cellular EC junctions enriched in ICAM-1 expression (14, 99). Together with the findings outlined above, this suggests that there are preferred locations for leukocyte TEM across the intact venular wall. It is thus reasonable to speculate that the portals in the endothelial monolayer may be aligned with gaps in the loose network of pericytes, and with the low-density regions in ECM proteins. If this is indeed the case, then through as yet undefined signaling mechanisms, ECs may have the capacity to indicate to migrating leukocytes (possibly through clustering of ICAM-1) the location of regions with preferred local morphology and protein expression such as will support their egress into the tissue. Whether these phenomena contribute to differences in permeability responses between arterioles and venules is not yet clear, but certainly EC morphology (discussed next) differs between arterioles and venules in a way that suggests that this factor has the capacity to be a contributing factor.
EC morphology
Morphometric analysis in both isolated cell systems and in intact blood perfused microvessels has revealed that size and shape of individual ECs varies in different sized vessels, and that ECs are distinctly different between arterioles and venules (4, 99, 111). In arterioles, EC surface area increases as a function of vessel size, from ~300 µm2 in small arterioles (10–30µm in diameter) to greater than 1500 µm2 in larger arterioles (> 100 µm in diameter). In contrast, the size of venular ECs does not change in different sized vessels (and remains ~1200 µm2) (95). Importantly, ECs in arterioles are distinctly long and narrow (length/width ratio ~ 8:1) compared to venules, which are significantly more rounded (length/width ratio ~ 2.5:1) (95, 99). Intriguingly in arterioles with larger diameters, ECs become more elongated, while in larger venules ECs become significantly wider (95).
This distinct EC morphology in arterioles is beneficial for rapid EC communication with SMCs as each elongated EC can directly interact with three or more SMCs to induce vasoactive responses. EC morphology in arterioles is also likely to affect the way leukocytes interact with the vessel wall. For example by default, EC morphology and alignment dictates the number of tri-cellular junctions per unit vessel length. As tri-cellular junctions are preferred locations for leukocyte transmigration (14, 99), the number of available locations will affect the efficiency of leukocyte TEM. Indeed we have recently shown that while the total junctional length per surface area of vessel wall is not different between arterioles and venules, the number of tri-cellular junctions is significantly lower in arterioles compared to venules (99). Furthermore, enhanced leukocyte TEM occurs in venular convergences (97), which due to their EC morphology have a significantly higher number of tri-cellular junctions per unit vessel length compared to straight venular regions (99). In contrast, the number of tri-cellular junctions in arterioles is not increased in divergences, presumably because the long and narrow arteriolar ECs are able to bend appropriately to properly accommodate vessel geometry (Fig 2). The specific characteristic of tri-cellular junctions that enables them to function preferentially as portals has yet to be determined; understanding what confers this specificity on some EC junctions and not others in neighboring cells is an important question that, when answered, should contribute significantly to a more integrated understanding of regulation of vessel wall permeability and/or TEM.
Figure 2. EC junctional morphology in arterioles and venules differs in a manner that will affect leukocyte interactions.
Intact blood perfused cremaster muscle venules (left panel) and arterioles (right panel) were immunofluorescently labeled for PECAM-1 and ICAM-1 respectively using local introduction and perfusion of the appropriate primary and fluorescently tagged secondary antibodies in sequence. As illustrated by the representative images, PECAM −1 is primarily localized to the EC junctions, while ICAM-1 is excluded from EC junctions. Staining for both molecules allows cell borders of individual ECs to be identified. Different morphology and alignment of ECs in arterioles and venules by default dictates the arrangement and the number of tri-cellular junctions per unit vessel length; this in turn affects leukocyte interactions. Moreover, while in venules, relatively round ECs simply “fill in” the space in the converging region, in arterioles, long narrow ECs appear to appropriately traverse the bifurcation to properly accommodate vessel geometry.
Adhesion molecules
Expression levels and spatial distribution of adhesion molecules on the endothelial surface will determine the timing and location of leukocyte interactions with ECs, and hence will significantly impact inflammatory responses. P-and E-selectins mediate leukocyte rolling interactions in venules (1, 39, 43, 49), and the roles of members of the CAM family in meditating leukocyte adhesion and transendothelial migration in postcapillary venules have also been well characterized (15, 99, 115). While the distribution of some these molecules axially along the venular tree has been described (21), local variations in adhesion molecule density on the EC surface, in relation to leukocyte interactions, have to our knowledge, only been functionally documented for P-selectin and ICAM-1 (43, 101). While this information is at present limited to these two adhesion molecules, it has facilitated an enhanced understanding of the roles of arterioles and venules in microvascular wall permeability to macromolecules and leukocytes, as outlined next.
Surface expression of P-selectin and ICAM-1 in unstimulated arterioles is significantly lower than in unstimulated venules (100), however after stimulation with inflammatory cytokines, expression of these molecules is increased not only in venules, as has been well recognized (12, 63, 101), but also in arterioles (69, 101). Interestingly, although perhaps not surprisingly, leukocyte-EC interactions closely correlate with the expression levels of adhesion molecules in these two microvessel types. Thus, leukocyte rolling interactions are readily observed in unstimulated venules, however unstimulated arterioles, where adhesion molecule expression on the EC surface is close to undetectable, do not support leukocyte interactions with the vessel wall. Activated arterioles show increased expression of P-selectin and ICAM-1 to levels that are typically observed in unstimulated venules (100). Intriguingly, under these conditions, leukocyte rolling interactions are now supported in arterioles, but firm adhesion is rarely observed. In activated venules, expression of both P-selectin and ICAM-1 is further increased over the levels seen in unactivated venules or activated arterioles, resulting in leukocyte adhesion and TEM. Thus in general, relatively low expression of these adhesion molecules in both arterioles and venules is sufficient to sustain leukocyte rolling, however, a significantly higher expression is needed to support firm adhesion and TEM. The inability of activated arteriolar ECs to raise adhesion molecule expression to levels typical of activated venules presumably reflects one aspect of the larger question of what are the mechanisms underlying the differentiation of arteriolar versus venular ECs into cells of differing phenotypes. Importantly, while significantly increased over unstimulated conditions, the low expression of adhesion molecules in activated arterioles relative to activated venules is likely to be one of explanations for the absence of leukocyte emigration from activated arterioles. However, while leukocyte interactions in arterioles do not ultimately lead to TEM, we have recently shown that leukocyte interactions with ICAM-1 (which is also upregulated in inflamed arterioles) serve a different purpose, which is contribution to the regulation of arteriolar barrier function. Specifically, following TNFα treatment, engagement of ICAM-1 by rolling leukocytes in arterioles as well as venules results in changes in permeability to albumin (96, 98). Depletion of circulating neutrophils prevents this TNFα mediated increase in arteriolar albumin permeability (96). Importantly, antibody crosslinking of ICAM-1 in unstimulated, neutrophil-depleted arterioles increases permeability to a level similar to that seen following TNFα treatment (96, 98). Together, these findings argue that there is a direct contribution of leukocyte-EC interactions to regulation of arteriolar permeability specifically through engagement of ICAM-1.
In addition to differences in the expression levels, the distribution of adhesion molecules also varies between arterioles and venules, both with respect to specific regions of the microcirculatory network, and among individual ECs in equivalent microvessels. Immunostaining of intact blood perfused microvessels revealed high heterogeneity in the surface expression of both P-selectin (43) and ICAM-1 (101). Expression of these adhesion molecules in some regions along the vessel wall was significantly higher than in other regions (illustrated in Fig 3A). Not surprisingly these regions support increased leukocyte interactions.
Figure 3. Microvessels exhibit regional variations in expression levels and distribution of surface adhesion molecules.
Intact blood perfused cremaster muscle arterioles were immunofluorescently labeled for ICAM-1 or P-selectin using local introduction and perfusion of the appropriate primary and fluorescently tagged secondary antibodies in sequence. (A) A representative image and a corresponding fluorescence intensity plot showing variability of ICAM-1 expression in an arteriole imaged at the central vessel plane (schematic at bottom of panel) using confocal microscopy. (B) An example of the expression patterns of P-selectin (upper panel) and ICAM-1 (lower panel) in TNFα stimulated cremaster arterioles, imaged at the upper plane of the vessel cross section (schematic at bottom of panel).
Similarly, the distribution patterns of adhesion molecules differ from one molecule to another. For example P-selectin is expressed as punctuate clusters over the EC surface (Fig 3B, and (29, 43) and thus mediates stochastic leukocyte rolling interactions (43, 46). Moreover, while overall expression levels increase, the expression patterns of P-selectin do not change with inflammatory stimulus (100). This is consistent with the role of P-selectin in mediating initial interactions between leukocytes and ECs. ICAM-1 expression on the other hand is more diffuse over the EC surface (Fig 3B, bottom panel), and exhibits great variability in expression density both between adjacent ECs, as well as across the surface of individual cells (101). Interestingly, in inflamed vessels ICAM-1 expression is clustered at the regions of migrating leukocytes (99) and around actively migrating leukocytes (15).
While clustering of ICAM-1 can be observed in individual venular ECs, the expression of ICAM-1 is increased in all venular ECs upon activation (Fig 4A, (101)). In contrast, upon activation arteriolar ECs show redistribution of ICAM-1 among cells, where some cells increase and some decrease their ICAM-1 surface expression, magnifying the overall variability of ICAM-1 in the arteriole (Fig 4B, (101)). We speculate from this finding that some arteriolar ECs play a more important role in the inflammatory response than do others, but this idea, along with elaboration of the mechanisms that enable some of them to upregulate ICAM-1 surface expression while others appear to downregulate it, remains to be tested. One possibility is that ECs that downregulate suface ICAM-1 have upregulated expression of another key molecule in the overall inflammatory response, arguing possible specialization of specific regions of the arteriolar wall to mediate distinct functions, but again this idea will require testing. This general concept, ie that different ECs are specialized to express different components of the inflammatory response is a compelling speculation that could be generalized to all regions of the microcirculation.
Figure 4. TNFα treatment enhances the heterogeneity in ICAM-1 expression patterns in arterioles but not venules.
Representative immunofluorescence images, and the corresponding surface plots, of intact blood perfused cremaster muscle microvessels, showing that in response to TNFα, ICAM-1 expression in venules (Panel A) is increased in all ECs. Arterioles on the other hand (Panel B), initially express much less ICAM-1, undergo redistribution of ICAM-1 among ECs where some cells increase and some cells decrease their ICAM-1 expression, thus magnifying the variability of ICAM-1.
Flow environment
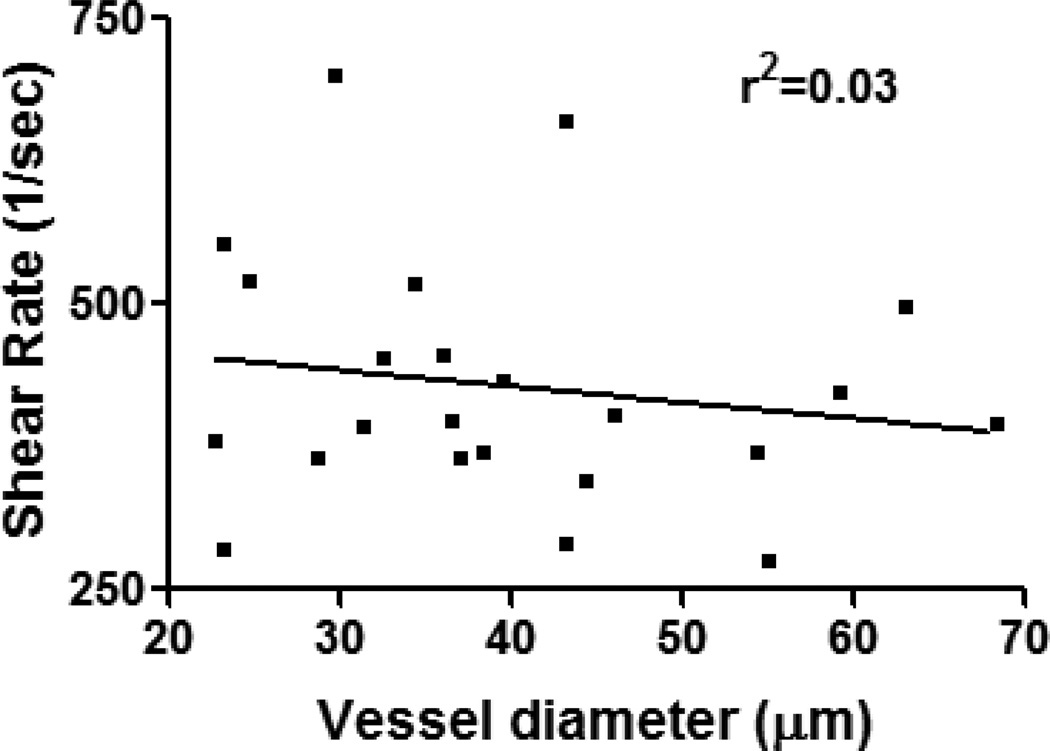
It is well established that ECs from different regions of the circulation are variously influenced by flow-dependent mechanical deformations, resulting in characteristically different gene expression and signaling in ECs residing in arterial versus venous vessels (reviewed by Chien (17)). In the microvasculature, shear rates in resistance arterioles are typically higher than in venules and range in general from 200 to 4000 sec−1 (89, 102). Interestingly, unlike in venules, where the shear environment can be predicted by vessel diameter (97), in arterioles, due to their dynamic nature and vasoactivity, shear rates cannot not be correlated to vessel size (Fig 5). These differences in shear environment may well contribute directly or indirectly to the differences in leukocyte-EC interactions and regulation of barrier function in arterioles and venules. Despite evidence that leukocyte interactions with venular ECs appear to be largely uninfluenced by the hemodynamic environment (52), particularly at higher shear rates (80), fluid shear forces can indeed affect leukocyte-EC interactions in venules (86), although the observed effects appear to vary with the experimental approach. For example, in isolated cell systems leukocyte delivery, hence leukocyte rolling and adhesion, is significantly decreased under high hydrodynamic flow (51). However we found that this is not the case in intact blood perfused venules where increased adhesion is observed in smaller venules (<40µm) that typically display an elevated shear environment (97). In these vessels, basal expression of ICAM-1 is significantly higher compared to larger venules (97), further supporting the finding that in vivo, adhesion molecules play a more prominent role in regulating leukocyte-EC interactions. Arterioles, as discussed above, have typically higher shear rates compared to venules, and expression of adhesion molecules in control and inflamed arterioles is significantly lower than in venules under similar conditions. Indeed leukocyte rolling is induced in inflamed but not unstimulated arterioles. To initiate contact with endothelial cells, leukocytes must exit the center line of the flow stream and marginate towards the vessel wall. Vessel bifurcations, characterized by stagnation regions (low blood flow, (97)) are the primary locations where initial contact between leukocytes and ECs occurs. We argue therefore that only in activated arterioles are the levels of surface adhesion molecules sufficient that leukocytes can remain in continuous contact with ECs. Further, we argue that in arterioles, the shear environment would be expected to have a more potent effect on leukocyte-EC interactions than in venules. Indeed under unactivated conditions, leukocyte adhesion in arterioles is rarely observed. It is however possible to induce adhesion in these vessels by locally reducing blood flow via partial occlusion of the inflow vessel (80, 100)), indicating that the shear environment does indeed contribute to the observed lack of leukocyte adhesion in these vessels. There is good evidence suggesting that variations in shear rate can affect expression of EC adhesion molecules. For example, expression of ICAM-1 is significantly increased when ECs in flow chambers are subjected to high shear environments (70). Interestingly however, we note that in small arterioles that typically support higher shear flow than their downstream venules, the expression of adhesion molecules is significantly lower than in venules, suggesting that in intact networks other factors must contribute to the regulation of adhesion molecules expression. In addition to lower expression of adhesion molecules, the elevated shear rate in arterioles is thus another cause for the lack of adhesion and hence leukocyte migration in arterioles.
Figure 5. Shear rate in arterioles cannot be predicted by vessel size.
Shear rate in TNFα stimulated (500ng, 4h, via intrascrotal injection) intact blood perfused cremaster muscle arterioles of various diameters was assessed by tracking the displacement of fluorescently labeled beads (0.5µm) which were injected into the blood stream via a catheter inserted into the jugular vein. No significant (p<0.05) correlation was found between vessel size and the prevailing shear rate in these vessels.
Hemodynamic factors can also affect endothelial barrier function. In arterioles, where flow patterns are more variable, in-vivo permeability to albumin is directly correlated to spatial variations in shear stress (32) and is sensitive to flow reversal (2). The effects of fluid dynamics on endothelial barrier function must be due, at least in part, to the specific effects of fluid shear on endothelial morphology, intracellular signaling and cytoskeletal and junctional organization (59, 75).
Physiological and pathological consequences of arteriolar responses to inflammatory stimuli
As has been discussed above, wall structure, EC morphology, shear environment and expression of adhesion molecules are different in arterioles compared to venules. However, inflammatory activation of arterioles results in increased expression of EC adhesion molecules, induction of leukocyte-EC interactions, and changes in barrier function (macromolecular permeability). While leukocytes do not appear to extravasate from arterioles to any significant extent, induction of leukocyte rolling in arterioles clearly has alternative physiologically relevant consequences, including increased arteriolar permeability and the potential priming of both leukocytes and the downstream venules (discussed below).
Physiology
As discussed earlier in this review, increased expression of ICAM-1 on the luminal surface of arteriolar ECs during inflammation and its engagement by interacting leukocytes results in increased endothelial permeability to macromolecules such as albumin (18, 96, 98). Given the surface area of resistance arterioles, their contribution to total solute exchange is potentially significant. We have not explored whether other molecules implicated in leukocyte-EC adhesive interactions may also directly activate ECs and hence affect arteriolar permeability, as has been shown for the products released by neutrophils following interactions between neutrophils and venular ECs (25, 53), but there is no reason to suppose that there are not other mediators of permeability changes in arterioles yet to be described. Certainly, as discussed earlier, coronary arterioles show both acute and adaptational changes in permeability in response to adenosine, further attesting to the potential contribution of these microvessels to regulation of fluid homeostasis.
An important consequence for leukocyte-EC interactions in arterioles is the potential for these interactions to “prime” both the leukocytes and the downstream capillaries and venules for subsequent inflammatory responses. For example, it is established that activation/conformational changes of leukocyte β2-integrins are initiated by leukocyte rolling (87, 88). As activation of β2-integrin is essential for binding of endothelial adhesion ligands, including ICAM-1 and VCAM-1 (22), and thus transitioning the leukocyte to firm adhesion, it is a reasonable speculation that rolling interactions in arterioles might initiate β2-integrin activation at this earlier stage in the leukocyte’s passage through the microvasculature, and thus support more efficient adhesion and TEM when these cells reach venules. Indeed, in parallel plate flow chamber assays, early leukocyte rolling on E-selectin is essential for β2-integrin activation and transitioning to firm adhesion, through interactions with ICAM-1 (93). As E-selectin is expressed in activated arterioles, and ICAM-1 is found in abundance in activated venules, the findings in flow chambers may thus be simulating consecutive events in an integrated in vivo vascular network.
There is accumulating evidence that leukocyte engagement of EC ligands results in release from leukocytes of a variety of signal molecules (of which CAP37 is a classic example (25)), that are potent mediators of enhanced solute permeability in venules. Similarly, the neutrophil products cathepsin G and elastase can both affect endothelial permeability (30), as do interleukins 1, 6, and 8 as well as TNFα (7, 74), among others. Clearly, leukocyte interactions with arteriolar ECs raise the possibility that released substances can be convectively carried in the blood stream to induce permeability changes in distal microvessels and thus contribute to inflammatory responses in venules.
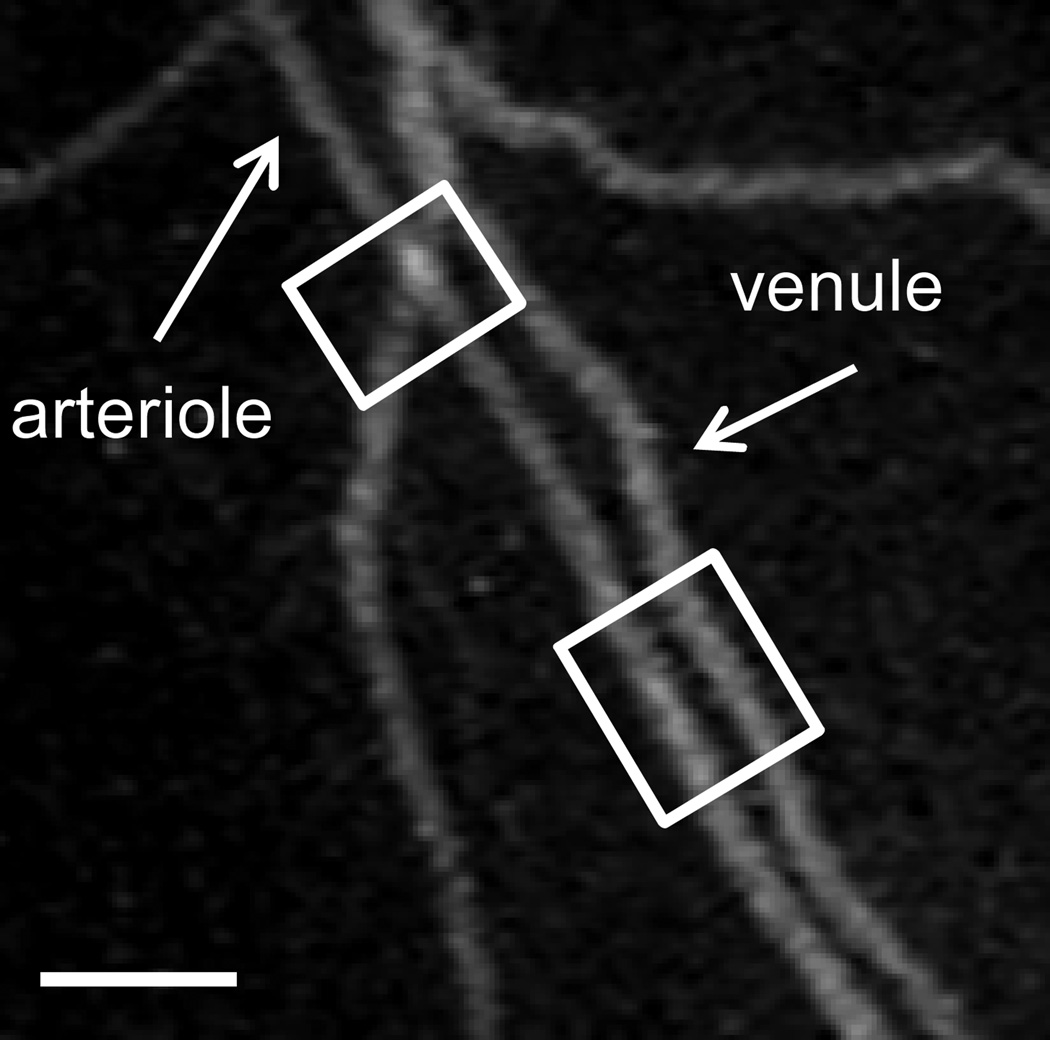
A common geometry in many microvascular beds is the parallel alignment of descending arterioles and ascending collecting venules (91), or arteriole-venule pairs crossing each other, illustrated in Fig 6. This geometry is reinforced by a complex network of capillaries that fills the spaces between parallel arterioles and venules. The proximity of these different vessel types creates a high likelihood that paracrine cross-talk may occur between them. Indeed, the dilatory effects of vasoactive substances that diffuse from venous blood to the adjacent arterioles have been demonstrated (28, 31). In some cases, such as in mesenteric arterioles of high cholesterol mice, it has been shown that arteriolar tone is dependent on leukocyte-EC interactions in nearby venules, and can be modulated by inhibition of leukocyte β2-integrins (45). Given that arterioles can change their barrier function in inflammation, thus contributing to solute and fluid exchange, it is clearly possible that inflammatory mediators released in activated arterioles could also diffuse towards neighboring capillaries and venules, thus affecting their function. Indeed it is well established that signaling molecules, including inflammatory mediators, released as a consequence of leukocyte-EC interactions in venules can affect arteriolar function (44, 45) hence the proposed reversal in signaling direction (from arterioles to venules to prime the inflammatory response) appears entirely reasonable, but will require further exploration.
Figure 6. Alignment of arterioles and venules in close proximity of each other may elicit paracrine talk between them.
To visualize the geometry of the microvascular bed in cremaster muscle of anesthetized mice, a feed arteriole to a region of the network was microinjected with fluorescently labeled BSA to perfuse the downstream section of the intact microvasculature. A representative field of view shows parallel alignment of descending arterioles and ascending collecting venules, as well as arterioles and venules pairs crossing each other (highlighted in white squares), suggesting that paracrine cross-talk is possible between these vessels.
Pathology
Increased vascular permeability and accumulation of immune cells is a hallmark of inflammation-based disorders, including chronic obstructive pulmonary disease, cystic fibrosis, inflammatory bowel diseases, arthritis, atherosclerosis and thrombosis. The microvasculature, and specifically arterioles, are affected by these disorders, and play a pivotal role in facilitating pathological symptoms. For example, thrombi can form in mesenteric and cremaster arterioles, leading to downstream occlusion and tissue ischemia (61, 72). Furthermore, thrombus formation has been observed in cerebral arterioles (26), increasing the risk for stroke. Intriguingly, both increased expression of EC adhesion molecules and leukocyte-EC interactions, as observed in inflamed arterioles are identified as risk factors for thrombosis (3). Under normal physiological conditions arterioles that express low levels of adhesion molecules do not support leukocyte interactions, and thus are protected from thrombus formation. However in inflammation, leukocyte interactions with ECs often result in de-endothelization and exposure of sub-endothelial matrix, thus promoting thrombus formation. One of the key players promoting pathological thrombus formation is circulating platelets (48). In inflamed arterioles, activated ECs upregulate the levels of P-selectin and release VWF (42, 72, 100). Intriguingly, P-selectin has been shown to mediate both platelet adhesion, as well as leukocyte recruitment towards the arteriolar wall during inflammation (24, 112). Similarly, VWF released by ECs can bind to the exposed sub-endothelial matrix, and serve as an adhesive ligand for both circulating platelets (90) and inflammatory monocytes (38). Moreover, platelets can independently promote leukocyte recruitment and accumulation at the vessel wall by directly mediating leukocyte attachment (77), and indirectly through secretion of leukocyte chemoattractants (116). Inhibition of both leukocyte-EC interactions and absence of either P-selectin or VWF can delay thrombus formation (72). These examples link inflammation and thrombosis, arguing that these are interdependent processes that activate and perpetuate each other in arterioles. While the underlying mechanisms are the subject of ongoing research, it is clear that upregulation of adhesion molecule and chemokine expression on the arteriolar wall during inflammation plays an important role in promoting this vascular pathology.
Another example of where arterioles play a critical role in manifestation of a disease occurs in diabetes. Diabetic patients in most cases also suffer from hypertension (103), both well-defined risk factors for atherosclerosis. While atherosclerosis is characterized by increased stiffness of large arteries, in both diabetic and hypertensive patients the microcirculation (specifically small arterioles) is also remodeled. Increased cross-sectional area (thickening of the arteriolar wall), and increased collagen deposition are characteristic of diabetic arterioles (84). These changes likely result from impaired myogenic responses (58), increased wall stresses and alterations in the vascular ECM leading to endothelial dysfunction (47). Components of the immune system including circulating blood cells, as well as expression levels of cytokines and chemokines are also altered in diabetic arterioles (19). Thus here too, increased expression of surface adhesion molecules hence increased leukocyte-EC interactions, are likely contributors to vascular dysfunction and disease.
In summary, recent findings suggest that arteriolar barrier properties are tightly regulated under normal physiological conditions, as well as during inflammation. Arterioles have been identified as potential contributors to the maintenance of proper tissue homeostasis via regulation of solute and fluid exchange. Moreover changes in expression of surface adhesion molecules and induction of leukocyte-EC interactions in arterioles, while directly linked to pathological phenotypes, can potentially prime and enhance the responsiveness of downstream capillaries and venules during inflammation, thus providing further support for the integrated nature of signaling and responses in the intact microcirculation.
Acknowledgments
Sources of Support: NIH HL 75186, HL18208 (to IHS) CCFA CDA 3597 (to RS)
List of abbreviations
- EC
endothelial cell
- ICAM-1
intercellular adhesion molecule 1
- VCAM-1
vascular cell adhesion molecule 1
- PECAM-1
platelet endothelial cell molecule 1
- TEM
transendothelial migration
- AngII
angiotensin II
- L-NAME
Nω-nitro-L-arginine methyl ester
- NOS (eNOS)
nitric oxide synthase (endothelial NOS)
- PAF
platelet activating factor
- SFK
src family kinases
- VEGF
vascular endothelial growth factor
- VEGFR-2
VEGF receptor 2
- SMC
smooth muscle cell
- ECM
extracellular matrix
- VWF
Von Willibrand factor
- BSA
bovine serum albumin
References
- 1.Abbassi O, Kishimoto TK, McIntire LV, Anderson DC, Smith CW. E-selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest. 1993;92:2719–2730. doi: 10.1172/JCI116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Sarai RK, Altangerel A, Clark JF, Weinbaum S, Curry FE. Microvascular permeability to water is independent of shear stress, but dependent on flow direction. Am J Physiol Heart Circ Physiol. 2013;304:H1077–H1084. doi: 10.1152/ajpheart.00956.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshar-Kharghan V, Thiagarajan P. Leukocyte adhesion and thrombosis. Curr Opin Hematol. 2006;13:34–39. doi: 10.1097/01.moh.0000190107.54790.de. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 5.Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation. Am J Physiol Cell Physiol. 2012;303:C385–C395. doi: 10.1152/ajpcell.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–2779. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol. 1999;277:L1057–L1065. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez A, Cerda-Nicolas M, Naim Abu Nabah Y, Mata M, Issekutz AC, Panes J, Lobb RR, Sanz MJ. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood. 2004;104:402–408. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- 9.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 10.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 11.Barber AJ, Antonetti DA. Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 2003;44:5410–5416. doi: 10.1167/iovs.03-0244. [DOI] [PubMed] [Google Scholar]
- 12.Briscoe DM, Cotran RS, Pober JS. Effects of tumor necrosis factor, lipopolysaccharide, IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992;149:2954–2960. [PubMed] [Google Scholar]
- 13.Bruijn JA, Dinklo NJ. Distinct patterns of expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and endothelial-leukocyte adhesion molecule-1 in renal disease. Lab Invest. 1993;69:329–335. [PubMed] [Google Scholar]
- 14.Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997;159:2893–2903. [PubMed] [Google Scholar]
- 15.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 18.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 20.Egan TM, Thomas Y, Gibson D, Funkhouser W, Ciriaco P, Kiser A, Sadoff J, Bleiweis M, Davis CE. Trigger for intercellular adhesion molecule-1 expression in rat lungs transplanted from non-heart-beating donors. Ann Thorac Surg. 2004;77:1048–1055. doi: 10.1016/j.athoracsur.2003.08.023. discussion 1055. [DOI] [PubMed] [Google Scholar]
- 21.Eppihimer MJ, Russell J, Langley R, Vallien G, Anderson DC, Granger DN. Differential expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1) in murine tissues. Microcirculation. 1998;5:179–188. [PubMed] [Google Scholar]
- 22.Fagerholm SC, Varis M, Stefanidakis M, Hilden TJ, Gahmberg CG. alpha-Chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood. 2006;108:3379–3386. doi: 10.1182/blood-2006-03-013557. [DOI] [PubMed] [Google Scholar]
- 23.Florey L, Sheppard BL. The permeability of arterial endothelium to horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1970;174:435–443. doi: 10.1098/rspb.1970.0003. [DOI] [PubMed] [Google Scholar]
- 24.Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–1127. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- 26.Gavins FN, Russell J, Senchenkova EL, De Almeida Paula L, Damazo AS, Esmon CT, Kirchhofer D, Hebbel RP, Granger DN. Mechanisms of enhanced thrombus formation in cerebral microvessels of mice expressing hemoglobin-S. Blood. 2011;117:4125–4133. doi: 10.1182/blood-2010-08-301366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- 28.Harris NR, First GA, Specian RD. Influence of arteriovenular pairing on PAF-induced capillary filtration. Am J Physiol. 1999;276:H107–H114. doi: 10.1152/ajpheart.1999.276.1.H107. [DOI] [PubMed] [Google Scholar]
- 29.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 30.Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem. 2003;278:14002–14012. doi: 10.1074/jbc.M300351200. [DOI] [PubMed] [Google Scholar]
- 31.Hester RL, Hammer LW. Venular-arteriolar communication in the regulation of blood flow. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1280–R1285. doi: 10.1152/ajpregu.00744.2001. [DOI] [PubMed] [Google Scholar]
- 32.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol. 2004;286:H1916–H1922. doi: 10.1152/ajpheart.00897.2003. [DOI] [PubMed] [Google Scholar]
- 33.Huang Q, Wu M, Meininger C, Kelly K, Yuan Y. Neutrophil-dependent augmentation of PAF-induced vasoconstriction and albumin flux in coronary arterioles. Am J Physiol. 1998;275:H1138–H1147. doi: 10.1152/ajpheart.1998.275.4.H1138. [DOI] [PubMed] [Google Scholar]
- 34.Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol. 2007;293:H1196–H1205. doi: 10.1152/ajpheart.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huxley VH, Williams DA. Basal and adenosine-mediated protein flux from isolated coronary arterioles. Am J Physiol. 1996;271:H1099–H1108. doi: 10.1152/ajpheart.1996.271.3.H1099. [DOI] [PubMed] [Google Scholar]
- 36.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000;278:H1177–H1185. doi: 10.1152/ajpheart.2000.278.4.H1177. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa M, Sekizuka E, Sato S, Yamaguchi N, Inamasu J, Bertalanffy H, Kawase T, Iadecola C. Effects of moderate hypothermia on leukocyte- endothelium interaction in the rat pial microvasculature after transient middle cerebral artery occlusion. Stroke. 1999;30:1679–1686. doi: 10.1161/01.str.30.8.1679. [DOI] [PubMed] [Google Scholar]
- 38.Isobe T, Hisaoka T, Shimizu A, Okuno M, Aimoto S, Takada Y, Saito Y, Takagi J. Propolypeptide of von Willebrand factor is a novel ligand for very late antigen-4 integrin. J Biol Chem. 1997;272:8447–8453. doi: 10.1074/jbc.272.13.8447. [DOI] [PubMed] [Google Scholar]
- 39.Jung U, Bullard DC, Tedder TF, Ley K. Velocity differences between L- and P-selectin-dependent neutrophil rolling in venules of mouse cremaster muscle in vivo. Am J Physiol. 1996;271:H2740–H2747. doi: 10.1152/ajpheart.1996.271.6.H2740. [DOI] [PubMed] [Google Scholar]
- 40.Kataoka H, Kim SW, Plesnila N. Leukocyte-endothelium interactions during permanent focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2004;24:668–676. doi: 10.1097/01.WCB.0000117812.35136.5B. [DOI] [PubMed] [Google Scholar]
- 41.Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, Swedenborg J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 42.Khandoga A, Biberthaler P, Enders G, Teupser D, Axmann S, Luchting B, Hutter J, Messmer K, Krombach F. P-selectin mediates platelet-endothelial cell interactions and reperfusion injury in the mouse liver in vivo. Shock. 2002;18:529–535. doi: 10.1097/00024382-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Kim MB, Sarelius IH. Role of shear forces and adhesion molecule distribution on P-selectin-mediated leukocyte rolling in postcapillary venules. Am J Physiol Heart Circ Physiol. 2004;287:H2705–H2711. doi: 10.1152/ajpheart.00448.2004. [DOI] [PubMed] [Google Scholar]
- 44.Kim MH, Carter PR, Harris NR. P-selectin-mediated adhesion impairs endothelium-dependent arteriolar dilation in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2007;292:H632–H638. doi: 10.1152/ajpheart.00780.2006. [DOI] [PubMed] [Google Scholar]
- 45.Kim MH, Granger DN, Harris NR. Mediators of CD18/P-selectin-dependent constriction of venule-paired arterioles in hypercholesterolemia. Microvasc Res. 2007;73:150–155. doi: 10.1016/j.mvr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King MR, kim MB, Sarelius IH. dynamics of leukocyte rolling on punctate distributions of P-selectin. Molecular Biology of the Cell. 2002;13:547a–548a. [Google Scholar]
- 47.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012:918267. doi: 10.1155/2012/918267. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–1540. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubes P, Kanwar S. Histamine induces leukocyte rolling in post-capillary venules A P-selectin-mediated event. J Immunol. 1994;152:3570–3577. [PubMed] [Google Scholar]
- 50.Kunkel EJ, Jung U, Ley K. TNF-alpha induces selectin-mediated leukocyte rolling in mouse cremaster muscle arterioles. Am J Physiol. 1997;272:H1391–H1400. doi: 10.1152/ajpheart.1997.272.3.H1391. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 52.Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- 53.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 54.Ley K, Tedder TF. Leukocyte interactions with vascular endothelium. New insights into selectin-mediated attachment and rolling. J Immunol. 1995;155:525–528. [PubMed] [Google Scholar]
- 55.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 56.Liu X, Peter FW, Barker JH, Pierangeli SS, Harris EN, Anderson GL. Leukocyte-endothelium interaction in arterioles after ischemia and reperfusion. J Surg Res. 1999;87:77–84. doi: 10.1006/jsre.1999.5725. [DOI] [PubMed] [Google Scholar]
- 57.Long EO. ICAM-1: getting a grip on leukocyte adhesion. J Immunol. 2011;186:5021–5023. doi: 10.4049/jimmunol.1100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorenzi M, Feke GT, Pitler L, Berisha F, Kolodjaschna J, McMeel JW. Defective myogenic response to posture change in retinal vessels of well-controlled type 1 diabetic patients with no retinopathy. Invest Ophthalmol Vis Sci. 2010;51:6770–6775. doi: 10.1167/iovs.10-5785. [DOI] [PubMed] [Google Scholar]
- 59.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109(Pt 4):713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 60.Marks J, Birkett DA, Shuster S. “Capillary permeability” in patients with collagen vascular diseases. Br Med J. 1972;1:782–784. doi: 10.1136/bmj.1.5803.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marx I, Christophe OD, Lenting PJ, Rupin A, Vallez MO, Verbeuren TJ, Denis CV. Altered thrombus formation in von Willebrand factor-deficient mice expressing von Willebrand factor variants with defective binding to collagen or GPIIbIIIa. Blood. 2008;112:603–609. doi: 10.1182/blood-2008-02-142943. [DOI] [PubMed] [Google Scholar]
- 62.McIntyre TM, Prescott SM, Weyrich AS, Zimmerman GA. Cell-cell interactions: leukocyte-endothelial interactions. Curr Opin Hematol. 2003;10:150–158. doi: 10.1097/00062752-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10:27–39. doi: 10.1016/s1359-6101(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 64.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 65.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 66.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 67.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 68.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nabah YN, Mateo T, Cerda-Nicolas M, Alvarez A, Martinez M, Issekutz AC, Sanz MJ. L-NAME induces direct arteriolar leukocyte adhesion, which is mainly mediated by angiotensin-II. Microcirculation. 2005;12:443–453. doi: 10.1080/10739680590960962. [DOI] [PubMed] [Google Scholar]
- 70.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr., Gimbrone MA., Jr. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nees S, Juchem G, Eberhorn N, Thallmair M, Forch S, Knott M, Senftl A, Fischlein T, Reichart B, Weiss DR. Wall structures of myocardial precapillary arterioles and postcapillary venules reexamined and reconstructed in vitro for studies on barrier functions. Am J Physiol Heart Circ Physiol. 2012;302:H51–H68. doi: 10.1152/ajpheart.00358.2011. [DOI] [PubMed] [Google Scholar]
- 72.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85:599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 74.Nooteboom A, Van Der Linden CJ, Hendriks T. Tumor necrosis factor-alpha and interleukin-1beta mediate endothelial permeability induced by lipopolysaccharide-stimulated whole blood. Crit Care Med. 2002;30:2063–2068. doi: 10.1097/00003246-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng. 2002;30:430–446. doi: 10.1114/1.1467924. [DOI] [PubMed] [Google Scholar]
- 76.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 77.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, Sajer SA, Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 78.Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, Ferri C, Desideri G, Gulino A, Santucci A. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- 79.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perry MA, Granger DN. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Invest. 1991;87:1798–1804. doi: 10.1172/JCI115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D'Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiao RL, Wang HS, Yan W, Odekon LE, Del Vecchio PJ, Smith TJ, Malik AB. Extracellular matrix hyaluronan is a determinant of the endothelial barrier. Am J Physiol. 1995;269:C103–C109. doi: 10.1152/ajpcell.1995.269.1.C103. [DOI] [PubMed] [Google Scholar]
- 83.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 84.Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103:1238–1244. doi: 10.1161/01.cir.103.9.1238. [DOI] [PubMed] [Google Scholar]
- 85.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 86.Russell J, Cooper D, Tailor A, Stokes KY, Granger DN. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol Gastrointest Liver Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- 87.Salas A, Shimaoka M, Chen S, Carman CV, Springer T. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin lymphocyte function-associated antigen-1. J Biol Chem. 2002;277:50255–50262. doi: 10.1074/jbc.M209822200. [DOI] [PubMed] [Google Scholar]
- 88.Salas A, Shimaoka M, Phan U, Kim M, Springer TA. Transition from rolling to firm adhesion can be mimicked by extension of integrin alphaLbeta2 in an intermediate affinity state. J Biol Chem. 2006;281:10876–10882. doi: 10.1074/jbc.M512472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sato M, Ohshima N. Effect of wall shear rate on thrombogenesis in microvessels of the rat mesentery. Circ Res. 1990;66:941–949. doi: 10.1161/01.res.66.4.941. [DOI] [PubMed] [Google Scholar]
- 90.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 91.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 92.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–2330. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 93.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 94.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. Embo J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sumagin R, Brown CW, 3rd, Sarelius IH, King MR. Microvascular endothelial cells exhibit optimal aspect ratio for minimizing flow resistance. Ann Biomed Eng. 2008;36:580–585. doi: 10.1007/s10439-008-9467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am J Physiol Cell Physiol. 2011;301:C804–C813. doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sumagin R, Lamkin-Kennard KA, Sarelius IH. A separate role for ICAM-1 and fluid shear in regulating leukocyte interactions with straight regions of venular wall and venular convergences. Microcirculation. 2009;16:508–520. doi: 10.1080/10739680902942271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol. 2010;184:5242–5252. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sumagin R, Sarelius IH. A role for ICAM-1 in maintenance of leukocyte-endothelial cell rolling interactions in inflamed arterioles. Am J Physiol Heart Circ Physiol. 2007;293:H2786–H2798. doi: 10.1152/ajpheart.00720.2007. [DOI] [PubMed] [Google Scholar]
- 101.Sumagin R, Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 2006;291:H2116–H2125. doi: 10.1152/ajpheart.00248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tangelder GJ, Slaaf DW, Arts T, Reneman RS. Wall shear rate in arterioles in vivo: least estimates from platelet velocity profiles. Am J Physiol. 1988;254:H1059–H1064. doi: 10.1152/ajpheart.1988.254.6.H1059. [DOI] [PubMed] [Google Scholar]
- 103.Tarnow L, Rossing P, Gall MA, Nielsen FS, Parving HH. Prevalence of arterial hypertension in diabetic patients before and after the JNC-V. Diabetes Care. 1994;17:1247–1251. doi: 10.2337/diacare.17.11.1247. [DOI] [PubMed] [Google Scholar]
- 104.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am J Physiol. 1997;272:H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- 105.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 106.Walmsley JG, Gore RW, Dacey RG, Jr., Damon DN, Duling BR. Quantitative morphology of arterioles from the hamster cheek pouch related to mechanical analysis. Microvasc Res. 1982;24:249–271. doi: 10.1016/0026-2862(82)90016-4. [DOI] [PubMed] [Google Scholar]
- 107.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weisberg HF. Osmotic pressure of the serum proteins. Ann Clin Lab Sci. 1978;8:155–164. [PubMed] [Google Scholar]
- 109.Weisiger RA, Pond SM, Bass L. Albumin enhances unidirectional fluxes of fatty acid across a lipid-water interface: theory and experiments. Am J Physiol. 1989;257:G904–G916. doi: 10.1152/ajpgi.1989.257.6.G904. [DOI] [PubMed] [Google Scholar]
- 110.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 111.Wojciechowski JC, Sarelius IH. Preferential binding of leukocytes to the endothelial junction region in venules in situ. Microcirculation. 2005;12:349–359. doi: 10.1080/10739680590934763. [DOI] [PubMed] [Google Scholar]
- 112.Wood KC, Hebbel RP, Granger DN. Endothelial cell P-selectin mediates a proinflammatory and prothrombogenic phenotype in cerebral venules of sickle cell transgenic mice. Am J Physiol Heart Circ Physiol. 2004;286:H1608–H1614. doi: 10.1152/ajpheart.01056.2003. [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yan SR, Sapru K, Issekutz AC. The CD11/CD18 (beta2) integrins modulate neutrophil caspase activation and survival following TNF-alpha or endotoxin induced transendothelial migration. Immunol Cell Biol. 2004;82:435–446. doi: 10.1111/j.0818-9641.2004.01268.x. [DOI] [PubMed] [Google Scholar]
- 115.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]