Abstract
The group of schizophrenia disorders affects approximately 1% of the population and has both genetic and environmental etiologies. Sufferers report various behavioral abnormalities including hallucinations and delusions (positive symptoms), reduced joy and amotivation (negative symptoms), plus inattention and poor learning (cognitive deficits). Despite the heterogeneous symptoms experienced, most patients smoke. The self-medication hypothesis posits that patients smoke to alleviate symptoms, consistent with evidence for nicotine-induced enhancement of cognition. While nicotine acts on multiple nicotinic acetylcholine receptors (nAChRs), the primary target of research is often the homomeric α7 nAChR. Given genetic linkages between schizophrenia and this receptor, its association with P50 sensory gating deficits, and its reduced expression in post-mortem brains, many have attempted to develop α7 nAChR ligands for treating schizophrenia. Recent evidence that ligands can be orthosteric agonists or positive allosteric modulators (PAMs) has revitalized the hope for treatment discovery. Herein, we present evidence regarding: 1) Pathophysiological alterations of α7 nAChRs that might occur in patients; 2) Mechanistic evidence for the normal action of α7 nAChRs; 3) Preclinical studies using α7 nAChR orthosteric agonists and type I/II PAMs; and 4) Where successful translational testing has occurred for particular compounds, detailing what is still required. We report that the accumulating evidence is positive, but that greater work is required using positron emission tomography to understand current alterations in α7 nAChR expression and their relationship to symptoms. Finally, cross-species behavioral tasks should be used more regularly to determine the predictive efficacy of treatments.
1. Introduction
Schizophrenia was first described in 1896 [1] and labeled as dementia praecox. Although cognitive dysfunction was initially the core component, in the 1950s more traditional diagnoses of schizophrenia became based on positive and negative symptoms [2, 3]. Positive symptoms include behaviors not normally present but appear due to the disorder. These symptoms include hallucinations, delusions, and bizarre behavior. Negative symptoms are behaviors that are normally present but are reduced due to the disease process, including alogia, affective flattening, anhedonia, and avolition [4]. More recently, the cognitive symptoms of these patients have again been recognized as core to the disorder since they correlate most closely with functional outcome [5, 6]. Currently approved treatments are primarily efficacious at treating positive symptoms and do not adequately improve cognition or negative symptoms in patients [7–10]. Recent focus has been to develop treatments to enhance cognition in patients on the premise that their functional outcome will also be improved.
The lack of approved treatment for cognition in schizophrenia has made developing such treatments more difficult because there are no positive controls with which to compare a test compound [11]. Research has focused therefore, on identifying aspects of schizophrenia that differ from healthy subjects in order to provide targets to develop treatments. Epidemiological evidence consistently report a higher proportion of smoking in patients with schizophrenia (40–90%) compared with the general population (15–25%) [12–14]. Furthermore, patients with schizophrenia also smoke more cigarettes per day. The primary psychoactive ingredient in cigarette smoke is nicotine and patients with schizophrenia also select cigarettes with higher nicotine content and extract more nicotine per cigarette than healthy controls. The increased smoking rate of patients may reflect self-medication [15–18]. Such an hypothesis is supported by evidence that nicotine improves cognitive functioning in patients, as well as healthy subjects. For example, nicotine-induced improvement in vigilance has been observed using the continuous performance test (CPT) in both healthy volunteers [19–21] and patients with schizophrenia [22], which may exert downstream beneficial effects on other cognitive domains [23] (see also a review by [24]). Nicotine may also have neuroprotective effects. For example, in 50,000 Swedish conscripts, smoking rates were inversely correlated with progression to schizophrenia [25], while smoking has also been associated with later onset [26]. An alternative hypothesis is however, that patients smoke to alleviate some of the negative side-effects of their antipsychotic medication. Because patients not taking antipsychotic medications also smoke at high rates however, it is unlikely patients smoke to alleviate medication side-effects [27]. Moreover, high smoking rates occur before a patient’s first episode, prior to treatment [28]. One final hypothesis is that there is shared susceptibility toward smoking and the development of schizophrenia, hence its comorbidity [29]. Given the evidence of nicotine-induced improvements in cognition even in non-smoking patients however, the interest in its beneficial qualities remains high. Since the negative effects of nicotine, such as nausea and addiction, make it an undesirable therapeutic [30, 31], focus has been placed on determining the mechanism underlying its beneficial cognitive action.
1.1. Diverse mechanisms of action of nicotine
Nicotine is the prototypical ligand of the nicotinic acetylcholine receptors (nAChRs). nAChRs are ligand-gated ion channels, existing as combinations from a family of similar but distinct subunits α1-α10, β1–4, γ, δ, and ε [32, 33]. The most predominant receptor in the mammalian brain is the α4β2 nAChR, while there is also high expression of the α7 nAChR [34]. Both receptors are widely expressed in areas of the brain important to cognition, such as the hippocampus, thalamus, frontal, cingulate, occipital cortices [35, 36]. Nicotine binds to heteromeric receptors such as the α4β2 nAChR at higher affinities compared with the homomeric receptors such as the α7 nAChR [37]. Studies support that the α4β2 nAChR is required for the initiation of smoking addiction [38], hence treatments targeted at the α7 nAChR receptor would be less likely to be addictive [39, 40]. Adler et al., [41] proposed that the significantly higher levels of smoking seen in patients with schizophrenia may be due to an implicit desire to activate the α7 nAChR. Certainly, reduced α7 nAChR function can increase an animal’s work rate to obtain nicotine once preference is acquired [42]. Geerts [43] has suggested however, that smoking levels may not be high enough to affect α7 nAChRs. Certainly, half a cigarette saturates the α4β2 nAChRs [44] and so it is unclear why patients smoke so much, commonly chain smoking one cigarette after another. Collectively, the evidence supports that the α7 nAChR receptor is likely important for the degree to which patients smoke, particularly given the work of Brunzell and McIntosh, [42] and the evidence of reduced α7 nAChR expression in patients with schizophrenia ([45], also see section 2.). This mini-review will focus on: 1) Pathophysiological alterations of the α7 nAChR that might occur in patients; 2) Mechanistic evidence for the normal action of α7 nAChR effects; 3) Preclinical studies using α7 nAChR orthosteric agonists as well as type I and II PAMs; and 4) Where successful translational testing has occurred for particular compounds and what is still required.
2. Genetic and pathophysiological linkage between the α7 nAChR and schizophrenia
The epidemiological, genetic, and pathophysiological evidence linking schizophrenia to the α7 nAChR is summarized in table 1. Schizophrenia carries a lifetime risk of 1% [46]. In monozygotic twins however, that risk is ~40%, [47, 48], indicative of a strong genetic contribution to schizophrenia. While numerous genetic studies have implicated diverse risk genes for schizophrenia [49], here we will focus on α7 nAChRs. Wallace and Bertrand provide a good schematic describing the genetic linkage of the α7 nAChR and schizophrenia [50]. Genome-wide association studies have associated copy number variations of a locus containing the α7 nAChR with high risk for schizophrenia [51]. Moreover, α7 nAChR mRNA expression may be regulated by neuregulin-1 genetic variation [52], an established genetic risk factor for schizophrenia [53–56]. More directly, Freedman et al., [57] identified a link between deficient gating of the P50 auditory event-related potential and a locus on chromosome 15q14, at a polymorphic marker <120 kb from the α7 nAChR gene with a LOD score of 5.3, Θ = 0.039, which was replicated in later studies [58, 59], although not all [60]. P50 gating refers to a paradigm in which 2 acoustic clicks are presented about 500 msec apart, resulting in a reduced (i.e. gated) P50 response to the second stimulus. Originally conceptualized as a measure of short-term habituation, P50 gating has been shown to be reduced in patients with schizophrenia and is affected by nicotine [61]. In rodents, the hippocampal P20/N40 potential is considered to provide a putative analog of human P50 gating [62]. Accordingly, the gating of the P20/N40 in various mouse strains correlated with their hippocampal α7 nAChR expression [63]. After administration of nicotine or other α7 nAChR agonists the P20/N40 gating of each strain was improved [63–66], providing converging support for the α7 nAChR as a viable therapeutic target in schizophrenia [43, 67, 68].
Table 1.
Pathophysiological linkage between the α7 nAChR and schizophrenia
| Linkage type | Evidence | References |
|---|---|---|
| Genetic | P50 deficit linked to chromosome 15q14 | [56] |
| Genome wide association studies | [50] | |
|
| ||
| Post-Mortem | Reduced binding in dentate gyrus and CA3 region | [69] |
| Reduced binding in the cingulate cortex | [46] | |
| Frontal lobe regions | [70–72] | |
|
| ||
| Epidemiology | High nicotine-seeking behavior | [15–18] |
| Treatment | Nicotine improves symptoms including cognitive functioning | [19–22] |
Further support for specifically targeting the α7 nAChR in treating schizophrenia comes from post-mortem studies examining the brains of patients. Reduced α7 nAChR protein levels have been observed in post-mortem brains of patients with schizophrenia in the dentate gyrus and CA3 region – though not CA1 [69]. Importantly, this reduced α7 nAChR expression has been linked to the degree of global cognitive dysfunction in these patients [69]. In other studies, Marutle et al., [45] observed reduced α7 nAChR binding in the cingulate cortex of patients, but not the orbitofrontal cortex, with no difference in the dorsolateral prefrontal cortex (PFC). Overall therefore, decreased α7 nAChR binding in post-mortem brains of patients have been noted in the reticular nucleus of the thalamus, the hippocampus, the cingulate cortex, and the frontal lobe regions [45, 70–72], although not all studies have replicated these findings [73]. The brains examined in patients are mostly from chronically ill patients whom have undergone chronic antipsychotic treatment, many with multiple concurrent treatments. Thus, the impact of antipsychotic medication or even smoking status on α7 nAChR binding levels cannot always be accounted for. The effect of chronic antipsychotic treatment can be examined at least using rodent studies. Terry et al., [74] demonstrated that 90-day chlorpromazine, risperidone, and olanzapine treatment significantly reduced α7 nAChR binding levels of rats in the posterior cortex and amygdala, while haloperidol did not affect binding levels. After 180 days, only risperidone treatment still significantly reduced α7 nAChR binding [74]. Furthermore, the negative association between age and α7 nAChR binding levels in the perirhinal cortex of humans [73] supports the need for further examination of α7 nAChR binding levels in patients across ages. These studies demonstrate the need to understand impact of the disease process on α7 nAChR expression that is untainted by treatment, age, or smoking effects.
One mechanism by which α7 nAChR expression could be examined in patients with fewer confounds than examining post-mortem brain tissue would be to use positron emission tomography (PET) or single-photon emission computed tomography (SPECT). The development of novel α7 nAChR ligands suitable for PET imaging will be a key step forward in understanding altered neurochemistry in patients with schizophrenia. With PET or SPECT studies, α7 nAChR levels could be examined in: a) subjects with a high-risk of psychosis; b) first-episode patients; c) never-medicated patients; d) non-smoking patients; e) chronically ill patients on various antipsychotic medications; and f) smoking vs. non-smoking patients. Furthermore, it would be extremely useful to assess the link between current binding levels in patients and their positive and negative symptoms, functional outcome, and cognitive performance specifically in cognitive domains identified by the National Institute of Mental Health funded initiatives Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS; [75, 76]) and Cognitive Neuroscience Treatment to Improve Cognition in Schizophrenia (CNTRICS; [77]). Unfortunately however, to date no PET or SPECT studies have been conducted examining α7 nAChR binding levels in patients. There are numerous complexities when developing PET or SPECT ligands. Toyohara et al., [78] detailed some complexities regarding the development of α7 nAChR radioligands for PET and SPECT imaging. Up until that point, there were limited options because of a scarcity of high-affinity ligands. By 2013, Horti and colleagues [79] described two novel radioligands [(11)C]A-833834 (5-(6-(5-[(11)C]methylhexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)pyridazin-3-yl)-1H-indole) and [(11)C]A-752274 (2-(6-[(11)C]methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)-7-(6-methyl-3,6-diazabicyclo[3.2.0]heptan-3-yl)-9H-fluoren-9-one), designed to image functioning α7 nAChR levels in the brain. While these molecules may have high-affinity binding, their low blood-brain barrier (BBB) permeability limits their use in humans [79]. Thus, in terms of α7 nAChR expression, post-mortem brain analysis supports reduced expression in key areas related to cognition and symptoms, but these analyses may be confounded by extraneous factors.
When examining nAChR binding levels using [3H]-nicotine and [3H]-epibatidine that target predominantly high-affinity nAChRs, Breese et al., [80] found that smoking up-regulated binding in control subjects, while this increase was not seen in the hippocampus or thalamus of patients. In contrast, another study observed increased [3H]-cytisine and [3H]-epibatidine binding in patients [45], though smoking status was not identified for all subjects. Importantly, SPECT ligands are available for high-affinity nAChRs and commonly used in smoking studies [81, 82]. When high-affinity nAChRs were examined using SPECT imaging with [123I]5-A-85380, decreased availability was observed in 1-week smoking abstinent patients in the frontal and parietal cortices as well as the thalamus in smoking patients compared with controls [83]. Because this study was conducted in living patients, it was established that their current high-affinity nAChR levels correlated inversely with negative symptom severity. These patients were currently being treated with a variety of antipsychotic medications that could impact nAChR expression. Administering a variety of antipsychotic treatments over 180 days to rats did not alter high-affinity nAChR expression however [84]. Although it was not established whether the medication interfered with chronic nicotine-induced up-regulation, these studies provide support that the lower up-regulation in smoking patients compared with controls is likely a result of changes germane to the disorder itself. The same control studies including patients at different stages of the disease described above for α7 nAChR radioligands would also be required for high-affinity nAChRs however.
2.1. The putative impact of reduced α7 nAChR expression
Recent reviews by Thomsen et al., [68] and Bencherif et al., [85] have identified meaningful connections between areas of α7 nAChR expression, their effect on other neurotransmitters, and how these changes might relate to the dopamine and glutamate hypotheses of schizophrenia. These connections reveal the impact reduced expression of the α7 nAChR might have on mechanisms likely to be important in the manifestation of schizophrenia. Key to these connections is that α7 nAChRs are ideally located to modulate neurotransmitter release in key regions related to the dopamine and glutamate hypotheses. For example, α7 nAChRs are located on midbrain dopamine cell bodies of the ventral tegmental area (VTA), subthalamic nuclei, presynaptically on dopamine terminal regions in the striatum, nucleus accumbens, and frontal cortex, as well as on glutamate and GABA neurons that project into dopamine regions and terminals [86–89]. Activation of α7 nAChRs can increase dopamine release in the striatum, VTA, nucleus accumbens, and PFC areas [90–92]. Evidence for the nAChR selectivity of agonist effects comes from knockout studies whereby α7 nAChR knockout mice do not exhibit choline-induced striatal dopamine release in mice [93]. Specific targeting of presynaptic α7 nAChRs can enhance AMPA-mediated excitatory postsynaptic currents in VTA [94, 95] and PFC [96] dopaminergic neurons, as well as facilitate glutamatergic synaptic currents in hippocampal neurons [97, 98] and pyramidal neurons in the auditory cortex [99]. α7 nAChR activation releases GABA from GABAergic interneurons [100, 101]. GABA acts on GABAB receptors leading to decreased striatal glutamate release, which may in turn result in the increased dopamine release [85]. Using an enzyme-based microelectrode in vivo, Kondardsson-Geuken and colleagues [102] demonstrated that the α7 nAChR modulates glutamate release in the PFC of rats. Interestingly, choline-induced stimulation of glutamate release was blocked by both α-bungarotoxin and kynurenine [102], a precursor of kynurenic acid. The importance of kynurenic acid relates to it being: 1) a negative modulator of α7 nAChR function; 2) elevated in patients with schizophrenia [103, 104]; and 3) disruptive to prepulse inhibition (PPI; [105], a measure of sensorimotor gating disrupted in patients with schizophrenia [106–108]) when elevated. In vitro stimulation of the septal cholinergic input induces CA1 hippocampal and VTA long term potentiation (LTP) via α7 nAChRs [94, 109], enhancing hippocampal LTP [110]. Moreover, LTP is reduced in mice with no α7 nAChR expression [111] as well as in patients with schizophrenia [112]. Because striatal dopaminergic neurons are involved in LTP, which may underlie aspects of learning [113], α7 nAChR-induced striatal dopamine release and LTP induction may be linked. Thus, α7 nAChRs can stimulate dopamine release and induce LTP. Such mechanisms can be important for ameliorating impaired cognition and behavioral abnormalities in patients with schizophrenia.
One example of the importance of striatal dopamine release is for reward-associative learning. Dopamine plays a key role in the reward-prediction error hypothesis [114–116], firing in response to an unpredicted but not predicted reward [117, 118]. Hence, dopamine strengthens the synaptic connection between reward and action, providing a mechanism for Thorndike’s Law of Effect. Patients with schizophrenia exhibit deficits in reward-related learning [119–121], likely associated with reduced brain activation following reward-predicting stimuli as seen in unmedicated patients [122]. Poor reward-related learning in patients may be impacted by lower motivational levels [123], but given the lack of associations with negative symptoms, this deficit is likely specific to reward-associative learning [119, 120, 124]. Interestingly, striatal DRD1 receptors are linked to the direct pathway that stimulates the thalamus and cortex [125, 126] and is important for the dopamine reward-prediction hypothesis [127]. DRD1 stimulation likely strengthens synaptic connections, promoting LTP [128]. Similarly, DRD1 knockout mice exhibit altered LTP and impaired associative learning [129–131]. In addition to impaired LTP, α7 nAChR knockout mice also exhibit impaired learning from reward-associative cues, from learning complex cognitive tasks such as the 5-choice serial reaction-time task ([132] as well as poor baseline performance), the odor span task [133], and radial arm maze [134] [135] to simple constitutive learning [134]. These mice exhibit normal learning when aversive motivators are used however, such as context fear conditioning or Barnes maze learning [134, 136]. Hence, it has been proposed that the α7 nAChR is key for learning using reward-associative cues [134]. Interestingly, mice with 50% reduced expression of the α7 nAChR exhibit a learning and attentional phenotype between that of knockout and wildtype mice [132, 134]. Given the putatively reduced α7 nAChR expression in patients with schizophrenia, it may be useful to assess the susceptibility of these mice to environmental factors linked with schizophrenia, such as vitamin D deficiency [137, 138] or maternal immune activation during prenatal development [139].
3. Ligands, their targets, and putative effects in preclinical studies
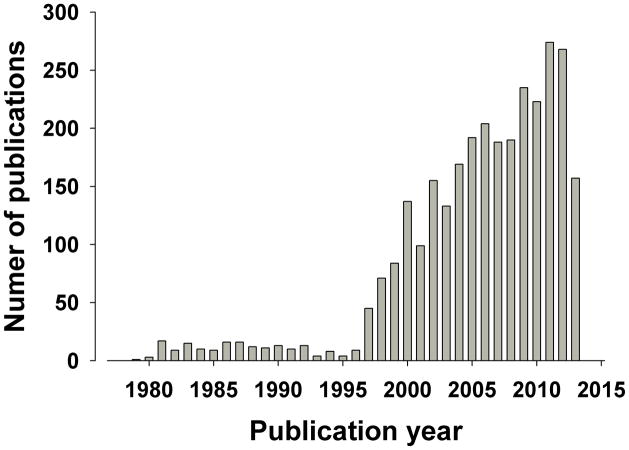
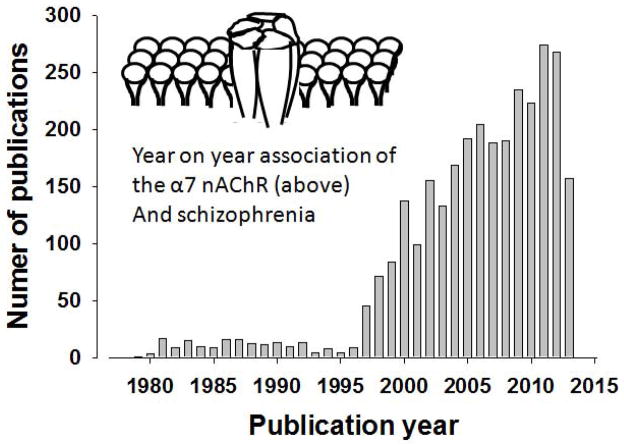
Our knowledge regarding the complexity of the α7 nAChR and its relationship to schizophrenia has increased year after year, consistent with the steady rise in publications on this nAChR, with only 9 in 1996, rising to 45 in 1997, to 268 in 2012 (Figure 1). Knowledge of presynaptic localization of α7 nAChRs have altered viewpoints on doses to be used. For example, presynaptic α7 nAChRs enables low concentrations of a drug to act specifically on this receptor while higher doses may block nAChRs [140, 141]. These findings support the putative U-shaped dose-response function of α7 nAChR treatments and highlight the need to test lower doses. Werkheiser and colleagues [142] discovered that low exposure to the α7 nAChR partial agonist AZD0328 and SSR180711 improved short-term memory (9 minute delay) novel object recognition task (NORT) performance in mice. A molecular mechanism of this effect was proposed whereby low doses of these treatments increased α7 nAChR binding in rats in the frontal cortex and hippocampus, while higher doses decreased binding and did not improve cognition [142]. Thus, dosing may critically relate to the site of action of treatment supporting the assessment of a wide dose range in studies.
Figure 1. Graphical representation of year-by-year publications involving the α7 nAChR when searched on Pubmed using the terms “alpha7 AND nicotinic”.
From few publications early on, maintaining between 0–10 until 1996, there has been a steady increase in publications citing the α7 nAChR. The figures for 2013 are only recorded as far as June 25th, 2013.
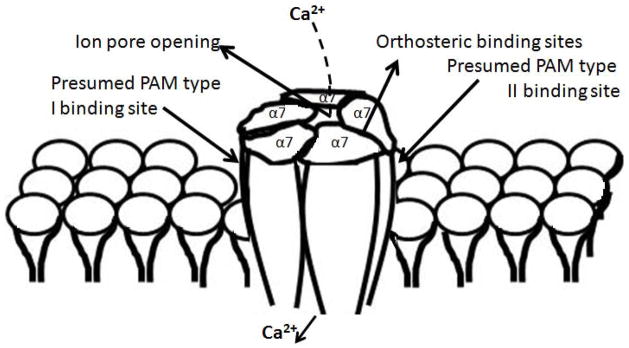
The discovery of positive allosteric modulators (PAMs) of α7 nAChR [143] has further opened treatment possibilities [144]. PAMs only function in the presence of the endogenous ligand, preserving the temporal and spatial integrity of neurotransmission. PAMs are also less prone to cause prolonged desensitization of the α7-nAChRs that reduces function, as may occur after chronic administration of orthosteric agonists [145, 146]. In terms of reward-associative learning, this difference could be important given that saliency should only be placed on those stimuli predicting reward, not on any stimuli encountered. Traditional agonists of α7 nAChR act at the extracellular orthosteric binding site located at the interface between two subunits, providing 4 binding sites per receptor. PAMs bind at a different site leading to the potentiation of effects of endogenous ligands. Interestingly, two types of PAM have been discovered that likely have different binding sites (Figure 2). Type I PAMs predominantly affect the peak current response, while type II PAMs increase the duration of the channel opening and are not accompanied by a profound retardation of the kinetics of desensitization [147]. The lack of desensitization in type II PAMs could mediate the lack of repeated treatment-induced up-regulation of α7 nAChRs that is seen in orthosteric modulators and type I PAMs [148]. Type I PAMs have been developed and include CCMI [144], and NS1738 [149]. Type II PAMs include PNU-120596 [150], TQS [147], A-867744 [151], and JNJ-1930942 [152]. Comparing and contrasting the effects of these PAMs by type will be useful for identifying their potential use as treatments by class.
Figure 2. Schematic representation of the α7 nAChR and its associated binding sites.
The homomeric α7 nAChR consists of 5 transmembrane spanning α7 subunits. The conjunction of each subunit is a potential binding site (4 in total) for orthosteric agonists, which includes the natural ligands acetylcholine and choline. When the ion channel is opened by an agonist, calcium (Ca2+) can enter the cell (see [68] for further downstream effects). The discovery of type I and II positive allosteric modulators (PAMs) has led to the hypothesis that they bind at different sites since they potentiate or prolong the effects of natural ligands (respectively).
3.1. Orthosteric agonists at the α7 nAChR
Early studies examining the cognitive effects of orthosteric agonists at α7 nAChRs in rat attention measured by the 5-choice serial reaction-time task were not positive [153–155], and could relate to the use of acute dosing or the poor BBB permeability of these compounds [156, 157]. This agonist, AR-R 17779, did improve long-term social recognition in rats at similar doses however [158]. More positive findings came from the partial agonist GTS-21 (DMXBA) which improved P20/N40 auditory gating in mouse strains with poor gating [64] and in isolation-reared rats [159]. More potent full agonist compounds with good BBB permeability have proven more efficacious. For example, the full orthosteric agonist 5-(6-[(3R)-1-azabicyclo[2.2.2]oct-3-yloxy] pyridazin-3-yl)-1H-indole (ABT-107) improved delay-dependent working memory in monkeys and social recognition in rats [160]. Such findings are particularly interesting given that ABT-107 is tolerable and crosses the BBB in healthy humans [161]. Tropisetron is an anti-emetic used during chemotherapy [162] and in addition to being a 5-HT3 antagonist, it is a partial orthosteric agonist at α7 nAChRs. Repeated treatment (14 days) with tropisetron improved NORT performance in mice [163], subjected to the sub-chronic phencyclidine (PCP) induced cognitive deficit model of schizophrenia [164, 165]. Kohnomi and colleagues [166] demonstrated that tropisetron blocked apomorphine-induced disruption of prepulse inhibition of startle (PPI) – a reliable model of antipsychotic efficacy [167]. This effect was blocked by the selective α7 nAChR antagonist methylycaconicitine (MLA), supporting an α7 nAChR not 5-HT3 receptor mechanism of action. Importantly, tropisetron attenuated apomorphine-induced increase in c-fos positive cells in the VTA, but not the nucleus accumbens or the dorsolateral striatum [166]. These data support α7 nAChR-induced changes that are mediated via the indirect dopamine release in the VTA. Moreover, these data would suggest tropisetron might improve positive and cognitive symptoms of patients with schizophrenia. Another orthosteric partial agonist with 5-HT3 receptor antagonist affinity is R3487 (otherwise known as MEM3454), which improved sustained attention task performance in female rats, driven primarily by responses to target stimuli [168]. Another full orthosteric agonist is compound A (R)-N-(1-azabicyclo[2.2.2]oct-3-yl)(5-(2-pyridyl)thiophene-2-carboxamide), the administration of which leads to dopamine release in the PFC [169] and improved non-spatial working memory as measured by the odor span task [170]. TC-5619 is a full orthosteric agonist at α7 nAChR and improved PPI and social approach in the th(tk−)/th(tk−) mouse model of schizophrenia, while improving NORT (24 hour delay) in rats [171]. Moreover, TC-5619 improved apomorphine-induced disruption in PPI, a test for antipsychotic efficacy [108]. These findings support the putative utility of TC-5619 at treating positive, negative, and cognitive symptoms of patients with schizophrenia. Low doses of the orthosteric α7 nAChR agonist AZD0328 increased dopamine release in mice [90] and monkeys [92]. Moreover, low doses of AZD0328 improved delay-dependent working memory in monkeys, delay-dependent memory in mice using the NORT [142], and delayed reinforcement learning in rats [90]. Higher doses tended to disrupt performance however [142], supporting the U-shaped dose response of α7 nAChR treatments. Direct PFC-induced administration of the orthosteric full agonist PNU 282987 improved radial arm maze learning in rats [172]. Systemic administration of PNU 282987 also reversed PCP-induced disruption of NORT in rats [173], although similar doses did not improve attentional functioning in normal or scopolamine-induced inattention in mice, unlike nicotine [174]. The orthosteric partial agonist EVP-6124 [(R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide] improved long-term (24 hour) NORT performance, perhaps by potentiating the actions of acetylcholine [175]. Since this effect was blocked by MLA, it was hypothesized that EVP-6124 could improve cognition in patients with memory disorders, such as schizophrenia. Finally, the orthosteric full agonist CP-810123 was noted to have high BBB permeability and bioavailability, and reversed amphetamine-induced disruption in sensory gating as well as scopolamine-induced disruption of 30-min delayed NORT in rats [176]. Thus, there is increasing preclinical evidence of novel orthosteric ligands that may improve some of the behavioral abnormalities associated with schizophrenia.
3.2. Positive allosteric modulators of the α7 nAChR
α7 nAChR orthosteric agonists and PAMs can exert similar effects, e.g. the orthosteric partial agonist SSR180711 enhanced LTP [177] as did the type II PAM JNJ-1930942 [152]. Direct comparisons between orthosteric agonists (partial or full), type I, or II PAMs have rarely been conducted in preclinical tests. Thomsen and colleagues [148] demonstrated that repeated administration of the full orthosteric agonist A-582941 increased α7 nAChR binding and improved long-term memory (24 hr) for social discrimination in rats, an effect not seen after single administration. Similarly, the α7 nAChR type I PAM AVL-3288 – but not the type II PAM PNU-120596 – improved long-term memory for social discrimination in rats only after a 7-day repeated administration. Such evidence could relate to the α7 nAChR up-regulation that occurs with orthosteric agonists and type I PAMs but not type II PAMs [178]. Other preclinical tests have supported the use of acute dosing of orthosteric agonists (described in 3.1.), type I, and II PAMs. The type I PAM CCMI improved the working memory performance of rats in the radial maze [144]. Another type I PAM, NS 1738, reversed scopolamine-induced deficits in water maze learning and social recognition memory in rats [149]. In another animal model of schizophrenia, kynurenic acid impaired set-shifting deficits in rats which was reversed by systemic or intra-PFC administration of the type I PAM galantamine [179], likely via enhanced PFC glutamate release [102]. Evidence supporting that these effects were α7 nAChR PAM-related and not from the acetylcholinesterase inhibition action of galantamine came from a lack of donepizil-induced (another acetylcholinesterase inhibitor) reversal of the same deficits [180]. In contrast, the type II PAM PNU-120596 improved long-term (48 hour) object recognition memory in rats only when co-administered with the acetylcholinesterase inhibitor donepezil, an affect blocked by the α7 nAChR antagonist MLA [181]. PNU-120596 also only improved water maze learning in age-induced cognitively impaired rats and delayed-matched to sample in monkeys over longer delays when co-administered with donepezil [181]. PNU-120596 treatment alone was successful in an animal model of schizophrenia, reversing the effects of PCP-induced set-shifting and NORT deficits of rats [182]. The mechanism underlying this acute type II PAM effect is unclear and while improvements have been observed in manipulation-induced deficits, donepezil improved performance alone in the same rats. Another type II PAM JNJ-1930942, improved P20/N40 sensory gating measured in DBA/2 mice, an effect blocked by α-bungarotoxin [152]. These type II PAMs therefore require further investigation and clinical trials (see 3.3 and table 2).
Table 2.
Preclinical to clinical development of α7 nAChR treatments to date
| Compound | Preclinical findings | Clinical findings & Comments | Reference |
|---|---|---|---|
| Partial Orthosteric agonists | |||
| DMXBA (GTS-21) | Acutely improved P20/N40 gating in mice and isolation-reared rats | Chronic treatment Improved P50 gating In large sample no cognitive but some negative symptom improvements, effects may be allelic influenced | [64, 154, 183–185] |
| AZD0328 | Improved NORT in mice (9 min delay) | Not yet tested clinically | [162] |
| SSR180711 | Improved NORT in mice (9 min delay) | Not yet tested clinically | [162] |
| Tropisetron | Repeated dosing improved NORT (24 hr) in normal and PCP-treated mice. Blocked apomorphine-induced PPI deficits | Chronic treatment improved P50 gating, sustained attention, and negative symptoms Did not improve short-term memory | [163–166 and 186–188] |
| R3487 | Improved sustained attention | Not yet tested clinically | [168] |
| Full Orthosteric agonists | |||
| ABT-107 | Improved delay-dependent and social recognition memory | BBB-penetrant in humans, other effects not yet tested | [160 and 161] |
| AR-R 17779 | No effect on sustained attention, improved social recognition (24 hr) | Unsuitable kinetic profile for humans | [153–155, 158] |
| Compound A | Improved non-spatial working memory span | Not yet tested clinically | [170] |
| TC-5619 | Improved PPI and social approach in th(tk−)/th(tk−) mouse model Improved NORT (24 hour) in rats Blocked apomorphine-induced PPI deficits | Improved maze learning and negative symptoms clinically No change in positive symptoms reported; may be influenced by co- treatment with antipsychotics | [108, 170, 185] |
| AZD0328 | Acutely improved delay-dependent memory in monkeys and NORT (15 min) in mice, improved delayed learning in rats | Disruptive at higher doses in animals Not yet tested clinically | [90 and 142] |
| PNU 282987 | Improved radial arm maze learning in rats Reversed PCP-induced deficits of NORT Did not prevent normal or scopolamine- induced deficits in attention/vigilance | Unsuitable kinetic profile for humans | [172–174] |
| A-582941 | Repeated treatment improved social discrimination (24 hr) in rats | Effects in animals not seen after single administration, not tested clinically | [148] |
| Type I Positive Allosteric Modulators | |||
| AVL-3288 | Repeated treatment improved social discrimination (24 hr) in rats | Effects in animals not seen after single administration, not tested clinically | [148] |
| CCMI | Improved working memory span in rats using the radial arm maze | Not yet tested clinically | [144] |
| NS 1738 | Reversed scopolamine-induced water- maze learning and social recognition memory | Not yet tested clinically | [149] |
| Type II Positive Allosteric Modulators | |||
| PNU-120596 | Acute nor chronic treatment affected social discrimination (24 hr) in rats Blocked NORT (24 hr), and age-induced water maze learning deficits Reversed PCP-induced NORT (1 min) and set-shifting deficits | Baseline improvement in rodents only seen with donepezil co-administration Not yet tested clinically | [148, 181, and 182] |
| JNJ-1930942 | Acutely improved P20/N40 sensory gating in mice | Did not improve any clinical sensory gating using acute doses | [152 and 191] |
NORT=novel object recognition task; PPI=prepulse inhibition; PCP=phencyclidine
In summary, α7 nAChR treatments have come a long way from their early development, with numerous groups and companies involved in developing novel α7 nAChR agonists. The focus on good BBB permeability has enhanced treatments available with which to test hypotheses and potential clinical efficacy. The focus on ensuring safety and tolerability has likewise improved the likelihood of testing the translational validity of early preclinical findings. Making these treatments available for independent testing in other domains would greatly improve the likelihood of identifying specific cognitive and behavioral domains that are relevant for testing in the clinic.
3.3. Translating preclinical evidence to clinical studies
The partial orthosteric agonist GTS-21 (DMXB-A) is probably the most widely tested α7 nAChR compound in patients with schizophrenia. To date, DMXB-A has had mixed results clinically. In a proof-of-concept study, Olincy et al., [183] reported that DMXB-A led to improvements in neurocognitive functioning and gating of P50 auditory evoked potentials in patients [183], which was consistent with improved P20/N40 in mice. In a larger double-blind follow-up study however, Freedman and colleagues [184] did not see the same cognitive enhancement from DMXB-A in patients as measured by the MATRICS Consensus Cognitive Battery in a crossover trial design. There were significant improvements at the higher dose on the Scale for the Assessment of Negative Symptoms (SANS), however [184]. Other small-scale fMRI studies (n=16) demonstrated that DMXB-A could improve the default network during a smooth pursuit eye-tracking task, which may be more prominent in people with a common vs. minor allele of the single nucleotide polymorphism (SNP), rs3087454 A/C [185]. Future studies will likely examine the impact this SNP has had on data collected to date. This SNP could also underlie the reason why in one study DMXB-A improved cognition, but in the larger sample at a higher dose it improved negative symptoms. Without further analyses however, these possibilities remain speculative.
Interestingly, and consistent with DMXB-A, the partial agonist TC-5619 developed by Targacept also improved negative symptoms as measured by the SANS in patients with schizophrenia (n=185) [186]. TC-5619 also improved maze learning of the CogState battery in a Phase II trial of patients with schizophrenia, possibly potentiated in smokers [186]. Given the success of TC-5619 in a model of positive symptoms [171], it is unfortunate that TC-5619 was only tested in combination with risperidone or quetiapine (chosen due to their common use in treating schizophrenia). Social cognition and memory data in humans were not presented in the Phase II trial [186]. Conducting such studies would have been useful for comparative purposes. In an 8-week double-blind study, the partial agonist tropisetron improved P50 gating and sustained attention of non-smoking patients with schizophrenia when tested in the Cambridge Neuropsychological Test Automated Battery (CANTAB) but did not affect positive or negative symptoms [187]. In a 10-day double-blind trial in nonsmoking patients with schizophrenia, tropisetron did improve immediate and delayed memory, as well as P50 gating in patients [188]. In a more recent 8-week double-blind trial as an add-on to risperidone treatment, tropisetron improved negative, but not positive, symptoms in patients with schizophrenia [189]. These data in part validate some of the preclinical evidence suggesting pro-memory efficacy of tropisetron, but not evidence for antipsychotic efficacy [166]. Studies specifically assessing negative symptom efficacy in animals, NORT-like memory in humans, and generating evidence for tropisetron-induced amelioration of positive symptoms, would provide greater support for cross-species translational validity of tropisetron trials. This latter inconsistency may be in part because as with the TC-5619 study, the patients were already receiving the atypical antipsychotic risperidone however [187]. In an as-yet not peer-reviewed published study, Envivo note that their α7 nAChR orthosteric partial agonist EVP-6124, when tested as an add-on to atypical antipsychotics in 319 patients, improved cognition and negative symptoms of patients with schizophrenia or schizoaffective disorder in a phase II trial as measured by Cogstate examination [190]. Once published, it will be interesting to see if the cognitive improvement is seen in visual memory, as was presented in preclinical studies [175]. Recently, the translational efficacy of JNJ-1930942 was assessed for improving sensory gating in patients as was discovered in DBA/2 mice [152]. Winterer et al., [191] examined whether acute doses of JNJ-1930942 would improve various markers of sensory gating in patients with schizophrenia in a within-subjects design (P50, P300, and mismatch negativity). No positive effect of JNJ-1930942 on any measure was observed however [191]. This lack of effect could relate to the use of patients that smoke as opposed to non-smokers. Given that in some preclinical studies the type II PAM PNU-120596 only improved performance in rodents when co-administered donepezil, further studies should examine the effects of co-treatment with donepezil. Alternatively, because the patients tested were being treated with a variety of antipsychotic treatments, preclinical studies should also be conducted in combination with antipsychotic treatment [11, 192, 193]. Finally, variations in the aforementioned SNP could preclude any positive effect of JNJ-1930942 in some of the patients.
Several α7 nAChR treatments (the partial and full orthosteric agonists DMXBA and TC-5619 respectively) have now demonstrated reductions in negative symptoms after chronic treatment in separate studies. Unfortunately, few preclinical studies have examined aspects of α7 nAChR contribution to behaviors relevant to negative symptoms. Conceivably however, these findings could relate to the putative action of the α7 nAChR on reward-associative learning, whereby activating the receptor has enhanced general reward-associations, perhaps via its indirect dopamine release and subsequent D1 activation in the striatum [194, 195]. Future studies would benefit from testing the effects of α7 nAChR treatments on reward-associative learning in cross-species tests, such as those described by CNTRICS [196]. Examining the correlation between any changes in reward-associative learning performances observed and negative symptoms would also be very useful. The evidence of preclinical to clinical testing effects for putative treatment compounds is summarized in table 2.
4. Conclusions and recommendations for future directions
The studies presented here are an overview of attempts to develop selective α7 nAChR treatments for the behavioral abnormalities seen in patients with schizophrenia. While many of these studies have been discussed elsewhere (in particular, see Geerts or Thomsen and colleagues [43, 68] for useful tables), this review has attempted to bring together: 1) Pathophysiological alterations that might occur in patients; 2) Mechanistic evidence for the normal action of α7 nAChR effects; 3) Preclinical studies using α7 nAChR orthosteric agonists as well as type I and II PAMs; and 4) Where successful translational testing has occurred for particular compounds and what is still required. It is clear that further assessments differentiating the role of partial and full agonists vs. type I or II PAMs are required in tests with translational validity for those used in humans. Presynaptic localization of α7 nAChR also supports assessing a wide dose range of α7 nAChR and may explain U-shaped dose responses observed in orthosteric agonist studies (see section 3.1). Wide dose response ranges may be needed given that higher doses of orthosteric agonists can be deleterious to rodent performance. These deleterious effects could result in adherence issues in clinical trials, although it is unclear whether chronic treatment – as will be used in clinical trials - will have the same deleterious effects. Chronic treatment results in receptor up-regulation, which may also underlie some of the beneficial effects of orthosteric agonists. Using PET or SPECT to determine α7 nAChR expression in patients in various states (see above) will also be critical given the putative importance of treatment-induced receptor up-regulation as well as the modeling of symptom domains relevant to schizophrenia. Moreover, demonstrating an α7 nAChR agonist-induced dopamine release in humans as is seen in rodents could be a vital biomarker for proof of consistency of action across species. Importantly, other nAChRs can increase dopamine release in the VTA, e.g. via α6β2 nAChR activation [197]. Nicotinic treatments may also need to be tested as more than simple add-ons to antipsychotic treatment to improve cognition alone, but possibly also as augmentation treatments to cognitive training therapies [198, 199]. In fact, given the putative role of the α7 nAChR in reward-associative learning and the use of positive feedback during such cognitive training, treatments targeted at this receptor may be ideal for augmenting cognitive training [192]
It has been previously suggested that there is a translational disconnect from preclinical to clinical studies, whereby the primary effect of α7 nAChR treatments is to improve attention in rodents, an effect not seen in humans [68]. The majority of studies leading to this critique however, are NORT or P20/N40 studies. Few claim NORT measures attention however, more likely aspects of short-term memory [200], while the P20/N40 is an analog of the P50 measure of sensory gating and is also not claimed to assess attention (see section 2.0). In fact, several positive preclinical P20/N40 effects of α7 nAChR agonists have often been replicated in human P50 studies (see section 3.3). As described above, for cognitive domains it is rare that clinical tests examine the same domain as have been tested in preclinical models. Tasks are being developed however, to assess similar cognitive domains such as attention in preclinical models as well as in man. For example, the development of cross-species tests of attention such as the mouse/rat/human sustained attention task and 5 choice continuous performance tests will provide a greater opportunity to test the translational validity such treatments. To date, α7 nAChR agonists have exhibited limited efficacy in either task [174], except the partial orthosteric agonist and 5HT3 antagonist R3487 [168]. Using such cross-species tests for cognition, and developing models for negative symptoms, may improve the translational capacity of treatment development.
Finally, it is important to note that our interest in nAChR stems from initial epidemiological studies demonstrating the preference of patients with schizophrenia to smoke at higher rates than the general population, followed by observations that smokers outperformed non-smokers in cognitive tasks. Experimental studies demonstrating that nicotine can improve attention/cognition in healthy subjects and patients with schizophrenia support the premise that the mechanism by which a treatment works remains intact in patients with schizophrenia. Hence, treatments aimed at nAChRs remain a viable target for development.
Acknowledgments
The authors would like to thank Ms. Mahalah Buell for her support. These studies were supported by NIH grants R01-MH071916 and R01-MH04228, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kraepelin E. Dementia praecox. 1896. [Google Scholar]
- 2.Andreasen NC, Flaum M, Schultz S, Duzyurek S, Miller D. Diagnosis, methodology and subtypes of schizophrenia. Neuropsychobiology. 1997;35:61–3. doi: 10.1159/000119390. [DOI] [PubMed] [Google Scholar]
- 3.Pearlson GD. Neurobiology of schizophrenia. Ann Neurol. 2000;48:556–66. [PubMed] [Google Scholar]
- 4.Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–33. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 (Suppl 9):3–8. discussion 36–42. [PubMed] [Google Scholar]
- 7.Carter CS. Applying new approaches from cognitive neuroscience to enhance drug development for the treatment of impaired cognition in schizophrenia. Schizophr Bull. 2005;31:810–5. doi: 10.1093/schbul/sbi046. [DOI] [PubMed] [Google Scholar]
- 8.Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–84. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- 9.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–47. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 10.Mintz J, Kopelowicz A. CUtLASS confirms CATIE. Arch Gen Psychiatry. 2007;64:978. doi: 10.1001/archpsyc.64.8.978-a. author reply 9–80. [DOI] [PubMed] [Google Scholar]
- 11.Floresco SB, Geyer MA, Gold LH, Grace AA. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull. 2005;31:888–94. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- 12.Dolan SL, Sacco KA, Termine A, Seyal AA, Dudas MM, Vessicchio JC, et al. Neuropsychological deficits are associated with smoking cessation treatment failure in patients with schizophrenia. Schizophr Res. 2004;70:263–75. doi: 10.1016/j.schres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62:1564–73. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwoskin LP, Pivavarchyk M, Joyce BM, Neugebauer NM, Zheng G, Zhang Z, et al. Targeting reward-relevant nicotinic receptors in the discovery of novel pharmacotherapeutic agents to treat tobacco dependence. Nebr Symp Motiv. 2009;55:31–63. doi: 10.1007/978-0-387-78748-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–72. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert DG, Gilbert BO. Personality, psychopathology, and nicotine response as mediators of the genetics of smoking. Behav Genet. 1995;25:133–47. doi: 10.1007/BF02196923. [DOI] [PubMed] [Google Scholar]
- 17.Leonard S, Gault J, Adams C, Breese CR, Rollins Y, Adler LE, et al. Nicotinic receptors, smoking and schizophrenia. Restor Neurol Neurosci. 1998;12:195–201. [PubMed] [Google Scholar]
- 18.Taiminen TJ, Salokangas RK, Saarijarvi S, Niemi H, Lehto H, Ahola V, et al. Smoking and cognitive deficits in schizophrenia: a pilot study. Addict Behav. 1998;23:263–6. doi: 10.1016/s0306-4603(97)00028-2. [DOI] [PubMed] [Google Scholar]
- 19.Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, et al. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–41. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- 20.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 21.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–43. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- 23.Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem. 2004;4:267–82. doi: 10.2174/1568026043451401. [DOI] [PubMed] [Google Scholar]
- 24.Newhouse PA, Potter AS, Dumas JA, Thiel CM. Functional brain imaging of nicotinic effects on higher cognitive processes. Biochem Pharmacol. 2011;82:943–51. doi: 10.1016/j.bcp.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingsson T, Lewis G. Investigating the association between cigarette smoking and schizophrenia in a cohort study. Am J Psychiatry. 2003;160:2216–21. doi: 10.1176/appi.ajp.160.12.2216. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Li C, Meng H, Du L, Wang Q, Wang Y, et al. Premorbid tobacco smoking is associated with later age at onset in schizophrenia. Psychiatry Res. 2010;178:461–6. doi: 10.1016/j.psychres.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 27.de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ. Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital. Schizophr Res. 2002;56:55–65. doi: 10.1016/s0920-9964(01)00192-x. [DOI] [PubMed] [Google Scholar]
- 28.Masterson E, O’Shea B. Smoking and malignancy in schizophrenia. Br J Psychiatry. 1984;145:429–32. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- 29.Wing VC, Wass CE, Soh DW, George TP. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Ann N Y Acad Sci. 2012;1248:89–106. doi: 10.1111/j.1749-6632.2011.06261.x. [DOI] [PubMed] [Google Scholar]
- 30.White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer’s disease. Psychopharmacology (Berl) 1999;143:158–65. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- 31.Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- 32.Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol. 2011;82:800–7. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- 34.Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and alpha-bungarotoxin. Mol Pharmacol. 1986;30:427–36. [PubMed] [Google Scholar]
- 35.Clarke PB. Chronic central nicotinic blockade after a single administration of the bisquaternary ganglion-blocking drug chlorisondamine. Br J Pharmacol. 1984;83:527–35. doi: 10.1111/j.1476-5381.1984.tb16517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Fukuyama H, et al. Quantification of human nicotinic acetylcholine receptors with 123I-5IA SPECT. J Nucl Med. 2004;45:1458–70. [PubMed] [Google Scholar]
- 37.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–78. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 38.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 39.Levin ED, Rezvani AH. Nicotinic-antipsychotic drug interactions and cognitive function. Exs. 2006;98:185–205. doi: 10.1007/978-3-7643-7772-4_10. [DOI] [PubMed] [Google Scholar]
- 40.Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 41.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–61. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 42.Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–43. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geerts H. alpha7 Nicotinic receptor modulators for cognitive deficits in schizophrenia and Alzheimer’s disease. Expert Opin Investig Drugs. 2012;21:59–65. doi: 10.1517/13543784.2012.633510. [DOI] [PubMed] [Google Scholar]
- 44.Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–15. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–26. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- 46.Cannon M, Jones P. Schizophrenia. J Neurol Neurosurg Psychiatry. 1996;60:604–13. doi: 10.1136/jnnp.60.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klaning U. Greater occurrence of schizophrenia in dizygotic but not monozygotic twins. Register-based study. Br J Psychiatry. 1999;175:407–9. doi: 10.1192/bjp.175.5.407. [DOI] [PubMed] [Google Scholar]
- 48.van Os J, McGuffin P. Can the social environment cause schizophrenia? Br J Psychiatry. 2003;182:291–2. doi: 10.1192/bjp.182.4.291. [DOI] [PubMed] [Google Scholar]
- 49.Allen NC, Bagade S, McQueen MB, Ioannidis JPA, Kavvoura FK, Khoury MJ, et al. Systemic Meta-Analyses and field synopsis of genetic association studies in schizophrenia: The SZGene database. Nature Genetics. 2008;40:827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 50.Wallace TL, Bertrand D. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin Ther Targets. 2013;17:139–55. doi: 10.1517/14728222.2013.736498. [DOI] [PubMed] [Google Scholar]
- 51.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew SV, Law AJ, Lipska BK, Davila-Garcia MI, Zamora ED, Mitkus SN, et al. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet. 2007;16:2921–32. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- 53.Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–40. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–52. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–92. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonard KE, Roberts LJ. The effects of alcohol on the marital interactions of aggressive and nonaggressive husbands and their wives. J Abnorm Psychol. 1998;107:602–15. doi: 10.1037//0021-843x.107.4.602. [DOI] [PubMed] [Google Scholar]
- 59.Tsuang DW, Skol AD, Faraone SV, Bingham S, Young KA, Prabhudesai S, et al. Examination of genetic linkage of chromosome 15 to schizophrenia in a large Veterans Affairs Cooperative Study sample. Am J Med Genet. 2001;105:662–8. [PubMed] [Google Scholar]
- 60.Curtis L, Blouin JL, Radhakrishna U, Gehrig C, Lasseter VK, Wolyniec P, et al. No evidence for linkage between schizophrenia and markers at chromosome 15q13-14. Am J Med Genet. 1999;88:109–12. doi: 10.1002/(sici)1096-8628(19990416)88:2<109::aid-ajmg1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–46. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 62.Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, et al. Mouse behavioral endophenotypes for schizophrenia. Brain Res Bull. 2010;83:147–61. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–7. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 64.Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiatry. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacology (Berl) 2005;183:13–9. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- 66.Wildeboer-Andrud KM, Stevens KE. The smoking cessation drug varenicline improves deficient P20-N40 inhibition in DBA/2 mice. Pharmacol Biochem Behav. 2011;100:17–24. doi: 10.1016/j.pbb.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hajos M, Rogers BN. Targeting alpha7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr Pharm Des. 2010;16:538–54. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- 68.Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–43. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 69.Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, et al. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–33. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- 70.Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 71.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 72.Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–82. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- 73.Thomsen MS, Weyn A, Mikkelsen JD. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011;13:701–7. doi: 10.1111/j.1399-5618.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- 74.Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Davis LW, Waller JL. Chronic exposure to typical or atypical antipsychotics in rodents: temporal effects on central alpha7 nicotinic acetylcholine receptors. Neuroscience. 2005;136:519–29. doi: 10.1016/j.neuroscience.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Marder SR. Drug initiatives to improve cognitive function. J Clin Psychiatry. 2006;67 (Suppl 9):31–5. discussion 6–42. [PubMed] [Google Scholar]
- 76.Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–7. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toyohara J, Wu J, Hashimoto K. Recent development of radioligands for imaging alpha7 nicotinic acetylcholine receptors in the brain. Curr Top Med Chem. 2010;10:1544–57. doi: 10.2174/156802610793176828. [DOI] [PubMed] [Google Scholar]
- 79.Horti AG, Ravert HT, Gao Y, Holt DP, Bunnelle WH, Schrimpf MR, et al. Synthesis and evaluation of new radioligands [(11)C]A-833834 and [(11)C]A-752274 for positron-emission tomography of alpha7-nicotinic acetylcholine receptors. Nucl Med Biol. 2013;40:395–402. doi: 10.1016/j.nucmedbio.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 81.Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, et al. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48:1829–35. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- 82.Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–14. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, et al. Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–34. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Waller JL. Chronic treatment with first or second generation antipsychotics in rodents: effects on high affinity nicotinic and muscarinic acetylcholine receptors in the brain. Neuroscience. 2006;140:1277–87. doi: 10.1016/j.neuroscience.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 85.Bencherif M, Stachowiak MK, Kucinski AJ, Lippiello PM. Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med Hypotheses. 2012;78:594–600. doi: 10.1016/j.mehy.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 86.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 87.Quik M, Polonskaya Y, Gillespie A, Jakowec M, Lloyd GK, Langston JW. Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J Comp Neurol. 2000;425:58–69. doi: 10.1002/1096-9861(20000911)425:1<58::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 88.Nirogi R, Kandikere V, Bhyrapuneni G, Saralaya R, Muddana N, Komarneni P. Methyllycaconitine: a non-radiolabeled ligand for mapping alpha7 neuronal nicotinic acetylcholine receptors - in vivo target localization and biodistribution in rat brain. J Pharmacol Toxicol Methods. 2012;66:22–8. doi: 10.1016/j.vascn.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Machaalani R, Kashi PK, Waters KA. Distribution of nicotinic acetylcholine receptor subunits alpha7 and beta2 in the human brainstem and hippocampal formation. J Chem Neuroanat. 2010;40:223–31. doi: 10.1016/j.jchemneu.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Sydserff S, Sutton EJ, Song D, Quirk MC, Maciag C, Li C, et al. Selective alpha7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem Pharmacol. 2009;78:880–8. doi: 10.1016/j.bcp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Thomsen MS, Hay-Schmidt A, Hansen HH, Mikkelsen JD. Distinct neural pathways mediate alpha7 nicotinic acetylcholine receptor-dependent activation of the forebrain. Cereb Cortex. 2010;20:2092–102. doi: 10.1093/cercor/bhp283. [DOI] [PubMed] [Google Scholar]
- 92.Castner SA, Smagin GN, Piser TM, Wang Y, Smith JS, Christian EP, et al. Immediate and sustained improvements in working memory after selective stimulation of alpha7 nicotinic acetylcholine receptors. Biol Psychiatry. 2011;69:12–8. doi: 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 93.Quarta D, Naylor CG, Barik J, Fernandes C, Wonnacott S, Stolerman IP. Drug discrimination and neurochemical studies in alpha7 null mutant mice: tests for the role of nicotinic alpha7 receptors in dopamine release. Psychopharmacology (Berl) 2009;203:399–410. doi: 10.1007/s00213-008-1281-x. [DOI] [PubMed] [Google Scholar]
- 94.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 95.Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–52. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–59. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- 97.Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–41. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 98.Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–64. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albuquerque EX, Pereira EF, Braga MF, Matsubayashi H, Alkondon M. Neuronal nicotinic receptors modulate synaptic function in the hippocampus and are sensitive to blockade by the convulsant strychnine and by the anti-Parkinson drug amantadine. Toxicol Lett. 1998;102–103:211–8. doi: 10.1016/s0378-4274(98)00309-9. [DOI] [PubMed] [Google Scholar]
- 101.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–95. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Konradsson-Geuken A, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, et al. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–82. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–22. doi: 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Erhardt S, Schwieler L, Engberg G. Kynurenic acid and schizophrenia. Adv Exp Med Biol. 2003;527:155–65. doi: 10.1007/978-1-4615-0135-0_18. [DOI] [PubMed] [Google Scholar]
- 105.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–60. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 106.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 107.Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–68. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- 108.Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–54. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 109.Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–65. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hunter BE, de Fiebre CM, Papke RL, Kem WR, Meyer EM. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci Lett. 1994;168:130–4. doi: 10.1016/0304-3940(94)90433-2. [DOI] [PubMed] [Google Scholar]
- 111.Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2009;29:8805–15. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, et al. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011;224:15–22. doi: 10.1016/j.bbr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 113.Surmeier DJ, Plotkin J, Shen W. Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr Opin Neurobiol. 2009;19:621–8. doi: 10.1016/j.conb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Human neural learning depends on reward prediction errors in the blocking paradigm. J Neurophysiol. 2006;95:301–10. doi: 10.1152/jn.00762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, et al. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–9. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 117.Enomoto K, Matsumoto N, Nakai S, Satoh T, Sato TK, Ueda Y, et al. Dopamine neurons learn to encode the long-term value of multiple future rewards. Proc Natl Acad Sci U S A. 2011;108:15462–7. doi: 10.1073/pnas.1014457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glimcher PW. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci U S A. 2011;108 (Suppl 3):15647–54. doi: 10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–64. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25:86–97. doi: 10.1037/a0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weiler JA, Bellebaum C, Brune M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23:571–80. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- 122.Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 123.Keefe RS, Vinogradov S, Medalia A, Silverstein SM, Bell MD, Dickinson D, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37:1057–65. doi: 10.1093/schbul/sbq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69:424–31. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerfen CR, Engber TM. Molecular neuroanatomic mechanisms of Parkinson’s disease: a proposed therapeutic approach. Neurol Clin. 1992;10:435–49. [PubMed] [Google Scholar]
- 126.Morris G, Schmidt R, Bergman H. Striatal action-learning based on dopamine concentration. Exp Brain Res. 2010;200:307–17. doi: 10.1007/s00221-009-2060-6. [DOI] [PubMed] [Google Scholar]
- 127.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47 (Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 129.Ortiz O, Delgado-Garcia JM, Espadas I, Bahi A, Trullas R, Dreyer JL, et al. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a−/− mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010;30:12288–300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Granado N, Ortiz O, Suarez LM, Martin ED, Cena V, Solis JM, et al. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb Cortex. 2008;18:1–12. doi: 10.1093/cercor/bhm026. [DOI] [PubMed] [Google Scholar]
- 131.Matthies H, Becker A, Schroeder H, Kraus J, Hollt V, Krug M. Dopamine D1-deficient mutant mice do not express the late phase of hippocampal long-term potentiation. Neuroreport. 1997;8:3533–5. doi: 10.1097/00001756-199711100-00023. [DOI] [PubMed] [Google Scholar]
- 132.Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- 133.Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, et al. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology. 2007;52:634–45. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 134.Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in alpha7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–33. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav Brain Res. 2009;196:207–13. doi: 10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–16. [PMC free article] [PubMed] [Google Scholar]
- 137.Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional Processing in C57BL/6J Mice Exposed to Developmental Vitamin D Deficiency. PLoS One. 2012;7:e35896. doi: 10.1371/journal.pone.0035896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Turner KM, Young JW, McGrath JJ, Eyles DW, Burne TH. Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behav Brain Res. 2013;242:47–53. doi: 10.1016/j.bbr.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 139.Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012;140:175–84. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lendvai B, Vizi ES. Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev. 2008;88:333–49. doi: 10.1152/physrev.00040.2006. [DOI] [PubMed] [Google Scholar]
- 141.Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Brain Res Rev. 1999;30:219–35. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 142.Werkheiser JL, Sydserff S, Hubbs SJ, Ding M, Eisman MS, Perry D, et al. Ultra-low exposure to alpha-7 nicotinic acetylcholine receptor partial agonists elicits an improvement in cognition that corresponds with an increase in alpha-7 receptor expression in rodents: implications for low dose clinical efficacy. Neuroscience. 2011;186:76–87. doi: 10.1016/j.neuroscience.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 143.Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, et al. 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharmacology. 2002;43:374–84. doi: 10.1016/s0028-3908(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 144.Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, et al. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A. 2007;104:8059–64. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 146.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. 2011;82:915–30. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gronlien JH, Hakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, et al. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–24. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 148.Thomsen MS, El-Sayed M, Mikkelsen JD. Differential immediate and sustained memory enhancing effects of alpha7 nicotinic receptor agonists and allosteric modulators in rats. PLoS One. 2011;6:e27014. doi: 10.1371/journal.pone.0027014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Timmermann DB, Gronlien JH, Kohlhaas KL, Nielsen EO, Dam E, Jorgensen TD, et al. An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- 150.Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Malysz J, Gronlien JH, Timmermann DB, Hakerud M, Thorin-Hagene K, Ween H, et al. Evaluation of alpha7 nicotinic acetylcholine receptor agonists and positive allosteric modulators using the parallel oocyte electrophysiology test station. Assay Drug Dev Technol. 2009;7:374–90. doi: 10.1089/adt.2009.0194. [DOI] [PubMed] [Google Scholar]
- 152.Dinklo T, Shaban H, Thuring JW, Lavreysen H, Stevens KE, Zheng L, et al. Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a novel positive allosteric modulator of the {alpha}7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 2011;336:560–74. doi: 10.1124/jpet.110.173245. [DOI] [PubMed] [Google Scholar]
- 153.Grottick AJ, Haman M, Wyler R, Higgins GA. Reversal of a vigilance decrement in the aged rat by subtype-selective nicotinic ligands. Neuropsychopharmacology. 2003;28:880–7. doi: 10.1038/sj.npp.1300102. [DOI] [PubMed] [Google Scholar]
- 154.Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- 155.Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–37. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- 156.Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP. A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods. 1995;61:113–8. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- 157.Cilia J, Cluderay JE, Robbins MJ, Reavill C, Southam E, Kew JN, et al. Reversal of isolation-rearing-induced PPI deficits by an alpha7 nicotinic receptor agonist. Psychopharmacology (Berl) 2005;182:214–9. doi: 10.1007/s00213-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 158.Van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R. AR-R 17779 improves social recognition in rats by activation of nicotinic alpha7 receptors. Psychopharmacology (Berl) 2004;172:375–83. doi: 10.1007/s00213-003-1668-7. [DOI] [PubMed] [Google Scholar]
- 159.O’Neill HC, Rieger K, Kem WR, Stevens KE. DMXB, an alpha7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology (Berl) 2003;169:332–9. doi: 10.1007/s00213-003-1482-2. [DOI] [PubMed] [Google Scholar]
- 160.Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, et al. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. J Pharmacol Exp Ther. 2010;334:875–86. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- 161.Othman AA, Lenz RA, Zhang J, Li J, Awni WM, Dutta S. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective alpha7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J Clin Pharmacol. 2011;51:512–26. doi: 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- 162.Simpson K, Spencer CM, McClellan KJ. Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2000;59:1297–315. doi: 10.2165/00003495-200059060-00008. [DOI] [PubMed] [Google Scholar]
- 163.Hashimoto K, Fujita Y, Ishima T, Hagiwara H, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of alpha7 nicotinic receptors. Eur J Pharmacol. 2006;553:191–5. doi: 10.1016/j.ejphar.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 164.Jentsch JD. Pre-clinical models of cognitive dysfunction in schizophrenia: new avenues to addressing unmet needs. Clinical Neuroscince Research. 2003;3:303–13. [Google Scholar]
- 165.Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol Ther. 2010 doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 166.Kohnomi S, Suemaru K, Goda M, Choshi T, Hibino S, Kawasaki H, et al. Ameliorating effects of tropisetron on dopaminergic disruption of prepulse inhibition via the alpha(7) nicotinic acetylcholine receptor in Wistar rats. Brain Res. 2010;1353:152–8. doi: 10.1016/j.brainres.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 167.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 168.Rezvani AH, Kholdebarin E, Brucato FH, Callahan PM, Lowe DA, Levin ED. Effect of R3487/MEM3454, a novel nicotinic alpha7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:269–75. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 169.Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, et al. alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci. 2009;29:539–50. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- 170.Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 2010;471:114–8. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 171.Hauser TA, Kucinski A, Jordan KG, Gatto GJ, Wersinger SR, Hesse RA, et al. TC-5619: an alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem Pharmacol. 2009;78:803–12. doi: 10.1016/j.bcp.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chan WK, Wong PT, Sheu FS. Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–9. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 173.McLean SL, Grayson B, Idris NF, Lesage AS, Pemberton DJ, Mackie C, et al. Activation of alpha7 nicotinic receptors improves phencyclidine-induced deficits in cognitive tasks in rats: implications for therapy of cognitive dysfunction in schizophrenia. Eur Neuropsychopharmacol. 2011;21:333–43. doi: 10.1016/j.euroneuro.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 174.Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013;240:119–33. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, et al. EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 176.O’Donnell CJ, Rogers BN, Bronk BS, Bryce DK, Coe JW, Cook KK, et al. Discovery of 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane (CP-810,123), a novel alpha 7 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders in schizophrenia: synthesis, SAR development, and in vivo efficacy in cognition models. J Med Chem. 2010;53:1222–37. doi: 10.1021/jm9015075. [DOI] [PubMed] [Google Scholar]
- 177.Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, Jegham S, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- 178.Thomsen MS, Mikkelsen JD. Type I and II positive allosteric modulators differentially modulate agonist-induced up-regulation of alpha7 nicotinic acetylcholine receptors. J Neurochem. 2012;123:73–83. doi: 10.1111/j.1471-4159.2012.07876.x. [DOI] [PubMed] [Google Scholar]
- 179.Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 2012;220:627–37. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–10. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Callahan PM, Hutchings EJ, Kille NJ, Chapman JM, Terry AV., Jr Positive allosteric modulator of alpha 7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–12. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McLean SL, Idris NF, Grayson B, Gendle DF, Mackie C, Lesage AS, et al. PNU-120596, a positive allosteric modulator of alpha7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol. 2012;26:1265–70. doi: 10.1177/0269881111431747. [DOI] [PubMed] [Google Scholar]
- 183.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–8. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 184.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69:7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, et al. A Randomized Exploratory Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) for Cognitive Enhancement in Schizophrenia. Neuropsychopharmacology. 2013;38:968–75. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shiina A, Shirayama Y, Niitsu T, Hashimoto T, Yoshida T, Hasegawa T, et al. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry. 2010;9:27. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang XY, Liu L, Liu S, Hong X, Chen da C, Xiu MH, et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012;169:974–81. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]
- 189.Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, et al. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3064-2. [DOI] [PubMed] [Google Scholar]
- 190.EnVivo. 2011 http://www.envivopharma.com/pipeline-nicotinic.php.
- 191.Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, et al. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study. Neuropharmacology. 2013;64:197–204. doi: 10.1016/j.neuropharm.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 192.Young JW, Amitai N, Geyer MA. Behavioral animal models to assess pro-cognitive treatments for schizophrenia. In: Geyer MA, Gross G, editors. Hanbook of Experimental Pharmacology. Heidelberg: Springer; 2012. [DOI] [PubMed] [Google Scholar]
- 193.Young JW, Jentsch JD, Bussey TJ, Wallace TL, Hutcheson DM. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–40. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- 195.Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem. 2004;90:1094–103. doi: 10.1111/j.1471-4159.2004.02574.x. [DOI] [PubMed] [Google Scholar]
- 196.Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C, et al. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35:197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA. Ventral tegmental area alpha6beta2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hahn B, Gold JM, Buchanan RW. The potential of nicotinic enhancement of cognitive remediation training in schizophrenia. Neuropharmacology. 2013;64:185–90. doi: 10.1016/j.neuropharm.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 199.Swerdlow NR. Are we studying and treating schizophrenia correctly? Schizophr Res. 2011;130:1–10. doi: 10.1016/j.schres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]