Abstract
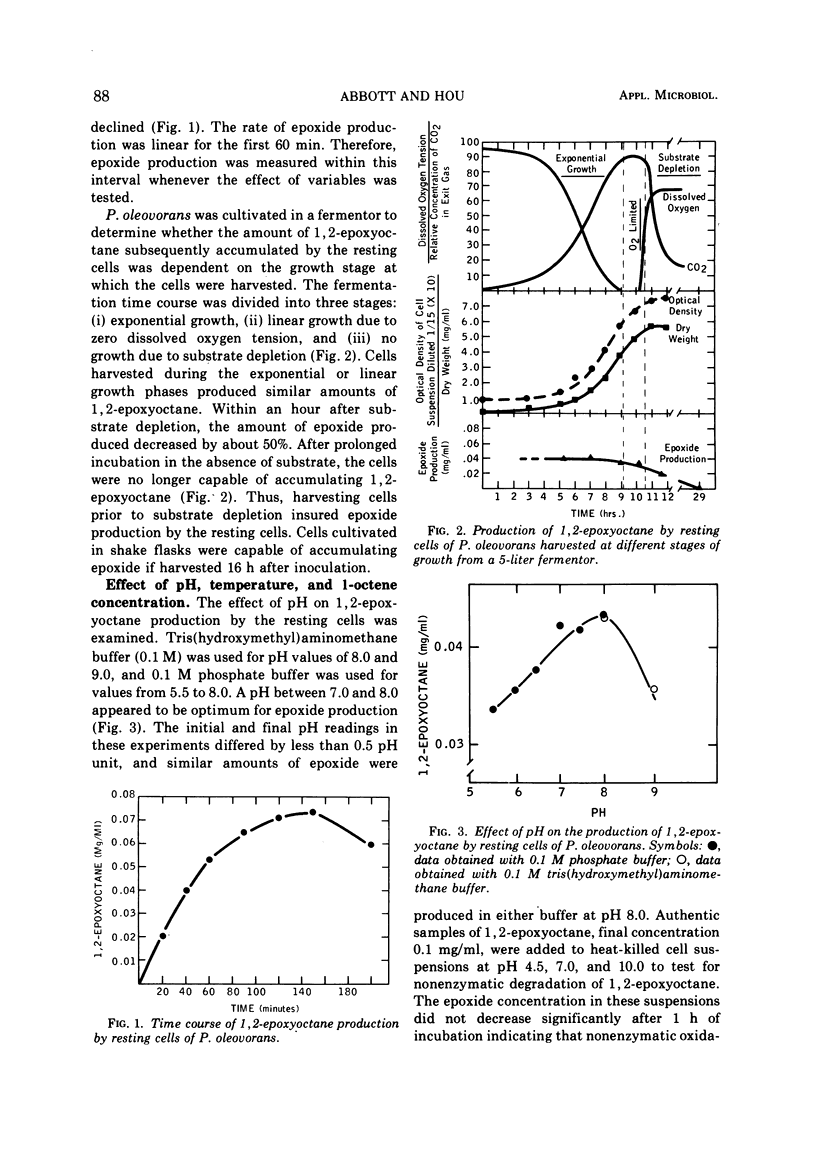
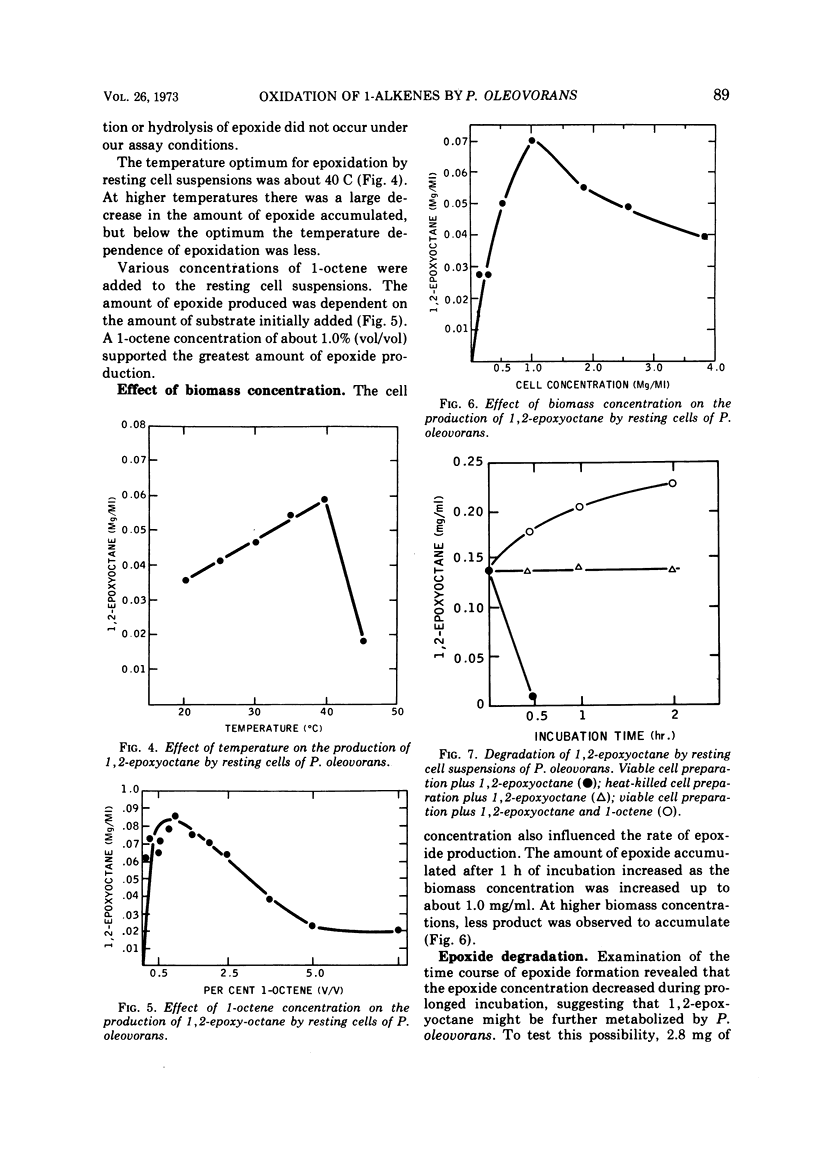
Resting cells of Pseudomonas oleovorans PO-1R that had been grown on octane oxidized 1-alkenes containing 6 to 12 carbon atoms and 1,7-octadiene to their corresponding 1,2-epoxides. The microorganism was capable of growing on 1-octene but not on 1,7-octadiene as a sole carbon source. The optimal temperature, pH, and 1-octene concentration for 1,2-epoxyoctane production by the resting cells were 34 to 40 C, pH 7 to 8, and 1.5 mg of 1-octene per ml, respectively. Epoxide concentration reached a maximum after 150 min of incubation and subsequently declined. In the absence of 1-octene, the epoxide was metabolized readily by the resting cells. The amount of 1,2-epoxyoctane produced was dependent on the initial cell concentration. With larger cell populations, the amount of epoxide present after 60 min of incubation was less than the amount observed at lower population densities after the same time period. This relationship was attributed to the rapid depletion of 1-octene at high biomass concentrations and the resultant early initiation of epoxide degradation by the resting cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Enzymatic -oxidation. VI. Isolation of homogeneous reduced diphosphopyridine nucleotide-rubredoxin reductase. J Biol Chem. 1972 Apr 10;247(7):2109–2116. [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Degradation of hydrocarbons by members of the genus Candida 3. Oxidative intermediates from 1-hexadecene and 1-heptadecene by Candida lipolytica. J Bacteriol. 1968 Oct;96(4):1115–1123. doi: 10.1128/jb.96.4.1115-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Fermentation of 1-hexadecene by Candida lipolytica. Biotechnol Bioeng. 1969 May;11(3):427–440. doi: 10.1002/bit.260110314. [DOI] [PubMed] [Google Scholar]
- Lode E. T., Coon M. J. Enzymatic omega-oxidation. V. Forms of Pseudomonas oleovorans rubredoxin containing one or two iron atoms: structure and function in omega-hydroxylation. J Biol Chem. 1971 Feb 10;246(3):791–802. [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. I. Alkene epoxidation by the -hydroxylation system of Pseudomonas oleovorans. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1230–1234. doi: 10.1016/0006-291x(72)90842-x. [DOI] [PubMed] [Google Scholar]
- May S. W., Abbott B. J. Enzymatic epoxidation. II. Comparison between the epoxidation and hydroxylation reactions catalyzed by the -hydroxylation system of Pseudomonas oleovorans. J Biol Chem. 1973 Mar 10;248(5):1725–1730. [PubMed] [Google Scholar]
- McKenna E. J., Coon M. J. Enzymatic omega-oxidation. IV. Purification and properties of the omega-hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1970 Aug 10;245(15):3882–3889. [PubMed] [Google Scholar]
- Peterson J. A., Basu D., Coon M. J. Enzymatic omega-oxidation. I. Electon carriers in fatty acid and hydrocarbon hydroxylation. J Biol Chem. 1966 Nov 10;241(21):5162–5164. [PubMed] [Google Scholar]
- Peterson J. A., Coon M. J. Enzymatic omega-oxidation. 3. Purification and properties of rubredoxin, a component of the omega-hydroxylation system of Pseudomonas oleovorans. J Biol Chem. 1968 Jan 25;243(2):329–334. [PubMed] [Google Scholar]
- Peterson J. A., Kusunose M., Kusunose E., Coon M. J. Enzymatic omega-oxidation. II. Function of rubredoxin as the electron carrier in omega-hydroxylation. J Biol Chem. 1967 Oct 10;242(19):4334–4340. [PubMed] [Google Scholar]
- SIH C. J. Microbiological epoxidation of steroids. J Bacteriol. 1962 Aug;84:382–382. doi: 10.1128/jb.84.2.382-382.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIJSSE G. J., van der LINDEN A. Pathways of hydrocarbon dissimilation by a Pseudomonas as revealed by chloramphenicol. Antonie Van Leeuwenhoek. 1963;29:89–100. doi: 10.1007/BF02046042. [DOI] [PubMed] [Google Scholar]
- VAN DER LINDEN A. C. EPOXIDATION OF ALPHA-OLEFINS BY HEPTANE-GROWN PSEUDOMONAS CELLS. Biochim Biophys Acta. 1963 Sep 3;77:157–159. doi: 10.1016/0006-3002(63)90484-0. [DOI] [PubMed] [Google Scholar]


