Abstract
Significance: Oxidative stress is a common denominator of various risk factors contributing to endothelial dysfunction and vascular diseases. Accumulated evidence suggests that sirtuin 1 (SIRT1) expression and/or activity is impaired by supraphysiological levels of oxidative stress, which in turn disrupts endothelial homeostasis. Recent Advances: Several microRNAs (miRNAs) are induced by oxidative stress and termed as oxidative stress-responsive miRNAs. They may play a role linking the imbalanced redox state with dysregulated SIRT1. Critical Issues: This review summarizes recent findings on oxidative stress-responsive miRNAs and their involvement in SIRT1 regulation. Because of the unique characteristics of miRNAs, research in this new area requires an integrative approach that combines bioinformatics and experimental validation. Thus, a research strategy is discussed to identify the SIRT1-regulating miRNAs under oxidative stress and their functional outcomes in relation to endothelial dysfunction. Additionally, the miRNAs implicated in vascular diseases such as atherosclerosis and abdominal aortic aneurysms are discussed along with the translational potential and challenges of using miRNAs and its analogs as therapeutic agents. Future Directions: Although at its infancy, research on oxidative stress-responsive miRNAs and their regulation of SIRT1 may provide new insights in understanding vascular disorders. Moreover, systematic approaches integrating in silico, in vitro, and in vivo observations can be useful tools in revealing the pathways modulating endothelial biology. Antioxid. Redox Signal. 19, 1522–1538.
Introduction
The imposition of excessive oxidative stress upon the vasculature is associated with many, if not all, pathophysiological processes leading to vascular impairments. Although a permissible level of free radicals may trigger protective adaptation, excessive oxidative stress often predisposes vasculature to various disorders. Pathophysiological conditions, including hyperglycemia, hyperlipidemia, hypertension, aging, and others, can all cause and/or exacerbate oxidative stress in the endothelium. The elevated levels of reactive oxygen species (ROS) alter the activity of vascular endothelial cells (ECs) through mechanisms at both the transcriptional and post-translational levels. High ROS levels damage mitochondrial DNA (mtDNA) and impair mitochondrial biogenesis, which subsequently inhibit the antioxidant defense processes (74). Additionally, supraphysiological levels of ROS induce the expression of the proinflammatory genes such as monocyte chemotactic protein-1, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1, via the nuclear factor-kappaB (NF-κB) pathways (24,143). At the post-translational level, excessive ROS can block the phosphorylation and activation of endothelial nitric oxide synthase (eNOS) (157). Excessive ROS can also cause dysfunctional eNOS through in part reactive nitrogen species (RNS), that is, peroxynitrite (ONOO−), a coupling product of O2− and NO. Peroxynitrite-uncoupled eNOS generates O2− rather than NO, which further augments oxidative stress. Collectively, these detrimental events lead to endothelial dysfunction hallmarked by attenuated NO bioavailability and an increased inflammatory response.
Silent mating-type information regulation 2 homolog (sirtuin 1 or SIRT1) is an NAD+-dependent class III histone deacetylase that is upregulated under caloric restriction (CR), extending the lifespan of many organisms (60,128,147). Activating via deacetylation of a myriad of stress-responsive transcription factors (TFs), coregulators, and enzymes, SIRT1 plays a key role in metabolic control at cellular, tissue, and whole-body levels (133). Because of its profound antioxidative and anti-inflammatory effects, a growing body of work focuses on SIRT1 in EC biology. In general, elevation of SIRT1 expression and/or activation exerts beneficial effects on ECs. In contrast, stress conditions such as excessive ROS and aging decrease SIRT1 expression/activity, which leads to endothelial dysfunction (114).
MicroRNAs (miRNAs), ranging from 18 to 24 nucleotides in length, belong to a class of noncoding small RNAs. The precursors of miRNAs, that is, primary transcripts (pri-miR), are induced transcriptionally in response to environmental stimuli in a manner similar to mRNA. miRNAs generally downregulate gene expression by binding to the complementary sequences in their mRNA targets at the 3′-untranslated region (3′UTR). As of yet, over 1500 human miRNAs have been reported (www.mirbase/org). Several miRNAs, including miR-200 and miR-34a, are induced by oxidative stress in various cell types (55,95), and therefore can be designated oxidative stress-responsive miRNAs. Because SIRT1 expression can be regulated at levels other than transcription and post-translation (1,164), it is probable that environmental stimuli such as oxidative stress can modulate SIRT1 through miRNAs as well.
As summarized in Table 1, alterations in miRNAs, SIRT1, and its regulators have been demonstrated in multiple conditions that involve oxidative stress and predispose to cardiovascular diseases. This review focuses on the interplay between miRNAs and SIRT1 in the context of oxidative stress-induced endothelial dysfunction, the pertinent approaches for experimental validation, and the translational implications of this supposition.
Table 1.
Association of Cardiovascular Risk Factors and Oxidative Stress, Alterations in Sirtuin 1 (Network) and microRNA, and Relevant Animal Models
| Conditions | Correlation with oxidative stress | Alteration in SIRT1 (network) | Alteration in miRNA | Relevant animal models |
|---|---|---|---|---|
| Hyperglycemia | Auto-oxidation of glucose, glycation of proteins AGEs, PKC activation of mitochondrial NOX (67) | SIRT1↓(109) p66shc↑(173) PARP↑(85) p53(+) (38) AMPK (−) (106) |
miR-150↑(33) miR-133a↑(39) miR-146a↑a(37) miR-103/107↑(148) miR-21↑a (30) miR-130, 132, 212, 335↑(36) miR-15a↑(142) miR93↑(90) miR-125a↑(50) miR-221↑(80) |
STZ-induced diabetes, db/db mice, GK nonobese Type 2 diabetic rats |
| Hyperlipidemia | Oxidation of LDL (ox-LDL), including lipids and proteins in subendothelial space (27) | SIRT1↓(76) PARP↑(48) |
miR-33↑(122) miR-181↑(159) miR-27b↑(155) miR-122, miR-370↑(42) miR-126↓(140) miR-155↓(32) Let-7g↓(19) |
LDLR−/−, ApoE−/−, ob/ob mice, HFD |
| Hypertension | AT1R-activation of NOX (92) | NAMPT↑(10) PARP1(+)(144) p53↑(87) |
miR-155↑(141) miR145↑(18) miR-328↑(45) miR-21↑a (111) miR-17↑(115) miR-424, miR-503↓(65) miR-150↓(124) |
AngII-infusion, high-salt diet |
| Aging/senescence | Electron leakage from mitochondria and mitochondrial DNA damage are contributing factors to oxidative stress (69) | SIRT1↓(116) p66shc(+)(100) |
miR-34↑(58) miR-371, miR-365-5p, miR-29c, miR-499, Let-7f↑(156) miR-10a, miR-21↑a (175) miR-200c↑a (95) miR-146a↑(153) miR-217(99) miR-17, miR-19b, miR-20a, miR-106a↓(47) |
p66shc−/− mice shown increased lifespan by 30% (100) Ames-dwarf mice has delayed aging (8) |
These miRNAs have been identified as H2O2-inducible miRNAs in vitro.
↑: upregulation;↓: downregulated; (+): activation; and (−): inactivation; SIRT1, sirtuin 1; miRNAs, microRNAs; AGEs, advanced glycation endproducts; PKC, protein kinase C; NOX, NAD(P)H oxidase; PARP, poly-ADP ribose polymerase; AMPK, AMP-activated protein kinase; STZ, streptozotocin; GK, Goto-Kakizaki; LDL, low-density lipoprotein; HFD, high-fat diet; AT1R, angiotensin II type 1 receptor; NAMPT, nicotinamide phosphoribosyl transferase; H2O2, hydrogen peroxide.
Oxidative Stress and SIRT1 in ECs
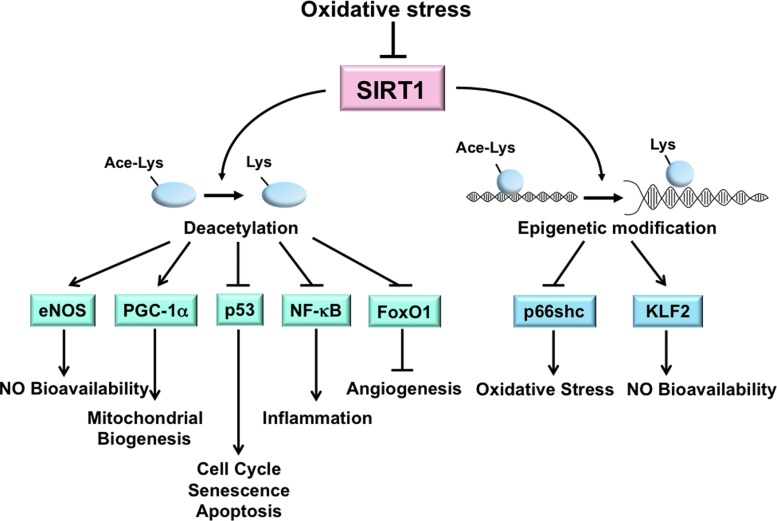
SIRT1 is highly sensitive to the cellular redox states (14). In ECs, SIRT1 activation can alleviate oxidative stress, prevent endothelial senescence, increase eNOS-derived NO bioavailability, and promote mitochondrial biogenesis (3,21,97,176). Transgenic mice with EC-specific SIRT1 overexpression on an ApoE−/− background ameliorates atherosclerosis, demonstrating the atheroprotective capacity of SIRT1 (170). Many of the effects of SIRT1 are due, in part, to its deacetylation of multiple targets, including eNOS, peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α), p53, Forkhead Box O family (FoxO), and NF-κB (15,91,97,127,154,167) (Fig. 1). Through the deacetylation of eNOS at Lys-496 and Lys-506, SIRT1 increases eNOS activity and hence contributes to endothelial homeostasis (97). The atheroprotective shear stress induces SIRT1 in ECs. In concert with AMP-activated protein kinase (AMPK) phosphorylation of eNOS, SIRT1 deacetylation of eNOS further enhances NO bioavailability (21). SIRT1 expression is upregulated in vivo in response to CR or resveratrol, an SIRT1 activator, and this is correlated with enhanced eNOS activity and reduced oxidative stress (103,125).
FIG. 1.
Oxidative stress-reduced SIRT1 expression or activity contributes to dysfunctional endothelium. SIRT1 directly deacetylates multiple targets, including eNOS, PGC-1α, p53, NF-κB, and FoxO1, and regulates the transcription of proteins such as p66shc and KLF2 via epigenetic modifications (i.e., histone deacetylation) in ECs. In sum, the functional outcome is enhanced NO bioavailability, mitochondrial biogenesis, and angiogenesis, but decreased senescence, apoptosis, inflammation, and oxidative stress. The increased oxidative stress associated with various pathophysiological conditions impairs SIRT1 deacetylation of these targets resulting in a dysfunctional endothelium. SIRT1, sirtuin 1; eNOS, endothelial nitric oxide synthase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1-alpha; p53, tumor protein 53; NF-κB, nuclear factor-kappaB; FoxO1, Forkhead box O family 1; KLF2, Krüppel-like factor 2; ECs, endothelial cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
PGC1α is a peroxisome proliferator-activated receptor-γ transcriptional coactivator and functions as a metabolic regulator. SIRT1 deacetylation of PGC1α is linked to mitochondrial biogenesis, which is essential for the maintenance of cellular redox homeostasis (99). PGC1α increases the level of mtDNA and upregulates many antioxidant enzymes, including manganese superoxide dismutase (MnSOD) and glutathione peroxidase 1, through PGC1α modulation of nuclear respiratory factors and mitochondrial transcription factor A (53,161). In ECs, shear stress-induced SIRT1 causes PGC1α induction and activation, and a concomitant increase in mitochondrial biogenesis (21). Additionally, SIRT1 induction by resveratrol or sera from CR rats significantly reduces mitochondrial ROS levels in cultured ECs (26,150).
SIRT1 can also regulate the cellular redox state through deacetylation of nuclear factor erythroid 2-related factor 2 (Nrf2), a master TF in the cellular response to oxidative stress. Under oxidative stress, Nrf2 binds to the antioxidant-responsive elements (AREs) in the promoter regions of many antioxidative genes, thus transactivating their expression (49,57). Resveratrol activates Nrf2 in cultured human coronary arterial ECs, which is associated with the upregulation of several Nrf2 target genes such as NAD(P)H:quinone oxidoreductase 1, γ-glutamylcysteine synthetase, and heme oxygenase-1 (149). However, SIRT1 probably deacetylates Nrf2 at Lys-588 and Lys-591 to promote its translocation from the nucleus to the cytoplasm in HepG2 cells. Such modification results in decreased binding of Nrf2 to AREs and therefore the inhibition of the expression of antioxidative genes. This regulation is to counteract the acetylation of Nrf2 by cAMP response element-binding protein (CREB)-binding protein (CBP), which results in nuclear retention of Nrf2 and its transactivation of the ARE-containing genes (63). Clearly, differential regulation of Nrf2 by SIRT1 in ECs warrants further examination.
SIRT1 activity also antagonizes the oxidative stress-induced senescence of ECs, largely through its negative modulation of p53 by deacetylation of Lys-373, Lys-382, and Lys-320. Such a response counteracts the effects of p300 and p300/CBP-associated factor (PCAF), which stabilizes p53 to promote cell cycle progression, senescence, and apoptosis (91,110). In line with this negative regulation of p53, the level of SIRT1 is related to premature senescence of human umbilical vein endothelial cells (110). The protective effects of SIRT1 on ECs can also be conferred by its deacetylation of NF-κB and FoxOs. SIRT1 deacetylates RelA/p65-NF-κB at Lys-310 and suppresses the NF-κB-mediated inflammatory and apoptotic processes (167). By deacetylating FoxO1 or FoxO3, SIRT1 increases the cell resistance to oxidative stress and promotes angiogenesis (15).
Because hypoxia affects the cellular redox state, SIRT1 is also likely to be modulated by changes in the NAD+/NADH ratio. Dioum et al. (31) demonstrated that SIRT1 activated by hypoxia deacetylates hypoxia-inducible factor (HIF)-2α to transactivate erythropoietin, an HIF-2α target gene. This is preceded by acetylation of HIF-2α by CBP, both of which are required for the maximal HIF-2 signaling under hypoxia (20). Conversely, HIF-1α has been shown to be inhibited by SIRT1 deacetylation (81). Because SIRT1 is downregulated under hypoxia (169), HIF-1α acetylation by PCAF should be enhanced and thus augmenting the transactivation of the HIF-1α target genes. These findings suggest a negative role of SIRT1 in tumor growth and angiogenesis. Although SIRT1 is an essential player in hypoxic response, the molecular basis for SIRT1 regulation of HIF-1α or HIF-2α remains to be determined.
Excessive oxidative stress may also cause the endothelial-to-mesenchymal transformation (EnMT), a process involved in cardiovascular development as well as in pathophysiological conditions such as pulmonary hypertension (4). Transforming growth factor-β (TGF-β) is a potent inducer of EnMT and can convert ECs to spindle-shaped α-SM actin-expressing cells. SIRT1 deacetylates Smad7 to causes its proteosomal degradation and thereby inhibit TGF-β signaling (72). Thus, SIRT1 may maintain the endothelial lineage and prevent the TGF-β-induced EnMT.
In addition to post-translational modification, SIRT1 can regulate several key molecules modulating endothelial homeostasis through transcriptional regulation. SIRT1 suppresses the transcription of the prooxidant molecule p66shc, which protects ECs from hyperglycemia-imposed oxidative damage (173). Moreover, resveratrol increases Krüppel-like factor 2 (KLF2), a critical transcriptional factor controlling eNOS expression in a SIRT1-dependent manner (44). Together, the critical role of SIRT1 in the regulation of endothelial homeostasis is manifested by its comprehensive regulation of molecules involved in the redox and inflammatory state and the endothelial phenotype. Given that SIRT1 can be regulated by miRNAs under oxidative stress, these oxidative stress-responsive miRNAs are likely to constitute the crosstalk between SIRT1 and its downstream target genes as well as between the deacetylation targets of SIRT1. For example, hypoxia-induced HIF-1 attenuates Nrf2-dependent transactivation in ECs (88). It is possible that oxidative stress-responsive miRNAs may regulate Nrf2 through SIRT1 and HIF-1.
Oxidative Stress Regulates SIRT1 Through miRNAs
Oxidative stress regulates a panel of miRNAs (95,136). Some of these miRNAs have been predicted in silico (computer-assisted bioinformatics analysis) and/or experimentally determined to target the 3′UTR of SIRT1 mRNA. In parallel to the direct regulation, miRNAs may suppress SIRT1 indirectly through the actions on SIRT1 regulators (Fig. 2). This section first focuses on miRNAs that directly target SIRT1 and then on those that indirectly regulate it. Given the unique characteristics of miRNAs, a research strategy that integrates bioinformatics predictions and experimental validation is discussed as well. Finally, because SIRT1 can regulate miRNAs through the modulation of their upstream TFs, the mechanisms by which SIRT1 regulates miRNAs under oxidative stress are discussed.
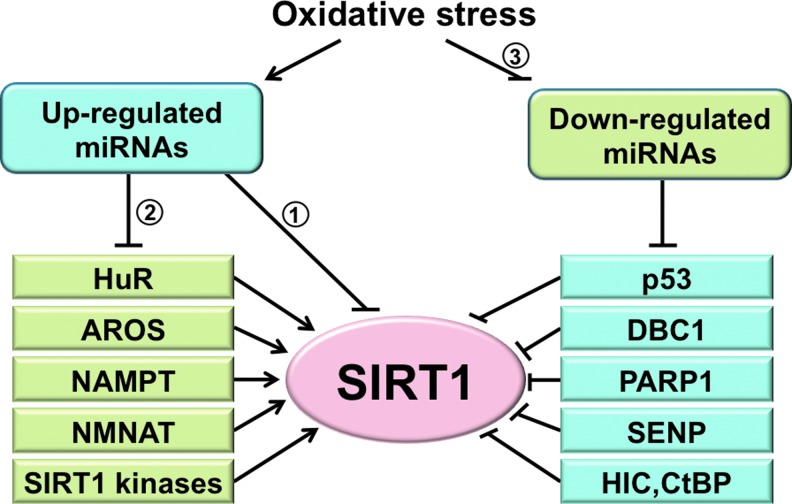
FIG. 2.
A proposed model of SIRT1 regulation by oxidative stress-responsive miRNAs. Oxidative stress may impair SIRT1 function by (1) upregulation of SIRT1-direct targeting miRNAs, (2) upregulation of miRNAs that can target the positive regulators of SIRT1, including HuR, AROS, NAMPT, NMNAT, and SIRT1 kinases, and (3) downregulation of miRNAs that target the negative regulators of SIRT1, that is, by desuppressing the SIRT1 inhibitors to cause SIRT1 inhibition. miRNAs, microRNAs; HuR, human antigen R; AROS, active regulator of SIRT1; NAMPT, nicotinamide phosphoribosyl transferase; NMNAT, nicotinamide mononucleotide adenylyl transferase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Direct targeting of SIRT1 by oxidative stress-induced miRNAs
Based on the complementary sequences of SIRT1 3′UTR, a panel of miRNAs that target SIRT1 under various physiological and pathophysiological conditions was predicted and experimentally validated. The first reported miRNA to downregulate SIRT1 during the senescence of human cell lines was miR-34a (165). The targeting SIRT1 by miR-34a was also observed in ECs and endothelial progenitor cells (EPCs) (58,171). Similarly, miR-217 was identified as an endogenous SIRT1 inhibitor, which promotes endothelial senescence (99). Several other miRNAs, including miR-181, miR-138, and miR-199, downregulate SIRT1 in various cell/tissue types. MicroRNA-181 is upregulated by a high-fat diet in the liver, resulting in decreased SIRT1 and impaired insulin sensitivity (172). Both miR-181 and miR-138 levels increase in senescent keratinocytes, and their targeting SIRT1 promotes senescence (126). In contrast, miR-199 is downregulated in hypoxic preconditioning, leading to SIRT1 desuppression and decreased apoptosis in cardiomyocytes (119). Table 2 summarizes the experimentally validated miRNAs that negatively regulate SIRT1 expression via 3′UTR targeting as well as experimental approaches employed in their validation. Although there are numerous studies on miRNA regulation of SIRT1, few studies correlated the oxidative stress-responsive miRNAs and their regulation of SIRT1 with functional consequences. Because oxidative stress is a primary cause of EC dysfunction, it is conceivable that the oxidative stress-induced miRNAs downregulate SIRT1 with dysregulated EC functions and phenotypes.
Table 2.
Summary of Experimentally Validated Sirtuin 1-Targeting microRNAs
| miRNA | Stimuli/model | Cell/tissue type | Approach | Functional consequences | TF | Reference |
|---|---|---|---|---|---|---|
| miR-9 | ↑ Glucose, ESC differentiation | Pancreatic β-cells, mESC | R; O; I | Insulin secretion, ESC differentiation | (118)(131) | |
| miR-22 | ↑ Breast cancer | Fibroblast | R; O | Senescence | (162) | |
| miR-34a | ↑ Senescence, aging, diet-induced obesity | HCT116, EC, EPC, hepatocytes |
R; O; I | Apoptosis, senescence, inhibition of angiogenesis | p53, FXR | (165)(58)(171) (75) |
| miR-93 | ↑ Aging | Rat liver | R; O | Senescence, prooxidative stress | (78) | |
| miR-132 | ↑ Low serum | Adipocytes | R; O | Proinflammatory response | (139) | |
| miR-138 | ↑ Aging | Keratinocytes | R; O | Senescence | p63 | (126) |
| miR-135, miR-204 | ↑ Differentiation | mESC | O | Differentiation | (131) | |
| miR-181 | ↑ Insulin resistance, high-fat diet, senescence, differentiation | Hepatocytes, keratinocytes, mESC | R; O; I | Insulin resistance, senescence, differentiation | (172)(126)(131) | |
| miR-195 | ↑ Free fatty acid palmitate | Cardiomyocytes | R; O; I | Increase ROS, apoptosis | (174) | |
| miR-199 | ↓ Hypoxia preconditioning, ischemia | Cardiomyocytes | R; O; I | Antiapoptosis | (119) | |
| miR-200a | ↑ Breast cancer | Epithelial cells | R; O; I | Metastasis | (34) | |
| miR-217 | ↑ Aging, atherosclerosisEthanol | EC, hepatocytes | R; O; I | Senescence, fat accumulation in the liver | (99)(168) | |
| miR-373, miR-520c | ↑ Highly aggressive cancer cells | HT1080 | R; O | Enhance migration and growth | (86) | |
| miR-449 | ↑ DNA damage, gastric cancer | HCT116, H1299 | O | Apoptosis | E2F1 | (13) |
| miR-486-5p | ↑ High glucose | Adipose tissue-derived MSC | R; O; I | Senescence | (66) | |
| miR-598 | ↑ Senescence | Adipose tissue-derived mesenchymal stem cells | R; O; I | (134) |
↑: miR upregulated and↓: miR downregulated by the stimuli/model; R, reporter assay; O, overexpression; I, inhibition; TF, transcription factor; ESC, embryonic stem cell; mESC, murine ESC; EC, endothelial cell; EPC, endothelial progenitor cell; p53, tumor protein 53; FXR, Farnesoid X receptor; p63, transformation-related protein 63; ROS, reactive oxygen species; MSC, mesenchymal stem cell.
Identification of SIRT1-targeting miRNAs under oxidative stress
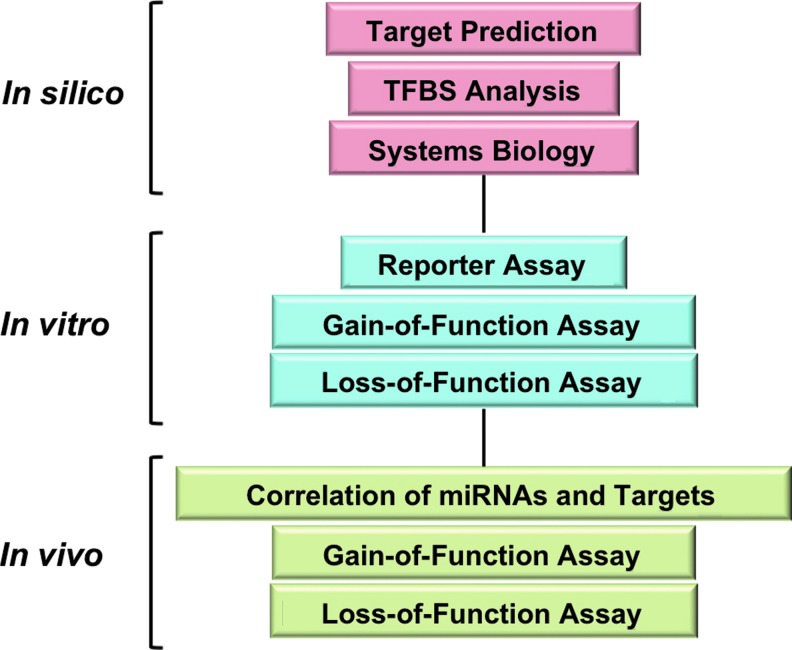
Bioinformatics algorithms have emerged as powerful tools to predict miRNAs that target SIRT1. For example, miR-92, a key regulator in neovascularization, was predicted to target SIRT1 mRNA (12). Such information is available in online databases, including microRNA.org (miRanda) (59), PicTar (70), and Targetscan (77), but it is important to note that all predictions need experimental validation. The approach to be used to study the regulation of SIRT1 by oxidative stress-responsive miRNAs, and its implication in endothelial biology arguably should combine the in silico, in vitro, and in vivo methods. An outline of such a strategy is illustrated in Figure 3, and the associated methods are listed in Table 3.
FIG. 3.
A research strategy to identify oxidative stress-responsive miRNAs. MicroRNAs of interest are predicted by in silico analyses, including target prediction and promoter analysis for putative transcription factor-binding sequences. These can be combined with systems biology approaches to map the miRNA target network. Results from in silico analyses need to be validated by in vitro and in vivo approaches consisting of the listed assays. Depending on the scope of study, this strategy may be expanded or simplified. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 3.
Approaches for Identification of Oxidative Stress-Responsive microRNAs That Target Sirtuin 1
| Approach | Purpose | Tool/method | Notes | |
|---|---|---|---|---|
| In silico | miRNA profile | Correlation of oxidative stress, miRNA and SIRT1 | miRbase; GEO datasets; literature search; experimental microarray or NGS | • Negative correlation of miRNA and SIRT1 in known conditions with oxidative stress; • MicroRNA abundance in ECs and vasculature; • Conservation across species (both for TFs and seed sequences) • Known cellular functions regulated by miRNA • Known molecular links between miRNA and SIRT1 • Possibility of circulating miRNA target endothelial SIRT1 |
| TFBS analysis | TF regulation of miRNA and sequence conservation | miRstart, TRANSFAC, Jaspar, UCSC genome browser | ||
| Target analysis | miRNA targeting of SIRT1 | miRanda, Pictar, Targetscan | ||
| In vitro | Reporter assay | Direct targeting of miRNA through seed sequence | Luciferase assay, tag reporter expression | • Inclusion of multiple putative target sites can increase the targeting efficiency of miRNAs • Construct with mutated seed sequence should be used as negative control |
| Gain of function | Mimic oxidative stress-induced miRNA | Pre-miR overexpression | • Straight-forward but with artefact: superphysiological induction of miRNA and substantial off-target effects (e.g., some pre-miRs may affect systemic miRNA biogenesis) • mRNA level may or may not change depending on the complementarity of miRNA-mRNA duplex |
|
| Loss of function | Blockage of oxidative stress-induced miRNA | LNA, anti-miR, miR eraser, miRNA sponge | • More definitive in determining of requirement of miRNA in oxidative stress-downregulated SIRT1 • Functional changes caused by miRNA inhibition should be simulated by SIRT1 overexpression |
|
| In vivo | Expression profile | Inverse correlation between miRNA and SIRT1 | Classic miRNA and SIRT1 detection methods | • Animal models to be considered: disease model; (tissue-specific) knockout/Tg model |
| Gain of function | Mimic oxidative stress-induced miRNA | Virus-driven overexpression of miRNA, local delivery of pre-miR | • Similar to gain-of-function approach in vitro. Note that viral overexpression will mainly be limited to the liver. | |
| Loss of function | Blockage of oxidative stress-induced miRNA | LNA, AntagomiR, viral expression of shRNA of miRNA | • Delivery approaches, i.e., local or systemic delivery needs to be considered to delineate tissue-specific targeting |
GEO, Gene Expression Omnibus; NGS, next-generation sequencing; TFBS, transcription factor-binding site; LNA, locked nucleic acid.
The discovery of stimulus-regulated miRNAs can start with miRNA profiles from a high-throughput screening, that is, microarrays or next-generation sequencing. This information can be obtained from published or experimentally derived datasets. Because hydrogen peroxide (H2O2), senescence, high glucose, oscillatory shear stress, and angiotensin II all trigger oxidative stress, miRNAs that are commonly induced by these stimuli are likely to be oxidative stress-responsive miRNAs. Importantly, because H2O2 is the direct agent that elevates cellular oxidative stress, H2O2 treatment offers arguably the most definitive stimuli for any screening approach. For example, miR-200c, miR-21, and miR-146a were identified from H2O2-treated cells and were also elevated in animal models associated with higher oxidative stress (11,82,95). The subsequent prediction of SIRT1 targeting by these miRNAs can be achieved by using the databases mentioned above. Additional considerations can be used to increase the validity of these predictions. These include, but are not limited to, (i) whether the induction of these miRNAs and suppression of SIRT1 are concurrent with a given cellular event, for example, the increased acetylation of SIRT1 targets, cellular senescence, impaired mitochondrial biogenesis, NO bioavailability, and angiogenesis; (ii) the conservation of miRNA-targeted sequences across species; and (iii) whether the TFs that putatively induce these miRNAs also downregulate SIRT1, which is facilitated by the TF-binding site (TFBS) analysis of the miRNA promoters.
Putative miRNA promoter regions can initially be obtained from miRStart (22) and then scanned for TFBSs. TFs such as NF-κB, activator protein 1, CREB1, signal transducer and activator of transcription 3, MYC, and specificity protein 1 are well-characterized TFs that are activated by oxidative stress. Their TFBS in the miRNA promoter regions can be predicted using position weight matrices available in databases such as TRANSFAC (158) and JASPAR (16). The results can be further subjected to University of California, Santa Cruz (UCSC) genome browser (http://genome.ucsc.edu/), for sequence conservation analysis. Presumably, miRNAs with TFBS conserved across species are more likely to be functional miRNAs.
In vitro and in vivo validation
A multitude of studies demonstrate in vitro and in vivo methods to validate the mechanisms by which miRNAs regulate their mRNA targets and hence produce functional changes. The methods employed to validate SIRT1 targeting by oxidative-responsive miRNAs are listed in Table 3. Several in vitro assays are routinely performed to examine whether SIRT1 is indeed a bona fide target of a given miRNA. Luciferase reporters, in which luciferase cDNA is linked to the SIRT1 3′UTR, can be cotransfected with putative pre-miR (miRNA mimetics) or anti-miR (miRNA inhibitors or antagomirs) to assess the functionality. The direct targeting of miRNA on the complimentary sequences of SIRT1 mRNAs can thus be tested. To confirm the direct targeting through the predicted binding sites, 3′UTR mutation experiments should be performed as a negative control.
To manipulate the levels of miRNAs, gain-of-function approaches, in which miRNAs are elevated by either pre-miR miRNA mimics or viral vectors, can be utilized. Additionally, loss-of-function assays where miRNAs are inhibited by locked nucleic acids (LNAs) or other types of antagomirs can be performed to examine whether the SIRT1 mRNA and/or protein levels change in response to perturbations in the miRNA levels. These experiments should be accompanied by the examination of the functional readouts of SIRT1, for example, the deacetylation of its targets. These gain- and loss-of-function approaches can also be used in vivo with additional considerations of the delivery efficiency and tissue specificity. In parallel to these functional studies, a biochemical approach is frequently used to provide more direct evidence in active miRNA–mRNA targeting. Instead of assessing the overall miRNA or mRNA changes in cells, one can immunoprecipitate miRNA-induced silencing complex components, typically, Ago proteins, and quantify the associated miRNA and mRNA species by using quantitative polymerase chain reaction or a microarray (9,160). With cross-linking immunoprecipitation–sequencing, RNAs can be crosslinked with RNA-binding proteins, and the Ago-bound miRNA–mRNA complexes can be comprehensively identified by subsequent deep sequencing. In addition to the approaches listed above, Thomson et al. have reviewed other useful methods with relevant examples (145).
Indirect inhibition of SIRT1 by oxidative stress-responsive miRNAs
Some of the oxidative stress-responsive miRNAs can modulate SIRT1 through the regulation of SIRT1 upstream regulators without directly targeting SIRT1. Recent advances in systems biology offer promising approaches to identify indirect regulators of SIRT1. For example, the miRNA targets predicted in silico can be categorized and stratified based on the existing information from the literature and on a network-based analysis. The network-based analysis tools include gene annotations (gene ontology) (5), Database for Annotation, Visualization and Integrated Discovery (54), Kyoto Encyclopedia of Genes and Genomes (107), and so on. The analyses of the protein–protein interactions (129,138), kinase–substrate interactions (51), and metabolic interactions (73) can be further applied to generate an interactome map of the target genes. Based on the visualized interactome and its relation to SIRT1, miRNAs and their putative targets can be selected for validation. Notably, miRNAs can target multiple genes within a network. Therefore, miRNAs predicted to target multiple genes that coordinately regulate SIRT1 (including those targeting SIRT1 mRNA) should be candidates for further study. An overview of SIRT1 regulators and their potential regulation by miRNAs is presented in Table 4. Such indirect regulation of SIRT1 by miRNAs is a part of oxidative stress-regulated SIRT1. Finally, although endothelial SIRT1 is most likely regulated by endogenous miRNAs produced by ECs, miRNAs released into the circulation from other tissues/organs; that is, circulating miRNAs might also regulate the SIRT1 network.
Table 4.
Overview of Sirtuin 1 Regulators and Their Potential Regulation by microRNAs
| Type of SIRT1 regulation | Regulating molecule | Molecular function | Putative up- or down-regulation of miRNA under oxidative stress | Example |
|---|---|---|---|---|
| Enzymatic activity | AROS (64) | Interaction with SIRT1 for activation | Up | |
| NAMPT, NMNAT (123), (40) | Control of cellular NAD+ level | Up | ||
| PARP (113) | Depletion of NAD+ to inhibit SIRT1 | Down | ||
| Post-transcriptional regulation | p53 (105) | Repression of SIRT1 transcription | Down | |
| HuR (1) | Stabilization of SIRT1 mRNA | Up | miR-519 (2) | |
| HIC1, CtBP (169) | Corepressor complex of SIRT1 transcription | Down | miR-141-200c (135) | |
| Post-translational regulation | SENP (166) | Destabilization of SIRT1 protein | Down | |
| Positive regulating kinases [JNK (104), CK2 (62), PKA (43), DYRK1, DYRK3 (46)] | Stabilization or activation of SIRT1 | Up | ||
| Negative regulating kinase [CDK1 (130)] | Inactivation of SIRT1 | Down | ||
| Other | AMPK (17) | Indirect activation of SIRT1, synergistic effect on SIRT1 deacetylation targets | Up | miR-33 (28) |
AROS, active regulator of SIRT1; NMNAT, nicotinamide mononucleotide adenylyl transferase; HuR, human antigen R; HIC1, hypermethylated in cancer 1; CtBP, carboxyl-terminal binding protein; SENP, sentrin-specific protease; JNK, c-Jun N-terminal kinase; CK2, casein kinase 2; PKA, protein kinase A; DYRK, dual-specificity tyrosine-phosphorylation regulated kinase; CDK1, cyclin-dependent kinase 1.
miRNA regulated by SIRT1 under oxidative stress
As discussed above, SIRT1 modulates many TFs and coactivator/repressors and hence regulates the transcription of their target genes. Thus, SIRT1 may also control the expression of an array of miRNAs that affects the EC biology. This aspect should be integrated into the network of oxidative stress-regulated SIRT1, and is exemplified by a recent study (41). Seeking miRNAs mediating SIRT1 downregulation of CREB in the brain, miRNAs in the mouse brain lacking SIRT1 activity were profiled and demonstrated that SIRT1 modulates synaptic plasticity and memory formation via its suppression of miR-134 by cooperating with YY1, a corepressor of miR-134 transcription. Conversely, loss of SIRT1 activity resulted in miR-134 induction, which in turn downregulated miR-134 targeting CREB and brain-derived neurotrophic factor to impair synaptic plasticity (41). Although there is as yet no supportive evidence, it is possible that miR-134 is oxidative stress-responsive in ECs and hence plays a functional role in oxidative stress-altered endothelial biology. The similar approach can be used to identify the oxidative stress-responsive miRNAs, in which transcription is regulated by SIRT1.
Oxidative Stress-miRNA-SIRT1 Pathway Is Implicated in Vascular Diseases
Oxidative stress and the associated endothelial dysfunction are common features of vascular diseases, including atherosclerosis, hypertension, aneurysm, stroke, and restenosis. Numerous animal and human studies support the hypothesis that oxidative stress is a major cause leading to inflammation and impaired NO bioavailability (112). In the dysfunctional ECs, the major sources of ROS/RNS are NAD(P)H oxidase (NOX), mitochondrial electron leakage, and uncoupled eNOS, which are opposed by antioxidant defense enzymes (29,101). Modulations of these molecules in vivo can alter vascular outcomes. Genetic ablation of p47phox, a key component of NOX, results in lower levels of superoxide with decreased atherosclerotic lesions in ApoE−/− mice (7). Similarly, NO induction decreases atherosclerosis in hypercholesterolemic animals (25). In contrast, ApoE−/− mice deficient in MnSOD, the antioxidant enzyme expressed in the mitochondria, show accelerated atherogenesis at the arterial branch points (6).
The extensive crosstalk between SIRT1 and these ROS-generating molecules reveals that SIRT1 plays a crucial role in maintaining endothelial homeostasis (126). Although their regulation appears to be fine-tuning, miRNAs could be an essential mechanism in SIRT1 regulation, as explored above. A role for the oxidative stress-miRNA-SIRT1 pathways in vascular diseases, particularly, atherosclerosis and abdominal aortic aneurysm (AAA), has been described in recent studies. It is likely that the miRNA regulation of SIRT1 is not limited to the pathobiology of these two diseases. For comprehensive review of miRNAs and cardiovascular diseases, readers are referred to Small et al. (137) and Ono et al. (108).
Oxidative stress is tightly associated with the initiation and progression of atherosclerosis, as well as plaque rupture (92). Many risk factors for atherosclerosis such as hyperglycemia, hypercholesterolemia, and atheroprone flow patterns are associated with enhanced oxidative stress and a decreased SIRT1 level in ECs and/or in the arterial wall (21,170,173). Thus, miRNAs capable of targeting SIRT1 are likely to be involved in the development of atherosclerosis. Among these, miR-34a and miR-217 were found to be upregulated in human atherosclerotic plaques (99,117). Other SIRT1-targeting miRNAs may also play a role in atherogenesis. For example, miR-520c and miR-373 increase matrix metallopeptidase 9 (MMP9) partially through targeting SIRT1 in human fibroscarcoma cells (86). Because MMP9 contributes to atherosclerosis and destabilization of the plaques (89), it is possible that the miR520c/miR373-SIRT1-MMP9 pathway is engaged in atherosclerosis.
AAA is caused by progressive weakening of the aortic wall, creating ballooning of the vessel. Supraphysiological oxidative stress, in concert with inflammatory processes and protease-mediated extracellular matrix (ECM) degradation, contributes to the pathogenesis of AAA (98,163). It is likely that SIRT1 is inhibited during the pathogenesis of AAA, and its impairment exacerbates the formation of aneurysms. Indeed, resveratrol, an SIRT1 activator, prevents AAA in mice, with a preserved ECM structure as well as attenuated inflammation, oxidative stress, and neovascularization (61). Data from a limited number of reports support the role of oxidative stress-responsive miRNA in AAA via SIRT1 targeting. Microarray profiling reveals that 10 miRNAs, including miR-92a and miR-132 (2 miRNAs potentially target SIRT1 in ECs), are upregulated in the aortic tissues of rat AAA (84). Because MMP activation, elastin breakdown, and collagen remodeling are common features of AAA, miRNAs involved in regulating MMP should be crucial in the pathogenesis of AAA. Maegdefessel et al. reported that miR-29b is downregulated in AAA development both in mouse and human tissue along with increased collagen levels (94). The inhibition of miR-29b using LNA reduces AAA progression in mice. In contrast, the overexpression of miR-29b leads to enlarged AAA (94). MiR-21 is also upregulated in AAA. By targeting phosphatase and tensin homolog protein, miR-21 overexpression increases proliferation and decreases apoptosis in the aorta, which are protective against aneurysm expansion. In contrast, systemic administration of LNAs antagonizing miR-21 worsened AAA (93). Due to the close correlation between MMP, vascular remodeling, and SIRT1 (102), the oxidative stress-miRNA-SIRT1 pathway may be involved in AAA pathogenesis. Considering that oxidative stress is a detrimental factor common to vascular dysfunction, it is reasonable to propose that miRNA regulation discussed above may be relevant for the onset of a wide spectrum of vascular diseases.
miRNA Therapeutics for Vascular Diseases
The reduced level of SIRT1 associated with various diseases and the beneficial effects of SIRT1 in alleviating endothelial dysfunction point to a therapeutic potential of SIRT1 activation for vascular diseases (52). However, the canonical SIRT1 activators (polyphenols) are plagued with pharmacokinetic properties (poor absorption and rapid metabolism) that limit their clinical utility (23). The discovery of miRNA opens a new window for the development of novel expression regulators in the form of oligonucleotides that either inhibit or mimic the actions of native miRNAs (14,68). Many reviews have summarized the potential use of miRNA in cardiovascular therapy (96,146,151,152). Here, we would like to make a few comments on these strategies in relation to miRNA in treating vascular disorders with SIRT1 aberration.
Conceptually, miRNAs can be used for intervention or palliation of vascular damage that involve oxidative stress-impaired SIRT1. An advantage of the miRNA strategy is that because miRNAs appear to act on functionally related targets in a coordinated fashion, their regulation is likely to produce pleotropic benefits with a minimum of side effects. Unfortunately, the downside of such large target spaces of miRNAs, that is, the lack of an absolute requirement for complementary target interaction, may enable the off-target effects (manipulating one miRNA may alter thousands of mRNA transcripts). Particularly, targeting some miRNAs may be tumorigenic.
There are additional challenges in the use of oligonucleotides as therapeutic agents. First of all, the size and polyanionic character of potential nucleotide agents limit their cell uptake, and they do not fit the drug-likeness rules of Lipinski (83). Their inherent metabolic instability imposes another challenge. However, these problems appear manageable through chemical modification, combination with lipid particles, and/or association with virus-based delivery systems. The recently developed antagomirs such as LNAs show great promise as therapeutic agents (35,71). They can be readily uptaken by cells due to their relatively small size and cholesterol conjugation. In addition, they are resistant to exo- and endonuclease, rendering them highly stable. Ideally, vascular tissue-specific delivering and targeting systems would greatly minimize untoward effects.
With this in mind and within the context of miRNA, SIRT1, and vascular diseases, there are a variety of strategies that in theory could utilize oligonucleotides to enhance SIRT1 activity and/or promote its downstream actions. For example, antisense oligonucleotides (antagomirs such as LNAs) could be administered to antagonize miRNAs that suppress SIRT1 expression and in turn increase its level. Of the currently known miRNAs that suppress SIRT1 expression (Table 2), all, but four, appear to be tumor suppressors. Therefore, if true in vivo, their antagomirs may be potential tumor promoters and of little therapeutic value. Antagomirs of the four miRNAs that suppress SIRT1 expression without tumor suppressor activity (i.e., miR-92, miR-93, miR-132, and miR-181) may be appropriate candidates to examine for their therapeutic potential for vascular diseases. Particularly, miR-92 may suppress the expression of both SIRT1 and KLF2 (12,132). Targeting miR-92 may hence exert pleotropic benefits to the vasculature.
miRNAs that modulate SIRT1 upstream regulators may also be considered as potential therapeutic targets. Notably, the activation or upregulation of most SIRT1 kinases appears to be associated with tumors and thus may not offer a safe approach to SIRT1 activation. Alternatively, increased expression of SIRT1 can be achieved by using pre-miR-targeting proteins that negatively regulate SIRT. Future studies elucidating the associated mechanism will reveal the possibility of such approaches.
Additional strategies enhancing the levels/activities of various kinases that act in concert with SIRT1 in imposing antioxidative and anti-inflammatory effects should be considered as well. One good example of such is AMPK. Because miR-33 may target AMPK (28), antagonizing miR-33 may enhance AMPK and consequentially enhance SIRT1 activity. Further, among the downstream effects of SIRT1 activation is the enhanced expression of the cholesterol transporter ATP-binding cassette, subfamily A, member 1 (ABCA1), which is also post-transcriptionally targeted by miR-33 (79,121). Therefore, antagomirs to miR-33, such as SIRT1, would increase the expression of ABCA1 leading to lower cellular cholesterol and higher high-density lipoprotein (HDL) levels, which is antiatherosclerotic. Indeed, the inhibition of miR33a/b has been proved to increase HDL and lower very low-density lipoprotein triglyceride, which is atheroprotective (120). However, because the miR-33a mimetic has antitumor effects (56), the oncogenic possibility of using miR-33 inhibitors warrants caution.
Conclusion and Perspectives
Clearly, increased SIRT1 expression and/or activity are indispensable in maintaining the endothelium in health. Oxidative stress, commonly exerted by pathophysiological conditions, on the other hand, leads to endothelial dysfunction. Given these opposing outcomes resulting from augmented SIRT1 versus oxidative stress, one should expect that an imbalanced redox state will negatively regulate SIRT1. Information provided in the review indicates that the oxidative stress-responsive miRNAs may play a role linking the increased oxidative stress and impaired SIRT1. It appears that miRNAs can both directly and indirectly regulate SIRT1 in ECs. Through coordinated regulation, such a network suppresses SIRT1 or desuppresses SIRT1 inhibitors, so that the aggravated inflammatory and oxidative statuses affect the endothelial function. This new paradigm of miRNA-involved EC biology not only is regulated at multiple levels (transcription, post-transcription, post-translation, etc.) but is also coordinated temporally and spatially. Because of this complexity, high-throughput screening, bioinformatics, and system biology should be integrated with experimental approaches in future studies. The usefulness of these state-of-the-art methods also include the identification of novel miRNAs and previous unidentified targets of miRNA involved in either pro- or antioxidative stress in ECs. The accuracy of this new approach heavily depends upon experimental validation in vitro and in vivo, which can be achieved by conventional methods. Because of the fine-tuning nature of miRNA regulation, the relative abundance of miRNA should be prudently considered. To avoid false-positive results obtained from miRNA overexpression experiments, genetic or siRNA deletion of the miRNA with ensuing gain of function should be considered. Notwithstanding, overexpression of miRNAs exceeding the physiological amounts may still have its therapeutic potential.
The approach outlined here can be applied to explore miRNA networks elicited by other environmental stimuli, which affect endothelial homeostasis either positively or negatively. To increase the translational potential, disease-based rather than cell culture-based screening (e.g., atherosclerotic tissues vs. H2O2-treated ECs) is more relevant to investigate the role of the miRNA-SIRT1 pathways in the pathophysiology of cardiovascular diseases. Last but not least, our understanding of miRNA in relation to the potential use in therapy is in its infancy. The above comments will hopefully spur future research on its therapeutic applications.
Abbreviations Used
- AAA
abdominal aortic aneurysm
- ABCA1
ATP-binding cassette, subfamily A, member 1
- AGEs
advanced glycation endproducts
- AMPK
AMP-activated protein kinase
- AREs
antioxidant-responsive elements
- AROS
active regulator of SIRT1
- AT1R
angiotensin II type 1 receptor
- CBP
CREB-binding protein
- CDK1
cyclin-dependent kinase 1
- CK2
casein kinase 2
- CR
caloric restriction
- CREB
cAMP response-element-binding protein
- CtBP
carboxyl-terminal binding protein
- DYRK
dual-specificity tyrosine-phosphorylation-regulated kinase
- EC
endothelial cell
- ECM
extracellular matrix
- EnMT
endothelial-to-mesenchymal transformation
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- FoxO
forkhead box O family
- FXR
Farnesoid X receptor
- GEO
Gene Expression Omnibus
- GK
Goto-Kakizaki
- H2O2
hydrogen peroxide
- HDL
high-density lipoprotein
- HFD
high-fat diet
- HIC1
hypermethylated in cancer 1
- HIF
hypoxia-inducible factor
- HuR
human antigen R
- JNK
c-Jun N-terminal kinases
- KLF2
Krüppel-like factor 2
- LDL
low-density lipoprotein
- LNA
locked nucleic acid
- mESCs
murine embryonic stem cells
- miR, miRNA
microRNA
- MMP
matrix metallopeptidase
- MnSOD
manganese superoxide dismutase
- MSC
mesenchymal stem cell
- mtDNA
mitochondrial DNA
- NAMPT
nicotinamide phosphoribosyl transferase
- NF-κB
nuclear factor-kappaB
- NGS
next-generation sequencing
- NMNAT
nicotinamide mononucleotide adenylyl transferase
- NOX
NAD(P)H oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- p53
tumor protein 53
- p63
transformation-related protein 63
- PARP
poly-ADP ribose polymerase
- PCAF
p300/CBP-associated factor
- PGC1α
peroxisome proliferator-activated receptor γ coactivator 1 alpha
- PKA
protein kinase A
- PKC
protein kinase C
- pri-miR
primary microRNA
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SENP
sentrin-specific protease
- SIRT1
sirtuin 1
- STZ
streptozotocin
- TF
transcription factor
- TFBS
transcription factor-binding site
- TGF-β
transforming growth factor-β
- 3′UTR
3′untranslated region
Acknowledgments
This work was supported in part by National Institutes of Health Research Grants HL106519 and HL105318 (to J.S.) and the American Heart Association Postdoctoral Fellowship Grant 11POST7590128 (to Z.C.). The authors wish to thank Dr. Wei Sun for his critical comments on the article.
References
- 1.Abdelmohsen K. Pullmann R., Jr. Lal A. Kim HH. Galban S. Yang X. Blethrow JD. Walker M. Shubert J. Gillespie DA. Furneaux H. Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cells. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmohsen K. Srikantan S. Kuwano Y. Gorospe M. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc Natl Acad Sci U S A. 2008;105:20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcendor RR. Gao S. Zhai P. Zablocki D. Holle E. Yu X. Tian B. Wagner T. Vatner SF. Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 4.Arciniegas E. Frid MG. Douglas IS. Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 5.Ashburner M. Ball CA. Blake JA. Botstein D. Butler H. Cherry JM. Davis AP. Dolinski K. Dwight SS. Eppig JT. Harris MA. Hill DP. Issel-Tarver L. Kasarskis A. Lewis S. Matese JC. Richardson JE. Ringwald M. Rubin GM. Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballinger SW. Patterson C. Knight-Lozano CA. Burow DL. Conklin CA. Hu Z. Reuf J. Horaist C. Lebovitz R. Hunter GC. McIntyre K. Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 7.Barry-Lane PA. Patterson C. van der Merwe M. Hu Z. Holland SM. Yeh ET. Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartke A. Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 9.Beitzinger M. Peters L. Zhu JY. Kremmer E. Meister G. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 2007;4:76–84. doi: 10.4161/rna.4.2.4640. [DOI] [PubMed] [Google Scholar]
- 10.Benigni A. Corna D. Zoja C. Sonzogni A. Latini R. Salio M. Conti S. Rottoli D. Longaretti L. Cassis P. Morigi M. Coffman TM. Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaumik D. Scott GK. Schokrpur S. Patil CK. Orjalo AV. Rodier F. Lithgow GJ. Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonauer A. Carmona G. Iwasaki M. Mione M. Koyanagi M. Fischer A. Burchfield J. Fox H. Doebele C. Ohtani K. Chavakis E. Potente M. Tjwa M. Urbich C. Zeiher AM. Dimmeler S. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 13.Bou Kheir T. Futoma-Kazmierczak E. Jacobsen A. Krogh A. Bardram L. Hother C. Gronbaek K. Federspiel B. Lund AH. Friis-Hansen L. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer. 2011;10:29. doi: 10.1186/1476-4598-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broderick JA. Zamore PD. MicroRNA therapeutics. Gene Ther. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet A. Sweeney LB. Sturgill JF. Chua KF. Greer PL. Lin Y. Tran H. Ross SE. Mostoslavsky R. Cohen HY. Hu LS. Cheng HL. Jedrychowski MP. Gygi SP. Sinclair DA. Alt FW. Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 16.Bryne JC. Valen E. Tang MH. Marstrand T. Winther O. da Piedade I. Krogh A. Lenhard B. Sandelin A. JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update. Nucleic Acids Res. 2008;36:D102–106. doi: 10.1093/nar/gkm955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canto C. Gerhart-Hines Z. Feige JN. Lagouge M. Noriega L. Milne JC. Elliott PJ. Puigserver P. Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caruso P. Dempsie Y. Stevens HC. McDonald RA. Long L. Lu R. White K. Mair KM. McClure JD. Southwood M. Upton P. Xin M. van Rooij E. Olson EN. Morrell NW. MacLean MR. Baker AH. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 19.Chen KC. Hsieh IC. Hsi E. Wang YS. Dai CY. Chou WW. Juo SH. Negative feedback regulation between microRNA let-7g and the oxLDL receptor LOX-1. J Cell Sci. 2011;124:4115–4124. doi: 10.1242/jcs.092767. [DOI] [PubMed] [Google Scholar]
- 20.Chen R. Xu M. Hogg RT. Li J. Little B. Gerard RD. Garcia JA. The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. J Biol Chem. 2012;287:30800–30811. doi: 10.1074/jbc.M111.244780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z. Peng IC. Cui X. Li YS. Chien S. Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien CH. Sun YM. Chang WC. Chiang-Hsieh PY. Lee TY. Tsai WC. Horng JT. Tsou AP. Huang HD. Identifying transcriptional start sites of human microRNAs based on high-throughput sequencing data. Nucleic Acids Res. 2011;39:9345–9356. doi: 10.1093/nar/gkr604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung S. Yao H. Caito S. Hwang JW. Arunachalam G. Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins T. Read MA. Neish AS. Whitley MZ. Thanos D. Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappaB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 25.Cooke JP. Singer AH. Tsao P. Zera P. Rowan RA. Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csiszar A. Labinskyy N. Jimenez R. Pinto JT. Ballabh P. Losonczy G. Pearson KJ. de Cabo R. Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dafforn A. Anderson M. Ash D. Campagna J. Daniel E. Horwood R. Kerr P. Rych G. Zappitelli F. The mode of binding of potential transition-state analogs to acetylcholinesterase. Biochim Biophys Acta. 1977;484:375–385. doi: 10.1016/0005-2744(77)90093-6. [DOI] [PubMed] [Google Scholar]
- 28.Davalos A. Goedeke L. Smibert P. Ramirez CM. Warrier NP. Andreo U. Cirera-Salinas D. Rayner K. Suresh U. Pastor-Pareja JC. Esplugues E. Fisher EA. Penalva LO. Moore KJ. Suarez Y. Lai EC. Fernandez-Hernando C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 30.Dey N. Das F. Mariappan MM. Mandal CC. Ghosh-Choudhury N. Kasinath BS. Choudhury GG. MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes. J Biol Chem. 2011;286:25586–25603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dioum EM. Chen R. Alexander MS. Zhang Q. Hogg RT. Gerard RD. Garcia JA. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 32.Donners MM. Wolfs IM. Stoger LJ. van der Vorst EP. Pottgens CC. Heymans S. Schroen B. Gijbels MJ. de Winther MP. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan Y. Zhou B. Su H. Liu Y. Du C. miR-150 regulates high glucose-induced cardiomyocyte hypertrophy by targeting the transcriptional co-activator p300. Exp Cell Res. 2013;319:173–184. doi: 10.1016/j.yexcr.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Eades G. Yao Y. Yang M. Zhang Y. Chumsri S. Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–26002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmen J. Lindow M. Schutz S. Lawrence M. Petri A. Obad S. Lindholm M. Hedtjarn M. Hansen HF. Berger U. Gullans S. Kearney P. Sarnow P. Straarup EM. Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 36.Esguerra JL. Bolmeson C. Cilio CM. Eliasson L. Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PLoS One. 2011;6:e18613. doi: 10.1371/journal.pone.0018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng B. Chen S. McArthur K. Wu Y. Sen S. Ding Q. Feldman RD. Chakrabarti S. miR-146a-mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–2984. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiordaliso F. Leri A. Cesselli D. Limana F. Safai B. Nadal-Ginard B. Anversa P. Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 39.Fred RG. Bang-Berthelsen CH. Mandrup-Poulsen T. Grunnet LG. Welsh N. High glucose suppresses human islet insulin biosynthesis by inducing miR-133a leading to decreased polypyrimidine tract binding protein-expression. PLoS One. 2010;5:e10843. doi: 10.1371/journal.pone.0010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulco M. Cen Y. Zhao P. Hoffman EP. McBurney MW. Sauve AA. Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J. Wang WY. Mao YW. Graff J. Guan JS. Pan L. Mak G. Kim D. Su SC. Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao W. He HW. Wang ZM. Zhao H. Lian XQ. Wang YS. Zhu J. Yan JJ. Zhang DG. Yang ZJ. Wang LS. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerhart-Hines Z. Dominy JE., Jr. Blattler SM. Jedrychowski MP. Banks AS. Lim JH. Chim H. Gygi SP. Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+) Mol Cells. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gracia-Sancho J. Villarreal G., Jr. Zhang Y. Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 2010;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo L. Qiu Z. Wei L. Yu X. Gao X. Jiang S. Tian H. Jiang C. Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 46.Guo X. Williams JG. Schug TT. Li X. DYRK1A and DYRK3 promote cell survival through phosphorylation and activation of SIRT1. J Biol Chem. 2010;285:13223–13232. doi: 10.1074/jbc.M110.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hackl M. Brunner S. Fortschegger K. Schreiner C. Micutkova L. Muck C. Laschober GT. Lepperdinger G. Sampson N. Berger P. Herndler-Brandstetter D. Wieser M. Kuhnel H. Strasser A. Rinnerthaler M. Breitenbach M. Mildner M. Eckhart L. Tschachler E. Trost A. Bauer JW. Papak C. Trajanoski Z. Scheideler M. Grillari-Voglauer R. Grubeck-Loebenstein B. Jansen-Durr P. Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hans CP. Feng Y. Naura AS. Zerfaoui M. Rezk BM. Xia H. Kaye AD. Matrougui K. Lazartigues E. Boulares AH. Protective effects of PARP-1 knockout on dyslipidemia-induced autonomic and vascular dysfunction in ApoE mice: effects on eNOS and oxidative stress. PLoS One. 2009;4:e7430. doi: 10.1371/journal.pone.0007430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He CH. Gong P. Hu B. Stewart D. Choi ME. Choi AM. Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 50.Herrera BM. Lockstone HE. Taylor JM. Wills QF. Kaisaki PJ. Barrett A. Camps C. Fernandez C. Ragoussis J. Gauguier D. McCarthy MI. Lindgren CM. MicroRNA-125a is over-expressed in insulin target tissues in a spontaneous rat model of type 2 diabetes. BMC Med Genomics. 2009;2:54. doi: 10.1186/1755-8794-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hornbeck PV. Chabra I. Kornhauser JM. Skrzypek E. Zhang B. PhosphoSite: a bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics. 2004;4:1551–1561. doi: 10.1002/pmic.200300772. [DOI] [PubMed] [Google Scholar]
- 52.Houtkooper RH. Pirinen E. Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsieh YC. Choudhry MA. Yu HP. Shimizu T. Yang S. Suzuki T. Chen J. Bland KI. Chaudry IH. Inhibition of cardiac PGC-1alpha expression abolishes ERbeta agonist-mediated cardioprotection following trauma-hemorrhage. FASEB J. 2006;20:1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- 54.Huang da W. Sherman BT. Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 55.Hulsmans M. De Keyzer D. Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25:2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim AF. Weirauch U. Thomas M. Grunweller A. Hartmann RK. Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71:5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda H. Serria MS. Kakizaki I. Hatayama I. Satoh K. Tsuchida S. Muramatsu M. Nishi S. Sakai M. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2(NF-E2-related factor 2) and androgen. Biochem J. 2002;364:563–570. doi: 10.1042/BJ20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito T. Yagi S. Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 59.John B. Sander C. Marks DS. Prediction of human microRNA targets. Methods Mol Biol. 2006;342:101–113. doi: 10.1385/1-59745-123-1:101. [DOI] [PubMed] [Google Scholar]
- 60.Kaeberlein M. McVey M. Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaneko H. Anzai T. Morisawa M. Kohno T. Nagai T. Anzai A. Takahashi T. Shimoda M. Sasaki A. Maekawa Y. Yoshimura K. Aoki H. Tsubota K. Yoshikawa T. Okada Y. Ogawa S. Fukuda K. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 62.Kang H. Jung JW. Kim MK. Chung JH. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One. 2009;4:e6611. doi: 10.1371/journal.pone.0006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawai Y. Garduno L. Theodore M. Yang J. Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim EJ. Kho JH. Kang MR. Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cells. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 65.Kim J. Kang Y. Kojima Y. Lighthouse JK. Hu X. Aldred MA. McLean DL. Park H. Comhair SA. Greif DM. Erzurum SC. Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim YJ. Hwang SH. Lee SY. Shin KK. Cho HH. Bae YC. Jung JS. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev. 2012;21:1749–1760. doi: 10.1089/scd.2011.0429. [DOI] [PubMed] [Google Scholar]
- 67.King GL. Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 68.Kole R. Krainer AR. Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11:125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kregel KC. Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 70.Krek A. Grun D. Poy MN. Wolf R. Rosenberg L. Epstein EJ. MacMenamin P. da Piedade I. Gunsalus KC. Stoffel M. Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 71.Krutzfeldt J. Rajewsky N. Braich R. Rajeev KG. Tuschl T. Manoharan M. Stoffel M. Silencing of microRNAs in vivo with “antagomirs.”. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 72.Kume S. Haneda M. Kanasaki K. Sugimoto T. Araki S. Isshiki K. Isono M. Uzu T. Guarente L. Kashiwagi A. Koya D. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282:151–158. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 73.Lee DS. Park J. Kay KA. Christakis NA. Oltvai ZN. Barabasi AL. The implications of human metabolic network topology for disease comorbidity. Proc Natl Acad Sci U S A. 2008;105:9880–9885. doi: 10.1073/pnas.0802208105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee HC. Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Lee J. Padhye A. Sharma A. Song G. Miao J. Mo YY. Wang L. Kemper JK. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lei J. Gu X. Ye Z. Shi J. Zheng X. Antiaging effects of simvastatin on vascular endothelial cells. Clin Appl Thromb Hemost. 2012 doi: 10.1177/1076029612458967. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Lewis BP. Burge CB. Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 78.Li N. Muthusamy S. Liang R. Sarojini H. Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech Ageing Dev. 2011;132:75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Li X. Zhang S. Blander G. Tse JG. Krieger M. Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cells. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 80.Li Y. Song YH. Li F. Yang T. Lu YW. Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun. 2009;381:81–83. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim JH. Lee YM. Chun YS. Chen J. Kim JE. Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cells. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 82.Lin Y. Liu X. Cheng Y. Yang J. Huo Y. Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lipinski CA. Lombardo F. Dominy BW. Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 84.Liu G. Huang Y. Lu X. Lu M. Huang X. Li W. Jiang M. Identification and characteristics of microRNAs with altered expression patterns in a rat model of abdominal aortic aneurysms. Tohoku J Exp Med. 2010;222:187–193. doi: 10.1620/tjem.222.187. [DOI] [PubMed] [Google Scholar]
- 85.Liu J. Wu Y. Wang B. Yuan X. Fang B. High levels of glucose induced the caspase-3/PARP signaling pathway, leading to apoptosis in human periodontal ligament fibroblasts. Cell Biochem Biophys. 2012 doi: 10.1007/S12013-012-9470-y. [DOI] [PubMed] [Google Scholar]
- 86.Liu P. Wilson MJ. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-κB factor in human fibrosarcoma cells. J Cell Physiol. 2012;227:867–876. doi: 10.1002/jcp.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Q. Wang G. Zhou G. Tan Y. Wang X. Wei W. Liu L. Xue W. Feng W. Cai L. Angiotensin II-induced p53-dependent cardiac apoptotic cell death: its prevention by metallothionein. Toxicol Lett. 2009;191:314–320. doi: 10.1016/j.toxlet.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Loboda A. Stachurska A. Florczyk U. Rudnicka D. Jazwa A. Wegrzyn J. Kozakowska M. Stalinska K. Poellinger L. Levonen AL. Yla-Herttuala S. Jozkowicz A. Dulak J. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxid Redox Signal. 2009;11:1501–1517. doi: 10.1089/ars.2008.2211. [DOI] [PubMed] [Google Scholar]
- 89.Loftus IM. Naylor AR. Goodall S. Crowther M. Jones L. Bell PR. Thompson MM. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31:40–47. doi: 10.1161/01.str.31.1.40. [DOI] [PubMed] [Google Scholar]
- 90.Long J. Wang Y. Wang W. Chang BH. Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 2010;285:23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo J. Nikolaev AY. Imai S. Chen D. Su F. Shiloh A. Guarente L. Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 92.Madamanchi NR. Vendrov A. Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 93.Maegdefessel L. Azuma J. Toh R. Deng A. Merk DR. Raiesdana A. Leeper NJ. Raaz U. Schoelmerich AM. McConnell MV. Dalman RL. Spin JM. Tsao PS. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012;4:122ra22. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maegdefessel L. Azuma J. Toh R. Merk DR. Deng A. Chin JT. Raaz U. Schoelmerich AM. Raiesdana A. Leeper NJ. McConnell MV. Dalman RL. Spin JM. Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magenta A. Cencioni C. Fasanaro P. Zaccagnini G. Greco S. Sarra-Ferraris G. Antonini A. Martelli F. Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18:1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin K. O'Sullivan JF. Caplice NM. New therapeutic potential of microRNA treatment to target vulnerable atherosclerotic lesions and plaque rupture. Curr Opin Cardiol. 2011;26:569–575. doi: 10.1097/HCO.0b013e32834b7f95. [DOI] [PubMed] [Google Scholar]
- 97.Mattagajasingh I. Kim CS. Naqvi A. Yamamori T. Hoffman TA. Jung SB. DeRicco J. Kasuno K. Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCormick ML. Gavrila D. Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:461–469. doi: 10.1161/01.ATV.0000257552.94483.14. [DOI] [PubMed] [Google Scholar]
- 99.Menghini R. Casagrande V. Cardellini M. Martelli E. Terrinoni A. Amati F. Vasa-Nicotera M. Ippoliti A. Novelli G. Melino G. Lauro R. Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 100.Migliaccio E. Giorgio M. Mele S. Pelicci G. Reboldi P. Pandolfi PP. Lanfrancone L. Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 101.Mueller CF. Laude K. McNally JS. Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 102.Nakamaru Y. Vuppusetty C. Wada H. Milne JC. Ito M. Rossios C. Elliot M. Hogg J. Kharitonov S. Goto H. Bemis JE. Elliott P. Barnes PJ. Ito K. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 103.Napoli C. Balestrieri ML. Sica V. Lerman LO. Crimi E. De Rosa G. Schiano C. Servillo L. D'Armiento FP. Beneficial effects of low doses of red wine consumption on perturbed shear stress-induced atherogenesis. Heart Vessels. 2008;23:124–133. doi: 10.1007/s00380-007-1015-8. [DOI] [PubMed] [Google Scholar]
- 104.Nasrin N. Kaushik VK. Fortier E. Wall D. Pearson KJ. de Cabo R. Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nemoto S. Fergusson MM. Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 106.Ning J. Xi G. Clemmons DR. Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology. 2011;152:3143–3154. doi: 10.1210/en.2011-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogata H. Goto S. Sato K. Fujibuchi W. Bono H. Kanehisa M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ono K. Kuwabara Y. Han J. MicroRNAs and cardiovascular diseases. FEBS J. 2011;278:1619–1633. doi: 10.1111/j.1742-4658.2011.08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orimo M. Minamino T. Miyauchi H. Tateno K. Okada S. Moriya J. Komuro I. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–894. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 110.Ota H. Akishita M. Eto M. Iijima K. Kaneki M. Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Parikh VN. Jin RC. Rabello S. Gulbahce N. White K. Hale A. Cottrill KA. Shaik RS. Waxman AB. Zhang YY. Maron BA. Hartner JC. Fujiwara Y. Orkin SH. Haley KJ. Barabasi AL. Loscalzo J. Chan SY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perez-Linares J. Ochoa JL. Gago-Martinez A. Effect of PSP toxins in white leg shrimp Litopenaeus vannamei Boone, 1931. J Food Sci. 2008;73:T69–73. doi: 10.1111/j.1750-3841.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 113.Pillai JB. Isbatan A. Imai S. Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 114.Potente M. Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle. 2008;7:2117–2122. doi: 10.4161/cc.7.14.6267. [DOI] [PubMed] [Google Scholar]
- 115.Pullamsetti SS. Doebele C. Fischer A. Savai R. Kojonazarov B. Dahal BK. Ghofrani HA. Weissmann N. Grimminger F. Bonauer A. Seeger W. Zeiher AM. Dimmeler S. Schermuly RT. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 116.Quintas A. de Solis AJ. Diez-Guerra FJ. Carrascosa JM. Bogonez E. Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp Gerontol. 2012;47:198–201. doi: 10.1016/j.exger.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 117.Raitoharju E. Lyytikainen LP. Levula M. Oksala N. Mennander A. Tarkka M. Klopp N. Illig T. Kahonen M. Karhunen PJ. Laaksonen R. Lehtimaki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 118.Ramachandran D. Roy U. Garg S. Ghosh S. Pathak S. Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic beta-islets. FEBS J. 2011;278:1167–1174. doi: 10.1111/j.1742-4658.2011.08042.x. [DOI] [PubMed] [Google Scholar]
- 119.Rane S. He M. Sayed D. Vashistha H. Malhotra A. Sadoshima J. Vatner DE. Vatner SF. Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rayner KJ. Esau CC. Hussain FN. McDaniel AL. Marshall SM. van Gils JM. Ray TD. Sheedy FJ. Goedeke L. Liu X. Khatsenko OG. Kaimal V. Lees CJ. Fernandez-Hernando C. Fisher EA. Temel RE. Moore KJ. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rayner KJ. Sheedy FJ. Esau CC. Hussain FN. Temel RE. Parathath S. van Gils JM. Rayner AJ. Chang AN. Suarez Y. Fernandez-Hernando C. Fisher EA. Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rayner KJ. Suarez Y. Davalos A. Parathath S. Fitzgerald ML. Tamehiro N. Fisher EA. Moore KJ. Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Revollo JR. Grimm AA. Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 124.Rhodes CJ. Wharton J. Boon RA. Roexe T. Tsang H. Wojciak-Stothard B. Chakrabarti A. Howard LS. Gibbs JS. Lawrie A. Condliffe R. Elliot CA. Kiely DG. Huson L. Ghofrani HA. Tiede H. Schermuly R. Zeiher AM. Dimmeler S. Wilkins MR. Reduced miR-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;187:294–302. doi: 10.1164/rccm.201205-0839OC. [DOI] [PubMed] [Google Scholar]
- 125.Rippe C. Lesniewski L. Connell M. LaRocca T. Donato A. Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rivetti di Val Cervo P. Lena AM. Nicoloso M. Rossi S. Mancini M. Zhou H. Saintigny G. Dellambra E. Odorisio T. Mahe C. Calin GA. Candi E. Melino G. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A. 2012;109:1133–1138. doi: 10.1073/pnas.1112257109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodgers JT. Lerin C. Haas W. Gygi SP. Spiegelman BM. Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 128.Rogina B. Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rual JF. Venkatesan K. Hao T. Hirozane-Kishikawa T. Dricot A. Li N. Berriz GF. Gibbons FD. Dreze M. Ayivi-Guedehoussou N. Klitgord N. Simon C. Boxem M. Milstein S. Rosenberg J. Goldberg DS. Zhang LV. Wong SL. Franklin G. Li S. Albala JS. Lim J. Fraughton C. Llamosas E. Cevik S. Bex C. Lamesch P. Sikorski RS. Vandenhaute J. Zoghbi HY. Smolyar A. Bosak S. Sequerra R. Doucette-Stamm L. Cusick ME. Hill DE. Roth FP. Vidal M. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- 130.Sasaki T. Maier B. Koclega KD. Chruszcz M. Gluba W. Stukenberg PT. Minor W. Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saunders LR. Sharma AD. Tawney J. Nakagawa M. Okita K. Yamanaka S. Willenbring H. Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2:415–431. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schreiber BE. Valerio CJ. Keir GJ. Handler C. Wells AU. Denton CP. Coghlan JG. Improving the detection of pulmonary hypertension in systemic sclerosis using pulmonary function tests. Arthritis Rheum. 2011;63:3531–3539. doi: 10.1002/art.30535. [DOI] [PubMed] [Google Scholar]
- 133.Schwer B. Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Shin KK. Kim YJ. Hong CP. Yang JW. Bae YC. Jung JS. Retracted article: miR-598 induces replicative senescence in human adipose tissue-derived mesenchymal stem cells via silent information regulator 1. Mol Cell Biochem. 2013;372:285. doi: 10.1007/s11010-012-1330-y. [DOI] [PubMed] [Google Scholar]
- 135.Simmons CR. Zou F. Younkin SG. Estus S. Evaluation of the global association between cholesterol-associated polymorphisms and Alzheimer's disease suggests a role for rs3846662 and HMGCR splicing in disease risk. Mol Neurodegener. 2011;6:62. doi: 10.1186/1750-1326-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Simone NL. Soule BP. Ly D. Saleh AD. Savage JE. Degraff W. Cook J. Harris CC. Gius D. Mitchell JB. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Small EM. Frost RJ. Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stelzl U. Worm U. Lalowski M. Haenig C. Brembeck FH. Goehler H. Stroedicke M. Zenkner M. Schoenherr A. Koeppen S. Timm J. Mintzlaff S. Abraham C. Bock N. Kietzmann S. Goedde A. Toksoz E. Droege A. Krobitsch S. Korn B. Birchmeier W. Lehrach H. Wanker EE. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–968. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 139.Strum JC. Johnson JH. Ward J. Xie H. Feild J. Hester A. Alford A. Waters KM. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun C. Alkhoury K. Wang YI. Foster GA. Radecke CE. Tam K. Edwards CM. Facciotti MT. Armstrong EJ. Knowlton AA. Newman JW. Passerini AG. Simon SI. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circ Res. 2012;111:1054–1064. doi: 10.1161/CIRCRESAHA.112.270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun HX. Zeng DY. Li RT. Pang RP. Yang H. Hu YL. Zhang Q. Jiang Y. Huang LY. Tang YB. Yan GJ. Zhou JG. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 142.Sun LL. Jiang BG. Li WT. Zou JJ. Shi YQ. Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011;91:94–100. doi: 10.1016/j.diabres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 143.Sung FL. Zhu TY. Au-Yeung KK. Siow YL O K. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB. Kidney Int. 2002;62:1160–1170. doi: 10.1111/j.1523-1755.2002.kid577.x. [DOI] [PubMed] [Google Scholar]
- 144.Szabo C. Pacher P. Zsengeller Z. Vaslin A. Komjati K. Benko R. Chen M. Mabley JG. Kollai M. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10:28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thomson DW. Bracken CP. Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–6853. doi: 10.1093/nar/gkr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med. 2012;4:3–14. doi: 10.1002/emmm.201100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tissenbaum HA. Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 148.Trajkovski M. Hausser J. Soutschek J. Bhat B. Akin A. Zavolan M. Heim MH. Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 149.Ungvari Z. Bagi Z. Feher A. Recchia FA. Sonntag WE. Pearson K. de Cabo R. Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ungvari Z. Labinskyy N. Mukhopadhyay P. Pinto JT. Bagi Z. Ballabh P. Zhang C. Pacher P. Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van Rooij E. Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Rooij E. Purcell AL. Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 153.Vasa-Nicotera M. Chen H. Tucci P. Yang AL. Saintigny G. Menghini R. Mahe C. Agostini M. Knight RA. Melino G. Federici M. miR-146a is modulated in human endothelial cell with aging. Atherosclerosis. 2011;217:326–330. doi: 10.1016/j.atherosclerosis.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 154.Vaziri H. Dessain SK. Ng Eaton E. Imai SI. Frye RA. Pandita TK. Guarente L. Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 155.Vickers KC. Shoucri BM. Levin MG. Wu H. Pearson DS. Osei-Hwedieh D. Collins FS. Remaley AT. Sethupathy P. MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology. 2012;57:533–542. doi: 10.1002/hep.25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wagner W. Horn P. Castoldi M. Diehlmann A. Bork S. Saffrich R. Benes V. Blake J. Pfister S. Eckstein V. Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Whitsett J. Picklo MJ., Sr. Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 158.Wingender E. Chen X. Hehl R. Karas H. Liebich I. Matys V. Meinhardt T. Pruss M. Reuter I. Schacherer F. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 2000;28:316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu C. Gong Y. Yuan J. Zhang W. Zhao G. Li H. Sun A. Zou Y. Ge J. microRNA-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. J Lipid Res. 2012;53:2355–2363. doi: 10.1194/jlr.M028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu W. Xiao H. Laguna-Fernandez A. Villarreal G., Jr. Wang KC. Geary GG. Zhang Y. Wang WC. Huang HD. Zhou J. Li YS. Chien S. Garcia-Cardena G. Shyy JY. Flow-dependent regulation of krüppel-like factor 2 is mediated by microRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu Z. Puigserver P. Andersson U. Zhang C. Adelmant G. Mootha V. Troy A. Cinti S. Lowell B. Scarpulla RC. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 162.Xu D. Takeshita F. Hino Y. Fukunaga S. Kudo Y. Tamaki A. Matsunaga J. Takahashi RU. Takata T. Shimamoto A. Ochiya T. Tahara H. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yajima N. Masuda M. Miyazaki M. Nakajima N. Chien S. Shyy JY. Oxidative stress is involved in the development of experimental abdominal aortic aneurysm: a study of the transcription profile with complementary DNA microarray. J Vasc Surg. 2002;36:379–385. doi: 10.1067/mva.2002.124366. [DOI] [PubMed] [Google Scholar]
- 164.Yamakuchi M. MicroRNA regulation of SIRT1. Front Physiol. 2012;3:68. doi: 10.3389/fphys.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamakuchi M. Ferlito M. Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang Y. Fu W. Chen J. Olashaw N. Zhang X. Nicosia SV. Bhalla K. Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yeung F. Hoberg JE. Ramsey CS. Keller MD. Jones DR. Frye RA. Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yin H. Hu M. Zhang R. Shen Z. Flatow L. You M. MicroRNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J Biol Chem. 2012;287:9817–9826. doi: 10.1074/jbc.M111.333534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Q. Wang SY. Fleuriel C. Leprince D. Rocheleau JV. Piston DW. Goodman RH. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci U S A. 2007;104:829–833. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Zhang QJ. Wang Z. Chen HZ. Zhou S. Zheng W. Liu G. Wei YS. Cai H. Liu DP. Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao T. Li J. Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299:E110–116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou B. Li C. Qi W. Zhang Y. Zhang F. Wu JX. Hu YN. Wu DM. Liu Y. Yan TT. Jing Q. Liu MF. Zhai QW. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia. 2012;55:2032–2043. doi: 10.1007/s00125-012-2539-8. [DOI] [PubMed] [Google Scholar]
- 173.Zhou S. Chen HZ. Wan YZ. Zhang QJ. Wei YS. Huang S. Liu JJ. Lu YB. Zhang ZQ. Yang RF. Zhang R. Cai H. Liu DP. Liang CC. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 174.Zhu H. Yang Y. Wang Y. Li J. Schiller PW. Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]
- 175.Zhu S. Deng S. Ma Q. Zhang T. Jia C. Zhuo D. Yang F. Wei J. Wang L. Dykxhoorn DM. Hare JM. Goldschmidt-Clermont PJ. Dong C. MicroRNA-10A* and microRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res. 2013;112:152–164. doi: 10.1161/CIRCRESAHA.112.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zu Y. Liu L. Lee MY. Xu C. Liang Y. Man RY. Vanhoutte PM. Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]