Abstract
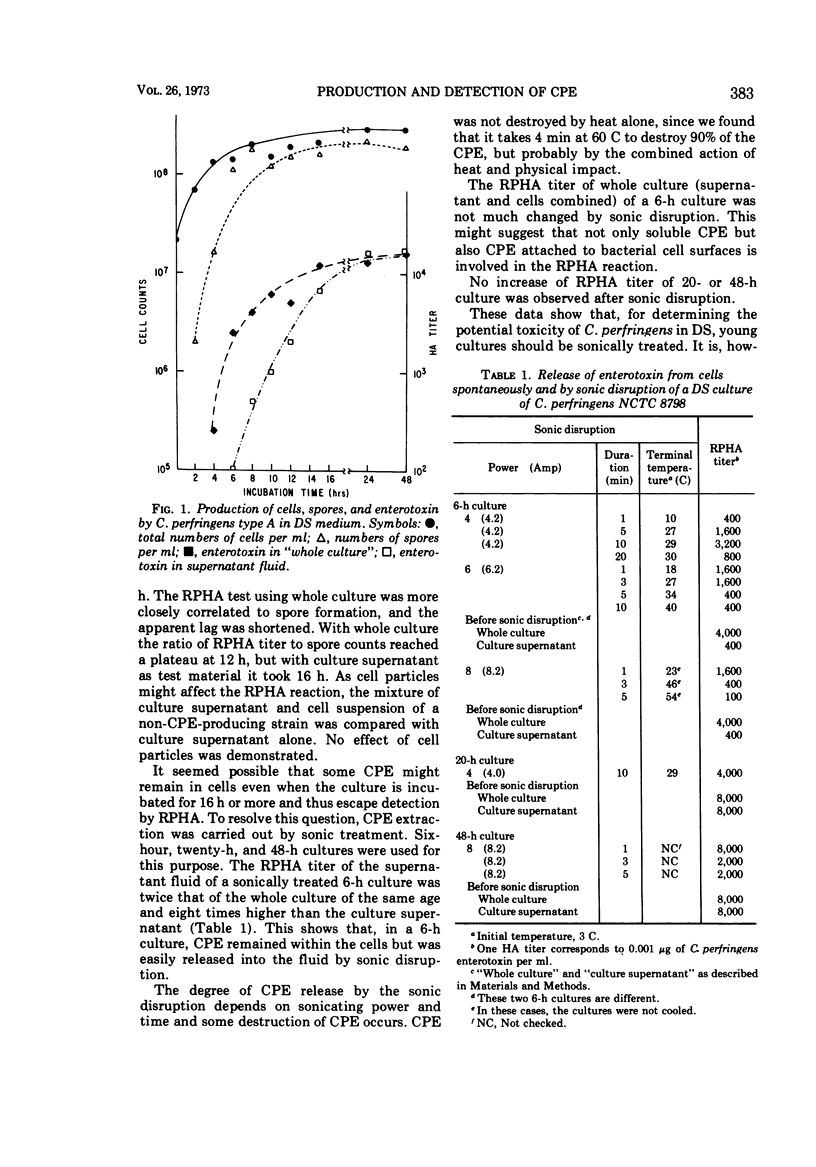
The reversed passive hemagglutination (RPHA) test yielded a positive reaction in 2 h with as little as 0.5 ng of purified Clostridium perfringens enterotoxin (CPE) per ml as well as with cultures of some C. perfringens grown in Duncan-Strong (DS) medium. This method is the most sensitive, the simplest, and the fastest among all reported. The time course of CPE production of Clostridium perfringens NCTC 8798 in DS was investigated by RPHA. CPE in culture was detectable at 4 h, increased gradually, reached a maximum at 12 to 14 h, and remained at a high level of 20 μg/ml through 48 h of incubation. CPE synthesized within cells is released easily by sonic disruption of young cultures and by aging the cultures 20 h or more. Heat shock of the cell inoculum was essential for CPE production by C. perfringens in DS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casman E. P., Bennett R. W., Dorsey A. E., Stone J. E. The micro-slide gel double diffusion test for the detection and assay of staphylococcal enterotoxins. Health Lab Sci. 1969 Oct;6(4):185–198. [PubMed] [Google Scholar]
- Duncan C. L., Somers E. B. Quantitation of Clostridium perfringens type A enterotoxin by electroimmunodiffusion. Appl Microbiol. 1972 Nov;24(5):801–804. doi: 10.1128/am.24.5.801-804.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Clostridium perfringens Type A Food Poisoning I. Response of the Rabbit Ileum as an Indication of Enteropathogenicity of Strains of Clostridium perfringens in Monkeys. Infect Immun. 1971 Jan;3(1):167–170. doi: 10.1128/iai.3.1.167-170.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Ileal loop fluid accumulation and production of diarrhea in rabbits by cell-free products of Clostridium perfringens. J Bacteriol. 1969 Oct;100(1):86–94. doi: 10.1128/jb.100.1.86-94.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H., Sebald M. Sporulation and enterotoxin production by mutants of Clostridium perfringens. J Bacteriol. 1972 Apr;110(1):378–391. doi: 10.1128/jb.110.1.378-391.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L. Time of enterotoxin formation and release during sporulation of Clostridium perfringens type A. J Bacteriol. 1973 Feb;113(2):932–936. doi: 10.1128/jb.113.2.932-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genigeorgis C., Sakaguchi G., Riemann H. Assay methods for Clostridium perfringens type A enterotoxin. Appl Microbiol. 1973 Jul;26(1):111–115. doi: 10.1128/am.26.1.111-115.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A. H. Erythemal activity of the cellular enteropathogenic factor of Clostridium perfringens type A. Can J Microbiol. 1970 Aug;16(8):651–654. doi: 10.1139/m70-112. [DOI] [PubMed] [Google Scholar]
- Hauschild A. H., Hilsheimer R. Purification and characteristics of the enterotoxin of Clostridium perfringens type A. Can J Microbiol. 1971 Nov;17(11):1425–1433. doi: 10.1139/m71-227. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Brenner K., Angelotti R., Hall H. E. Serological studies of types A, B, and E botulinal toxins by passive hemagglutination and bentonite flocculation. J Bacteriol. 1966 Mar;91(3):967–974. doi: 10.1128/jb.91.3.967-974.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Silverman S. J., Knott A. R., Howard M. Rapid, sensitive assay for staphylococcal enterotoxin and a comparison of serological methods. Appl Microbiol. 1968 Jul;16(7):1019–1023. doi: 10.21236/ad0838753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R. L., Duncan C. L. Transient increase in capillary permeability induced by Clostridium perfringens type A enterotoxin. Infect Immun. 1972 Jan;5(1):147–150. doi: 10.1128/iai.5.1.147-150.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D. H., Duncan C. L., Perna G. Clostridium perfringens Type A Food Poisoning II. Response of the Rabbit Ileum as an Indication of Enteropathogenicity of Strains of Clostridium perfringens in Human Beings. Infect Immun. 1971 Jan;3(1):171–178. doi: 10.1128/iai.3.1.171-178.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Ito A., Soda S., Sato H., Sadahiro S. Bioassay for -toxin of Clostridium perfrigens using survival time in mice. Jpn J Med Sci Biol. 1972 Feb;25(1):15–23. [PubMed] [Google Scholar]


