Abstract
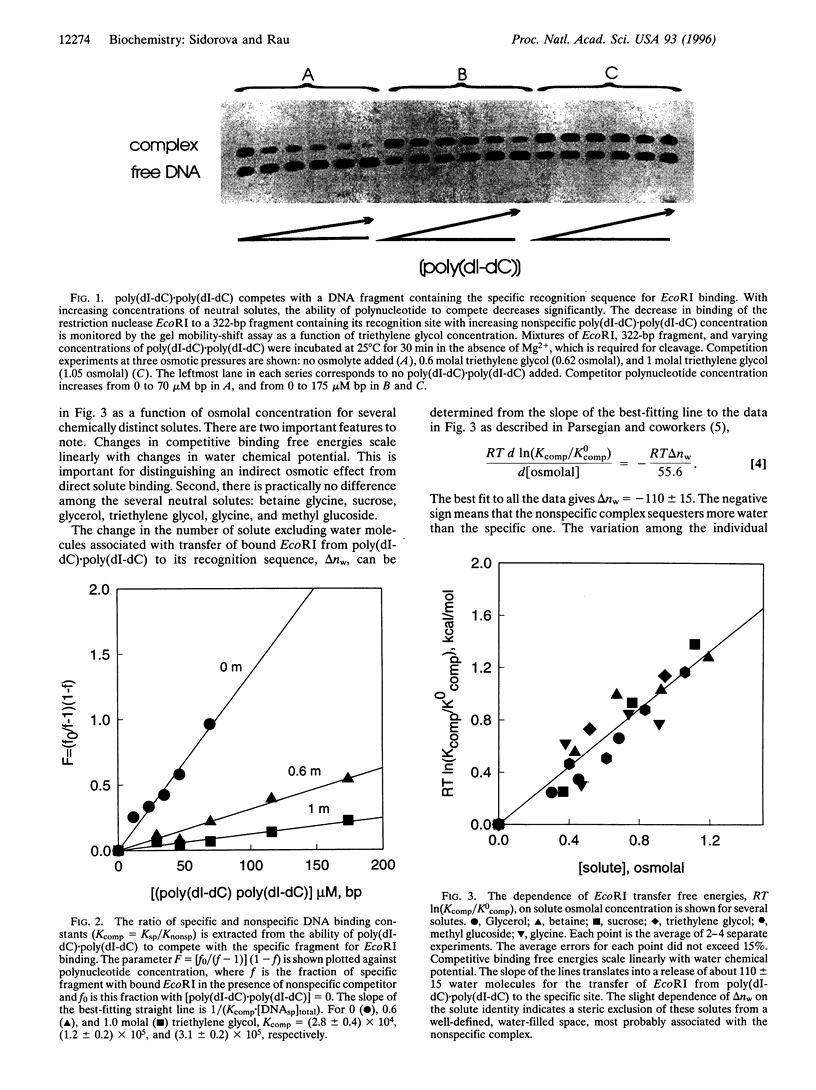
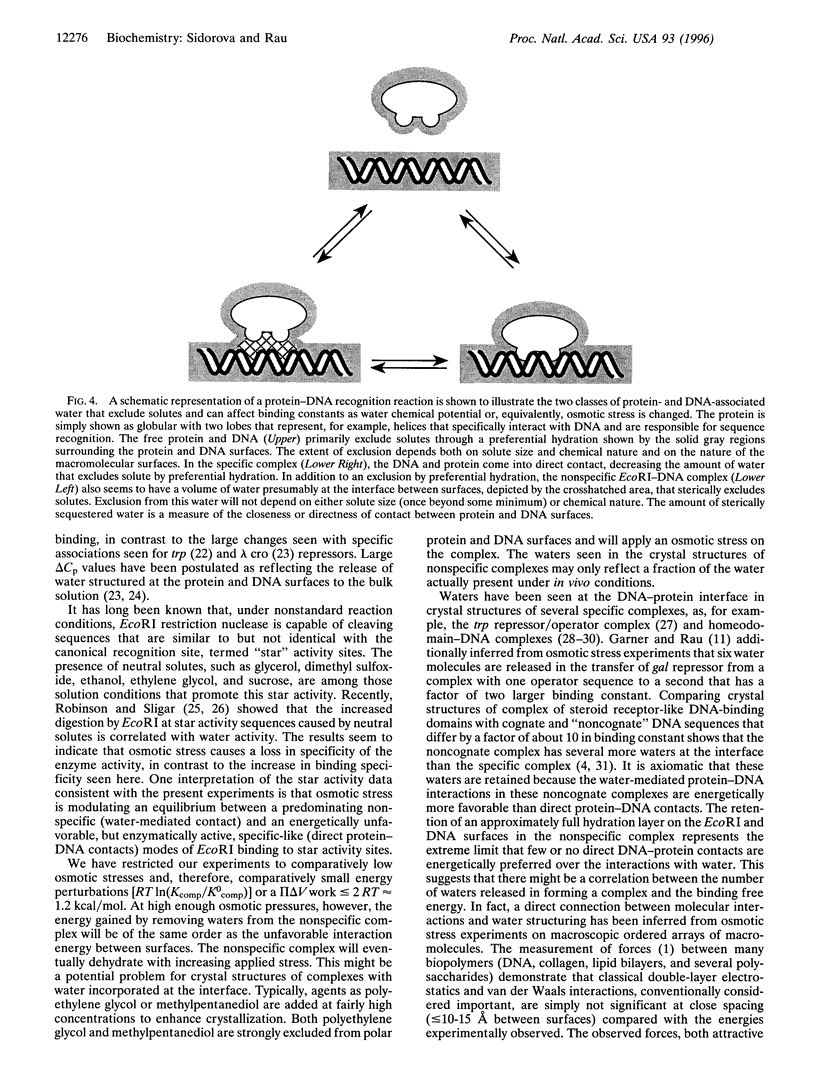
The free energy difference between complexes of the restriction nuclease EcoRI with nonspecific DNA and with the enzyme's recognition sequence is linearly dependent on the water chemical potential of the solution, set using several very different solutes, ranging from glycine and glycerol to triethylene glycol and sucrose. This osmotic dependence indicates that the nonspecific complex sequesters some 110 waters more than the specific complex with the recognition sequence. The insensitivity of the difference in number of waters released to the solute identity further indicates that this water is sequestered in a space that is sterically inaccessible to solutes, most likely at the protein-DNA interface of the nonspecific complex. Calculations based on the structure of the specific complex suggest that the apposing DNA and protein surfaces in the nonspecific complex retain approximately a full hydration layer of water.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbuckle N. D., Luisi B. A recipe for specificity. Nat Struct Biol. 1995 May;2(5):341–346. doi: 10.1038/nsb0595-341. [DOI] [PubMed] [Google Scholar]
- Bezrukov S. M., Vodyanoy I., Parsegian V. A. Counting polymers moving through a single ion channel. Nature. 1994 Jul 28;370(6487):279–281. doi: 10.1038/370279a0. [DOI] [PubMed] [Google Scholar]
- Colombo M. F., Rau D. C., Parsegian V. A. Protein solvation in allosteric regulation: a water effect on hemoglobin. Science. 1992 May 1;256(5057):655–659. doi: 10.1126/science.1585178. [DOI] [PubMed] [Google Scholar]
- Eisenberg H. Protein and nucleic acid hydration and cosolvent interactions: establishment of reliable baseline values at high cosolvent concentrations. Biophys Chem. 1994 Dec;53(1-2):57–68. doi: 10.1016/0301-4622(94)00076-x. [DOI] [PubMed] [Google Scholar]
- Fried M. G. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis. 1989 May-Jun;10(5-6):366–376. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Rau D. C. Water release associated with specific binding of gal repressor. EMBO J. 1995 Mar 15;14(6):1257–1263. doi: 10.1002/j.1460-2075.1995.tb07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirth D. T., Sigler P. B. The basis for half-site specificity explored through a non-cognate steroid receptor-DNA complex. Nat Struct Biol. 1995 May;2(5):386–394. doi: 10.1038/nsb0595-386. [DOI] [PubMed] [Google Scholar]
- Ha J. H., Capp M. W., Hohenwalter M. D., Baskerville M., Record M. T., Jr Thermodynamic stoichiometries of participation of water, cations and anions in specific and non-specific binding of lac repressor to DNA. Possible thermodynamic origins of the "glutamate effect" on protein-DNA interactions. J Mol Biol. 1992 Nov 5;228(1):252–264. doi: 10.1016/0022-2836(92)90504-d. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Aggarwal A. K. Structure of the even-skipped homeodomain complexed to AT-rich DNA: new perspectives on homeodomain specificity. EMBO J. 1995 Dec 15;14(24):6280–6291. doi: 10.1002/j.1460-2075.1995.tb00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbury J. E., Wright J. G., Sturtevant J. M., Sigler P. B. A thermodynamic study of the trp repressor-operator interaction. J Mol Biol. 1994 May 20;238(5):669–681. doi: 10.1006/jmbi.1994.1328. [DOI] [PubMed] [Google Scholar]
- Leikin S., Parsegian V. A., Rau D. C., Rand R. P. Hydration forces. Annu Rev Phys Chem. 1993;44:369–395. doi: 10.1146/annurev.pc.44.100193.002101. [DOI] [PubMed] [Google Scholar]
- Lesser D. R., Kurpiewski M. R., Jen-Jacobson L. The energetic basis of specificity in the Eco RI endonuclease--DNA interaction. Science. 1990 Nov 9;250(4982):776–786. doi: 10.1126/science.2237428. [DOI] [PubMed] [Google Scholar]
- Li T., Stark M. R., Johnson A. D., Wolberger C. Crystal structure of the MATa1/MAT alpha 2 homeodomain heterodimer bound to DNA. Science. 1995 Oct 13;270(5234):262–269. doi: 10.1126/science.270.5234.262. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- McClarin J. A., Frederick C. A., Wang B. C., Greene P., Boyer H. W., Grable J., Rosenberg J. M. Structure of the DNA-Eco RI endonuclease recognition complex at 3 A resolution. Science. 1986 Dec 19;234(4783):1526–1541. doi: 10.1126/science.3024321. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Rau D. C. Macromolecules and water: probing with osmotic stress. Methods Enzymol. 1995;259:43–94. doi: 10.1016/0076-6879(95)59039-0. [DOI] [PubMed] [Google Scholar]
- Preisler R. S., Chen H. H., Colombo M. F., Choe Y., Short B. J., Jr, Rau D. C. The B form to Z form transition of poly(dG-m5dC) is sensitive to neutral solutes through an osmotic stress. Biochemistry. 1995 Nov 7;34(44):14400–14407. doi: 10.1021/bi00044a017. [DOI] [PubMed] [Google Scholar]
- Robinson C. R., Sligar S. G. Hydrostatic and osmotic pressure as tools to study macromolecular recognition. Methods Enzymol. 1995;259:395–427. doi: 10.1016/0076-6879(95)59054-4. [DOI] [PubMed] [Google Scholar]
- Robinson C. R., Sligar S. G. Molecular recognition mediated by bound water. A mechanism for star activity of the restriction endonuclease EcoRI. J Mol Biol. 1993 Nov 20;234(2):302–306. doi: 10.1006/jmbi.1993.1586. [DOI] [PubMed] [Google Scholar]
- Sidorova NYu, Gazoni P., Rau D. C. Competition between netropsin and restriction nuclease EcoRI for DNA binding. J Biomol Struct Dyn. 1995 Oct;13(2):367–385. doi: 10.1080/07391102.1995.10508846. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Record M. T., Jr Coupling of local folding to site-specific binding of proteins to DNA. Science. 1994 Feb 11;263(5148):777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- Steitz T. A. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q Rev Biophys. 1990 Aug;23(3):205–280. doi: 10.1017/s0033583500005552. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ross P. D., Mudd C. P. Thermodynamics of Cro protein-DNA interactions. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8180–8184. doi: 10.1073/pnas.89.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff S. N. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- Timasheff S. N. Water as ligand: preferential binding and exclusion of denaturants in protein unfolding. Biochemistry. 1992 Oct 20;31(41):9857–9864. doi: 10.1021/bi00156a001. [DOI] [PubMed] [Google Scholar]
- Wilson D. S., Guenther B., Desplan C., Kuriyan J. High resolution crystal structure of a paired (Pax) class cooperative homeodomain dimer on DNA. Cell. 1995 Sep 8;82(5):709–719. doi: 10.1016/0092-8674(95)90468-9. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]