Abstract
Background:
Natural products might alter the labeling of blood constituents with technetium-99m (99mTc) and these results may be correlated with modifications of the shape of the red blood cells (RBC). The biodistribution of radiopharmaceuticals can be also altered.
Objective:
This investigation aimed to determine biological effects of an aqueous extract of chamomile (CE).
Materials and Methods:
To study the effect of the CE on the labeling of blood constituents with 99mTc, in vitro and in vivo assays were performed. The effect of the CE on the morphology of RBC was observed under light microscope. The images were acquired, processed, and the perimeter/area ratio of the RBC determined. To analyze the effect of the CE on biodistribution of the sodium pertechnetate (Na99mTcO4) in Wistar rats, these animals were treated or not with a CE. Na99mTcO4 was injected, the rats were sacrificed, the organs were removed, weighted and percentage of radioactivity/gram calculated.
Result:
In the in vitro experiment, the radioactivity on blood cells compartment and on insoluble fractions of plasma was diminished. The shape and the perimeter/area ratio of the RBC were altered in in vitro assays. An increase of the percentage of radioactivity of Na99mTcO4 was observed in stomach after in vivo treatment.
Conclusion:
These results could be due to substances of the CE or by the products of the metabolism of this extract in the animal organism. These findings are examples of drug interaction with a radiopharmaceutical, which could lead to misdiagnosis in clinical practice with unexpected consequences.
Keywords: Biodistribution, Matricaria recutita, radiolabeling, red blood cells, sodium pertechnetate, technetium-99m
INTRODUCTION
One the most commonly consumed single ingredient herbal tea or tisane is chamomile (Matricaria recutita) which is prepared with dried flowers. Infusions and essential oils from fresh or dried flower heads have aromatic, flavoring, and coloring properties. Both are used in a number of commercial products.[1,2]
Chamomile seems to have antioxidant activity[3] and a significant effect in the red blood cell membrane resistance.[4] Studies with animal model indicate potent anti-inflammatory action,[5] some anti-mutagenic properties,[6] gastro-protective effect,[7] and anxiolytic effects.[8] Chamomile also has acaricidal effect,[9] anti-microbial activity,[10] anti-viral activity,[11] wound healing activity,[12] and anti-hyperglycemic activity.[3] Anti-proliferative and apoptotic effects were observed too.[13] Segal and Pilote[14] have suggested that chamomile could increase the anti-coagulant effect of warfarin. Many of these biological properties are due to the action of some flavonoids and terpenoids compounds in the chamomile.[1,15]
Radiopharmacy plays an important role in the development and in the research of radiopharmaceutical. Radiopharmaceuticals (molecular or cellular structures) are designed to have specific bio-distribution and uptake in determined targets and elimination patterns when administered to normal subjects.[16] Sodium pertechnetate (Na99mTcO4) is a radiopharmaceutical used to obtain images from thyroid, gastric mucosa, salivary glands, and choroids plexus of the brain.[16,17] It has already been reported that biodistribution of radiopharmaceuticals and radiolabeling process can be modified by a wide variety of conditions, such as, drug therapy, radiation therapy, disease and medicinal plants therapy.[18,19]
There are many applications of red blood cells (RBC) labeled with 99mTc, as radiopharmaceutical, and this labeling process depends on a reducing agent; stannous chloride (SnCl2) has been usually used. When whole blood is utilized on the labeling of RBC with 99mTc, radioactivity is mainly found inside of RBC (beta-chain of the hemoglobin). Furthermore, it is also bound outside of the RBC on plasma proteins.[16] The band-3 anion transport system[20] and calcium channels[21] may be the ways that pertechnetate and stannous ion have, respectively, to reach the interior of the RBC. Drugs (patient medications) and some natural products have being reported to decrease the labeling of blood constituents with 99mTc, and this change would be caused by alterations in the RBC plasma membrane.[22,23,24,25,26]
The purpose of this work was to evaluate the effects of an aqueous extract of chamomile (CE) on the labeling of blood constituents with 99mTc (using an in vitro and an in vivo/in vitro experiment), on the morphology of the RBC and on the biodistribution of the radiopharmaceutical sodium pertechnetate in organs isolated from Wistar rats.
MATERIALS AND METHODS
Animals
Adult male Wistar naive rats (three months old, 250±15g) were housed, five per cage, in an environmental-controlled room. Animals had free access to water and food, and ambient temperature was kept at 25 ± 2°C. This study was performed in accordance with the guidelines of “Care and Use of Laboratory Animals” (US National Institutes of Health 85-23, revised 1996). Handling and experimentation protocols were approved by the Ethics Committee for the Use and Care of Experimental Animals of the Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro.
Extract preparation and spectrophotometric measurements
An aqueous extract was freshly prepared (just before the assay) mixing 3g of crushed and dried flowers of chamomile (Leão Junior S.A, lot number D124/06) in 50ml of 0.9% NaCl (saline). The mixture was centrifuged (clinical centrifuge, 1500rpm, 5min). The supernatant was separated and had the optical density (OD) analyzed in a spectrophotometer (Analyser Comércio e Indústria LTDA, model Analyser 800M, São Paulo, Brazil) that was recorded in the range from 400 up to 700nm with intervals of 20nm, using a tube with 1cm of path length. All the experiments were performed in validity of the product as it was informed by the manufacturer. This solution was considered to have a final concentration of 60mg/ml and was diluted to 30, 15, 7.5, 3.75mg/ml. Saline solution was used in all experiments as a control. The highest value of OD (0.884±0.074) in 500nm of the prepared extract was considered to be a quality and reproducibility marker for the preparation of each extract.[27,28]
In vitro treatment of blood samples with chamomile extract and 99mTc-labeling of blood constituents
Heparinized whole blood was withdrawn by cardiac puncture from anesthetized Wistar male rats (n=10) and immediately used to experimental assays. Blood samples (0.5ml) were incubated with 100μl of the aqueous chamomile extract at different concentrations (60, 30, 15, 7.5, 3.75mg/ml) or saline as control, for 60 min.
Blood samples treated with CE were incubated with stannous chloride (1.2μg/ml; 500μl) (Sigma-Aldrich, USA) during 1 hour. After this period of time, 99mTc (100μl, 3.7MBq/ml) as sodium pertechnetate (recently milked from 99Mo/99mTc generator, Instituto de Pesquisas Energéticas e Nucleares, Comissão Nacional de Energia Nuclear, São Paulo, Brazil), was added, and the incubation continued for another 10 minutes. These samples were centrifuged (3500rpm, 5minutes) in a clinical centrifuge, and plasma (P) and blood cells (BC) were isolated. Samples (20μl) of P and BC were also precipitated with 1.0ml of trichloroacetic acid (TCA 5%), centrifuged, and insoluble (IF) and soluble (SF) fractions were separated. The procedures were carried out at ambient temperature. The radioactivity in P, BC, IF-P, SF-P, IF-BC, and SF-BC was counted in a well counter (DPC, model Gamma-C12, Los Angeles, USA), and the percentage of radioactivity (%ATI) was calculated as described: (i) % ATI BC = CPM (BC)/CPM (P) + CPM (BC); (ii) %ATI IF-P = CPM (IF-P)/CPM (SF-P) + CPM (IF-P) e (iii) % ATI (IF-BC) = CPM (IF-BC)/CPM (SF-BC) + CPM (IF-BC).
In vivo treatment of rats with chamomile extract and 99mTc-labeling of blood constituents
The animals received a CE (60 mg/kg, n=10) or saline (control, n=10) for 7 days by orogastric gavage. On the 8th day, Na99mTcO4 (7.4 MBq, 0.3ml) recently milked from 99Mo/99mTc generator (Instituto de Pesquisas Energéticas e Nucleares, Comissão Nacional de Energia Nuclear, São Paulo, Brazil) was injected through the ocular plexus of the anesthetized animals.
Samples (500μl) of heparinized whole blood were withdrawn and incubated with stannous chloride (1.2μg/ml; 500μl) (Sigma-Aldrich, USA) during 1 hour. These samples were centrifuged (3500rpm, 5minutes) in a clinical centrifuge, and plasma (P) and blood cells (BC) were isolated. Samples (20μl) of P and BC were also precipitated with 1.0ml of trichloroacetic acid (TCA 5%), centrifuged, and insoluble (IF) and soluble (SF) fractions were separated. The procedures were carried out at ambient temperature. The radioactivity in P, BC, IF-P, SF-P, IF-BC, and SF-BC was counted in a well counter (DPC, model Gamma-C12, Los Angeles, USA), and the percentage of radioactivity (%ATI) was calculated as described: (i) % ATI BC = CPM (BC)/CPM (P) + CPM (BC); (ii) %ATI IF-P = CPM (IF-P)/CPM (SF-P) + CPM (IF-P) e (iii) % ATI (IF-BC) = CPM (IF-BC)/CPM (SF-BC) + CPM (IF-BC).
Light microscopy of blood treated in vitro with chamomile
Histological evaluations were performed with blood samples treated with various concentrations (60, 30, 15, 7.5, 3.75mg/ml) of an aqueous extract of chamomile or saline for 60 min at room temperature. Blood smears were prepared, dried, fixed and staining with May-Grunwald/Giemsa stain (Isofar, Brazil). After that, the morphology of the RBC was observed under light microscope (Eclipse E400, ×100). The images were captured with a video camera (CCD Sony DXC-151 A), processed, and the morphometry (perimeter/area ratio) of the RBC determined with an image analyzer using Image Pro Plus 4.5 (Media Cybernetics, San Diego, USA).
Light microscopy of blood isolated from animals treated with chamomile
Histological evaluations were performed with heparinized blood samples withdrawn from Wistar rats that received the extract (n=5) or saline (n=5, as a control). Blood smears were prepared, dried, fixed, and staining with May-Grunwald/Giemsa stain (Isofar, Brazil). After that, the morphology of the RBC was observed under light microscope (Eclipse E400, ×100). The images were captured with a video camera (CCD Sony DXC-151 A), processed, and the morphometry (perimeter/area ratio) of the RBC determined with an image analyzer using Image Pro Plus 4.5.
Determination of the biodistribution of the radiopharmaceutical Na99mTcO4
On the 8th day, Na99mTcO4 (7.4 MBq, 0.3ml) recently milked from 99Mo/99mTc generator (Instituto de Pesquisas Energéticas e Nucleares, Comissão Nacional de Energia Nuclear, São Paulo, Brazil) was injected through the ocular plexus of the anesthetized animals (n=10). After 10 min, the rats were sacrificed, the organs (pancreas, testis, stomach, kidney, spleen, duodenum, heart, lung, liver, thyroid, bone, muscle, and brain) were removed, isolated, weighted, and the radioactivity of each organ was obtained in a well counter (DPC, model Gamma-C12, Los Angeles, USA). The radioactivity percentage per gram (%ATI/g) of each organ was determined.
Statistical analysis
Results were reported as means ± standard deviation of %ATI, %ATI/g, and perimeter/area. The comparison between groups were performed by ANOVA and post-hoc Dunnett's test in radiolabeling evaluation and in RBC morphometry of blood treated in vitro with CE and Student's t test in biodistribution of the radiopharmaceutical and in RBC morphometry of blood from animals treated in vivo with CE. A P value ≤ 0.05 was considered statistically significant (Graph-Pad Prism version 5.03, San Diego, USA).
RESULTS
Spectrophotometric measurements
An absorption spectrum of the CE was obtained, and the highest value to OD of this extract (0.884 ± 0.07) was in the wavelength of 500nm [Figure 1]. This data was considered as quality marker of the extract preparation.
Figure 1.
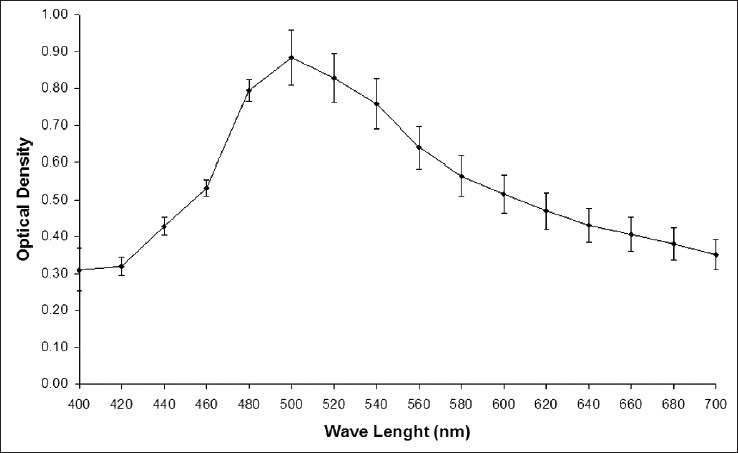
Optical density measurements of an aqueous extract of chamomile obtained by spectrophotometer
In vitro treatment of blood samples with chamomile extract and 99mTc-labeling of blood constituents
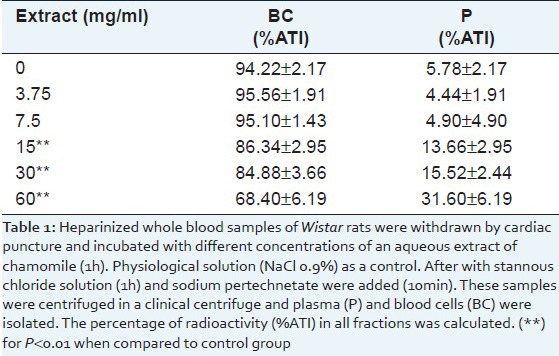
There was a significant decrease (P < 0.01) in the distribution of 99mTc in the blood cells compartment from 94.22±2.17 to 86.34±2.95, 84.88±3.66, and 68.40±6.19 when the whole blood cells was treated with 15, 30 and 60 mg/ml of CE, respectively [Table 1].
Table 1.
Distribution of the radioactivity in plasma (P) and cellular (BC) compartments of blood incubated with different concentrations of an aqueous extract of chamomile
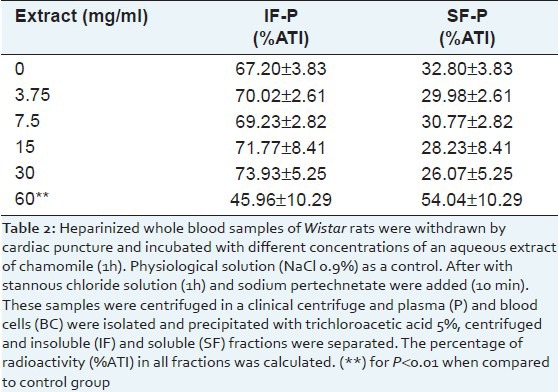
There was a significant decrease (P < 0.01) on the fixation of 99mTc from 67.20 ± 3.83 to 45.96 ± 10.29 in plasma proteins (insoluble fraction of the plasma) in blood cells treated with 60 mg/ml of CE [Table 2].
Table 2.
Distribution of the radioactivity on insoluble fraction of plasma (IF-P) and soluble fraction of plasma (SF-P) of blood incubated with different concentrations of an aqueous extract of chamomile
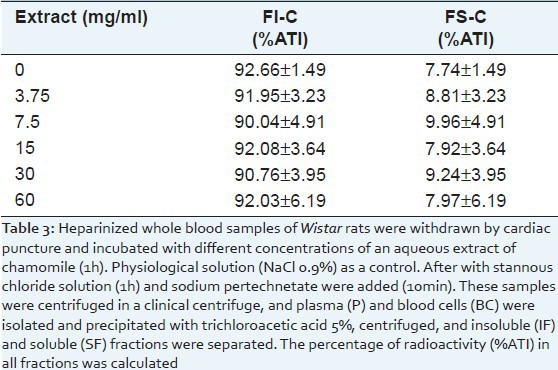
There was no difference (P > 0.05) in the fixation of the radioactivity in the insoluble fraction of samples of blood cells obtained from whole blood treated with different concentrations of CE [Table 3].
Table 3.
Distribution of the radioactivity on insoluble fraction of cell (IF-C) and soluble fraction of cell (SF-C) of blood incubated with different concentrations of an aqueous extract of chamomile
In vivo treatment of rats with chamomile extract and 99mTc-labeling of blood constituents
There was no difference (P>0.05) on the labeling of the blood constituents with 99mTc that whole blood was withdrawn of Wistar rats treated with the CE by 7 days [Table 4].
Table 4.
Distribution of radioactivity on blood cells (BC), plasma (P), insoluble fraction, and soluble fraction of the blood cells (IF-BC and SF-BC) and of the plasma (IF-P and SF-P) of samples of blood of the animal treated with a an aqueous extract of chamomile

Light microscopy of blood treated in vitro with chamomile
The RBC from blood treated with CE showed a crenate shape [Figure 2a] when compared with RBC from blood treated with saline [Figure 2b].
Figure 2.
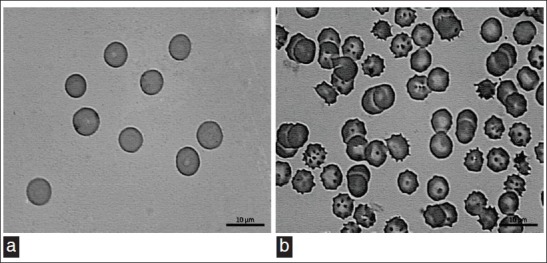
Photomicrography of blood smears incubated with saline (a) or with 60 mg/ml CE (b). Samples of blood of Wistar rats were incubated with saline or CE. After that, blood smears were prepared, dried, fixed, and stained. Finally, the morphology of red blood cells was evaluated in a light microscope (H and E, ×100)
This qualitative data was confirmed by a morphometric evaluation, which shows that the perimeter/area ratio of the RBC from blood treated with all different concentrations of CE increased significantly (P<0.01) when compared with RBC from blood treated with saline [Figure 3].
Figure 3.
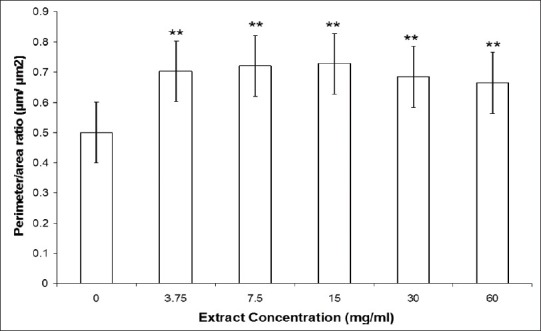
Perimeter/area (μm/μm2) ratio of RBC of Wistar rats blood samples incubated with different concentrations of CE. RBC from Wistar rats were incubated with different concentrations of CE or saline. After that, blood smears were prepared, dried, fixed, and stained. Finally, the morphology of red blood cells was evaluated in a light microscope (1000x). The photomicrography was captured for analysis and of the perimeter/area ratio of the RBC was determined. (**) for P<0.01 when compared to control group
Light microscopy of blood isolated from animals treated with chamomile
The RBC from animals treated with 60 mg/kg of CE showed a preserved shape [Figure 4a] when compared with RBC from animals treated with saline [Figure 4b].
Figure 4.
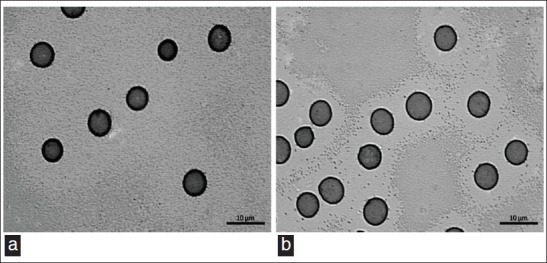
Photomicrography of a blood smears from Wistar rats treated with saline (a) or 60 mg/kg CE (b) for 7 days. Samples of heparinized whole blood were withdrawn, after that, blood smears were prepared, dried, fixed, and stained. Finally, the morphology of red blood cells was evaluated in a light microscope (H and E, ×100)
This qualitative data was confirmed by a morphometric evaluation, which shows that CE did not alter significantly (P>0.05) the perimeter/area ratio of the RBC [Figure 5].
Figure 5.
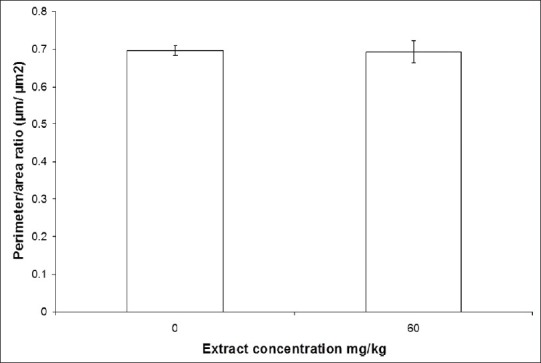
Perimeter/area ratio (μm/μm2) of RBC from Wistar rats treated with CE (60mg/kg) or saline (control). Blood smears were prepared, dried, fixed, and stained with May-Grunwald/Giemsa stain. Finally, the morphology of red blood cells was evaluated in a light microscope (1000 ×). The photomicrography was captured for analysis, and of the perimeter/area ratio of the RBC was determined
Determination of the bio-distribution of the radiopharmaceutical Na99mTcO4
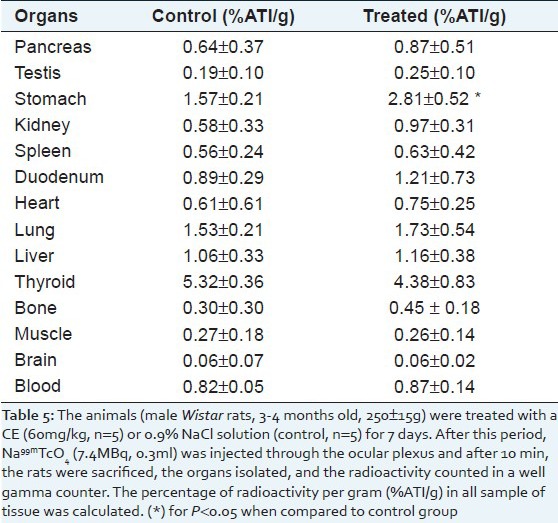
The uptake (%ATI/g) of Na99mTcO4 significantly (P<0.05) increased from 1.57±0.21 to 2.81±0.52 in stomach from animals that received the CE by 7 days [Table 5].
Table 5.
Effect of CE on the bio-distribution of Na99mTcO4, in organs isolated from Wistar rats treated with an aqueous extract of chamomile
DISCUSSION
Studies with natural products are not easy to be developed due to the necessity to choose an adequate form of extracting of the substances that there are on the natural products. The absorption spectrum of chemical products has been used for authors, as a tool, to evaluate the characteristics of these products. In this work we have used a spectrophotometric evaluation for determination of a marker of the preparation of this aqueous extract of chamomile to be used, and the highest value to OD obtained with this extract was 0.884±0.074 in the wavelength of 500nm.[27,28]
Different classes of bioactive constituents are present in chamomile, which have been isolated and used as medicinal preparations. Approximately 120 secondary metabolites have been identified in chamomile, including 28 terpenoids and 36 flavonoids.[1] The aim components of the essential oil extracted from the chamomile flowers are the terpenoids α-bisabolol and its oxide azulenes including chamazulene and acetylene derivatives. Flavonoids and terpenoids are responsible for several biological properties attributed to the chamomile.[4,5,6,7,14] but the action mechanism of the chamomile is not totally explained yet. The use of experimental models to study the effects of chamomile has been proposed.[8,12,13]
The effect of drugs on the labeling of blood constituents with 99mTc might be explained by: (i) a direct inhibition (chelating action) of the stannous and pertechnetate ions, (ii) damage induced in the cell membrane, (iii) competition of the cited ions for the same binding sites, (iv) possible generation of reactive oxygen species that could oxidize the stannous ions, and/or (v) cause a direct oxidation of the stannous ions.[18]
In the in vitro study, the decreasing of the radioactivity on the cellular compartment could be explained for the possible action of substances presents in the CE on the RBC plasma membrane, on the other hand, this finding could be justified by the alterations on the morphology of the erythrocyte membrane. Garcia-Pinto et al.[4] have also reported that an aqueous extract of chamomile cause an increase in RBC osmotic fragility. We speculate that the effect of the chamomile extract happens throw the cellular membrane proteins, weaken and/or altering the membrane transport throw ionic channels.
In the in vivo studies, no alterations on the labeling of the RBC were found. This fact could be justified by (i) possible generation of inactive metabolites or by (ii) ingestion of the small amount of chamomile that would be unable to reach blood concentration as high as in in vitro study.
Ganzera et al.[29] observed that chamomile major constituents (chamazulene, cis-spiroether and trans-spiroether) inhibited four human cytochrome P450 enzymes (CYP1A2, CYP2C9, CYP2D6 and CYP3A4). These findings are in agreement with our results, which could be explained in part for the substances present on the CE that could form complexes with stannous and/or pertechnetate ions (quelating action), to making difficult the fixation of the radioactivity on the insoluble fraction of the plasma. The fact of the fixation of radioactivity had not decreased in the insoluble fraction of the blood cell, it is possible propose that this quelating action of the chemical compounds present in the CE is not so effective and would not have action on the ions that reached in the interior of the RBC.
The qualitative analysis showed a crenate shape of the RBC from blood treated in vitro with CE, and the morphometric analysis confirmed this alteration indicating an increased perimeter/area ratio. These alterations on the morphology of the RBC could also justify the decrease of the labeling in RBC and on the insoluble fraction of the cells with 99mTc in the in vitro investigation. It is possible that these alterations could interfere with the transport of the stannous and/or pertechnetate ions trough the RBC cell membrane since it was demonstrated that chamomile exert effect in RBC plasma membrane.[4]
Radiopharmaceuticals are designed to have specific bio-distribution and uptake in determined targets and/or elimination patterns when administered to normal subjects.[17] The evaluation of the effects of an aqueous extract of Matricaria recutita on biodistribution of Na99mTcO4 suggests an increase on uptake of radioactivity on stomach, kidney, lung, and liver; however, there was only a significant alteration in the uptake of Na99mTcO4 in the stomach. The alteration of uptake of this radiopharmaceutical on the stomach of the animals, which were treated with Matricaria recutita extract, could also be related to the clinical application of this natural product in gastric disturbs. Khayyal et al.[7] observed that Matricaria recutita extracts could have gastro-protective action, which would be in relation with an increased production of mucin and prostaglandin E2 and a decrease in leukotrienes. Rocha et al.[30] described that α-bisabolol may be associated with an increase of gastric sulfydryl groups bioavailability, leading to a reduction of gastric oxidative injury. These results could be due to substances present in the Matricaria recutita extract or by the products of the metabolism of this extract in the animal organism.
The evidence that natural and synthetic drugs can affect the biodistribution of radiopharmaceuticals in setting of nuclear medicine clinic is already known for some chemical products. However, this drug interaction with radiopharmaceutical is not fully understood. Several authors have described the effect of drugs on biodistribution of radiopharmaceuticals.[25,26,27] When the drug interaction with radiopharmaceuticals is known, if desirable or undesirable, the natural consequence is a correct diagnosis. However, when it is unknown, it is undesirable and the consequences are the possibility of misdiagnosis and/or the repetition of the examination with an increase of radiation dose to the patient. The possible explanation to the appearance of drug interaction with radiopharmaceuticals are (i) modification of the radiopharmaceuticals, (ii) alteration of the labeling efficiency of the radiopharmaceuticals, (iii) modification of the target organ, (iv) modification of no target organ, and/or the (v) alteration of the biding of the radiopharmaceuticals with the blood proteins.[19] Thus, the development of biological models to study the drug interactions with radiopharmaceuticals is highly relevant. Our findings demonstrated the relevance of these experimental models.
In conclusion, the substances present in an extract of Matricaria recutita were capable to alter the morphology of RBC and, moreover, in in vitro studies has altered the labeling of these RBC. In addition, this extract was capable to interfere in the uptake of a radiopharmaceutical in the stomach. Despite, although our results have been obtained with animals, we suggest paying attention with the nuclear medicine procedures in patients who are undertaking Matricaria recutita.
ACKNOWLEDGMENTS
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de nível Superior (Capes) and Universidade do Estado do Rio de Janeiro (UERJ).
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Mol Med Report. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82–95. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cemek M, Kağa S, Simşek N, Büyükokuroğlu ME, Konuk M. Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. Nat Med (Tokyo) 2008;62:284–93. doi: 10.1007/s11418-008-0228-1. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Pinto AB, Pereira LD, Santos-Filho SD. Effects of the Matricaria recutita L. (German Chamomile) on sheep erythrocyte osmotic fragility. Phcog Mag. 2006;2:48–51. [Google Scholar]
- 5.Schempp H, Weiser D, Kelber O, Elstner EF. Radical scavenging and anti-inflammatory properties of STW 5 (Iberogast) and its compounds. Phytomedicine. 2006;5:33–44. doi: 10.1016/j.phymed.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Ceruelos A, Madrigal-Bujaidar V, de la Cruz C. Inhibitory effect of chamomile essential oil on the sister chromatid exchanges in mouse bone marrow. Toxicol Lett. 2002;135:103–10. doi: 10.1016/s0378-4274(02)00253-9. [DOI] [PubMed] [Google Scholar]
- 7.Khayyal MT, Seif-El-Nasr M, El-Ghazaly MA, Merali Z, Trudeau VL, Arnason JT. Mechanisms involved in the gastro-protective effect of STW 5 (Iberogast) and its components against ulcers and rebound acidity. Phytomedicine. 2006;5:56–66. doi: 10.1016/j.phymed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Awad R, Levac D, Cybulska P, Merali Z, Trudeau VL, Arnason JT. Effects of traditionally used anxiolytic botanicals on enzymes of the gamma-aminobutyric acid (GABA) system. Can J Physiol Pharmacol. 2007;85:933–42. doi: 10.1139/Y07-083. [DOI] [PubMed] [Google Scholar]
- 9.Khodadad PK, Mehdi RA. Biological activities of chamomile (Matricaria chamomile) flowers extract against the survival and egg laying of the cattle fever tick (Acari Ixodidae) J Zhejiang Univ Sci B. 2007;8:693–96. doi: 10.1631/jzus.2007.B0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira JC, Diniz Mde F, Lima EO. In vitro antimicrobial activity of plants in Acute Otitis Externa. Rev Bras Otorrinolaringol. 2008;74:118–24. doi: 10.1016/S1808-8694(15)30761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch C, Reichling J, Schneele J, Schnitzler P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine. 2008;15:71–8. doi: 10.1016/j.phymed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Jarrahi M. An experimental study of the effects of Matricaria chamomilla extract on cutaneous burn wound healing in albino rats. Nat Prod Res. 2008;22:422–7. doi: 10.1080/14786410701591713. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007;55:9470–8. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 14.Segal R, Pilote L. Warfarin interaction with Matricaria chamomilla. CMAJ. 2006;174:1281–2. doi: 10.1503/cmaj.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tschiggerl C, Bucar F. Guaianolides Volatile Compounds in Chamomile Tea. Plant Foods Hum Nutr. 2012;67:129–35. doi: 10.1007/s11130-012-0277-1. [DOI] [PubMed] [Google Scholar]
- 16.Saha GB. 6th ed. New York: Springer; 2010. Fundamental of Nuclear Pharmacy. [Google Scholar]
- 17.Bernardo-Filho M, Santos-Filho SD, Moura EG, Maiworm AI, Orlando MMC, Penas ME, et al. Drug interaction with radiopharmaceuticals: A review. Braz Arch Biol Technol. 2005;48:13–28. [Google Scholar]
- 18.Rebello BM, Moreno SR, Godinho CR, Neves RF, Fonseca AS, Bernardo-Filho M, et al. Effects of Passiflora edulis flavicarpa on the radiolabeling ofblood constituents, morphology of red blood cells and on the biodistribution of sodium pertechnetate in rats. Appl Radiat Isot. 2008;6:1788–92. doi: 10.1016/j.apradiso.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Souza DE, Pereira MO, Bernardo LC, Carmo FS, Fonseca Ade S, Bernardo-Filho M. An experimental model to study the effects of a senna extract on the blood constituent labeling and biodistribution of a radiopharmaceutical in rats. Clinics (Sao Paulo) 2011;66:483–6. doi: 10.1590/S1807-59322011000300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan RJ, Rabito CA. Radiolabeling of erythrocytes with technetium-99m: Role of band-3 protein in the transport of pertechnetate across the cell membrane. J Nucl Med. 1990;31:2004–9. [PubMed] [Google Scholar]
- 21.Gutfilen B, Boasquevisque EM, Bernardo-Filho M. Calcium channel blockers: Interference on red blood cells and plasma proteins labeling with Tc-99m. Rev Esp Med Nucl. 1992;11:195–9. [Google Scholar]
- 22.Fonseca AS, Frydman JN, Santos R, Bernardo-Filho M. Influence of antipyretic drugs on the labeling of blood elements with technetium-99m. Acta Biol Hung. 2005;56:275–82. doi: 10.1556/ABiol.56.2005.3-4.10. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca AS, Frydman JN, Rocha VC, Bernardo-Filho M. Acetylsalicylic acid decreases the labeling of blood constituents with technetium-99M. Acta Biol Hung. 2007;58:187–98. doi: 10.1556/ABiol.58.2007.2.5. [DOI] [PubMed] [Google Scholar]
- 24.Benarroz MO, Fonseca AS, Rocha GS, Frydman JN, Rocha VC, Pereira MO, et al. Cinnamomum zeylanicum extract on the radiolabelling of blood constituents and the morphometry of red blood cells: In vitro assay. Appl Radiat Isot. 2008;66:139–46. doi: 10.1016/j.apradiso.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Frydman JN, Rocha VC, Benarroz MO, Rocha GS, Pereira MO, de Souza da Fonseca A, et al. Assessment of effects of a Cordia salicifolia extract on the radiolabeling of blood constituents and on the morphology of red blood cells. J Med Food. 2008;11:767–72. doi: 10.1089/jmf.2008.0045. [DOI] [PubMed] [Google Scholar]
- 26.Braga AC, Gomes ML, Santos JS, Oliveira JF, Machado EF, Oliveira MP, et al. Alteration of the labeling of blood constituents with technetium-99m and the morphology of red blood cells by Baccharis trimera extract. Afr J Pharm Pharmacol. 2012;4:228–34. [Google Scholar]
- 27.Carmo FS, Diniz CL, Pereira MO, Santos-Filho SD, Bernardo-Filho M. Characterization of physicochemical parameters and the effect on the labeling of blood constituents with technetium-99m of a Solanum melongena commercial extract. J Med Plants Res. 2011;23:5598–604. [Google Scholar]
- 28.Pinto NS, Carmo FS, Diniz CL, Almeida DS, Pereira MO, Santos-Filho SD, et al. Effects of an aqueous extract of Three Ballerinaon the survival of Escherichia coli AB1157 cultures and in the action of stannous chloride. J Med Plants Res. 2011;14:3256–9. [Google Scholar]
- 29.Ganzera M, Schneider P, Stuppner H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci. 2006;78:856–61. doi: 10.1016/j.lfs.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 30.Moura Rocha NF, Venâncio ET, Moura BA, Gomes Silva MI, Aquino Neto MR, Vasconcelos Rios ER, et al. Gastroprotection of (-)-alpha-bisabolol on acute gastric mucosal lesions in mice: The possible involved pharmacological mechanisms. Fundam Clin Pharmacol. 2010;4:63–71. doi: 10.1111/j.1472-8206.2009.00726.x. [DOI] [PubMed] [Google Scholar]


