Abstract
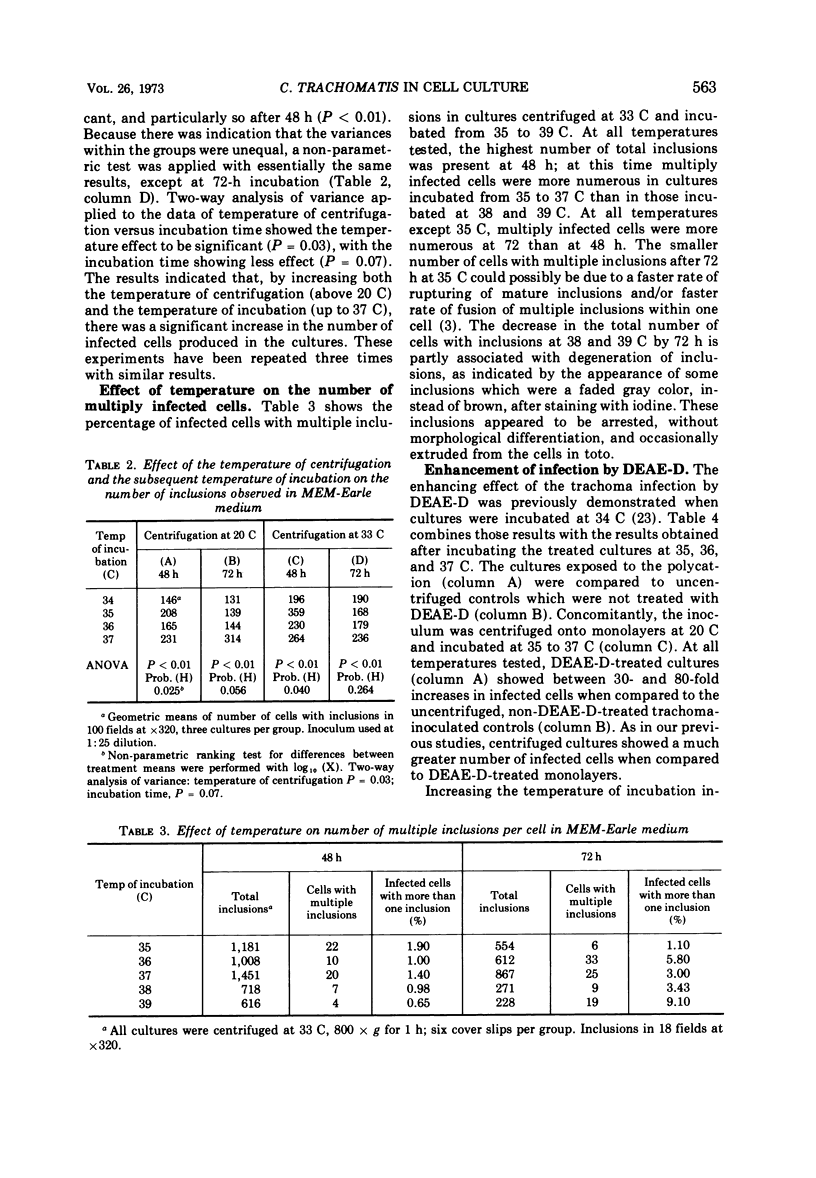
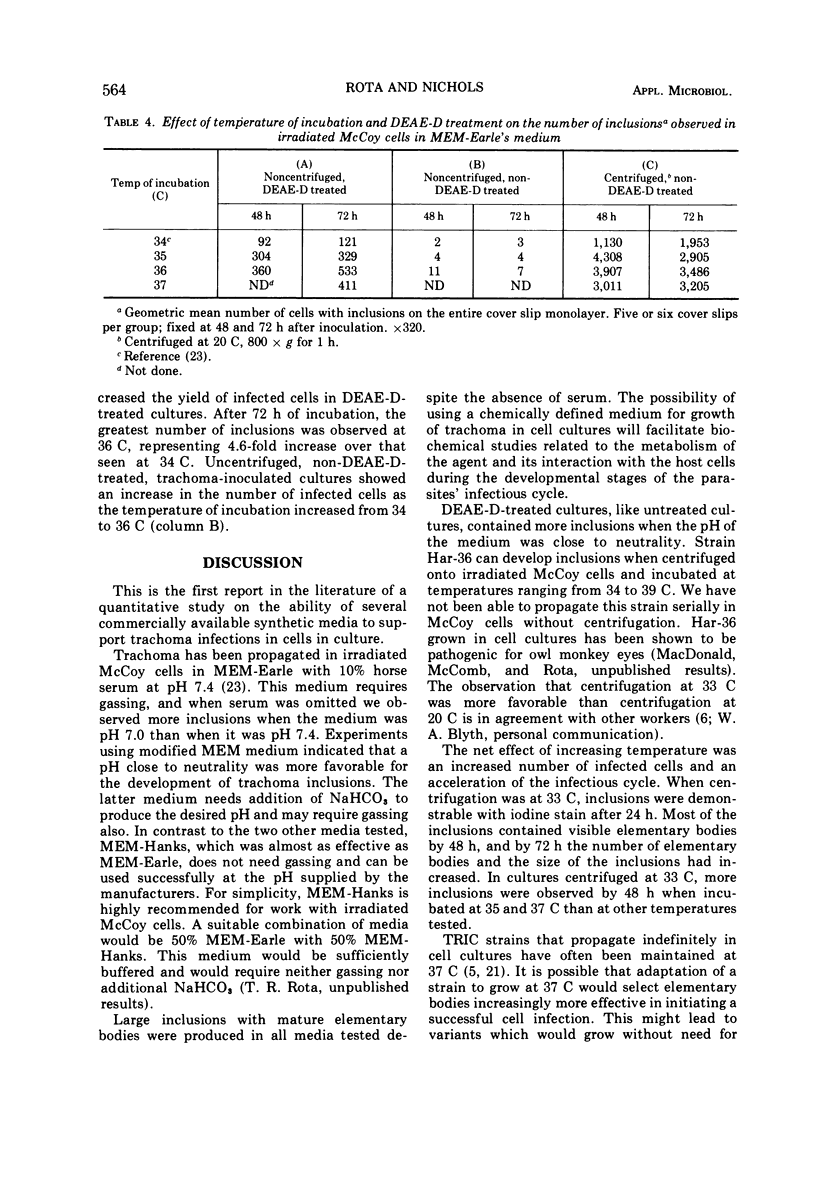
Three chemically defined cell culture media, Eagle minimum essential medium (MEM) with Earle basal salt solution, Eagle MEM with Hanks basal salt solution, and a modified Eagle MEM, were tested and found capable of supporting the development of Chlamydia trachomatis in 60Co-treated McCoy cells. The enhancement of trachoma infection by diethylaminoethyl-dextran (DEAE-D) was greater at pH values closer to neutrality than at any other pH values measured at the start of the experiments. Centrifugation of the trachoma inoculum onto cell monolayers at 33 C increased the number of inclusions when compared to centrifugation at 20 C. When the inoculum was centrifuged onto cell monolayers and subsequent incubation was at temperatures ranging from 34 to 39 C, the greatest number of inclusions was observed after incubation from 35 through 37 C. Enhancement of the trachoma infection by DEAE-D was tested at temperatures ranging from 35 to 37 C. These cultures had three- to fivefold increases in inclusions when compared to previously reported experiments in which DEAE-D-treated cultures were incubated at 34 C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. D., McComb D. E., Nichols R. L., Roca-Garcia M. Studies on trachoma. VII. Isolation of a mixture of type 1 and type 2. Trachoma strains from a child in Saudi Arabia. Am J Trop Med Hyg. 1970 Sep;19(5):842–845. [PubMed] [Google Scholar]
- Blyth W. A., Taverne J. Some consequences of the multiple infection of cell cultures by TRIC organisms. J Hyg (Lond) 1972 Mar;70(1):33–37. doi: 10.1017/s0022172400022063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER L. H. Growth characteristics of inclusion blennorrhea virus in cell cultures. Ann N Y Acad Sci. 1962 Mar 5;98:42–49. doi: 10.1111/j.1749-6632.1962.tb30530.x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Gordon F. B., Dressler H. R., Quan A. L. Relative sensitivity of cell culture and yolk sac for detection of TRIC infection. Am J Ophthalmol. 1967 May;63(5 Suppl):1044–1048. doi: 10.1016/0002-9394(67)94080-9. [DOI] [PubMed] [Google Scholar]
- Harrison M. J. Enhancing effect of DEAE-Dextran on inclusion counts of an ovine Chlamydia (Bedsonia) in cell culture. Aust J Exp Biol Med Sci. 1970 Apr;48(2):207–213. doi: 10.1038/icb.1970.20. [DOI] [PubMed] [Google Scholar]
- Kuo C., Wang S., Grayston J. T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972 Mar;125(3):313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- LITWIN J. Growth of the agent of trachoma in the embryonated egg. Ann N Y Acad Sci. 1962 Mar 5;98:145–162. doi: 10.1111/j.1749-6632.1962.tb30539.x. [DOI] [PubMed] [Google Scholar]
- MURRAY E. S., GUERRA P., ABBOTT A. G., McCOMB D. E. Isolation of viruses of trachoma from patients in Ethiopia. Ann N Y Acad Sci. 1962 Mar 5;98:14–23. doi: 10.1111/j.1749-6632.1962.tb30526.x. [DOI] [PubMed] [Google Scholar]
- McComb D. E., Bell S. D., Jr The application of an in vitro typing test to TRIC Bedsoniae. Am J Ophthalmol. 1967 May;63(5 Suppl):1429–1437. doi: 10.1016/0002-9394(67)94127-x. [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Jenkin H. M. Growth of Chlamydia psittaci strain meningopneumonitis in mouse L cells cultivated in a defined medium in spinner cultures. In Vitro. 1972 Sep-Oct;8(2):94–100. doi: 10.1007/BF02615966. [DOI] [PubMed] [Google Scholar]
- OSSOWSKI L., BECKER Y., BERNKOPF H. AMINO ACID REQUIREMENTS OF TRACHOMA STRAINS AND OTHER AGENTS OF THE PLT GROUP IN CELL CULTURE. Isr J Med Sci. 1965 Mar;1:186–193. [PubMed] [Google Scholar]
- POLLARD M., TANAMI Y. Cytochemistry of trachoma virus replication in tissue cultures. Ann N Y Acad Sci. 1962 Mar 5;98:50–61. doi: 10.1111/j.1749-6632.1962.tb30531.x. [DOI] [PubMed] [Google Scholar]
- Reeve R., Taverne J. Strain differences in the behavior of TRIC agnets in cell cultures. Am J Ophthalmol. 1967 May;63(5 Suppl):1167–1173. doi: 10.1016/0002-9394(67)94099-8. [DOI] [PubMed] [Google Scholar]
- Rota T. R., Nichols R. L. Infection of cell cultures by trachoma agent: enhancement by DEAE-dextran. J Infect Dis. 1971 Oct;124(4):419–421. doi: 10.1093/infdis/124.4.419. [DOI] [PubMed] [Google Scholar]
- Yamane I., Matsuya Y., Jimbo K. An autoclavable powdered culture medium for mammalian cells. Proc Soc Exp Biol Med. 1968 Jan;127(1):335–336. doi: 10.3181/00379727-127-32685. [DOI] [PubMed] [Google Scholar]


