Abstract
There is a global epidemic of obesity, and obesity is known to inhibit AMP-activated protein kinase (AMPK) activity and impairs myogenesis. Myogenin mediates the fusion of myoblasts into myotubes, a critical step in myogenesis. We observed that inhibition of AMPKα1 downregulates myogenin expression and myogenesis, but the underlying mechanisms are unclear. We postulated that AMPK regulates myogenin expression through phosphorlytion of histone deacetylase 5 (HDAC5). In C2C12 cells, HDAC5 knockdown increased while HDAC5 stablization by MC1568 reduced myogenin expression. Consistently, using luciferase assay, we observed that myogenin promoter activity was negatively regulated by HDAC5. Using RNA interference and primary myoblasts prepared from wild-type and AMPKα1 knockout mice, we further demonstrate that AMPKα1 regulates HDAC5 phosphorylation at Ser 259 and 498. Mutation of these two Ser to Ala in HDAC5 abolished the regulatory role of AMPKα1 on myogenin expression, clearly showing the necessity of these phosphorylation sites in mediating myogenin expression. In aggregate, these data show that AMPK inhibition downregulates myogenin transcription and myogenesis through phosphorylation of HDAC5, mediated mainly by AMPKα1. These data demonstrate that AMPK is a key molecular target for promoting myogenesis and muscular regeneration. Because drugs activating AMPK activity, such as metformin, are widely available, our finding has critical clinical implications to ensure proper muscle development and regeneration in obese subjects and under other pathophysiological conditions where AMPK activity is attenuated.
Keywords: AMPK, histone deacetylase, myogenin, myogenesis, obesity, phosphorylation
skeletal muscle, which comprises ∼40% of the body mass of adults, is the main peripheral tissue responsive to insulin stimulated uptake of glucose (21) and critical in the development of type 2 diabetes (23). Skeletal muscle hyperplasia occurs during the fetal stage while postnatal growth is primary due to hypertrophy, both of which involve extensive myogenesis. Downregulation of myogenesis during the fetal stage permanently reduces the number of muscle fibers in later life, reducing muscle mass and contraction force (1, 3, 15). Consistently, attenuated myogenic differentiation and fusion of satellite cells lead to muscular atrophy, impairing normal muscular functions (2, 6, 10).
Obesity is increasing at an alarming pace with a current obesity rate at >30% and another 30% overweight in the United States (12). It has been well demonstrated that obesity and its associated chronic inflammation inhibit AMP-activated protein kinase (AMPK) activity (9, 11, 20, 41). Our previous studies show that low AMPK activity is correlated with attenuated myogenic differentiation during early muscle development (43, 46, 49). In addition, AMPK constitutively active mutation in Rendement Napole (RN) pigs is associated with enhanced muscle growth in this pig breed (29, 32). These data show the positive association between AMPK and myogenesis, but the causal relationship remains to be established. AMPK is a heterotrimeric enzyme, composed of α-, β-, and γ-subunits, which plays an important role in energy metabolism (16, 19, 36). The catalytic α-subunit of AMPK has two isoforms, α1 and α2, which display differential expression during myogenic cell differentiation (31). Knockout of either α1- or α2-subunit results in visually normal mice, but knockout of both α1- and α2-subunits is lethal around embryonic day 9.5 (45), showing that α1 and α2 have compensatory roles in regulating fetal growth and development. Currently, there is no direct evidence detailing the AMPK isoform-specific role in myogenesis.
Cell differentiation and tissue development are controlled by epigenetic modifications, including histone and DNA modifications (33, 38). Histone acetylation activates gene expression (24, 28), which is regulated by histone acetyltransferase and histone deacetylase (HDAC; Ref. 40). HDAC5 belongs to the class IIa HDAC family and acts as a conserved transcriptional repressor through interaction with myocyte enhancer factor-2 (MEF2) (4). The activity of HDAC5 is mainly regulated through phosphorylation by several kinases (14, 27, 48). Myogenin is necessary for myoblast fusion into myotubes, a critical step in myogenesis (8, 37). The myogenin promoter contains a MEF2 binding site (17), which prompted us to hypothesize that AMPK activity is necessary for myogenesis through a process mediated by HDAC5 and MEF2 and that an AMPK isoform-specific mechanism exists. Our data demonstrated that AMPKα1 but not AMPKα2 stimulates myogenin expression and myogenesis via phosphorylation of HDAC5 at Ser 259 and 498, which provides an important mechanisms linking AMPK to myogenic differentiation.
MATERIALS AND METHODS
Animal experiments.
All animals were handled in accordance with protocols approved by the Animal Use and Care Committees of Washington State University (Permit No. 04158). Wild-type (WT) C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). AMPKα1−/− 129S2/SvPas [AMPKα1 knockout (KO)] and AMPKα2−/− C57BL/6 (AMPKα2 KO) mice were generated as previously described (18, 44).
Antibodies and chemicals.
Antibodies against HDAC5 (no. 2082), phospho-HDAC5 at Ser 259 (no. 3443), phospho-HDAC5 at Ser 498 (no. 3424), tag (no. 2368), mouse IgG (no. 7076), β-tubulin (no. 2146), histone H3K9 (no. 9649), goat anti-mouse Alexa Fluor 555 (no. 4409), goat anti-rabbit Alexa Fluor 488 (no. 4412), and goat anti-rabbit Alexa Fluor 555 (no. 4413) antibodies were purchased from Cell Signaling (Danvers, MA). Rabbit anti-desmin antibody (ab15200) was purchased from Abcam (Cambridge, MA). Anti-myogenin (F5D) and anti-myosin heavy chain (anti-MHC; MF20) mouse monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). IRDye 800CW goat anti-rabbit secondary antibody and IRDye 680 goat anti-mouse secondary antibody were purchased from LI-COR Biosciences (Lincoln, NE). Puromycin were purchased from Sigma (St. Louis, MO). MC1568 was purchased from Selleck (Houston, TX). pGL4 promoter luciferase plasmid was purchased from Promega (Madison, WI). Lipofectamine was purchased from Invitrogen (Carlsbad, CA).
Cell culture.
Myogenic C2C12 cells were grown at 37°C with 5% CO2 in DMEM supplemented with 10% FBS and 1% antibiotic mixture. Primary myoblasts were extracted from neonatal mice following a procedure described previously with modifications (35). Briefly, muscle from hindlimbs was minced and digested in DMEM with collagenase D and Dispase II (Roche Diagnostics, Mannheim, Germany) at 37°C for ∼30 min. The slurry was then passed through a 100-μm cell strainer. Cells were collected by centrifuge at 350 g for 5 min. The cell pellet was then resuspended in F-10 with 20% FBS and 1% antibiotic mixture. Primary myoblasts were seeded on collagen-coated plates and enriched by preplating. The purity of enriched primary myoblasts was checked by FACS and immunocytochemistry using anti-desmin antibody. When cells reached 100% confluence, the culture medium was switched to DMEM supplemented with 2% horse serum and 1% antibiotic mixture to induce myogenic differentiation. Myogenin mRNA expression, myogenin protein content, and MHC were analyzed at 2, 3, and 6 days after inducing myogenesis respectively.
Immunoblotting analyses.
Immunoblotting analysis was performed as previously described using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE; Ref. 48). Band density was normalized to β-tubulin content.
Real-time quantitative PCR.
Total RNA was extracted using Trizol (Sigma) followed by DNase (NEB, Ipswich, MA) treatment, and cDNA was synthesized using a reverse transcription kit (Bio-Rad, Hercules, CA). Real-time quantitative PCR (RT-PCR) was carried out using CFX RT-PCR detection system (Bio-Rad) with a SYBR Green RT-PCR kit from Bio-Rad. The following cycle parameters were used: 34 three-step cycles of 95°C, 20 s; 55°C, 20 s; and 72°C, 20 s. Primer sequences and their respective PCR fragment lengths were as follows: Myogenin (97 bp), forward 5′-GAGATCCTGCGCAGCGCCAT-3′, and reverse 5′-CCCCGCCTCTGTAGCGGAGA-3′; and 18S rRNA (110 bp), forward 5′-TGCTGTCCCTGTATGCCTCT-3′, and reverse 5′-TGTAGCCACGCTCGGTCA-3′. After amplification, a melting curve (0.01°C/s) was used to confirm product purity, and agarose gel electrophoresis was performed to confirm that only a single product of the right size was amplified. Relative mRNA content was normalized to 18S rRNA content.
Construction of expression vector.
The myogenin promoter fragment containing the MEF2 binding site was amplified from mouse genomic DNA using the following primers: forward 5′- CTAGCTAGCCGTCCGTCCAAGACAACCC-3′, and reverse 5′- CCGCTCGAGCAGGTCGGAAAAGGCTTGTT-3′. PCR products were subcloned between Xhol and Nhel sites of the pGL4-promoter-luciferase plasmid. The construct was verified by digestion with restriction enzymes and sequencing.
Transfection.
Plasmid transfection of C2C12 was performed using Lipofectamine according to the manufacturer's instructions. Briefly, 12 h before transfection, cells were switched to medium without antibiotics. Transfections were carried out when cells reached 95% confluence, using a 1:3 ratio of DNA (μg):Lipofectamine (μl); medium was switched to DMEM medium containing 10% FBS and 1% antibiotics 12 h following transfection. For short hairpin (sh)RNA interference, AMPKα1 shRNA, AMPKα2 shRNA, HDAC5 shRNA, and control shRNA (Santa Cruz Biotech, Santa Cruz, CA) were delivered into cells, and transfected cells were selected with puromycin (2 μg/ml). For HDAC5 overexpression, C2C12 cells were transfected with HDAC5 expression vectors, FLAG-HDAC5-WT, FLAG-HDAC5-S259A, FLAG-HDAC5-S498A, and FLAG-HDAC5-S259A/S498A (catalog no. 32213, 32214, 32215, and 32216; Addgene, Cambridge, MA). C2C12 cells transfected with green fluorescent protein (GFP) expression vector and nontransfected C2C12 cells were used as controls.
Plasmid transfection of primary myoblasts was performed using neon transfection system from Life Technologies (Grand Island, NY) following the manufacturer's suggested protocol with slight modifications. Briefly, 5 × 105 primary myoblasts were resuspended in 100 μl resuspension buffer and mixed with 5 μg of each plasmid. The mixture was then loaded to the system. Transfection was performed with the following parameters: pulse voltage (1,500 V), pulse width (10 ms), and pulse number (3). Transfected primary myoblasts were then cultured in F-10 with 20% FBS and 1% antibiotic mixture.
Luciferase reporter activity assay.
To measure the transcriptional activity of the myogenin promoter, C2C12 cells were transfected with different expression vectors. Renilla luciferase vector (Promega, Madison, WI) was transfected as an internal control, and each transfection assay was performed at least six times. An equal amount of plasmids was used for all treatments. At 24 h following transfection, cells were harvested, and luciferase activity was measured using a dual luciferase assay kit (Promega) according to the manufacturer's instructions. Data from each experiment were normalized to the Renilla luciferase activity (48).
FACS.
Cells were fixed in 4% PFA for 10 min at 37°C, permeabilized in ice-cold methanol for 30 min, and washed in PBS with 1% BSA. Then, 5 × 105 cells in PBS with 1% BSA were stained with rabbit anti-desmin primary antibody for 1 h, washed, and stained with goat anti-rabbit Alexa Fluor 555 secondary antibody for 30 min. Stained cells were sorted on FACSaria (BD Biosciences, San Jose, CA) and analyzed by FlowJo (Treestar, San Carlos, CA).
Immunocytochemical staining.
Cells grown on coverslips were fixed in 4% paraformaldehyde for 10 min, permeabilized with ice-cold methanol for 5 min, quenched with 0.1% sodium borohydride for 5 min, and incubated with anti-MHC, anti-desmin, or mouse IgG (1:100) at 4°C overnight. Fluorescent secondary antibody (1:1,000) was then added, and stained cells were incubated at room temperature for 1 h. Fluorescence was examined using a Leica inverted microscope (48).
Muscle fiber counting, muscle fiber area measure, muscle mass measure, and head-to-tail length measure.
Two-month-old WT mice, AMPKα1 KO mice, and AMPKα2 KO mice were killed using CO2. Body weight and head-to-tail length were measured. Tibialis anteria (TA) muscle were weighed. Soleus muscle were isolated, fixed in 4% paraformaldehyde, and embedded in paraffin. Embedded tissue were sectioned (6-μm thick) and stained with hematoxylin and eosin. Cross sections with similar distance to the distal end of muscle were analyzed using Image pro plus (Media Cybernetics, Rockville, MD) to determine muscle fiber number and area.
Statistics.
For all studies, at least three independent experiments were conducted. All data are expressed as means ± SE. Data were analyzed using the general linear model of SAS (SAS Institute, Cary, NC), and Tukey's Studentized range test was used to determine significance of differences among means. P < 0.05 was considered significant.
RESULTS
HDAC5 regulates myogenin expression and myogenesis.
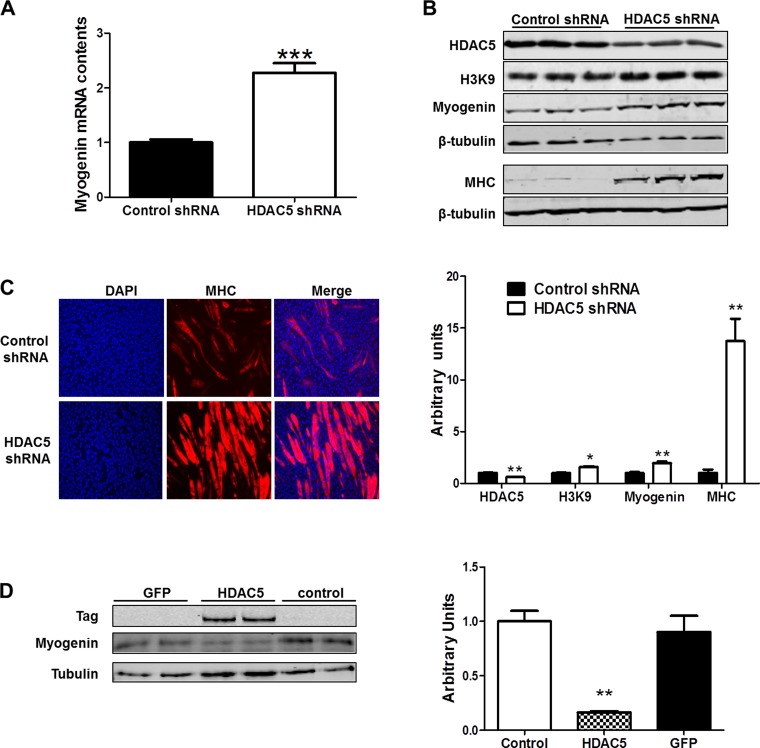
HDAC5 regulates gene transcription through interaction with MEF2 (22), and a conserved MEF2 binding site on the myogenin promoter has been identified (17). To investigate whether HDAC5 regulates myogenin mRNA transcription, we used HDAC5 shRNA to knock down endogenous HDAC5. We found that HDAC5 knockdown increased myogenin mRNA levels (Fig. 1A). Myogenin and MHC protein levels were also increased as was H3K9 acetylation (Fig. 1B). Immunocytochemical staining further confirmed that more myotubes were formed by myocytes following HDAC5 knockdown compared with control shRNA transfected cells (Fig. 1C). To further investigate the role of HDAC5 on myogenin expression, HDAC5 was then overexpressed in C2C12 cells. As expected, myogenin protein level was decreased pronouncedly (Fig. 1D).
Fig. 1.
Histone deacetylase 5 (HDAC5) regulated myogenin expression and myogenesis. A: myogenin mRNA content in C2C12 cells following HDAC5 knockdown. B: HDAC5, H3K9, myogenin, and myosin heavy chain (MHC) protein content in C2C12 cells following HDAC5 knockdown. C: MHC immunocytochemical staining of myotubes formed by C2C12 transfected with control short hairpin (sh)RNA and HDAC5 shRNA. D: myogenin protein level in C2C12 following HDAC5 overexpression. GFP, green fluorescent protein. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.0001 vs. control; means ± SE; n ≥ 3.
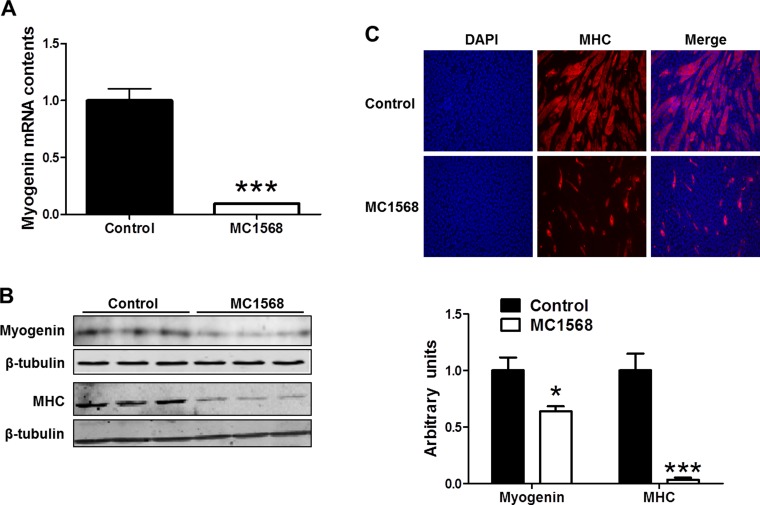
C2C12 cells were treated with MC1568 (5 μM), a specific class II HDAC inhibitor, which stabilizes the repressor structure of HDAC5 (30). The myogenin mRNA level was decreased dramatically by the addition of MC1568 (Fig. 2A). In addition, MC1568 treatment decreased myogenin and MHC protein levels (Fig. 2B). As expected, MC1568 also decreased the number of mature myotubes compared with the control group (Fig. 2C).
Fig. 2.
HDAC5 stabilization by MC1568 reduced myogenin expression and myogenesis. C2C12 cells in myogenic differentiation medium supplemented with MC1568 (5 μM) or vehicle (PBS). A: myogenin mRNA content analyzed by RT-PCR. B: myogenin and MHC contents analyzed by immunoblotting. C: MHC immunocytochemical staining of myotubes. *P < 0.05 vs. control; ***P < 0.0001 vs. control; means ± SE; n ≥ 3.
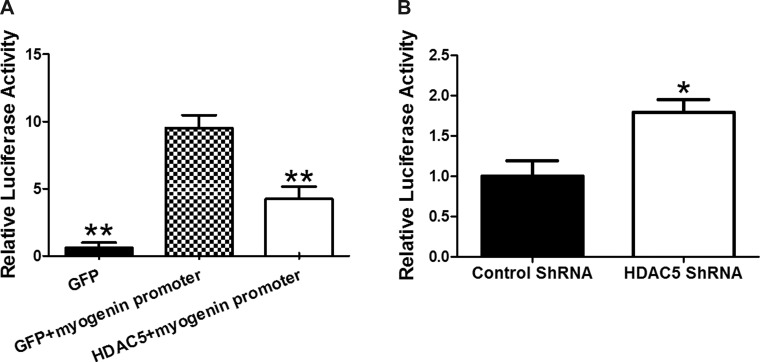
The gene-specific deacetylation of HDAC5 is mediated by MEF2. To determine if HDAC5 regulates the transcriptional activity of the myogenin promoter, we cloned the myogenin promoter containing the MEF2 binding site into pGL4 luciferase vector to construct a myogenin promoter-driven luciferase reporter vector. C2C12 cells transfected with the myogenin promoter-luciferase vector were cotransfected with the HDAC5 expression vector or GFP vector. Cells receiving the myogenin promoter-luciferase vector and GFP vector expressed higher luciferase activity than cells receiving myogenin promoter-luciferase vector and HDAC5 vector (Fig. 3A). Knockdown of endogenous HDAC5 by HDAC5 shRNA resulted in elevated luciferase activity (Fig. 3B), demonstrating that HDAC5 downregulates myogenin expression and myogenesis.
Fig. 3.
HDAC5 regulated myogenin promoter activity. A: luciferase activity of C2C12 cells transfected with myogenin promoter reporter, together with or without HDAC5 vector. B: luciferase activity of C2C12 cells with HDAC5 knockdown or control shRNA transfection plus myogenin promoter reporter. *P < 0.05 vs. control; **P < 0.01 vs. control; means ± SE; n ≥ 3.
AMPKα1 phosphorylates HDAC5, necessary for mediating myogenin expression.
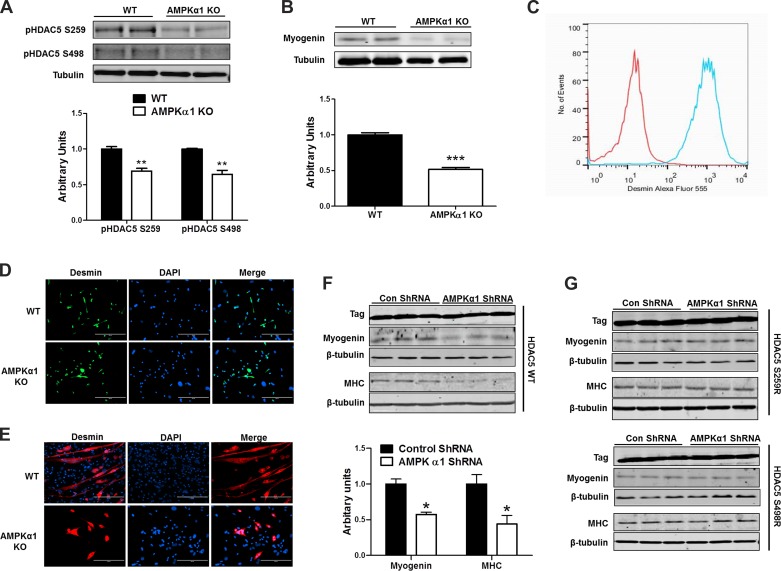
To test the effects of reduced AMPK activity on phosphorylation of HDAC5, we isolated myoblasts from WT and AMPKα1 KO mice and tested the phophorylation level of HDAC5 Ser 259 and HDAC5 Ser 498, which are known to be phosphorylated by AMPK (26). We found that the phosphorylation of both HDAC5 Ser 259 and Ser 498 were reduced in AMPKα1 KO myoblasts (Fig. 4A). As expected the myogenin protein level was lower in TA muscle isolated from 2-mo-old AMPKα1 KO mice than that from 2-mo-old WT mice (Fig. 4B). WT and AMPKα1 KO primary myoblasts were isolated, enriched, and tested for purity by FACS and ICC using anti-desmin antibody (Fig. 4, C and D). Enriched myoblasts were then induced for myogenic differentiation. We found that the myotube formation of AMPKα1 KO myoblasts was greatly reduced (Fig. 4E). To further test whether AMPK mediates myogenesis through HDAC5 in an isoform-specific manner, we transfected control cells and AMPKα1 knockdown cells with vectors expressing WT HDAC5, HDAC5 with Ser 259 mutated to Ala, or HDAC5 with Ser 498 mutated to Ala; Ser 259 and Ser 498 are two putative phosphorylation sites by AMPK. When transfected with WT HDAC5, AMPKα1 knockdown was associated with a lower myogenin content compared with the control group (Fig. 4F). In contrast, transfection with mutant HDAC5s blocked the downregulation of myogenin expression by AMPK knockdown, clearly showing the necessity of these phosphorylation sites in AMPK-regulated myogenin expression (Fig. 4G).
Fig. 4.
AMP-activated protein kinase (AMPK)α1 enhances myogenesis through phosphorylating HDAC5 at Ser 259 and 498. A: levels of HDAC5 phosphorylated at Ser 259 (S259) and Ser 498 (S259) in wild-type (WT) and AMPKα1 knockout (KO) myoblasts. B: myogenin protein level in tibialis anteria (TA) muscle isolated from 2-mo-old WT and AMPKα1 KO mice. C–D: enriched myoblasts were tested for purity by FACS (C) and immunocytochemistry (D) using anti-desmin antibody. E: MHC immunocytochemical staining of myotubes formed by WT and AMPKα1 KO myoblasts. F: myogenin and MHC levels in control, C2C12, and C2C12 cells with AMPKα1 knockdown and transfected with WT HDAC5. G: myogenin and MHC levels in control, C2C12, and C2C12 cells with AMPKα1 knockdown and transfected with HDAC5 S259A (Ser 259 to Ala) and HDAC5 S498A (Ser 498 to Ala). *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.0001 vs. control; means ± SE; n ≥ 3.
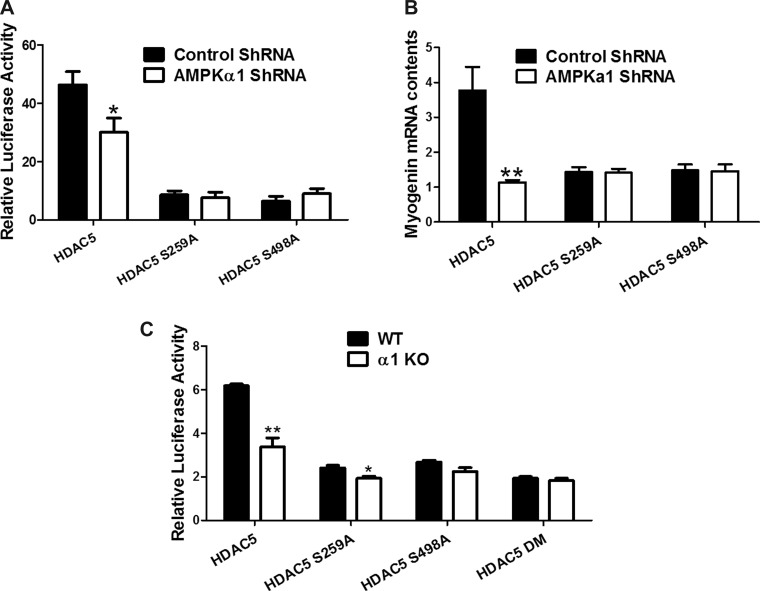
We further analyzed myogenin promoter-mediated luciferase activity. HDAC5 suppressed the transcriptional activity of the myogenin promoter in AMPKα1 knockdown cells; this effect was absent in cells transfected with the HDAC5 mutants, further demonstrating that AMPK regulates myogenin expression through phosphorylation of HDAC5 (Fig. 5A).
Fig. 5.
Mutation on HDAC5 phosphorylation sites abolished the effect of AMPK on myogenin promoter activity. A: C2C12 cells were transfected with pGL4 myogenin + luciferase vector, either scramble or AMPKα1 ShRNA, together with HDAC5, HDAC5 S259A, or HDAC5 S498A. Myogenin promoter activity was monitored by luciferase activity. B: C2C12 cells were transfected with either scramble or AMPKα1 ShRNA, together with HDAC5, HDAC5 S259A, or HDAC5 S498A. Myogenin mRNA contents were tested by RT PCR. C: WT and AMPKα1 KO primary myoblasts were transfected with HDAC5, HDAC5 S259A, HDAC5 S498A, or HDAC5 DM (with both Ser 259 and Ser 498 mutated to Ala), together with pGL4 myogenin promoter reporter vector. Luciferase activity was analyzed. *P < 0.05 vs. control; **P < 0.01 vs. control; means ± SE; n ≥ 3.
We also analyzed myogenin mRNA expression. Consistently, AMPKα1 knockdown cells transfected with HDAC5 exhibited reduced myogenin mRNA contents; however, cells transfected with either HDAC5 mutant showed no change in myogenin mRNA contents (Fig. 5B). In aggregate, these data show that AMPK inhibition downregulates myogenin transcription and myogenesis through HDAC5 in C2C12 cells.
Enriched primary myoblasts were further transfected with myogenin promoter-luciferase vector together with WT HDAC5, HDAC5 S259A, HDAC5 S498A, or HDAC5 with both sites mutated (HDAC5 DM). WT HDAC5 significantly suppressed the activity of myogenin promoter in AMPKα1 KO primary myoblasts compared with WT primary myoblasts (P < 0.01). HDAC5 S259A suppressed the activity of myogenin promoter in AMPKα1 KO primary myoblasts but to a lesser extent (P < 0.05). HDAC5 S498A caused a slight but not significant reduction in the activity of myogenin promoter. The HDAC5 double mutant failed to achieve any suppression in the activity of myogenin promoter compared with WT and AMPKα1 KO primary myoblasts (Fig. 5C). These data further confirmed that AMPK regulates HDAC5 through phosphorylation at S259 and S498 and these phosphorylations induce myogenin expression.
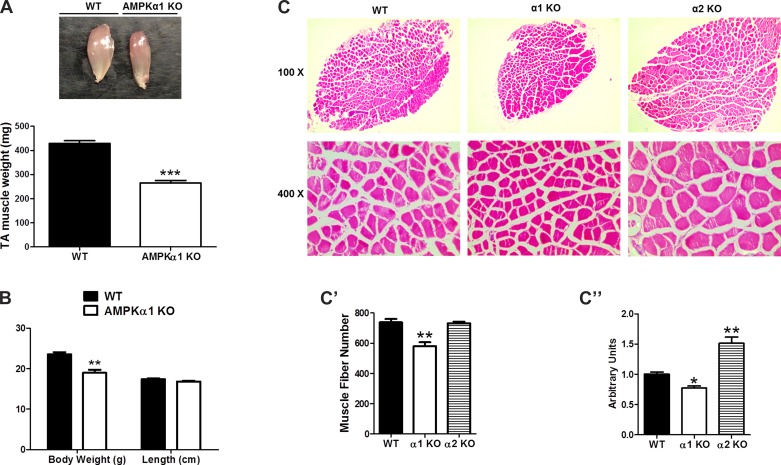
AMPKα1 KO mice had reduced muscle mass, less muscle fiber number and smaller muscle fiber diameter.
Our in vitro studies have clearly established that AMPKα1 KO attenuated myogenesis via phosphorylation of HDAC5. If this notion is correct, we expect to see that AMPKα1 KO mice has reduced muscle mass, less muscle fibers, and small muscle fiber size compared with those of WT mice. Indeed, the TA muscle in AMPKα1 KO mice was significantly smaller than that in WT mice. It should be noticed that the reduced muscle mass was due to smaller muscle diameter rather than reduced muscle length (Fig. 6A). The body weight of AMPKα1 KO mice was also found to be smaller than that of WT mice but no difference in head-to-tail length, indicating that the reduced body weight was mainly due to reduced muscle mass (Fig. 6B). As expected, the total number of fibers in the whole soleus muscle cross section of AMPKα1 KO mice was ∼25% less than that of WT mice, and muscle fibers in AMPKα1 KO mice were ∼20% smaller in diameter than muscle fibers in WT mice (Fig. 6C). However, an increased muscle fiber diameter (Fig. 6C) and muscle mass (data not shown) was observed in AMPKα2 KO mice, albeit no change in muscle fiber number, which further suggests an AMPKα1-specific role in myogenesis.
Fig. 6.
Effects of AMPK KO on muscle mass, muscle fiber number, body weight and head-to-tail length of mice. A: weight of TA muscle of WT and AMPKα1 KO mice. B: body weight and head-to-tail length of WT and AMPKα1 KO mice. C: hematoxylin and eosin staining of soleus muscle cross sections of WT mice, AMPKα1 KO mice and AMPKα2 KO mice. C′: muscle fiber numbers in soleus muscle from WT mice, AMPKα1 KO mice and AMPKα2 KO mice. C": average area of muscle fibers in soleus muscle from WT mice, AMPKα1 KO mice, and AMPKα2 KO mice. *P < 0.05 vs. control; **P < 0.01 vs. control; means ± SE; n ≥ 3.
DISCUSSION
Obesity and T2D are closely linked metabolic complications, both of which are increasing at alarming rates (34). Pathophysiological changes such as obesity and chronic inflammation are known to inhibit, rather than activate, AMPK (41), suggesting that inhibition, rather than activation, is more relevant to the pathophysiological roles of AMPK (7, 47). AMPK is phosphorylated by a constitutively active kinase, LKB1, which suggests that dephosphorylation rather than phosphorylation may be the main mechanism for regulating AMPK activity (39). In our previous studies, we observed that low AMPK activity correlated with impaired myogenesis (50), despite of undefined mechanisms.
To explore, we analyzed the structure of myogenin promoter and found a conserved MEF2 binding site on the myogenin promoter (17). It is well established that HDAC5 interacts with MEF2 to regulate gene expression (24, 25). We asked whether HDAC5 was involved in the regulation of myogenin expression. Indeed, HDAC5 knockdown increased H3K9 acetylation, which was associated with enhanced myogenin expression and myogenesis. We also treated C2C12 cells with MC1568, which stabilizes the repressor structure of HDAC5 (30); consistently, the myogenin level and myogenesis were decreased. In addition, overexpression of HDAC5 in C2C12 reduced myogenin content. In combination, these data indicate that HDAC5 negatively regulates myogenin transcription and myogenesis.
To further determine if HDAC5 directly regulates the transcriptional activity of the myogenin promoter, we cloned the myogenin promoter into pGL4 luciferase vector to construct a myogenin promoter-driven luciferase reporter. In line with above data, HDAC5 negatively regulates the transcriptional activity of myogenin promoter.
Phosphorylation of HDAC5 by protein kinases leads to its translocation from the nucleus to the cytoplasm. This represents a conserved mechanism by which HDAC class II family members regulate gene transcription (13). Our previous study showed that AMPK phosphorylates HDAC5 at Ser 259 and 498, promoting its nuclear translocation to induce β-catenin expression (48). Consistently, experiment showed that phosphorylation of HDAC5 is reduced in AMPKα1 KO myoblasts. We then tested whether the phosphorylation of HDAC5 by AMPK also regulates myogenin expression.
AMPK contains two catalytic subunit isoforms, α1 and α2. To further test whether this regulation is in an isoform-specific manner, we separated primary myoblasts from WT, AMPKα1 KO mice, and AMPKα2 KO mice and induced fusion. AMPKα1 KO but not AMPKα2 KO (data not shown) dramatically reduced myotube formation. A reduced myogenin level was found in AMPKα1 KO muscle compared with WT muscle. In addition, overexpression of HDAC5 mutants abolished the effect AMPKα1 knockdown on myogenin expression, myogenin promoter activity, and myogenesis in C2C12 cells. Furthermore, mutation of either HDAC5 Ser 259 or Ser 498 abolished the regulatory effects of AMPKα1 on myogenin in C2C12 cells, indicating that the phosphorylations of both Ser 259 and Ser 498 by AMPKα1 might be required for the efficient nuclear export of HDAC5.
Because RNA interference only partially knockdown AMPK catalytic α-subunit, to clearly elucidate the role of AMPKα1 on myogenin expression, we isolated primary myoblasts from neonatal WT and AMPKα1 KO mice, and these myoblasts were transfected with myogenin promoter-luciferase vector or with HDAC5 WT or HDAC5 mutants (Ser 259 and Ser 498 to Ala). HDAC5 WT dramatically suppressed myogenin promoter activity in AMPKα1 KO compared with WT primary myoblasts, showing that the absence of AMPKα1 activity attenuates the inhibitory effect on HDAC5. These mutants (Ser259Ala, Ser498Ala, and DM) suppressed myogenin promoter activity in both AMPKα1 KO and WT myoblasts to a similar extent, showing that these sites are necessary for induction of HDAC5 translocation by AMPKα1.
Our observation that AMPK activity is necessary to promote myogenin expression and myogenesis via phosphorylation of HDAC5 has important physiological implications. The obesity epidemic is becoming increasingly serious, and it is well demonstrated that obesity inhibits AMPK activity via TNFα (41). In addition, AMPK activity is also regulated by adipokines, such as leptin, adiponectin, and resistin, and cytokines, such as interleukin-6 (42). Therefore, obesity, inflammation, and other physiological factors that inhibit AMPK activity are likely to negatively affect myogenesis through downregulation of myogenin transcription, which may exert long-term negative effects on the mass and properties of skeletal muscle. Indeed, in this study, we observed that the fiber number in soleus muscle was reduced for ∼25% and muscle fiber diameter was reduced for ∼20% due to AMPKα1 KO, and TA muscle was found to be 30% smaller in AMPKα1 KO mice than in WT mice, clearly showing the importance of AMPK in the regulation of myogenesis. In addition, a reduced body weight but a normal head-to-tail length was observed in AMPKα1 KO, which could be mostly due to the reduced muscle mass. Hence, drugs activating AMPK, such as metformin, a common antidiabetic drug, can be used to activate AMPK to promote muscle development and regeneration. In addition, the demonstration of HDAC5 in the mediation of myogenin expression and myogenesis facilitates the exploration of HDAC5 inhibitors as additional chemical agents to enhance muscular development and regeneration, improving overall muscle properties. Interestingly, AMPKα2 KO was associated with increased muscle fiber diameter, which could be due to the inhibitory effects of AMPKα2 on protein synthesis suggesting that AMPKα1 and AMPKα2 have different roles in myogenesis and muscle hypertrophy (5).
In summary, AMPK phosphorylates HDAC5, which promotes the expression of myogenin and myogenesis. These data in combination with our previous observation in the regulation of β-catenin expression by HDAC5 (48) bring a uniform mechanisms linking AMPK to myogenesis through phosphorylation of HDAC5, a notion corroborated by the wide presence of MEF2 binding sites in the promoters of myogenic genes. Furthermore, in this study, we demonstrate that such promoting effect on myogenesis is mainly mediated by AMPKα1 subunit.
GRANTS
The work was supported by National Institute of Child Health and Human Development Grants 1R01-HD-067449 and 1R03-HD-057506 and US Department of Agriculture National Research Initiative Grant USDA-NRI-2008-35206-18826.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.F., J.-X.Z., J.L., M.-J.Z., and B.V. performed experiments; X.F., J.-X.Z., J.L., and M.-J.Z. analyzed data; X.F. interpreted results of experiments; X.F. prepared figures; X.F. and M.D. drafted manuscript; J.-X.Z. and M.D. conception and design of research; B.V. and M.D. edited and revised manuscript; M.D. approved final version of manuscript.
REFERENCES
- 1.Aberle ED. Myofiber differentiation in skeletal muscles of newborn runt and normal weight pigs. J Anim Sci 59: 1651–1656, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Allen RE, Merkel RA, Young RB. Cellular aspects of muscle growth: myogenic cell proliferation. J Anim Sci 49: 115–127, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Bayol SA, Macharia R, Farrington SJ, Simbi BH, Stickland NC. Evidence that a maternal “junk food” diet during pregnancy and lactation can reduce muscle force in offspring. Eur J Nutr 48: 62–65, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Bertos NR, Wang AH, Yang XJ. Class II histone deacetylases: structure, function, and regulation. Biochem Cell Biol 79: 243–252, 2001 [PubMed] [Google Scholar]
- 5.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Brameld JM, Mostyn A, Dandrea J, Stephenson TJ, Dawson JM, Buttery PJ, Symonds ME. Maternal nutrition alters the expression of insulin-like growth factors in fetal sheep liver and skeletal muscle. J Endocrinol 167: 429–437, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res 69: 5392–5399, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Brunetti A, Goldfine ID. Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J Biol Chem 265: 5960–5963, 1990 [PubMed] [Google Scholar]
- 9.Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 22: 1846–1855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2: 499, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Du M, Yan X, Tong JF, Zhao J, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod 82: 4–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci USA 97: 7835–7840, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, McKinsey TA, Olson EN, Jin ZG. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem 283: 14590–14599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handel SE, Stickland NC. The effects of low birthweight on the ultrastructural development of two myofibre types in the pig. J Anat 150: 129–143, 1987 [PMC free article] [PubMed] [Google Scholar]
- 16.Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc 36: 28–34, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Johanson M, Meents H, Ragge K, Buchberger A, Arnold HH, Sandmoller A. Transcriptional activation of the myogenin gene by MEF2-mediated recruitment of myf5 is inhibited by adenovirus E1A protein. Biochem Biophys Res Commun 265: 222–232, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Solis RS, Arias EB, Cartee GD. Postcontraction insulin sensitivity: relationship with contraction protocol, glycogen concentration, and 5′ AMP-activated protein kinase phosphorylation. J Appl Physiol 96: 575–583, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes 58: 2536–2546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RC, Wang ZM, Heymsfield SB. Skeletal muscle mass and aging: regional and whole-body measurement methods. Can J Appl Physiol 26: 102–122, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Lemercier C, Verdel A, Galloo B, Curtet S, Brocard MP, Khochbin S. mHDA1/HDAC5 histone deacetylase interacts with and represses MEF2A transcriptional activity. J Biol Chem 275: 15594–15599, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell 6: 233–244, 2000 [DOI] [PubMed] [Google Scholar]
- 25.McGee SL. Exercise and HDAC interactions. Appl Physiol Nutr Metab 32: 852–856, 2007 [DOI] [PubMed] [Google Scholar]
- 26.McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57: 860–867, 2008 [DOI] [PubMed] [Google Scholar]
- 27.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci USA 97: 14400–14405, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 11: 497–504, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundstrom K, Reinsch N, Gellin J, Kalm E, Roy PL, Chardon P, Andersson L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 288: 1248–1251, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Nebbioso A, Manzo F, Miceli M, Conte M, Manente L, Baldi A, De Luca A, Rotili D, Valente S, Mai A, Usiello A, Gronemeyer H, Altucci L. Selective class II HDAC inhibitors impair myogenesis by modulating the stability and activity of HDAC-MEF2 complexes. EMBO Rep 10: 776–782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niesler CU, Myburgh KH, Moore F. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp Physiol 92: 207–217, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Park SK, Sheffler TL, Spurlock ME, Grant AL, Gerrard DE. Chronic activation of 5′-AMP-activated protein kinase changes myosin heavy chain expression in growing pigs. J Anim Sci 87: 3124–3133, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Perdiguero E, Sousa-Victor P, Ballestar E, Munoz-Canoves P. Epigenetic regulation of myogenesis. Epigenetics 4: 541–550, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125: 1275–1287, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298: E751–E760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Saccone V, Puri PL. Epigenetic regulation of skeletal myogenesis. Organogenesis 6: 48–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab 287: E310–E317, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Selvi RB, Kundu TK. Reversible acetylation of chromatin: implication in regulation of gene expression, disease and therapeutics. Biotechnol J 4: 375–390, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4: 465–474, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Steinberg GR, Watt MJ, Febbraio MA. Cytokine Regulation of AMPK signalling. Front Biosci 14: 1902–1916, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Tong JF, Yan X, Zhao JX, Nathanielsz PW, Du M. Metformin mitigates the impaired development of skeletal muscle in the offspring of obese mice. Nutr Diabetes 1: e7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111: 91–98, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: Lessons from transgenic and knockout animals. Front Biosci 14: 19–44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, Du M. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 151: 380–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem 285: 19051–19059, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao JX, Yue WF, Zhu MJ, Du M. AMP-activated protein kinase regulates beta-catenin transcription via histone deacetylase 5. J Biol Chem 286: 16426–16434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586: 2651–2664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586: 2651–2664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]