Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder consisting of chronic bronchitis and/or emphysema. COPD patients suffer from chronic infections and display exaggerated inflammatory responses and a progressive decline in respiratory function. The respiratory symptoms of COPD are similar to those seen in cystic fibrosis (CF), although the molecular basis of the two disorders differs. CF is a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene encoding a chloride and bicarbonate channel (CFTR), leading to CFTR dysfunction. The majority of COPD cases result from chronic oxidative insults such as cigarette smoke. Interestingly, environmental stresses including cigarette smoke, hypoxia, and chronic inflammation have also been implicated in reduced CFTR function, and this suggests a common mechanism that may contribute to both the CF and COPD. Therefore, improving CFTR function may offer an excellent opportunity for the development of a common treatment for CF and COPD. In this article, we review what is known about the CF respiratory phenotype and discuss how diminished CFTR expression-associated ion transport defects may contribute to some of the pathological changes seen in COPD.
Keywords: COPD, cystic fibrosis, CFTR, mucus stasis, respiratory infections, epithelial sodium channel (ENaC), calcium-activated chloride channel
chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) are airway diseases that are characterized by airflow limitation and a progressive loss of lung function (127, 130, 159). CF is an autosomal recessive disease and affects ∼70,000 people worldwide. COPD is caused by both environmental and genetic factors and affects more than 64 million people in the world (http://www.who.int/mediacentre/factsheets/fs315/en/). The leading risk factor for COPD is cigarette smoking (95), and presently there are more than 1 billion cigarette smokers worldwide (http://www.who.int/mediacentre/factsheets/fs339/en/). Most COPD patients have smoked for more than 20 years. Male smokers are 12 times more likely to die from COPD compared with men who do not smoke (2). Furthermore, a large population is exposed to secondhand smoke, which is associated with considerable morbidity both in adults and children (1). Other causes of COPD include occupational exposures to dust, chemical fumes (∼15–20% of the cases), and genetic factors such as mutations in the α1-antitrypsin gene (∼3%) (94). As would be predicted, α1-antitrypsin gene mutations exacerbate the progression of COPD in smokers (112).
The symptoms of COPD involve airflow limitation, strong inflammatory responses, and protease imbalance (107). Approximately two-thirds of patients with COPD exhibit chronic bronchitis, characterized by excess mucus production and recurrent cough. Chronic bronchitis displays the classic features of the CF respiratory phenotype, which include mucus accumulation (12, 117, 127), chronic bacterial infections, and persistent neutrophilic airway inflammation (134, 140). Mucus hypersecretion and stasis directly contribute to bacterial infections and subsequent inflammatory responses in COPD and are therefore considered a major contributor to lung function decline seen in these patients (64, 65, 153). The other main symptom of COPD is emphysema. Most COPD patients present a combination of emphysema and inflammation with a relatively high incidence of bronchiectasis (51, 91). Although the bacterial pathogens found in COPD lungs differ from those found in CF patients, it is important that the lung tissue microbiome is altered in both conditions compared with control subjects (144). Furthermore, lower airway mucus obstruction is present in both diseases (24, 39, 96, 99), leading to an accelerated loss of lung function. Even COPD patients with emphysema but without chronic bronchitis display mucus obstruction in the small airways (63, 64, 154).
The Roles of Proper Sodium and Chloride Transport in the Regulation of the Airway Surface Liquid
Because of the similar symptoms of CF lung disease and COPD, it is tempting to consider a common factor that can be affected by both cystic fibrosis transmembrane conductance regulator (CFTR) mutations and environmental insults. Indeed, physiological respiratory functions are tightly linked with epithelial ion and water transport pathways (80). Ion channels that regulate Na+ and Cl− transport in the lung are required for the proper hydration and ionic composition of airway surface liquid (ASL). For Cl− transport, CFTR is the primary apical ion channel in the lung epithelium. In fact, CFTR was one of a number of candidate genes suggested as a risk factor for COPD (82). Inhibition of CFTR function has been shown to reduce ASL thickness and hydration, ciliary beat frequency, and mucociliary clearance and results in decreased clearance of respiratory pathogens (38). Interestingly, however, Cftr knockout mice lack respiratory pathology, suggesting that alternative Cl− channels in the murine lung may compensate for the loss of CFTR (35). Increased Na+ absorption through epithelial sodium channels (ENaC) in the proximal airways of CF patients was proposed more than three decades ago by Knowles et al. (73) when they reported a higher in vivo nasal and tracheobronchial potential difference in CF patients than in normal volunteers (73). Furthermore Jiang et al. (68) demonstrated that confluent monolayers of airway cells from patients with CF had higher levels of amiloride-sensitive Na+ reabsorption compared with those from normal individuals (68). In vitro studies in human airway epithelial cells indicate that CFTR regulates the functional and surface expression of ENaC (128). ENaC activity has been shown to be inversely correlated to predicted CFTR levels, and, more importantly, CFTR heterozygous and homozygous knockout mice have higher levels of proteolytically processed ENaC subunit levels in the lungs (77). Interestingly, the β-ENaC transgenic mouse, which overexpresses β-subunit of the sodium channel, develops CF-like lung disease (88) and therefore serves as a model for both CF (86) and COPD (88). Furthermore, complete loss of CFTR in the β-ENaC mouse has been shown to exacerbate the extent of lung injury (81). Interestingly, overexpression of transgenic human CFTR in the lung fails to correct the β-ENaC mouse lung disease (56). This is consistent with another report demonstrating that elevations in ENaC activity caused by β-subunit mutation exhibit CF-like symptoms (121). However, it needs to be emphasized that the CF pig model [Cftr−/−] with normal ENaC function still develops lung disease, suggesting that enhanced ENaC function is not necessary for the development of the CF respiratory phenotype in this model (30, 38)]. Thus ENaC overexpression contributes to abnormal mucus rheology and may result in significant pathology due to mucus stasis and airway obstruction. However, whether this occurs in patients with CF and COPD disease needs to be determined. This short summary clearly demonstrates that the causative roles of chloride and sodium transport in the development of the CF lung disease and COPD remain controversial. What is clear is that a disturbance in the delicate balance between Na+ reabsorption and Cl− secretion may contribute to alterations in the thickness and/or ionic composition of the ASL with pathological consequences. Because of their location, airway epithelial cells are exposed to large concentrations of environmental pollutants including cigarette smoke. Indeed, studies from several laboratories have demonstrated that sustained exposure to cigarette smoke, or cigarette smoke extract, containing reactive intermediates (free radicals) reduces CFTR expression and function (16, 21, 26, 36, 138). Considering the experimental data available to date and space limitations, we opted to concentrate on the role of CFTR in the development of the COPD and CF respiratory disease. We also discuss how therapies directed toward augmenting CFTR function may decrease the severity of COPD.
CFTR and Cystic Fibrosis
CF is an autosomal recessive disorder caused by mutations in the CFTR gene (reviewed in Ref. 127). CFTR is a cAMP-activated anion channel expressed on the apical surface of a number of epithelial cell types including airway cells. CFTR belongs to the ATP-binding cassette (ABC) family of transporters and is composed of two halves that are connected by a regulatory domain. Each half consists of six transmembrane segments and a nucleotide-binding domain.
More than 1,900 mutations have been identified in the CFTR gene. The most common mutation, loss of phenylalanine at position 508 (F508del), is found in ∼90% of the patients with CF. This mutation yields a CFTR protein that is retained in the endoplasmic reticulum and degraded. Similarly, another mutation, G551D, yields a protein that is transported to the cell surface but is unable to transport anions and thus has a channel-gating defect. Only six other mutations have a frequency of greater than 1% in the CF population (G542X, W1282X, G551D, 621 + G→T, N1303K, and R553X) (157). The loss of CFTR function results in altered exocrine secretion and pathological changes in the airways, gastrointestinal tract, pancreas, sweat glands, hepatobiliary system, and genital tract. Although there is no cure for CF, current therapies, particularly with regard to the gating mutants (G551D and others), are highly effective toward augmenting CFTR function and improving clinical outcome (5, 48, 120, 151). The leading cause of death in CF is respiratory failure caused by persistent airway infections, exaggerated inflammatory responses, and bronchiectasis. Loss of CFTR function leads to viscous secretions of the exocrine glands in multiple organs and results in chronic lung infections, recurrent wheeze, bronchiectasis, nasal polyposis, chronic sinusitis, cor pulmonale, reduced exocrine pancreatic functions, and inadequate intestinal absorption (127). The gastrointestinal symptoms are generally well controlled with modern therapy, whereas the respiratory symptoms persist and lead to progressive respiratory failure.
Mucociliary Clearance Defects in CF and COPD
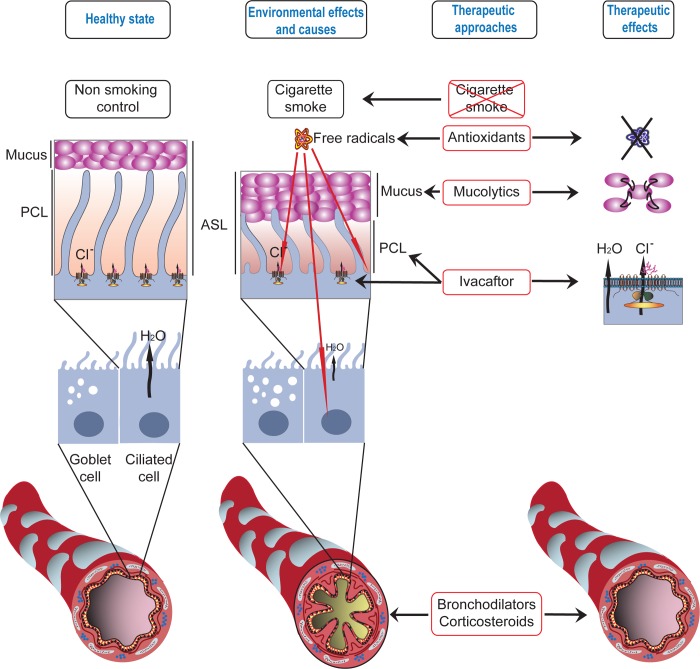
Compromised mucociliary clearance is a central feature of the respiratory pathology both in CF and COPD. A breakdown in this component of the innate immune response results in inefficient removal of inhaled chemicals, particles, and pathogens from the airways and contributes to chronic inflammation (for a review see Ref. 80). Airway surfaces are coated with a thin mucous fluid layer called the ASL. The ASL is continuously being transported toward the pharynx by ciliary movements. Both the composition of the ASL and efficient ciliary action are critical for cleansing the airways for maintaining normal respiratory function (80). Pathogens bind to the surface mucus layer, whereas the underlying fluid, the periciliary layer (PCL), is necessary to support ciliary beat (53, 80). Under physiological conditions, the PCL is the same height as the cilia (∼7 μM). A common feature of COPD and CF is that the PCL height is reduced in experimental models (Fig. 1), leading to compromised mucus clearance (23, 36, 138) and chronic bacterial infections (22). The reduction in ASL height has been attributed to sodium hyperabsorption caused by dysregulated ENaC function (22, 23, 84, 85, 87), although it is possible that the reduction of CFTR-mediated chloride secretion may be sufficient to mediate this effect. Other reports, however, disagree whether the ASL height is affected in CF (30, 44). Nevertheless, recent studies indicate that newborn pigs lacking CFTR (Cftr−/−) do not present enhanced ENaC function but develop airway pathology similar to CF (30). In addition, the majority of studies agree that mucociliary clearance is compromised in CF through the loss of CFTR function.
Fig. 1.
Environmental effects on airway surface liquid and therapeutic approaches for correcting these defects. Chronic obstructive pulmonary disease is often caused by years of cigarette smoke or exposure to environmental pollutants. These environmental insults lead to decreases in the periciliary layer (PCL) that lines the lungs and increases in mucus production that lead to compromised bacterial clearance, hyperinflammatory responses, and chronic bacterial infections. Mounting evidence also suggests that ion channels, including the chloride channel defective in cystic fibrosis, are decreased in cigarette smokers. Potential therapies are directed toward 1) breaking up the elevated mucus with mucolytics, 2) treatments with antioxidants to diminish the harmful effects of free radicals, 3) the use of bronchodilators and corticosteroids to open up the airways and decrease inflammation, and, most recently, 4) the concept of increasing chloride channel function to increase airway hydration.
Inflammatory Responses in CF and COPD
Both CF and COPD are characterized by recurring bacterial and viral infections that activate innate immune responses and promote mucus cell hyperplasia, recruitment of neutrophils and macrophages, and activation of the unfolded protein response (UPR) (for a review see Ref. 123). There is also evidence that UPR markers such as the endoplasmic reticulum resident chaperone, the binding immunoglobulin protein (BiP, hsp70, grp78) and protein disulfide isomerase (PDI) are elevated in lung samples from smokers (72). Although it is not clear how smoking induces the UPR (123), the reactive species present in cigarette smoke could lead to oxidative stress and induction of the UPR (70). Cigarette smoke has also been shown to elevate IL-8, a neutrophil chemoattractant, by increasing IL-8 mRNA stability (98). Furthermore, T cell responses have been shown to be important in COPD. Podolin et al. (113) found that T cell depletion in a mouse model of COPD inhibited alveolar destruction caused by chronic cigarette smoke exposure. Cigarette smoke has been shown to induce reversible changes in energy metabolism and the cellular redox status that are not related to the inflammatory responses in mouse lung (6). In these studies, microarray analysis in mouse lungs after exposure to cigarette smoke for 8 wk revealed that genes involved in metabolism, mitochondrial function, and redox regulation were all upregulated compared with controls (6). Other important proteins, however, are downregulated in response to cigarette smoke. Histone deacetylase 2 (HDAC2) regulates a number of important cellular processes including inhibiting inflammatory responses and is posttranslationally modified by oxidative stress caused cigarette smoke exposure. This leads to HDAC2 reduction in patients with COPD, and loss of this protein exacerbates the clinical pathology of this disease (reviewed in Ref. 162).
Reduced ASL pH has been shown to impair bacterial killing in the CF pig model (110). This suggests that the initial insult in CF is caused by loss of bicarbonate transport across CFTR and leads to reduced ASL pH (110). Impaired bacterial killing and/or clearance would certainly exacerbate the symptoms by amplifying the inflammatory response, which in turn would activate the UPR (123). Furthermore, cigarette smoke and the reactive species present in cigarette smoke (see Effects of Cigarette Smoke on Anion Transport) have been shown to activate the UPR (123), leading to decreased CFTR expression and function (13, 14, 22, 116, 117). The exaggerated inflammatory responses sustain the UPR and lead to apoptosis, tissue injury, fibrosis, and a continuous decline in lung function (33). Toll-like receptor 4 (TLR4) has been shown to provide a protective effect against emphysema-like changes in the lung after exposure to cigarette smoke since TLR4-deficient mice show more cigarette smoke-induced autophagy compared with controls (10).
Interestingly, it has been proposed that loss of CFTR function itself leads to lung inflammation (109). Perez et al. (109) showed that treatment of primary cultures of polarized human tracheal epithelial cells with a specific CFTR inhibitor, CFTRinh-172, for 5 days mimicked the inflammatory profile seen in CF patients and included an increase in IL-8 secretion. Because removal of the inhibitor reversed this effect, it suggested that loss of CFTR activity was responsible for the inflammatory cascade observed in CF (109). The elevated levels of secreted IL-8 in CF results in neutrophil recruitment and a subsequent increase in inflammation that exacerbates the problem (18, 33). Further support for the importance of CFTR in maintaining the physiological environment of tissues comes from studies on the pancreas of newborn CF pigs that indicate the presence of increased neutrophil, macrophage, and lymphocyte infiltration in dilated acini and in the interstitium, without an apparent bacterial infection. This suggests the proinflammatory nature of mucus stasis itself (3). Despite these reports, whether loss of CFTR itself or the subsequent infection promotes the inflammatory response remains controversial. For example, a recent in vitro study demonstrated that airway epithelial cells expressing ΔF508 mount a less pronounced inflammatory response to Pseudomonas aeruginosa (59) compared with cells expressing the wild-type protein. Furthermore, lung infection preceding the onset of inflammation has been reported in CF pigs (143).
CF Therapies
Considering the monogenetic nature of CF, gene replacement therapy showed great promise in the 1990s. However, progress in gene therapy has been much slower than anticipated (55). Alternative approaches have included high-throughput screens to identify small molecules that promote either 1) F508del CFTR rescue and delivery to the cell surface (108), 2) read-through of premature stop mutations to override nonsense mutations and help translation to produce full-length proteins (e.g., G542X, W1282X, etc.) (160), or 3) potentiation of channel-gating mutations such as G551D (151). The last class of compounds is particularly interesting since drugs such as ivacaftor (formerly VX-770) that potentiate the activity of CFTR mutants that have channel-gating defects have also been shown to dramatically increase the channel activity of wild-type CFTR (138, 151). Channel-gating mutations reach the apical cell surface but fail to conduct ions in response to elevations in cAMP (164). Ivacaftor has been shown to be a very effective activator of all of these CFTR mutant variants (164). Unfortunately, only ∼5% of the CF cases consist of gating mutations, even including the 10 newly identified mutations (164). However, considering that ivacaftor potentiates wild-type CFTR also, this drug may be beneficial in acquired CFTR deficiencies such as COPD that have decreased wild-type CFTR activity.
CFTR mutations are known to play a causative role in a number of other diseases including recurrent idiopathic pancreatitis (37, 135), congenital bilateral absence of vas deferens (115), bronchopulmonary aspergillosis (78), chronic sinusitis (83), and idiopathic bronchiectasis (54, 111). The fact that even mild CFTR dysfunction contributes to disease pathology supports the hypothesis that decreases in wild-type CFTR activity, if severe enough, could have detrimental effects and contribute to disorders such as COPD.
Effects of Cigarette Smoke on Anion Transport
Inhaled tobacco smoke consists of gas and solid (tar) phases. The gas phase contains very high concentrations of short-lived reactive intermediates such as superoxide, hydrogen peroxide, and nitric oxide, and a very large number of volatile compounds (150). The reactive species in cigarette smoke are detoxified by antioxidants (ascorbate and reduced glutathione) in the respiratory epithelial lining fluid. When antioxidants are depleted, reactive species interact with biological targets on respiratory epithelial cells. The solid phase of gas smoke (tar) contains very high concentrations of stable free radicals such as semiquinones, hydroquinones, and carbon-centered free radicals (114). Cigarette tar can produce large amounts of H2O2 in aqueous extracts (101), cause the release of iron, and lead to the production of hydroxyl radicals (97). In addition, cigarette smoke and reactive intermediates have high inflammatory potential and promote recruitment of activated inflammatory cells in the lung interstitial and air spaces, which further contribute to tissue injury (118). Recent studies by Thatcher et al. (148) showed that Neu-164 and Neu-107, two small molecule inhibitors of myeloperoxidase and potent antioxidants, inhibited cigarette-induced inflammation through scavenging reactive oxygen species and reducing the accumulation of inflammatory cells.
In 1983, prior to the identification of the CFTR gene, Michael Welsh demonstrated that cigarette smoke inhibited anion transport in canine tracheal epithelium in vitro and in vivo and first suggested that this effect might explain the abnormal mucociliary clearance and airway disease seen in cigarette smokers (158). Cantin and colleagues (26) tested the effects of cigarette smoke exposure in human airway cells (Calu-3) and demonstrated that CFTR expression and function were decreased by cigarette smoke. Using nasal potential difference (NPD) measurements, they demonstrated that healthy smokers without CFTR mutations displayed an NPD pattern consistent with CFTR deficiency. More importantly, they made the initial suggestion that this could contribute to the pathophysiology of chronic bronchitis seen in cigarette smokers (26). In more recent studies, Kreindler and colleagues (75) demonstrated that cigarette smoke extract inhibited chloride secretion and increased mucin secretion in human bronchial epithelial cells and suggested that this ion transport phenotype was similar to that of CF epithelia. Rowe, Dransfeld, and colleagues confirmed that CFTR dysfunction is present in smokers with and without COPD in the nasal airway (138) and lung (46), establishing the presence of abnormal chloride transport in individuals with clinical disease. Furthermore, in both the nose and lung, CFTR dysfunction was associated with chronic bronchitis, indicating the clinical relevance of the findings (46, 138). Tarran and colleagues (36) showed that cigarette smoke exposure induces CFTR internalization and delivery to an aggresome-like compartment and that loss of CFTR results in ASL volume depletion and mucus dehydration. ASL dehydration caused by smoke exposure was also shown by Rowe and colleagues (138), which caused delayed mucociliary transport in vitro. Both groups suggested that these effects contribute to the development of chronic bronchitis that is frequently seen in smokers (36, 138).
To better understand the mechanisms by which smoke alters CFTR function, a number of investigators exposed epithelial cells to reactive species and assessed their effects on CFTR function. Regulation of CFTR by nitric oxide (NO) and its congeners is complex: whereas S-nitrosation (i.e., addition of a nitrosonium to a reduced cysteine) of CFTR increases maturation and function of both wild-type and ΔF508 CFTR (165, 166), glutathionylation, oxidation, and nitration of CFTR are associated with loss of function and decreased levels of CFTR (16, 156). In addition, activation of cGMP-dependent protein kinase type II (PKGII) is associated with phosphorylation of CFTR and increased Cl− secretion across intestinal and airway cells (42, 149). However, other studies have shown that cGMP-dependent phosphorylation of CFTR in airway cells was not associated with increased activity as a Cl− channel (17). In a series of studies, Matalon and coworkers (31, 32) showed that very small concentrations of NO (generated by NO donors) stimulated Cl− secretion across Calu-3 cells by stimulating CFTR activity via cGMP-dependent mechanisms. The NO-induced increase in the Cl− current was reversed by addition of two phosphatases, suggesting that phosphorylation of CFTR by cGMP may be responsible for the CFTR activation. Indeed PKGII, but not PKGI, phosphorylated CFTR immunoprecipitated from Calu-3 cells in vitro (31). Furthermore, measurements of NPD in anesthetized mice showed that perfusion of the nares with an NO donor compound, (Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl) amino] diazen-1-ium-1, 2-diolate (DETANONOate), activated glybenclamide-sensitive Cl− secretion. Interestingly, PKGII did not phosphorylate CFTR from alveolar type II (ATII) cells in vitro, and NO or cGMP analogs did not increase Cl− secretion across amiloride-treated ATII cells in vitro. These findings suggest that small concentrations of NO donors may prove beneficial in stimulating Cl− secretion across airway cells without promoting alveolar edema. However, in a recent study, Kuebler and colleagues (141) showed that acute increases of left atrial pressure stimulated eNOS that resulted in decreased Na+ absorption and increased Cl− secretion that led to pulmonary edema (141).
In contrast to the acute changes caused by NO, more prolonged exposures to reactive intermediates inhibits CFTR function. Exposure of confluent monolayers of Calu-3, 16HBE, or mouse tracheal epithelial cells to low levels of NO generated by DETANONOate decreases CFTR expression and Cl− secretion in response to cAMP. These changes were found to be due to posttranslational modification of CFTR by reactive oxygen nitrogen intermediates. All of these studies suggest that exposure to reactive species compromises CFTR function (16, 69).
Treatments for COPD
COPD is among the most common causes of death worldwide and current pharmacological treatments offer no significant benefits concerning disease outcome (89, 100, 117). Although smoking cessation is essential to slow the progressive decline of respiratory function in COPD, novel treatments are necessary for both current and ex-smokers to reduce disease progression. The need for new COPD therapies is obvious, despite clear evidence that cigarette smoking is a major contributing and modifiable risk factor (71, 92). Fortuitously, it is very significant that improving COPD outcome leads to increases in the function of other organ systems, including the kidneys and heart (8).
The obvious first step in COPD treatment is smoking cessation. However, many COPD patients continue to suffer from chronic bronchitis (90), most likely due to irreversible functional and anatomic changes. Bronchodilators, frequently in combination with inhaled corticosteroids, remain the mainstay of treatment (Fig. 1). Although these treatments usually reduce the number of acute exacerbations, improve dyspnea, and increase quality of life, they do not significantly improve the mortality associated with COPD (25, 47, 147). Furthermore, inhaled steroids enhance the risk of pneumonia and have no effect on mucus obstruction or its clearance (40, 47, 137). Supplemental oxygen, although life saving in those who are hypoxic, does not alter COPD pathogenesis.
The connection between therapeutic approaches for CF and COPD has frequently involved the repurposing of agents used in one disease for treatment of the other. For example, intravenous corticosteroids during acute exacerbations of respiratory inflammation (45, 104), chest physiotherapy (28, 60, 93), and chronic administration of azithromycin (9, 131) have been shown improve the symptoms in both patient populations. An important exception is that dornase alpha, which clinically improves lung function and acute respiratory exacerbation frequency in CF patients (34), was found to be harmful in COPD patients (105). Nonantibiotic macrolides have also been suggested to limit human neutrophil activation of ENaC in both CF and COPD and may improve mucus stasis by limiting airway surface liquid depletion (146).
Mucus hypersecretion and stasis directly contribute to bacterial infections and subsequent inflammatory responses in both diseases and are therefore considered causative for the lung function decline seen in both patient populations (64, 65, 125, 153). Current mucolytics, however, have provided only marginal benefits with the exception of N-acetyl-cysteine and carbocysteine in some COPD patients (43, 52, 103, 167). This last example points to the involvement of reactive intermediates in disease progression. The lack of efficacy provided by mucolytics has been attributed to poor bioavailability and failure to deliver the drug to the distal airways where mucus obstruction is observed (19, 124, 126, 129). A novel approach that targets the small airways could overcome these limitations and improve mucus stasis, decrease airway obstruction, and inhibit lung function decline.
The limited benefit of mucolytic therapy in COPD patients supports an obvious need for alternative therapies to combat mucus stasis (50). The discovery of potent CFTR activators such as ivacaftor for CF patients with CFTR mutations leading to functional defects such as G551D and the observed CFTR dysfunction seen in COPD patients prompted some investigators to test the compound for both disorders (138). Nevertheless, ivacaftor effectively recovered the cigarette smoke-induced CFTR functional defect and partially restored ASL depth and mucociliary clearance in airway epithelial cells (138). Given that the Cl− conductance defect was CFTR specific (138) and most COPD patients do not have mutations in their CFTR gene, pharmacological approaches aimed at enhancing wild-type CFTR activity could be potentially beneficial in COPD by addressing mucus accumulation through a systemic, rather than an inhaled route. There are, however, limitations to this type of approach and presumably other medications including bronchodilators would still be required.
The use of CFTR potentiators or any drugs that affect CFTR expression and/or function are certainly not expected to correct all of the defects associated with COPD. However, they may be particularly helpful in COPD patients that exhibit CFTR dysfunction and manifestations of delayed mucociliary clearance such as those with chronic bronchitis. In contrast, those with emphysema or other irreversible lung remodeling are much less likely to benefit. It is also important to examine how persistent the loss of CFTR is after smoking cessation to understand the long-term consequences of CFTR dysfunction in COPD. Although smoking has a profound effect on CFTR expression immediately after exposure (36), it is unclear how long or how profound this effect lasts. Are there chronic effects that cannot be reversed with cessation? Previous studies suggest recovery of CFTR function in the nose following at least one year of smoking cessation (138), whereas the lung may be slower to recover (46). These and other concerns need to be considered. Although CFTR potentiators may be especially useful for those patients with reduced CFTR activity even in the absence of congenital mutations of the CFTR gene, they may not be efficacious in patients with extremely low levels of surface CFTR expression, in patients with other ion transport abnormalities, or in those without cigarette smoke-induced injury, such as individuals with α1-antitrypsin gene mutations. Would enhancing CFTR function reverse the effects of ENaC overactivity? Clearly in a complex disorder such as COPD increasing CFTR function is just one aspect to test of many alternative strategies. In that regard, some CF therapies such as chronic azithromycin have translated well in COPD patients (9), whereas others like dornase alpha have not, emphasizing that important differences in the pathogenesis of these two diseases can affect the success of mechanism-based therapeutics. With this in mind, until CFTR potentiators or other ion transport strategies are tested in patients, this type of therapy will remain speculative.
Alternative Chloride Channels (Calcium-Activated Chloride Channels)
One potential therapeutic approach to treat CF has been to stimulate alternative chloride channels in airway epithelial cells (168, 169). One promising candidate is the transmembrane protein 16A [TMEM16A; also called anoctamin 1 (ANO1)]. TMEM16A is a calcium-activated Cl− channel that is activated by intracellular calcium and calcium-mobilizing stimuli (27, 133, 161). TMEM16A was identified in 2008 and predicted to have eight transmembrane domains and activated by G protein-coupled receptors (GPCRs) (161). A number of GPCRs can activate Ca2+-dependent Cl− currents including ATP, acetylcholine, and histamine (76). For that reason, purinergic receptor activation (P2Y) has been tested as a therapeutic approach in CF (4, 163).
Given the properties of TMEM16A as an alternative chloride channel, Veit et al. (152) tested whether activation of TMEM16A could reduce the expression of proinflammatory cytokines IL-6, IL-8, and CXCL1/2 in two human airway epithelial models (152). Their results indicated that direct activation of TMEM16A with a small-molecule activator, activator F, inhibited IL-8 secretion in a human CF bronchial epithelial cell line (CFBE), whereas P2Y receptor agonists did not. These data suggest that direct activation of the channel was necessary for this effect (152). Using an inducible expression system, Veit et al. demonstrated that wild-type CFTR expression could also attenuate proinflammatory cytokine secretion, whereas G551D CFTR could not, indicating that chloride channel activity was necessary for this effect (152). Veit et al. suggest that the inability to activate TMEM16A with a P2Y receptor agonist may explain the limited efficacy of denufosol (152) in a 24-wk Phase 3 clinical trial for CF patients (4). However, despite considering TMEM16A as an alternative target, TMEM16A is only a minor component of calcium-activated chloride channel activity in airway epithelial cells (102).
CFTR is a major chloride channel in airway epithelium and its expression is inhibited by cigarette smoke. However, the effects of cigarette smoke on calcium-activated chloride channels remained unknown until recently. Using primary murine nasal septum epithelial cells and human sinonasal epithelial (HSNE) cultures, Virgin et al. (155) monitored P2Y purinergic receptor activation with UTP or ATP along with simultaneous inhibition of CFTR (152). These results indicated that cigarette smoke extract inhibited calcium-activated chloride channels in sinonasal epithelial cultures. Therefore, both CFTR and calcium-activated chloride channels are compromised by cigarette smoke extract in the airways (155), suggesting a severe chloride/bicarbonate transport defect.
MicroRNA Networks in COPD
Smoking undoubtedly affects gene expression in human airway epithelium (15, 29, 49, 58, 61, 142), and at least part of these changes can be attributed to differential expression patterns of microRNAs (miRs) (49, 132). miRs are noncoding RNAs that posttranslationally downregulate gene expression by inhibiting translation or degrading mRNA (79). Animal studies using mice and rats indicated that the expression of a number of miRs is decreased in response to smoke exposure (66, 67), suggesting an increase in mRNA and protein of the miR targets. In human studies, Schembri et al. (132) compared whole-genome miR and mRNA expression in bronchial airway epithelium between smokers and nonsmokers and found that 28 miRs were differentially expressed (132). They confirmed that when the levels of these miRs were reduced, the levels of target mRNAs were increased. More importantly, similar to the animal studies, most of the miRs were also reduced in the smokers' airway epithelial cells.
In an intriguing study, Ezzie et al. (49) examined the differential expression of miRs and mRNAs in whole lung tissue extracts from smokers with and without COPD (49). Using miR and mRNA arrays, they identified 70 miRs and 2,667 mRNAs that were differentially expressed (at least ±1.5-fold) (49). In this comparison, 13 miRs were reduced and 57 were increased in COPD. Interestingly, the levels of two miRs, miR-223 and miR-1274a, were higher in most COPD patients compared with smokers without obstruction (49). This is particularly noteworthy given that studies by Oglesby et al. (106) suggest that 1) miR-223 is increased in vivo in bronchial epithelium of individuals carrying the ΔF508 mutation, 2) functional inhibition of CFTR in vitro increases the expression of miR-223, and most importantly, 3) miR-223 downregulates CFTR expression (106). On the basis of the increased levels of miR-223 in COPD patients, it is tempting to speculate that miR-223 contributes to reduced CFTR expression in COPD patients.
Using the previously generated miR profiles, Ezzie et al. (49) examined their predicted targets and associated regulatory pathways. They identified the transforming growth factor (TGF-β) pathway as a potential player in the pathogenesis of COPD. TGF-β1 levels have been shown to be elevated in the small airway epithelium of smokers and COPD patients (145). Furthermore, TGF-β1 elevation appears to be important for recruiting macrophages into the airway epithelium of COPD patients (11, 41). Again, the connection to CFTR is apparent given the recent data indicating TGF-β1-mediated reduction of CFTR biogenesis in primary HBEs (139). Taken together, the results from several laboratories suggest that miRs may play an important role in regulating COPD and CF associated cellular pathways.
Recently, Hassan et al. (62) demonstrated that cigarette smoke and cadmium negatively regulate CFTR expression by affecting several steps in CFTR biogenesis (62). Both cigarette smoke and cadmium upregulate miR-101 and miR-144, two miRs that they demonstrate directly target the 3′ untranslated region of CFTR (62). And finally, they demonstrated that miR-101 is more highly expressed in lung samples from COPD patients than controls (62), providing another linkage between cigarette smoke, miRs, and decreased CFTR expression.
Summary and Future Directions
It is clear from the discussed studies that cigarette smoking leads to decreases in CFTR mRNA, protein, and function and contributes to mucus transport defects in patients with COPD and chronic bronchitis. The first in the sequence of events is the loss of chloride/bicarbonate channel activity, which alters mucus stasis and contributes to chronic bronchitis. Given the relatively moderate decrement in CFTR protein and function seen after cigarette smoke exposure, it is surprising that the effects on mucus clearance are so profound. Certainly the problem is exacerbated by the concomitant increase in mucin production that is also induced by cigarette smoking. However, airway hydration (23, 36, 138), airway pH (110), and viscosity of the airway mucus (44) are tightly regulated by CFTR-mediated chloride/bicarbonate secretion, and these results may reflect that mucus transport and susceptibility to infection are critically tied to even modest CFTR functional abnormalities.
Understanding the underlying defect may lead to the development of several treatment strategies for COPD. The complicating factor is trying to understand all of the causative effects that cigarette smoke has on ion channel activity and how these alterations affect respiratory function. Although there is evidence that CFTR, calcium-activated chloride channels, and possibly ENaC activities are affected, questions remain as to what other ion channels are involved. Would stimulation of CFTR and inhibition of ENaC be a viable approach in conditions like chronic bronchitis or would stimulation of CFTR be sufficient to balance the normal hydration properties of airway epithelia? These and other questions need to be answered to fully identify potential treatment opportunities. Blocking the dehydration effects of cigarette smoke is one approach that has been tested. Clunes et al. (36) demonstrated that hypertonic saline restored ASL height in cigarette smoke-exposed, dehydrated airway cultures, and this treatment could be potentially employed in COPD patients (36).
What other conditions are known to decrease CFTR activity? Heavy metals (62, 74, 122), hypoxia (57), endoplasmic reticulum stress and the UPR (116), accumulation of ceramide during the UPR (20, 64), and miRs (106, 119) regulate CFTR expression. Data from these studies suggest that proinflammatory conditions reduce CFTR expression. Therefore, maneuvers to elevate CFTR expression or activity could be potentially therapeutic. In that regard, Ramachandran et al. (119) showed that treating airway cells with a miR-138 mimic enhanced CFTR mRNA levels, supporting the view that miRs could be therapeutic. To assist in prioritizing these strategies, a better understanding of CFTR dysfunction in vivo as well as a closer examination of former smokers with COPD that continue to exhibit chronic bronchitis are required.
Currently, there appears to be limited treatments for mucus hypersecretion since the mucolytic agents have failed to demonstrate any clinical benefit (43, 167). There is evidence, however, that MARCKS-related peptide improves airway obstruction in a mouse model of mucus hypersecretion (7, 136). This suggests that alternative approaches may provide some clinical benefit. Another recent study indicates that smokers with and without COPD have reduced lower airway CFTR activity compared with healthy nonsmokers (46), lending further support for the therapeutic usefulness of CFTR activity enhancement protocols in diseases such as COPD. Clearly, fully understanding the regulatory mechanisms controlling ASL, mucus clearance, and the role of CFTR in the different regions of the lung should provide a roadmap for potential therapeutic interventions in future COPD therapies. Nevertheless, these recent studies are very encouraging and could open novel therapeutic avenues for this common and devastating disease.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01 DK060065 (J. F. Collawn), R01 HL076587 (Z. Bebok) and R01HL105487 (S. M. Rowe), 2R01HL031197 (S. Matalon), and by an American Lung Association Senior Research Fellowship (RT-219427-N; S. V. Raju). This work was also supported by Dr. Eric Sorscher and grants to the Gregory Fleming James Cystic Fibrosis Research Center (P30 DK 072482 and R474-CR11, from NIH and the CF Foundation, respectively).
DISCLOSURES
A. Rab, S. V. Raju, Z. Bebok, and J. F. Collawn declare no conflicts of interest, financial or otherwise. S. M. Rowe is the Principal Investigator of research grants to the University of Alabama at Birmingham (UAB) from Vertex Pharmaceuticals for the design and conduct of clinical trials in cystic fibrosis, including the evaluation of ivacaftor. Dr. Rowe received no personal income related to this activity. Dr. Rowe is also the principal investigator of basic research grants from National Heart, Lung, and Blood Institute and Forest Research Institute to UAB that are related to the work discussed herein. All perceived conflicts are managed and endorsed by the Conflict of Interest Review Board at UAB. S. Matalon has a grant from GlaxoSmithKline on understanding how TRPA1 antagonists modulate chlorine induced lung injury. The subject matter of this work is not related to the work presented in this review. Dr. Matalon is not receiving any personal payments (honoraria or stock options) from GHK.
AUTHOR CONTRIBUTIONS
A.R., S.M.R., and Z.B. prepared figures; A.R. and J.F.C. drafted manuscript; A.R., S.M.R., Z.B., S.M., and J.F.C. edited and revised manuscript; A.R., S.M.R., S.V.R., Z.B., S.M., and J.F.C. approved final version of manuscript; S.M.R. interpreted results of experiments; S.V.R. performed experiments.
REFERENCES
- 1.The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, 2006 [PubMed] [Google Scholar]
- 2.The 2004 United States Surgeon General's Report: The Health Consequences of Smoking. N S W Public Health Bull 15: 107, 2004 [PubMed] [Google Scholar]
- 3.Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB, Jr, Butler J, Uc A. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology 11: 506–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Accurso FJ, Moss RB, Wilmott RW, Anbar RD, Schaberg AE, Durham TA, Ramsey BW. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med 183: 627–634, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordonez CL, Campbell PW, Ashlock MA, Ramsey BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 363: 1991–2003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol 303: L889–L898, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Rengarajan S, Adler KB, Ram A, Ghosh B, Fahim M, Dickey BF. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J Appl Physiol 102: 399–405, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Akcay M, Yeter E, Durmaz T, Keles T, Akar Bayram N, Uyar M, Davutoglu V, Yuksel I, Kurt M, Bozkurt E. Treatment of acute chronic obstructive pulmonary disease exacerbation improves right ventricle function. Eur J Echocardiogr 11: 530–536, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365: 689–698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An CH, Wang XM, Lam HC, Ifedigbo E, Washko GR, Ryter SW, Choi AM. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol 303: L748–L757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 41: 631–638, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. Small airways in COPD. N Engl J Med 350: 2635–2637, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 39: 448–457, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartoszewski R, Rab A, Twitty G, Stevenson L, Fortenberry J, Piotrowski A, Dumanski JP, Bebok Z. The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem 283: 12154–12165, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol 8: R201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl− secretion in airway epithelia. J Biol Chem 277: 43041–43049, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Berger HA, Travis SM, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by specific protein kinases and protein phosphatases. J Biol Chem 268: 2037–2047, 1993 [PubMed] [Google Scholar]
- 18.Berger M. Lung inflammation early in cystic fibrosis: bugs are indicted, but the defense is guilty. Am J Respir Crit Care Med 165: 857–858, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Black PN, Morgan-Day A, McMillan TE, Poole PJ, Young RP. Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med 4: 13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J Immunol 186: 602–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodas M, Min T, Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. Am J Physiol Lung Cell Mol Physiol 300: L811–L820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med 13: 231–240, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 261: 5–16, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Brown JS, Zeman KL, Bennett WD. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med 166: 1240–1247, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775–789, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 173: 1139–1144, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Chakravorty I, Chahal K, Austin G. A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion. Int J Chron Obstruct Pulmon Dis 6: 693–699, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chari R, Lonergan KM, Ng RT, MacAulay C, Lam WL, Lam S. Effect of active smoking on the human bronchial epithelium transcriptome. BMC Genomics 8: 297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fortenberry JA, Lancaster JR, Jr, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol 39: 150–162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR, Jr, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione. J Biol Chem 281: 9190–9199, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol 23: 5–27, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Christopher F, Chase D, Stein K, Milne R. rhDNase therapy for the treatment of cystic fibrosis patients with mild to moderate lung disease. J Clin Pharm Ther 24: 415–426, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(−/−) mice. Proc Natl Acad Sci USA 91: 479–483, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 339: 653–658, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Collawn JF, Lazrak A, Bebok Z, Matalon S. The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302: L1141–L1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Com G, Clancy JP. Adenosine receptors, cystic fibrosis, and airway hydration. Hand Exp Pharmacol 193: 363–381, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Willits LR, Yates JC, Vestbo J. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 34: 641–647, 2009 [DOI] [PubMed] [Google Scholar]
- 41.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 1951–1957, 1998 [DOI] [PubMed] [Google Scholar]
- 42.de Jonge HR. Cyclic nucleotide-dependent phosphorylation of intestinal epithelium proteins. Nature 262: 591–593, 1976 [DOI] [PubMed] [Google Scholar]
- 43.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, van Schayck CP, Olivieri D, Del Donno M, De Backer W, Lankhorst I, Ardia A. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 365: 1552–1560, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Derichs N, Jin BJ, Song Y, Finkbeiner WE, Verkman AS. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J 25: 2325–2332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dovey M, Aitken ML, Emerson J, McNamara S, Waltz DA, Gibson RL. Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. Chest 132: 1212–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, Rowe SM. Acquired CFTR dysfunction in the lower airways in COPD. Chest 144: 498–506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 300: 2407–2416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckford PD, Li C, Ramjeesingh M, Bear CE. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J Biol Chem 287: 36639–36649, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezzie ME, Crawford M, Cho JH, Orellana R, Zhang S, Gelinas R, Batte K, Yu L, Nuovo G, Galas D, Diaz P, Wang K, Nana-Sinkam SP. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 67: 122–131, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flume PA. Pulmonary complications of cystic fibrosis. Respir Care 54: 618–627, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 176: 957–969, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Girod S, Zahm JM, Plotkowski C, Beck G, Puchelle E. Role of the physiochemical properties of mucus in the protection of the respiratory epithelium. Eur Respir J 5: 477–487, 1992 [PubMed] [Google Scholar]
- 54.Girodon E, Cazeneuve C, Lebargy F, Chinet T, Costes B, Ghanem N, Martin J, Lemay S, Scheid P, Housset B, Bignon J, Goossens M. CFTR gene mutations in adults with disseminated bronchiectasis. Eur J Hum Genet 5: 149–155, 1997 [PubMed] [Google Scholar]
- 55.Griesenbach U, Alton EW. Progress in gene and cell therapy for cystic fibrosis lung disease. Curr Pharm Des 18: 642–662, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Grubb BR, O'Neal WK, Ostrowski LE, Kreda SM, Button B, Boucher RC. Transgenic hCFTR expression fails to correct β-ENaC mouse lung disease. Am J Physiol Lung Cell Mol Physiol 302: L238–L247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guimbellot JS, Fortenberry JA, Siegal GP, Moore B, Wen H, Venglarik C, Chen YF, Oparil S, Sorscher EJ, Hong JS. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol 39: 514–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hackett NR, Heguy A, Harvey BG, O'Connor TP, Luettich K, Flieder DB, Kaplan R, Crystal RG. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol 29: 331–343, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Hampton TH, Ballok AE, Bomberger JM, Rutkowski MR, Barnaby R, Coutermarsh B, Conejo-Garcia JR, O'Toole GA, Stanton BA. Does the F508-CFTR mutation induce a proinflammatory response in human airway epithelial cells? Am J Physiol Lung Cell Mol Physiol 303: L509–L518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy KA, Anderson BD. Noninvasive clearance of airway secretions. Respir Care Clin N Am 2: 323–345, 1996 [PubMed] [Google Scholar]
- 61.Harvey BG, Heguy A, Leopold PL, Carolan BJ, Ferris B, Crystal RG. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med 85: 39–53, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Hassan F, Nuovo GJ, Crawford M, Boyaka PN, Kirkby S, Nana-Sinkam SP, Cormet-Boyaka E. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS One 7: e50837, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Sharafkhaneh A, Luketich JD, Coxson HO, Elliott WM, Sciurba FC. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 176: 454–459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 4: 435–459, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 23: 806–812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izzotti A, Calin GA, Steele VE, Croce CM, De Flora S. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J 23: 3243–3250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang C, Finkbeiner WE, Widdicombe JH, McCray PB, Jr, Miller SS. Altered fluid transport across airway epithelium in cystic fibrosis. Science 262: 424–427, 1993 [DOI] [PubMed] [Google Scholar]
- 69.Jilling T, Haddad IY, Cheng SH, Matalon S. Nitric oxide inhibits heterologous CFTR expression in polarized epithelial cells. Am J Physiol Lung Cell Mol Physiol 277: L89–L96, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 8: 229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kandel DB, Kiros GE, Schaffran C, Hu MC. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multilevel analysis. Am J Public Health 94: 128–135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol 38: 541–550, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Knowles MR, Carson JL, Collier AM, Gatzy JT, Boucher RC. Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am Rev Respir Dis 124: 484–490, 1981 [DOI] [PubMed] [Google Scholar]
- 74.Koblizek V, Tomsova M, Cermakova E, Papousek P, Pracharova S, Mandalia RA, Ceral J, Novosad J, Fila L, Sedlak V, Ruta J, Bartos V, Salajka F, Hrnciarik M. Impairment of nasal mucociliary clearance in former smokers with stable chronic obstructive pulmonary disease relates to the presence of a chronic bronchitis phenotype. Rhinology 49: 397–406, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 288: L894–L902, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol Cell Physiol 271: C435–C454, 1996 [DOI] [PubMed] [Google Scholar]
- 77.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lebecque P, Pepermans X, Marchand E, Leonard A, Leal T. ABPA in adulthood: a CFTR-related disorder. Thorax 66: 540–541, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 35: 116–129, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O'Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na+ hyperabsorption in murine airways. Am J Physiol Lung Cell Mol Physiol 304: L469–L480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luisetti M, Pignatti PF. The search for susceptibility genes of COPD. Monaldi Arch Chest Dis 50: 28–32, 1995 [PubMed] [Google Scholar]
- 83.Mainz JG, Koitschev A. Management of chronic rhinosinusitis in CF. J Cyst Fibros 8, Suppl 1: S10–S14, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest 102: 15–21, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 277: G709–G716, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O'Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice. J Biol Chem 285: 26945–26955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O'Neal WK, Boucher RC. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med 177: 730–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 121: 121S–126S, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 160: 1683–1689, 2000 [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Garcia MA, Soler-Cataluna JJ, Donat Sanz Y, Catalan Serra P, Agramunt Lerma M, Ballestin Vicente J, Perpina-Tordera M. Factors associated with bronchiectasis in patients with COPD. Chest 140: 1130–1137, 2011 [DOI] [PubMed] [Google Scholar]
- 92.McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J 32: 581–589, 2011 [DOI] [PubMed] [Google Scholar]
- 93.McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long-term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. J Pediatr 131: 570–574, 1997 [DOI] [PubMed] [Google Scholar]
- 94.Mehta AJ, Miedinger D, Keidel D, Bettschart R, Bircher A, Bridevaux PO, Curjuric I, Kromhout H, Rochat T, Rothe T, Russi EW, Schikowski T, Schindler C, Schwartz J, Turk A, Vermeulen R, Probst-Hensch N, Kunzli N. Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults. Am J Respir Crit Care Med 185: 1292–1300, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Miglino N, Roth M, Tamm M, Borger P. Asthma and COPD — the C/EBP connection. Open Respir Med J 6: 1–13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med 177: 426–432, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Moreno JJ, Foroozesh M, Church DF, Pryor WA. Release of iron from ferritin by aqueous extracts of cigarette smoke. Chem Res Toxicol 5: 116–123, 1992 [DOI] [PubMed] [Google Scholar]
- 98.Moretto N, Bertolini S, Iadicicco C, Marchini G, Kaur M, Volpi G, Patacchini R, Singh D, Facchinetti F. Cigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. Am J Physiol Lung Cell Mol Physiol 303: L929–L938, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Morgan L, Pearson M, de Iongh R, Mackey D, van der Wall H, Peters M, Rutland J. Scintigraphic measurement of tracheal mucus velocity in vivo. Eur Respir J 23: 518–522, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349: 1498–1504, 1997 [DOI] [PubMed] [Google Scholar]
- 101.Nakayama T, Church DF, Pryor WA. Quantitative analysis of the hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic Biol Med 7: 9–15, 1989 [DOI] [PubMed] [Google Scholar]
- 102.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem 286: 2365–2374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nash EF, Stephenson A, Ratjen F, Tullis E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev CD007168, 2009 [DOI] [PubMed] [Google Scholar]
- 104.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, Anderson P, Morgan NA. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 340: 1941–1947, 1999 [DOI] [PubMed] [Google Scholar]
- 105.O'Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 113: 1329–1334, 1998 [DOI] [PubMed] [Google Scholar]
- 106.Oglesby IK, Chotirmall SH, McElvaney NG, Greene CM. Regulation of cystic fibrosis transmembrane conductance regulator by microRNA-145, -223, and -494 is altered in DeltaF508 cystic fibrosis airway epithelium. J Immunol 190: 3354–3362, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Pandey R, Singh M, Singhal U, Gupta KB, Aggarwal SK. Oxidative/nitrosative stress and the pathobiology of chronic obstructive pulmonary disease. J Clin Diagn Res 7: 580–588, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest 115: 2564–2571, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol 292: L383–L395, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB, Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pignatti PF, Bombieri C, Benetazzo M, Casartelli A, Trabetti E, Gile LS, Martinati LC, Boner AL, Luisetti M. CFTR gene variant IVS8–5T in disseminated bronchiectasis. Am J Hum Genet 58: 889–892, 1996 [PMC free article] [PubMed] [Google Scholar]
- 112.Piitulainen E, Eriksson S. Decline in FEV1 related to smoking status in individuals with severe alpha1-antitrypsin deficiency (PiZZ). Eur Respir J 13: 247–251, 1999 [DOI] [PubMed] [Google Scholar]
- 113.Podolin PL, Foley JP, Carpenter DC, Bolognese BJ, Logan GA, Long E, 3rd, Harrison OJ, Walsh PT. T cell depletion protects against alveolar destruction due to chronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol 304: L312–L323, 2013 [DOI] [PubMed] [Google Scholar]
- 114.Pryor WA. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: the use of electron spin resonance. Free Radic Biol Med 13: 659–676, 1992 [DOI] [PubMed] [Google Scholar]
- 115.Quinzii C, Castellani C. The cystic fibrosis transmembrane regulator gene and male infertility. J Endocrinol Invest 23: 684–689, 2000 [DOI] [PubMed] [Google Scholar]
- 116.Rab A, Bartoszewski R, Jurkuvenaite A, Wakefield J, Collawn JF, Bebok Z. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol 292: C756–C766, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol Lung Cell Mol Physiol 277: L1067–L1088, 1999 [DOI] [PubMed] [Google Scholar]
- 119.Ramachandran S, Karp PH, Jiang P, Ostedgaard LS, Walz AE, Fisher JT, Keshavjee S, Lennox KA, Jacobi AM, Rose SD, Behlke MA, Welsh MJ, Xing Y, McCray PB., Jr A microRNA network regulates expression and biosynthesis of wild-type and DeltaF508 mutant cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 109: 13362–13367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 365: 1663–1672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rauh R, Soell D, Haerteis S, Diakov A, Nesterov V, Krueger B, Sticht H, Korbmacher C. A mutation in the β-subunit of ENaC identified in a patient with cystic fibrosis-like symptoms has a gain-of-function effect. Am J Physiol Lung Cell Mol Physiol 304: L43–L55, 2013 [DOI] [PubMed] [Google Scholar]
- 122.Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol Sci 116: 349–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ribeiro CM, O'Neal WK. Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med 12: 872–882, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Rogers DF. Mucociliary dysfunction in COPD: effect of current pharmacotherapeutic options. Pulm Pharmacol Ther 18: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care 52: 1134–1146; discussion 1146–1149, 2007 [PubMed] [Google Scholar]
- 126.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med 38: 116–125, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 128.Rubenstein RC, Lockwood SR, Lide E, Bauer R, Suaud L, Grumbach Y. Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L88–L101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sadowska AM, Manuel YKB, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 20: 9–22, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 161: 1016–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 131.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW., 3rd Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290: 1749–1756, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Schembri F, Sridhar S, Perdomo C, Gustafson AM, Zhang X, Ergun A, Lu J, Liu G, Bowers J, Vaziri C, Ott K, Sensinger K, Collins JJ, Brody JS, Getts R, Lenburg ME, Spira A. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci USA 106: 2319–2324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 991–998, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, Braganza J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med 339: 645–652, 1998 [DOI] [PubMed] [Google Scholar]
- 136.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med 10: 193–196, 2004 [DOI] [PubMed] [Google Scholar]
- 137.Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med 16: 118–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, Byan-Parker S, Grizzle W, Sorscher EJ, Dransfield MT, Rowe SM. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 7: e39809, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Snodgrass SM, Cihil KM, Cornuet PK, Myerburg MM, Swiatecka-Urban A. Tgf-β1 inhibits Cftr biogenesis and prevents functional rescue of ΔF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS One 8: e63167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 14: 1015–1022, 1999 [DOI] [PubMed] [Google Scholar]
- 141.Solymosi EA, Kaestle-Gembardt SM, Vadasz I, Wang L, Neye N, Chupin CJ, Rozowsky S, Ruehl R, Tabuchi A, Schulz H, Kapus A, Morty RE, Kuebler WM. Chloride transport-driven alveolar fluid secretion is a major contributor to cardiogenic lung edema. Proc Natl Acad Sci USA 110: E2308–E2316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA 101: 10143–10148, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, 4th, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: 29ra31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 185: 1073–1080, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 163: 1476–1483, 2001 [DOI] [PubMed] [Google Scholar]
- 146.Tarran R, Sabater JR, Clarke TC, Tan CD, Davies CM, Liu J, Yeung A, Garland AL, Stutts MJ, Abraham WM, Phillips G, Baker WR, Wright CD, Wilbert S. Nonantibiotic macrolides prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion. Am J Physiol Lung Cell Mol Physiol 304: L746–L756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359: 1543–1554, 2008 [DOI] [PubMed] [Google Scholar]
- 148.Thatcher TH, Hsiao HM, Pinner E, Laudon M, Pollock SJ, Sime PJ, Phipps RP. Neu-164 and Neu-107, two novel antioxidant and anti-myeloperoxidase compounds, inhibit acute cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 305: L165–L174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vaandrager AB, Smolenski A, Tilly BC, Houtsmuller AB, Ehlert EM, Bot AG, Edixhoven M, Boomaars WE, Lohmann SM, de Jonge HR. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activation. Proc Natl Acad Sci USA 95: 1466–1471, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 6: 445–462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 106: 18825–18830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, Lukacs GL. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell 23: 4188–4202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vestbo J. Epidemiological studies in mucus hypersecretion. Novartis Found Symp 248: 3–12; discussion 12–19, 277–282, 2002 [PubMed] [Google Scholar]
- 154.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 153: 1530–1535, 1996 [DOI] [PubMed] [Google Scholar]
- 155.Virgin FW, Azbell C, Schuster D, Sunde J, Zhang S, Sorscher EJ, Woodworth BA. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope 120: 1465–1469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang W, Oliva C, Li G, Holmgren A, Lillig CH, Kirk KL. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol 125: 127–141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watson MS, Cutting GR, Desnick RJ, Driscoll DA, Klinger K, Mennuti M, Palomaki GE, Popovich BW, Pratt VM, Rohlfs EM, Strom CM, Richards CS, Witt DR, Grody WW. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet Med 6: 387–391, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Welsh MJ. Cigarette smoke inhibition of ion transport in canine tracheal epithelium. J Clin Invest 71: 1614–1623, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wenzel RP, Fowler AA, 3rd, Edmond MB. Antibiotic prevention of acute exacerbations of COPD. N Engl J Med 367: 340–347, 2012 [DOI] [PubMed] [Google Scholar]
- 160.Wilschanski M, Miller LL, Shoseyov D, Blau H, Rivlin J, Aviram M, Cohen M, Armoni S, Yaakov Y, Pugatsch T, Cohen-Cymberknoh M, Miller NL, Reha A, Northcutt VJ, Hirawat S, Donnelly K, Elfring GL, Ajayi T, Kerem E. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J 38: 59–69, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Yao H, Rahman I. Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammaging and COPD. Am J Physiol Lung Cell Mol Physiol 303: L557–L566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, Abraham WM, Boucher RC. Pharmacology of INS37217 [P(1)-(uridine 5′)-P(4)-(2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther 302: 871–880, 2002 [DOI] [PubMed] [Google Scholar]
- 164.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, Hoffman BJ, Van Goor F. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros 11: 237–245, 2012 [DOI] [PubMed] [Google Scholar]
- 165.Zaman K, McPherson M, Vaughan J, Hunt J, Mendes F, Gaston B, Palmer LA. S-nitrosoglutathione increases cystic fibrosis transmembrane regulator maturation. Biochem Biophys Res Commun 284: 65–70, 2001 [DOI] [PubMed] [Google Scholar]
- 166.Zaman K, Palmer LA, Doctor A, Hunt JF, Gaston B. Concentration-dependent effects of endogenous S-nitrosoglutathione on gene regulation by specificity proteins Sp3 and Sp1. Biochem J 380: 67–74, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, Bai CX, Wang CZ, Wang C, Chen BY, Shi Y, Liu CT, Li Q, Wang ZS, Huang YJ, Luo ZY, Chen FP, Yuan JZ, Yuan BT, Qian HP, Zhi RC, Zhong NS. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet 371: 2013–2018, 2008 [DOI] [PubMed] [Google Scholar]
- 168.Zsembery A, Hargitai D. Role of Ca2+-activated ion transport in the treatment of cystic fibrosis. Wien Med Wochenschr 158: 562–564, 2008 [DOI] [PubMed] [Google Scholar]
- 169.Zsembery A, Strazzabosco M, Graf J. Ca2+-activated Cl− channels can substitute for CFTR in stimulation of pancreatic duct bicarbonate secretion. FASEB J 14: 2345–2356, 2000 [DOI] [PubMed] [Google Scholar]