Abstract
Mounting evidence suggests that the circadian clock plays an integral role in the regulation of many physiological processes including blood pressure, renal function, and metabolism. The canonical molecular clock functions via activation of circadian target genes by Clock/Bmal1 and repression of Clock/Bmal1 activity by Per1–3 and Cry1/2. However, we have previously shown that Per1 activates genes important for renal sodium reabsorption, which contradicts the canonical role of Per1 as a repressor. Moreover, Per1 knockout (KO) mice exhibit a lowered blood pressure and heavier body weight phenotype similar to Clock KO mice, and opposite that of Cry1/2 KO mice. Recent work has highlighted the potential role of Per1 in repression of Cry2. Therefore, we postulated that Per1 potentially activates target genes through a Cry2-Clock/Bmal1-dependent mechanism, in which Per1 antagonizes Cry2, preventing its repression of Clock/Bmal1. This hypothesis was tested in vitro and in vivo. The Per1 target genes αENaC and Fxyd5 were identified as Clock targets in mpkCCDc14 cells, a model of the renal cortical collecting duct. We identified PPARα and DEC1 as novel Per1 targets in the mouse hepatocyte cell line, AML12, and in the liver in vivo. Per1 knockdown resulted in upregulation of Cry2 in vitro, and this result was confirmed in vivo in mice with reduced expression of Per1. Importantly, siRNA-mediated knockdown of Cry2 and Per1 demonstrated opposing actions for Cry2 and Per1 on Per1 target genes, supporting the potential Cry2-Clock/Bmal1-dependent mechanism underlying Per1 action in the liver and kidney.
Keywords: per1, cry2, clock, pparα, kidney, liver, gene regulation
the circadian clock is an important regulator of many physiological functions including blood pressure; metabolism; and endocrine, immune, vascular, and renal function (1, 6, 30, 40, 43, 44, 49). The canonical circadian clock molecular mechanism, known as the transcription translational oscillating (TTO) loop, involves four core proteins that interact with each other to regulate transcription of circadian target genes (3). The four major canonical clock proteins are Bmal1, Clock, Period (Per) (homologs 1–3), and Cryptochrome (Cry) (homologs 1–2). Clock and Bmal1 form a heterodimer, interacting with E-Box response elements in circadian target genes (2, 13). Two of these genes include Per and Cry. Per and Cry are believed to interact and then repress the transcriptional activity of Clock/Bmal1. In addition to transcriptional regulation, the circadian clock also undergoes posttranslational modifications through the phosphorylation of the Per proteins by casein kinase 1 isoforms δ/ε (CK1δ/ε). Phosphorylation by CK1δ/ε allows Per1 entry into the nucleus (28, 42).
It was initially assumed that Per1 and Per2 shared similar responsibilities in the TTO loop, but recent work by researchers in our laboratory and others has begun to shed light on the possibility that Per1 and Per2 have distinct functions. Recent work in vivo and in vitro has shown that Per2 bridges the Clock/Bmal1 complex to Cry, allowing Cry to repress Clock/Bmal1 transcriptional activity in liver (8). This complex did not appear to require Per1. Studies using Per1/Cry2 knockout (KO) mice showed that Per1 represses Cry2 (36, 37). Another study showed that this repression occurred via a transcriptional mechanism, which involved promotion of transcriptional termination and prevented transcriptional reinitiation of the Cry2 gene (39). Our work has recently highlighted the role of Per1 in the basal and aldosterone-mediated regulation of αENaC, a subunit of the renal epithelial sodium channel (ENaC) (17–19, 50). Per1 also coordinately regulates multiple genes involved in sodium reabsorption in the kidney (50). These include the positive regulation of Fxyd5, a positive regulator of Na, K-ATPase (31) and the negative regulation of Endothelin-1 (ET-1), which is a powerful inhibitor of ENaC activity via an Endothelin-B receptor and nitric oxide–dependent mechanism (7). This coordinate regulation predicts that loss of Per1 should result in renal sodium wasting, leading to decreased plasma volume and subsequent decreased blood pressure (BP). Indeed, we have recently shown that Per1 KO mice have lower BP compared with wild-type (WT) controls (50). Cry1/2 KO mice exhibited salt-sensitive hypertension due to an upregulation of aldosterone production (14). Clock KO mice, however, were relatively hypotensive and exhibited dysregulated sodium excretion and mild diabetes insipidus (34, 62). Cry1/2 KO mice are lean (24), whereas Clock (Δ19) mutant animals, in which the Clock/Bmal1 heterodimer cannot bind DNA, are obese (53). Interestingly, even though Per1 is assumed to be a repressor, the Per1 KO BP phenotype appears to more closely resemble that of the Clock KO, whereas the Per1 KO BP phenotype appears to be opposite that of the Cry1/2 KO.
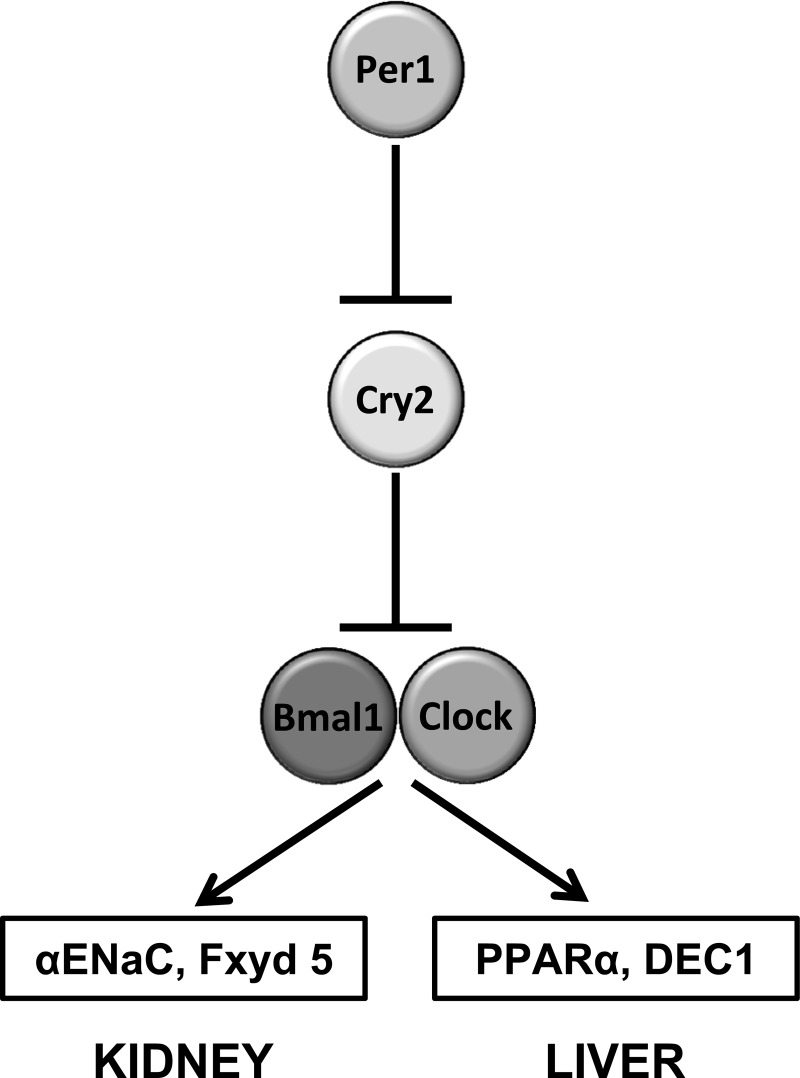
The goal of the present study was to investigate the mechanism underlying our previously observed noncanonical actions of Per1 in the positive regulation of gene expression in the kidney. In light of the recent work highlighting the possible roles of Per1 in the transcriptional repression of Cry2, we postulated that a potential mechanism for Per1 activation of renal sodium handling genes is through a Cry2-Clock/Bmal1-dependent mechanism. Specifically, and consistent with the findings of others (33, 36, 37, 39), we hypothesized that Per1 would repress Cry2, preventing its repression of Clock/Bmal1 (Fig. 1). However, because most of these studies have been performed in other tissue systems, it was important to test our hypothesis in the kidney and to compare those results with the more thoroughly characterized liver peripheral Clock. Much of the mechanism for circadian action has been identified in liver. Therefore, in this study we tested the hypothesis that Per1 action on target genes occurs through a Cry2-Clock/Bmal1-dependent mechanism in both liver and kidney. Our results indicate that Cry2 is upregulated in the absence of Per1, and that Per1 and Cry2 appear to mediate opposing action on target gene expression. These findings may help explain the mechanism by which Per1 appears to activate gene expression.
Fig. 1.
Hypothetical model in which Per1 represses Cry2, preventing its repression of Clock/Bmal1, resulting in activation of Per1 target genes in the kidney (αENaC and Fxyd5) and liver (PPARα and DEC1).
MATERIALS AND METHODS
Cell culture.
Alain Vandewalle (INSERM, Paris, France) kindly provided the mpkCCDc14 cells (5). AML12 cells were a gift from Yuri Sautin (University of Florida, Gainesville, FL) (46). mpkCCDc14 cells were maintained in DMEM-F12 plus 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin. AML12 cells were maintained in DMEM-F12 plus 10% heat-inactivated FBS, 5 μg/ml insulin, 5 ng/ml sodium selenite, 5 μg/ml apo-transferrin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 40 ng/ml dexamethasone.
For RNA silencing experiments, Non-Target-2 and SMARTpool small interfering RNA (siRNA) for CK1δ, CK1ε, Clock, and Cry2 were purchased from Dharmacon (catalog numbers D-001206-14-20, L-044377-00-0005, L-040108-00-0005, L-040484-01-0005, and L-040486-00-0005, respectively). A previously characterized individual siRNA for Per1 was purchased from Dharmacon (J-040487-08-0005) (19). siRNA was used according to the manufacturer's instructions. The mpkCCDc14 and AML12 cells were transfected using Dharmafect 4, as described previously (17, 19, 42).
Casein kinase 1δ/ε inhibitor experiments were performed as described previously (42). Twenty-four hours after cells reached 100% confluence, they were treated with 10 μM PF670462 (Santa Cruz) or vehicle (DMSO) for 72 h. Final DMSO concentration in both vehicle and inhibitor-treated cells was 0.1%.
Generation of Per1 shRNA in mpkCCDc14 cells.
SMART vector 2.0 Lentiviral particles containing Per1 small hairpin RNA (shRNA) or Non-Target shRNA were purchased from Dharmacon and used per the manufacturer's instructions. The vector contained a puromycin resistance cassette and green fluorescent protein (GFP) for selection. Lentivirus particles either containing nonarget or Per1 shRNA were transduced into 40,000 mpkCCDc14 cells. Cells that were successfully transduced were first selected using puromycin. Cells were then further assessed using fluorescence-activated cell sorting analysis through the Interdisciplinary Center for Biotechnology Research at the University of Florida (http://www.biotech.ufl.edu/) to determine percent positive GFP. Cells that were both puromycin-resistant and GFP-positive were then plated, and individual clones were separated and grown to confluence. Per1 mRNA knockdown was assessed by quantitative real-time polymerase chain reaction (QPCR). Clones that had sufficient knockdown (greater than 75%) were used for further experiments.
Animals.
All animal use protocols were approved by the University of Florida and North Florida/South Georgia Veterans Administration institutional animal care and use Committees in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Per1 KO and WT mice (129/sv) were originally provided by Dr. David Weaver [University of Massachusetts (4)]. WT and Per1 heterozygote (het) mice were bred in-house by University of Florida Animal Care Services staff. Animals were maintained on a normal 12-h light:dark cycle. Mice were fed normal laboratory chow and given free access to water. At midnight, mice were anesthetized, and tissues were collected and snap-frozen in liquid nitrogen. Kidneys were later dissected and cortex was removed for protein or RNA isolation.
RNA isolation and QPCR.
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA (10 μg) was treated with DNA-free DNaseI (Ambion). DNaseI-treated RNA (2 μg) samples were used as template for reverse transcription with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The resulting cDNAs (20 ng) were then used as template in QPCR reactions (Applied Biosystems) to evaluate changes in Per1, Clock, Bmal1, Cry2, αENaC, ET-1, Fxyd5, peroxisome proliferator–activated receptor α (PPARα), and differentiated embryo chondrocyte 1 (DEC1) mRNA levels. Cycle threshold (Ct) values were normalized against β-actin and relative quantification was performed using the ΔΔCt method (29). Fold change values were calculated as the change in mRNA expression levels relative to the control. TaqMan primer/probe sets were purchased from Applied Biosystems.
Protein isolation and Western blot analysis.
Nuclear and cytosolic extracts were isolated using the NE-PER kit (Pierce) according to the manufacturer's instructions. Whole tissue extracts were isolated using IP Lysis Buffer (Pierce) according to the manufacturer's instructions. Protein concentrations were then quantified by bicinchoninic acid assay (Pierce). Proteins were separated on a 4–20% Tris·HCl Ready Gel (BioRad), and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 2% nonfat dry milk in TBS-S (TBS plus 0.05% Rodeo Saddle Soap; USB) and incubated overnight at 4°C with anti-Clock (1:1,000) (Pierce), anti-Per1(1:500; Pierce), anti-Bmal1 (1:500; Pierce), anti-Cry2 (1:500; Thermo Scientific), anti-Per2 (1:500; Santa Cruz), anti-PPARα (Santa Cruz), anti-DEC1 (Santa Cruz), or anti-β-actin (1:500; Santa Cruz) antibodies. β-Actin was used as a loading control. The membrane was washed with 2% nonfat dry milk in TBS-S for 15 min and then incubated with horseradish peroxidase conjugate anti-rabbit secondary antibody and incubated in 2% nonfat dry milk in TBS-S for 1 h at 4°C. After incubation, the blot was washed with TBS-S for 15 min. Detection was performed using Novex ECL Chemiluminescent Substrate reagents (Invitrogen). Densitometry was performed using ImageJ (http://rsbweb.nih.gov/ij).
Coimmunoprecipitation.
Coimmunoprecipitations were performed using the Pierce coimmunoprecipitation kit according to the manufacturer's instructions. Anti-Per1 antibody columns were created using 40 μg of antibody (Thermo Scientific) and 500 μg of nuclear extract loaded onto the column. Nuclear extracts were precleared with control agarose resin (Pierce) per the manufacturer's instructions.
Statistical analysis.
A Student's unpaired t-test (Graphpad) was used to compare two data sets. Data are presented as means ± SE. P < 0.05 was considered significant.
RESULTS
Conserved canonical clock interactions are preserved in kidney cortex and liver.
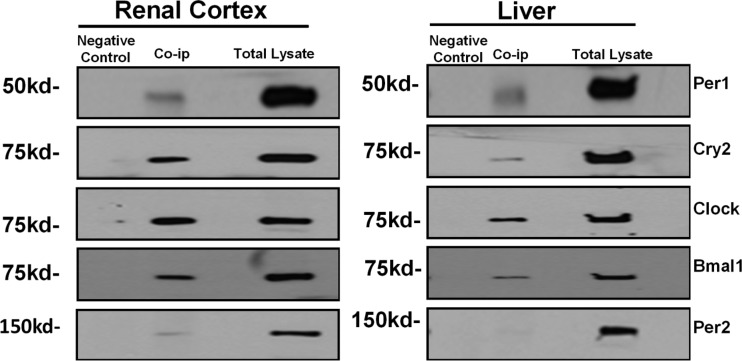
The core circadian clock complex consists of four major circadian proteins (Per, Cry, Clock, and Bmal1) that have been shown to interact in mouse liver and mouse embryonic fibroblasts (8). Although the existence of circadian rhythms in the kidney has been well established [reviewed in (49)], the presence of the core circadian complex has not been confirmed. Therefore, we performed coimmunoprecipitations in kidney cortex and liver to test which of the core Clock proteins associated with Per1 at midnight. Because mice are nocturnal, midnight is the peak of their active phase, when circadian proteins are activated (2, 25, 27). We and others have observed the existence and action of a smaller nuclear Per1 fragment (∼50 kDa) (9, 17, 42, 50); this nuclear form of Per1 interacts with E-boxes from the αENaC and ET-1 promoters (17, 39, 46). As shown, Per1 associates with Clock, Bmal1, Cry2, and Per2 in nuclear extracts derived from the renal cortex and the liver of WT mice euthanized at midnight (Fig. 2). It is not surprising that Per1 associates with itself because it has been shown previously that both Per1 and Per2 can homodimerize (21, 26, 61). Two recent studies have previously demonstrated the interaction of these proteins in mouse liver (8, 25).
Fig. 2.
Dissected cortex and whole liver were harvested from wild-type (WT) 129/sv mice euthanized at midnight. Nuclear extracts were collected and coimmunoprecipitations (Co-ip) were performed using anti-Per1 antibody with respective positive and negative controls. Positive control consisted of protein input. Negative controls consisted of replacing anti-Per1 resin with inactive cross-linked resin. Data are representative of three independent experiments. Western blot analysis was performed using anti-Per1, anti-Cry2, anti-Clock, anti-Bmal1, and anti-Per2 antibodies.
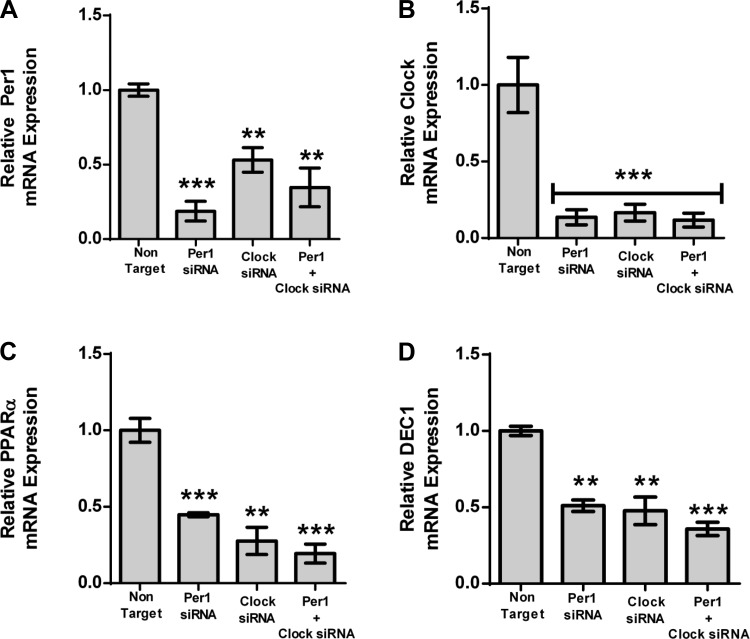
αENaC and Fxyd5 are Per1 and Clock target genes.
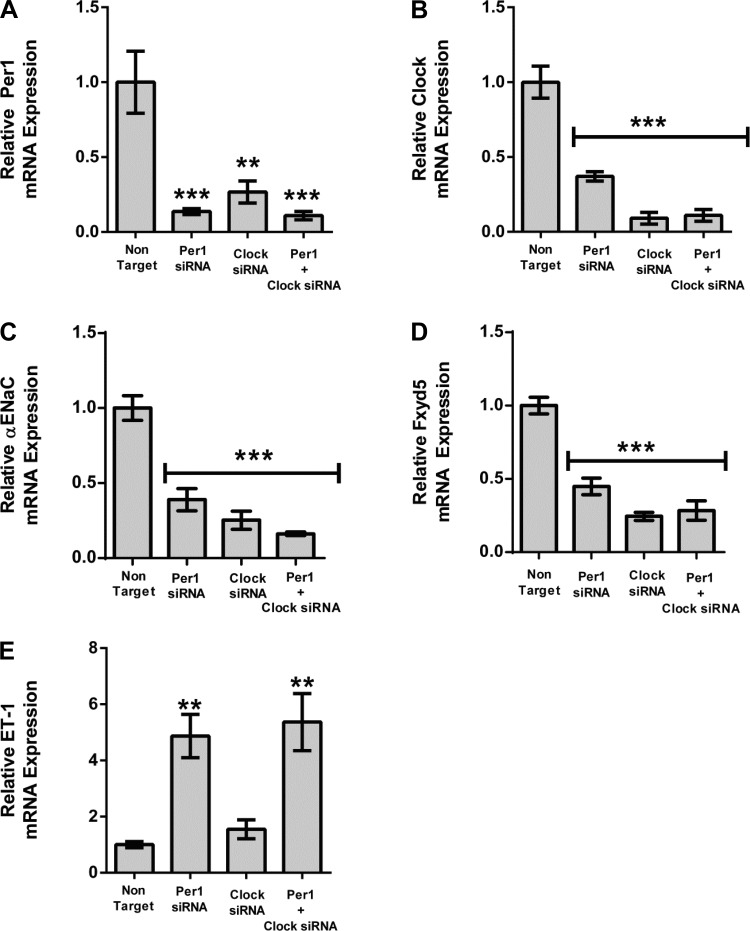
We have previously shown that αENaC, Fxyd5, and Endothelin-1 are Per1 target genes (17, 18, 50). To determine whether they were also Clock target genes, mpkCCDc14 cells, a model of the renal cortical collecting duct (5), were treated with Per1 siRNA, Clock siRNA, or a combination of Per1 and Clock siRNA (Fig. 3). As expected, mRNA expression of both Per1 and Clock was decreased with the respective siRNAs (Fig. 3, A and B). Interestingly, in both cases, Per1 siRNA resulted in reduced Clock mRNA, and Clock siRNA resulted in reduced Per1 mRNA. It is not surprising that Clock siRNA positively affects Per1 levels, because Clock/Bmal1 activity is known to increase Per1 expression (2, 22, 58). As we have shown previously (17, 50), both αENaC and Fxyd5 were significantly decreased with Per1 siRNA (Fig. 3, C and D). Fxyd5 and αENaC were also affected by Clock siRNA. Combination treatment resulted in knockdown that was not statistically different from individual treatments. Taken together, these data suggest that both Fyxd5 and αENaC are Clock target genes. Interestingly, ET-1 was not significantly affected by Clock siRNA (Fig. 3E), suggesting a potential Clock-independent mechanism for the repression of ET-1 by Per1.
Fig. 3.
αENaC and Fxyd5 are Clock targets, whereas ET-1 is Clock-independent. mpkCCDc14 cells were treated with nontarget, Per1, or Clock siRNA or a combination of Per1 and Clock short interfering RNA (siRNA) for 48 h. Quantitative real-time polymerase chain reaction (QPCR) was used to evaluate changes in Per1 (A), Clock (B), αENaC (C), Fxyd5 (D), or ET-1 (E) gene expression in Per1, Clock, or Clock/Per1 siRNA treated cells vs. nontarget siRNA control. *P < 0.05, **P < 0.01, ***P < 0.001; n = 4. Values are means ± SE.
Cry2 protein is upregulated following Per1 knockdown in mpkCCDc14 cells.
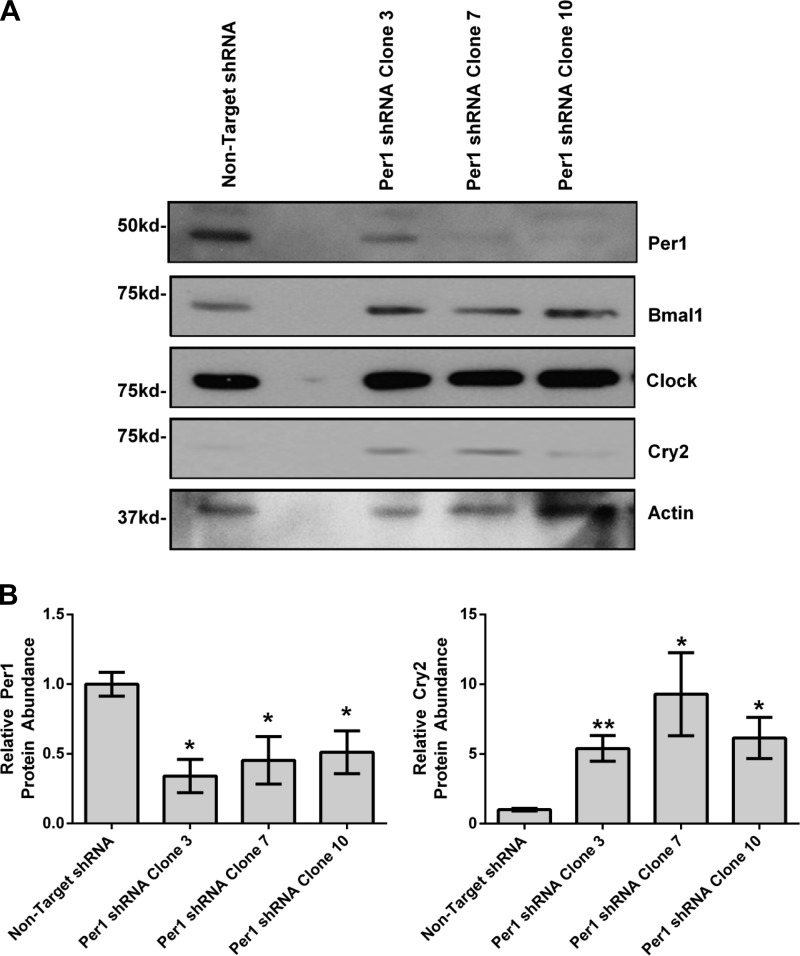
As described above, αENaC and Fxyd5 appear to be target genes of both Per1 and Clock. Our hypothesis is that this mechanism of regulation is Cry2-dependent. Previous work has shown that Per1 transcriptionally suppresses Cry2 (15, 36, 37); these findings predict that Per1 knockdown should result in upregulation of Cry2. To test this in mpkCCDc14 cells, Per1 was stably knocked down using lentiviral particles containing Per1 or nontarget shRNA. Cells were then selected using puromycin resistance and GFP cell sorting. Remaining cells were plated, clones were isolated, grown to confluence, and selected on the basis of reduction in Per1 and αENaC mRNA expression determined by QPCR (data not shown). Per1 protein levels were assessed in Per1 shRNA clones 3, 7, and 10 by Western blot. As mentioned above, the existence and action of a smaller nuclear Per1 fragment (∼50 kDa) has been observed by researchers in our laboratory and others (9, 17, 42, 50); this nuclear form of Per1 interacts with E-boxes from the αENaC and ET-1 promoters (17, 39, 46). As shown, nuclear Per1 protein levels were decreased in all three clones (Fig. 4A, quantified in Fig. 4B). Protein levels of Clock, Bmal1, and Cry2 were also assessed by Western blot (Fig. 4A). Consistent with our hypothesis, Western blot analysis showed that nuclear Cry2 levels were increased in all three Per1 shRNA clones (Fig. 4B). Nuclear Clock and Bmal1 protein levels did not appear to be altered.
Fig. 4.
Per1 knockdown results in upregulation of Cry2 in vitro in mpkCCDc14 cells. Lentiviral particles either containing nontarget or Per1 shRNA were transfected into 40,000 mpkCCDc14 cells; puromycin- and GFP-selected; and 10 individual clones were randomly selected, separated, and grown to confluence. Western blot analysis was performed using anti-Per1, anti-Bmal1, anti-Clock, anti-Cry2 antibodies. Anti-β-actin was used as a loading control. Densitometry analysis was used to quantitate the level of Per1 and Cry2 in (A). *P < 0.05 relative to nontarget short hairpin RNA (shRNA); n = 3. Values are means ± SE.
Identification of PPARα and DEC1 as Per1 target genes in AML12 cells and in liver.
PPARα is a lipid sensor that is activated by excess fatty acids and regulates lipoprotein synthesis and gluconeogenesis in the liver (41). Once activated, PPARα induces expression of downstream genes that metabolize fatty acids. It has been recently shown to be a circadian target gene (35, 55–57). Differentiated embryo chondrocyte 1 (DEC1), also known as basic helix-loop-helix protein 2, Sharp-2, or STRA13, is a basic helix-loop-helix transcriptional factor that binds to E-box response elements. DEC1 has been implicated in circadian control by competing for binding to E-boxes with the Clock-Bmal1 complex (23). It has also been implicated in regulation of adipogenesis (38), regulation of metabolite-sensing nuclear receptors (10), chondrogenesis (47), neurogenesis (45), T-lymphocyte activation (51), and cell growth arrest (52). DEC1 has also been identified as a circadian target gene (54).
We tested whether PPARα and DEC1 were Per1 targets in the mouse hepatocyte cell line, AML12 (46). AML12 cells were treated with Per1 siRNA, Clock siRNA, or a combination of both (Fig. 5). As was observed in the mpkCCDc14 cells, both Per1 and Clock were decreased following treatment of AML12 cells with the respective siRNAs (Fig. 5, A and B). Per1 siRNA resulted in decreased Clock expression, and Clock siRNA resulted in decreased Per1 expression. This result was also observed in the mpkCCDc14 cells, as described above. PPARα and DEC1 mRNA were both decreased following Per1 knockdown (Fig. 5, C and D), suggesting that they are Per1 target genes. Both were decreased with Clock siRNA, confirming that they are Clock target genes as well. A further decrease in expression was not observed following treatment with the combination of Per1 and Clock siRNA.
Fig. 5.
PPARα and DEC1 are Per1 and Clock target genes. AML12 cells were treated with nontarget, Per1, Clock siRNA or a combination of Per1 and Clock siRNA for 48 h. QPCR was used to evaluate changes in Per1 (A), Clock (B), PPARα (C), and DEC1 (D) expression in Per1, Clock, or Clock/Per1 siRNA treated cells vs. nontarget siRNA control; n = 4; *P < 0.05, **P < 0.01, ***P < 0.001. Values are means ± SE.
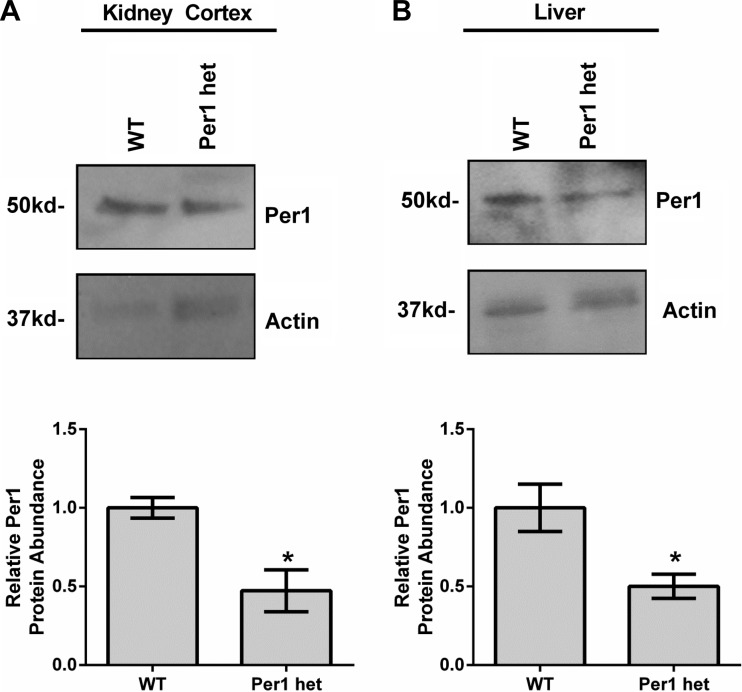
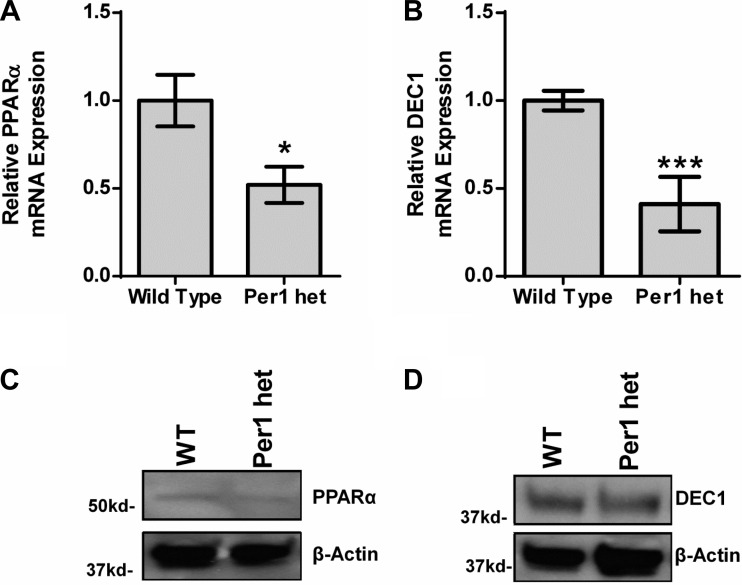
To determine whether PPARα and DEC1 are Per1 targets in vivo, expression levels were determined in available WT and Per1 het tissue. First, Per1 protein expression was measured in whole tissue extracts from liver and kidney cortex (Fig. 6, A and B). As expected, Per1 het mice exhibited ∼50% less Per1 in both the kidney cortex (Fig. 6A) and liver (Fig. 6B) compared with WT animals (note that circadian behavioral analysis has not been performed in Per1 het mice). Next, confirming our in vitro findings, mRNA expression of both PPARα and DEC1 was measured and found to be decreased in Per1 het mice compared with WT (Fig. 7, A and B). Consistent with our mRNA data, reduced Per1 levels resulted in a modest decrease in PPARα and DEC1 nuclear protein levels (Fig. 7, C and D).
Fig. 6.
Per1 heterozygote (het) mice exhibit reduced levels of Per1 protein. Whole-tissue extracts were isolated from kidney cortex (A) and liver (B) from WT 129/sv and Per1 het mice euthanized at midnight. Western blot analysis was performed using an anti-Per1 antibody to evaluate Per1 protein expression with β-actin as a loading control. Data are representative of four independent experiments. Densitometry analysis was used to quantitate expression (bottom); n = 4; *P < 0.05. Values are means ± SE.
Fig. 7.
Reduced Per1 levels in vivo results in decreased levels of PPARα and DEC1. Whole livers were harvested from WT 129/sv and Per1 het mice euthanized at midnight; nuclear extracts and RNA were collected. A and B: changes in PPARα and DEC1 mRNA were determined by QPCR. *P < 0.05; n = 4. Western blot analysis was performed using anti-PPARα, or anti-DEC1 to evaluate changes in PPARα (C) and DEC1 (D) protein expression with β-actin as a loading control. Data are representative of four independent experiments. Values are means ± SE.
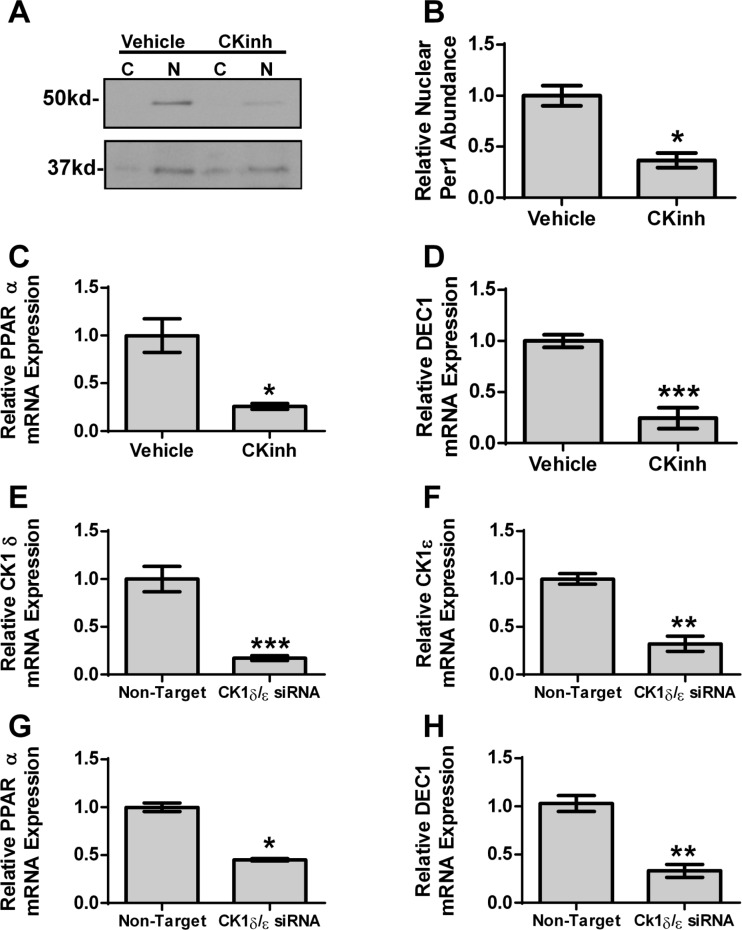
Per1 must be phosphorylated by CK1δ/ε to enter the nucleus (28). We recently showed that pharmacological inhibition of CK1δ/ε with PF670462 in mpkCCDc14 cells recapitulated the effects of Per1 knockdown, including decreased αENaC mRNA and protein expression; treatment with this drug decreased ENaC channel activity as well (42). Therefore, to further confirm the effects of Per1 on PPARα and DEC1, AML12 cells were treated with PF670462 (8). As expected, CK1δ/ε inhibition resulted in a decrease in nuclear Per1 abundance (Fig. 8A, quantified in Fig. 8B). CK1δ/ε inhibition resulted in decreased PPARα and DEC1 mRNA (Fig. 8, C and D). To further confirm this effect, AML12 cells were transfected with CK1δ/ε siRNA (Fig. 8, E and F). PPARα and DEC1 were decreased following CK1δ/ε knockdown (Fig. 8, G and H). Taken together, these data demonstrate that Per1 knockdown, either in vitro with siRNA, indirectly with CK1δ/ε inhibition, or in vivo, resulted in decreased PPARα and DEC1 mRNA.
Fig. 8.
Inhibition of CK1δ/ε reduces PPARα and DEC1 expression. A: nuclear and cytosolic extracts were collected from AML12 cells treated with 10 μM casein kinase 1δ/ε inhibitor PF670462 for 72 h (CKinh). Western blot analysis was performed using anti-Per1 or anti-β-actin antibodies as a loading control. Data are representative of three independent experiments. B: densitometry analysis was used to quantitate the level of Per1 in (A); n = 3. AML12 cells were treated with 10 μM casein kinase 1δ/ε inhibitor PF670462 for 72 h. QPCR was used to evaluate changes in PPARα (C) and DEC1 (D) gene expression in PF670462-treated cells vs. DMSO-treated cells; n = 3. AML12 cells were treated with nontarget or casein kinase 1δ and ε siRNA for 48 h. Real-time PCR was used to evaluate changes in CK1δ (E), CK1ε (F), PPARα (G), or DEC1 (H) gene expression in casein kinase 1δ/ε siRNA-treated cells vs. nontarget. *P < 0.05, **P < 0.01, ***P < 0.001; n = 4. Values are means ± SE.
Cry2 protein is upregulated following pharmacological inhibition of Per1 nuclear entry in AML12 cells.
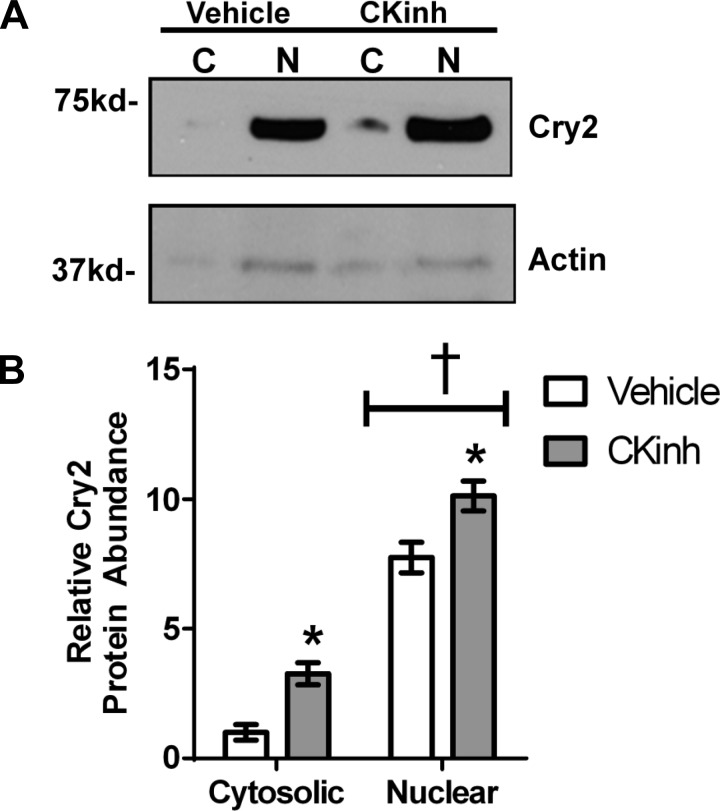
To test the effect of Per1 knockdown on Cry2 protein levels in AML12 cells, cells were treated with CK1δ/ε inhibitor (PF670462) to reduce nuclear Per1, and protein levels were assessed by Western blot (Fig. 9). Cry2 protein levels were significantly elevated in both the cytosol and nucleus following CK1δ/ε inhibition (Fig. 9A). This result was quantified (Fig. 9B), demonstrating that indirect inhibition of Per1 resulted in significant increases in cytosolic and nuclear Cry2 levels. Thus, in both mpkCCDc14 and AML12 cells, CK1δ/ε-mediated Per1 knockdown resulted in increased Cry2 levels, a result that is consistent with suppression of Cry2 by Per1.
Fig. 9.
Per1 knockdown results in upregulation of Cry2 in vitro in AML12 cells. A: nuclear and cytosolic extracts were collected from AML12 cells treated with 10 μM casein kinase 1δ/ε inhibitor PF670462 for 72 h. Western blot analysis was performed using anti-Cry2 or anti-β-actin antibodies as a loading control. Data are representative of three independent experiments. B: densitometry analysis was used to quantitate the level of Cry2 in (A). *P < 0.05 relative to vehicle, †P < 0.5 relative to cytosolic; n = 3. Values are means ± SE.
Per1 suppresses Cry2 in vivo in kidney cortex and liver.
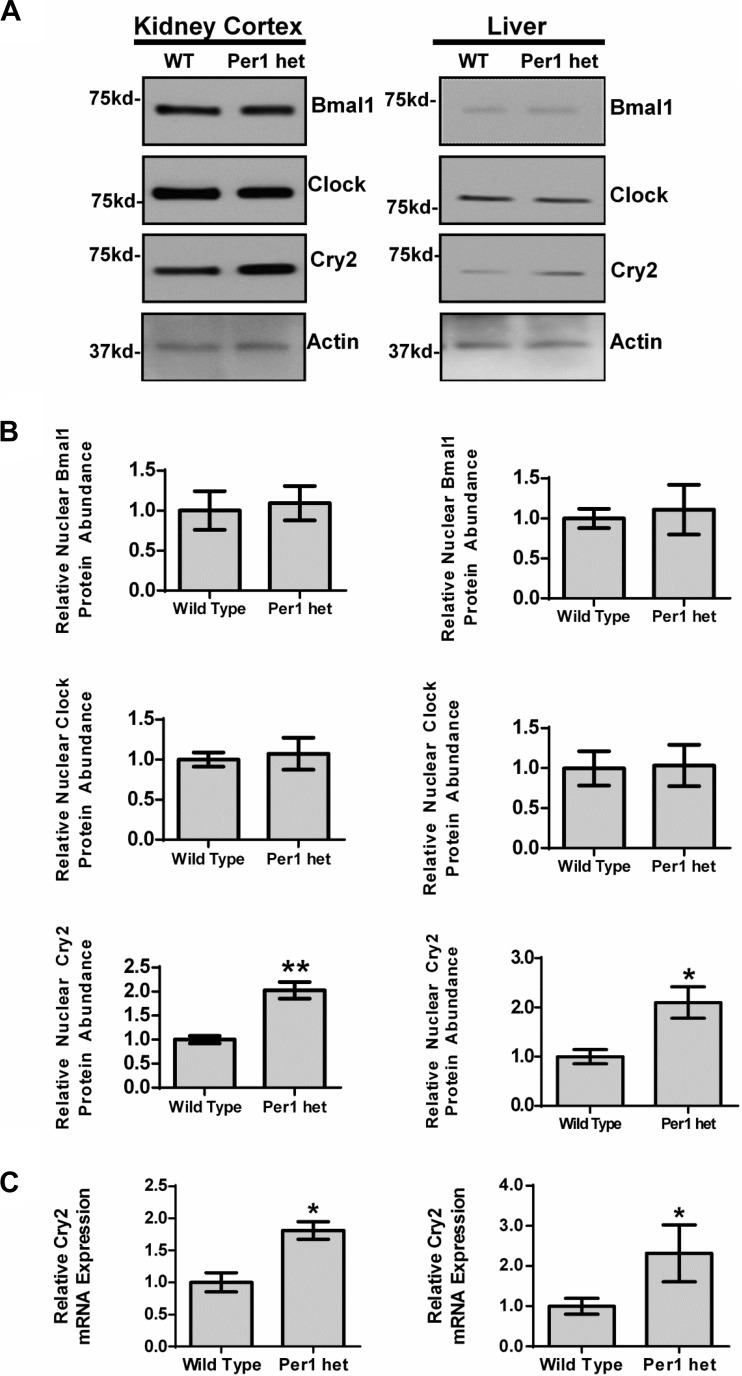
To determine whether Per1 suppresses Cry2 in vivo, nuclear Cry2 protein levels were measured by Western blot analysis of nuclear fractions from kidney cortex and liver of WT and Per1 het mice euthanized at midnight (Fig. 10). In kidney cortex and liver, nuclear Cry2 levels were significantly elevated compared with those of WT mice (Fig. 10A). Densitometry analysis indicated that nuclear Cry2 levels were increased twofold in both kidney cortex and in liver (Fig. 10B). Nuclear Clock and Bmal1 protein levels were not significantly altered in Per1 het compared with WT (Fig. 10, A and B). To determine whether suppression of Cry2 by Per1 occurred at the level of mRNA, Cry2 mRNA levels were measured in kidney cortex and liver by QPCR. Cry2 mRNA levels were significantly upregulated in both tissues, supporting the hypothesis that suppression of Cry2 by Per1 is at least partially dependent on regulation of mRNA expression (Fig. 10C).
Fig. 10.
Reduced Per1 expression results in upregulation of Cry2 in vivo in mouse kidney cortex and liver. Dissected cortex and whole liver were harvested from WT 129/sv and Per1 het mice euthanized at midnight, and nuclear extracts were collected. A: Western blot analysis was performed using anti-Bmal1, anti-Clock, or Anti-Cry2 to evaluate changes in Bmal1, Clock, and Cry2 protein expression with β-actin as a loading control. Data are representative of five independent experiments. B: densitometry analysis was used to quantitate the level of Bmal1, Clock, and Cry2 in (A); n = 5. C: QPCR was used to evaluate changes in Cry2 gene expression in WT vs. Per1 het mice; n = 4. *P < 0.05, **P < 0.01. Values are means ± SE.
Per1 and Cry2 mediate opposing actions on Per1 target genes in mpkCCDc14 and AML12 cells.
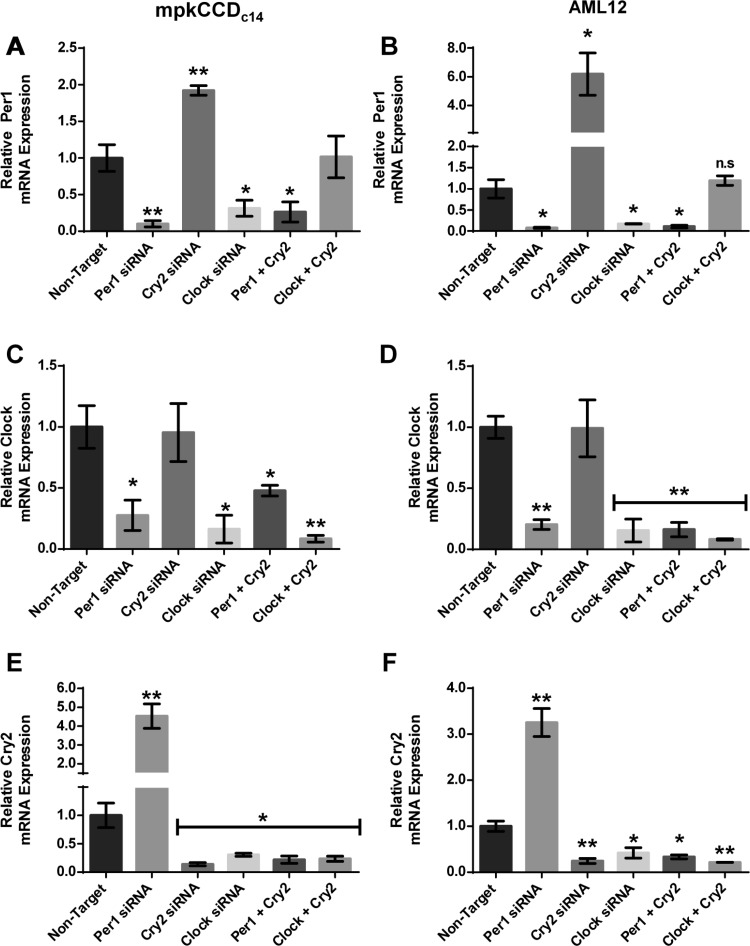
We hypothesized that Per1 action is mediated through a Cry2-Clock/Bmal1-dependent mechanism in which Per1 represses Cry2, preventing Cry2 from repressing Clock/Bmal1 action (Fig. 1). This hypothesis predicts that Per1 and Cry2 may mediate opposing actions on Per1 target gene expression. To test this hypothesis, mpkCCDc14 and AML12 cells were treated with Per1 siRNA, Cry2 siRNA, or Clock siRNA or a combination of either Per1 and Cry2 siRNA or Clock and Cry2 siRNA. The effect of these siRNAs on Per1, Clock, and Cry2 was assessed using QPCR (Fig. 11). As expected, Per1 mRNA levels in mpkCCDc14 and AML12 cells were decreased with either Per1 or Clock siRNA. Per1 mRNA levels were increased with Cry2 siRNA. Per1 levels were decreased with combination treatment of Cry2 and Per1 siRNA. Interestingly, a combination of Clock and Cry2 siRNA resulted in normalization of Per1 mRNA expression to control values in both mpkCCDc14 and AML12 cells (Figs. 10B and 11A). Clock mRNA levels in mpkCCDc14 and AML12 cells decreased with either Per1 siRNA or Clock siRNA (Fig. 11, C and D), as shown previously (see Figs. 3 and 5). Cry2 siRNA had no effect on Clock mRNA levels. The combination of Per1 siRNA and Clock siRNA resulted in decreased Clock mRNA expression. A combination of siRNA against Clock and Cry2 also resulted in decreased Clock mRNA expression in both cell types (Fig. 11, C and D). In both cell lines, treatment with Per1 siRNA resulted in increased Cry2 mRNA levels (Fig. 11, E and F), supporting the hypothesis that Per1-mediated suppression of Cry2 may be mRNA-dependent. Cry2 siRNA, Clock siRNA, or the combination of Clock and Cry2 siRNAs resulted in decreased Cry2 expression in mpkCCDc14 and AML12 cells (Fig. 11, E and F).
Fig. 11.
Effect of Per1, Cry2, and Clock on circadian gene expression in mpkCCDc14 and AML12 cells. mpkCCDc14 or AML12 cells were treated with nontarget, Per1, Cry2, or Clock siRNA or a combination of both Per1 and Cry2 or Cry2 and Clock for 48 h. A–C: QPCR was used to evaluate changes in Per1 (A and B), Clock (C and D), Cry2 (E and F) gene expression in mpkCCDc14 and AML12 siRNA treated cells vs. nontarget. P < 0.05, **P < 0.01; n = 3. Values are means ± SE.
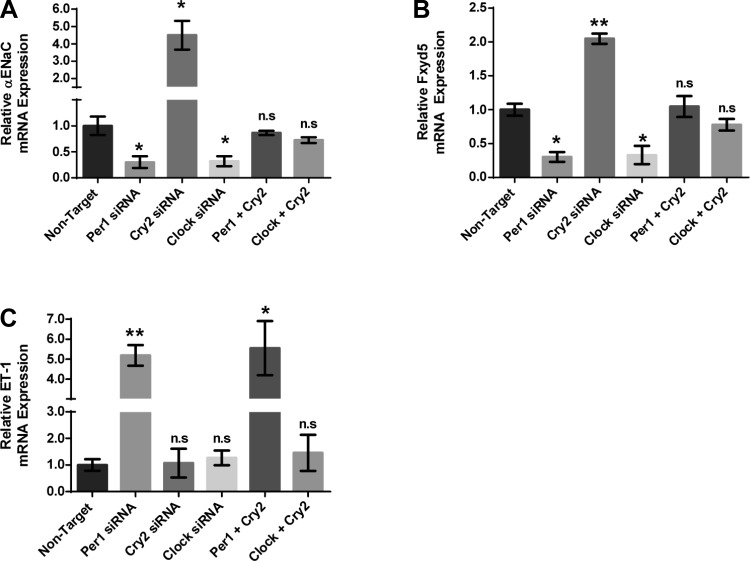
We next assessed the effect of Per1, Clock, and Cry2 action on Per1 target gene expression in mpkCCDc14 cells (Fig. 12). As we have shown previously, αENaC mRNA expression decreased with Per1 or Clock knockdown in mpkCCDc14 cells. Importantly, αENaC mRNA levels were increased with Cry2 siRNA-mediated knockdown, suggesting a role for Cry2 in repression of this Per1 target gene. The combination of Per1 and Cry2 siRNA returned expression of αENaC to control levels, supporting our hypothesis that Per1 and Cry2 mediate opposing actions on αENaC gene expression. A combination of Clock and Cry2 siRNA also resulted in control levels of αENaC gene expression (Fig. 12A). These effects were observed for Fxyd5 mRNA levels as well (Fig. 12B). ET-1, which did not appear to be a Clock target gene, was not affected by Cry2, Clock, or a combination of Clock and Cry2 siRNA, highlighting the potential Clock/Bmal1 independent effect of Per1 on ET-1 (Fig. 12C). Per1 or Per1 and Cry2 siRNA resulted in similar increases in ET-1 gene expression (Fig. 12C).
Fig. 12.
Per1 and Cry2 mediate opposing action on Per1 target genes in mpkCCDc14 cells. mpkCCDc14 cells were treated with nontarget, Per1, Cry2, or Clock siRNA or a combination of both Per1 and Cry2 or Cry2 and Clock for 48 h. QPCR was used to evaluate changes in αENaC (A), Fxyd5 (B), and ET-1 (C) gene expression in mpkCCDc14 siRNA-treated cells vs. nontarget. P < 0.05, **P < 0.01; n = 3. Values are means ± SE.
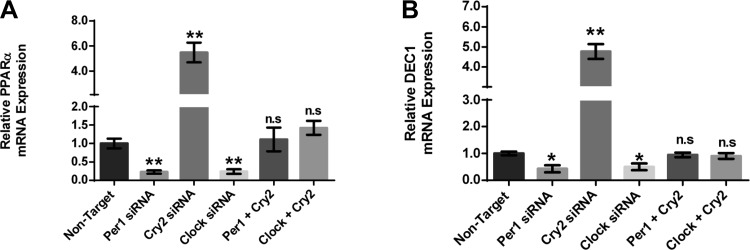
Next, we investigated the effect of Per1, Clock, and Cry2 action on Per1 target gene expression in AML12 cells. The effects on αENaC and Fxyd5 expression were mirrored in the AML12 cells for the target genes PPARα and DEC1 (Fig. 13). As shown above (see Fig. 5), PPARα mRNA expression decreased with Per1 or Clock knockdown (Fig. 13A). In contrast, PPARα mRNA levels were increased with Cry2 siRNA-mediated knockdown. The combination of Per1 and Cry2 siRNA returned expression of PPARα to control levels, supporting our hypothesis that Per1 and Cry2 mediate opposing actions on PPARα gene expression as well. A combination of Clock and Cry2 siRNA also resulted in control levels of PPARα gene expression (Fig. 13A). These effects were observed for DEC1 mRNA levels as well (Fig. 13B). Taken together, the results from both mpkCCDc14 and AML12 cells demonstrate that Per1 and Cry2 mediate opposing actions on Per1 target genes. These data are consistent with our proposed mechanism of a Cry2-Clock/Bmal1-dependent mechanism for Per1 action in liver and kidney.
Fig. 13.
Per1 and Cry2 mediate opposing action on Per1 target genes in AML12 cells. AML12 cells were treated with nontarget, Per1, Cry2, or Clock siRNA or a combination of both Per1 and Cry2 or Cry2 and Clock for 48 h. QPCR was used to evaluate changes in PPARα (A), and DEC1 (B) gene expression in AML12 siRNA-treated cells vs. nontarget. P < 0.05, **P < 0.01; n = 3. Values are means ± SE.
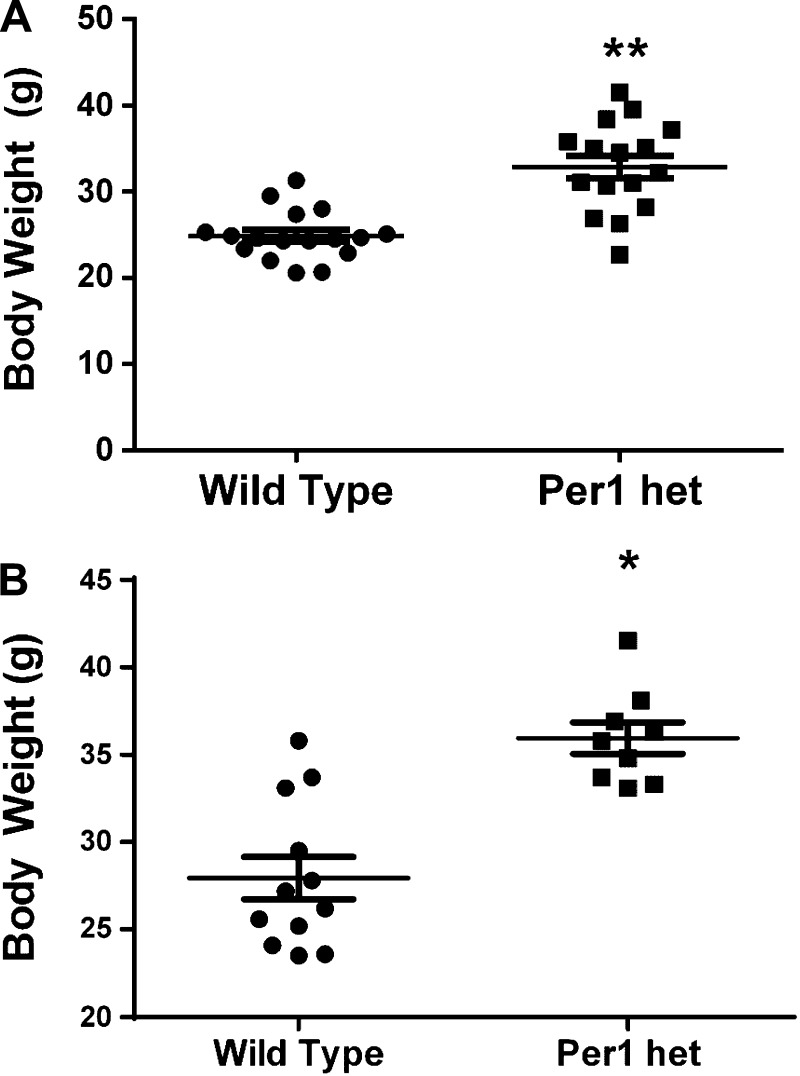
Per1 het mice weigh more than WT controls.
As shown above, Per1 positively regulates mRNA expression of PPARα. PPARα is critical for metabolic control; PPARα KO mice display increased body weight, hyperlipidemia, decreased serum insulin, and increased inflammation in adipose tissue (60). Per1/2/3 triple KO mice gain significantly more weight on normal or high-fat diets than WT controls (12), but such a phenotype has not been assessed for Per1 het mice. To compare body weight between Per1 het and WT mice, weight measurements were made in two independent sets of age-matched (16 or 28 wk) WT and Per1 het mice. Indeed, Per1 het mice weigh significantly more than WT controls at both 16 and 28 wk (Fig. 14), a result that is consistent with a role for Per1 in the regulation of PPARα, but likely involves multiple signaling pathways. Importantly, the heavier body weight phenotype of Per1 het provides further support for the hypothesis that Per1 and Cry2 mediate opposing action because Cry1/2 KO exhibit a lean body weight (24). Thus, the blood pressure (50) and body weight phenotype resulting from reduced Per1 expression in vivo resembles that of the Clock KO and is opposite that of the Cry1/2 KO.
Fig. 14.
Per1 het mice weigh more than WT controls. Body weight measurements of an independent set of 16- (A) and 28- (B) wk-old WT or Per1 het mice. *P < 0.05; n = 9–12. Values are means ± SE.
DISCUSSION
The goal of this study was to investigate the mechanism underlying our previously observed noncanonical actions of Per1 in the positive regulation of gene expression in the kidney. We tested whether Per1 action in the liver and kidney was due to a Cry2-Clock/Bmal1-dependent mechanism in which Per1 suppresses Cry2, preventing Cry2 from suppressing Clock/Bmal1 action (Fig. 1). In support of this hypothesis, we found that Per1 target genes in mpkCCDc14 cells were also Clock target genes. We identified two new Per1 target genes in AML12 cells and in liver, PPARα and DEC1. We confirmed the existence of the canonical clock complex in the kidney in vivo. Both in vitro and in vivo, Per1 knockdown resulted in upregulation of Cry2 at the mRNA and protein level, demonstrating a potential role for Per1 in Cry2 suppression. Importantly, we showed that Per1 and Cry2 mediate opposing actions on Per1 target genes in the liver and kidney.
Our results provide evidence for a Cry2-dependent mechanism underlying Per1 action on target genes. Our data are consistent with what has been observed using behavioral outputs for the role of Per1 in the suppression of Cry2 (36, 37). In those studies, it was demonstrated that Per1/Cry2 double-KO mice exhibited normalization of the behavioral phenotype of each single KO, as determined by actogram analysis. This is consistent with our observations that knockdown of both Per1 and Cry2 resulted in normalization of Per1 target gene expression in mpkCCDc14 and AML12 cells. As mentioned above, we have previously shown that Per1 KO mice have significantly lower BP than WT controls (50). This phenotype is opposite that of the Cry1/2 double KOs that exhibit salt-sensitive hypertension (14) and similar to that of Clock KO mice, which also display a relatively hypotensive phenotype (62). Furthermore, Cry1/2 KO mice exhibit a lean body weight phenotype (24), whereas Clock (Δ19) mutant mice are obese (53); the results of the current study provide evidence that Per1 het mice, similar to Clock KO mice, exhibit a heavier body weight phenotype. These physiological findings are consistent with our observation that Per1 and Cry2 exert opposing action on target gene regulation. However, the present study focuses on the opposing actions of Per1 and Cry2, and our results do not preclude the involvement of Per2 and Cry1 in the regulation of circadian target genes.
Interestingly, repression of ET-1 by Per1 appears to be independent of Bmal1/Clock or Cry2. ET-1 is a powerful inhibitor of ENaC and thus behaves as a natriuretic hormone in the renal collecting duct (7). Outside of the kidney, however, ET-1 acts as a potent vasoconstrictor (59). The tissue-specific mechanisms for the actions of Per1 on ET-1 are unknown and further investigation is required to define the mechanism by which Per1 regulates ET-1 expression in the kidney.
We have identified two new Per1 targets in the liver, PPARα and DEC1. PPARα is involved in fatty acid utilization and metabolism. We show that Per1 het mice weigh more than age-matched WT controls at 16 and 28 wk, a result that is consistent with the work of others. Per1/2/3 triple-KO mice gain significantly more weight on normal or high-fat diets than WT controls (12). Clock (Δ19) mutant animals, in which the Clock/Bmal1 heterodimer cannot bind DNA, exhibited metabolic syndrome with hyperlipidemia and obesity (53). Bmal1 KO mice are obese and exhibit hyperlipidemia (20). In support of the opposing actions of Per1 and Cry2, Cry1/2 double-KO mice are leaner than WT controls (24). Both Clock KO and Bmal1 KO mice are diabetic with reduced plasma insulin levels (32). As mentioned above, PPARα KO mice display increased body weight, hyperlipidemia, decreased plasma insulin, and increased adipose inflammation (60). It will be interesting to determine whether downregulation of PPARα in the circadian mutant animals could be a partial reason for the obesity and hypoinsulinemia observed in the Clock KO and Bmal1 KO animals. A recent study demonstrated that mice that were fed a high-fat diet during the evening had altered circadian clock gene expression and PPARα expression. This led to modulation of expression of the PPARα target genes, cholesterol-7-alpha-hydroxylase and diglyceride acyltransferase (56). These findings demonstrate that circadian clock modulation of PPARα expression leads to altered expression of PPARα target genes.
DEC1 has been implicated in adipogenesis (38). To the best of our knowledge, a metabolic phenotype has not been reported for DEC1 KO mice. DEC1 has been implicated in circadian control by competing for binding to E-boxes with the Clock-Bmal1 complex (23). It is important to note that due to the implications of DEC1 in the regulation of the circadian clock, it would be difficult to ascertain differences between the effects of the circadian proteins vs. DEC1 in the causality of a metabolic phenotype.
It is highly likely that Per1, as part of the circadian clock mechanism, regulates a multitude of other genes in the liver that pertain to metabolism. Studies have shown that more than 25% of metabolites display circadian oscillations in mice and humans (11, 16). Around 10% of genes in the liver, including many rate-limiting metabolic enzymes, are rhythmically expressed (48). A recent global ChIP-sequencing study examining DNA binding for Per1, Per2, Cry1, Cry2, Clock, and Bmal1 showed high enrichment of these proteins at multiple genes involved in common metabolic pathways (25).
Perspectives and Significance
In summary, the results of the present study support the hypothesis that Per1 target gene activation potentially occurs through a Cry2-Clock/Bmal1-dependent mechanism in which Per1 action de-represses Clock/Bmal1 (Fig. 1). These data are consistent with those of previous studies demonstrating that Per1 transcriptionally suppresses Cry2 (33, 36, 37, 39). Taken together, our observations lend support to a hypothetical model in which Per1 action on target genes may occur via a potential Cry2-Clock/Bmal1-dependent mechanism.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-085193 and by University of Florida Division of Nephrology funds to M. L. Gumz.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R., L.R.S., and M.L.G. conception and design of research; J.R., S.C.A., G.S., K.-Y.C., B.C., N.S., L.R.S., and L.A.J. performed experiments; J.R., S.C.A., K.-Y.C., N.S., L.R.S., L.A.J., and M.L.G. analyzed data; J.R., B.C., L.A.J., and M.L.G. interpreted results of experiments; J.R. prepared figures; J.R. drafted manuscript; J.R., S.C.A., G.S., L.R.S., and M.L.G. edited and revised manuscript; J.R. and M.L.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alain Vandewalle and Yuri Sautin for kindly providing cell lines.
Present address for L. Stow: Champions Oncology, 855 N. Wolfe St., Suite 619, Baltimore, MD 21205 (e-mail: lstow@championsoncology.com).
REFERENCES
- 1.Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens 19: 51–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht U. The mammalian circadian clock: a network of gene expression. Front Biosci 9: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Albrecht U, Eichele G. The mammalian circadian clock. Curr Opin Genet Dev 13: 271–277, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology 56: 574–580, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Schirmer A, Lee Y, Lee H, Kumar V, Yoo SH, Takahashi JS, Lee C. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell 36: 417–430, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M. Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB J 15: 2613–2622, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cho Y, Noshiro M, Choi M, Morita K, Kawamoto T, Fujimoto K, Kato Y, Makishima M. The basic helix-loop-helix proteins differentiated embryo chondrocyte (DEC) 1 and DEC2 function as corepressors of retinoid X receptors. Mol Pharmacol 76: 1360–1369, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci USA 109: 2625–2629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallmann R, Weaver DR. Altered body mass regulation in male mPeriod mutant mice on high-fat diet. Chronobiol Int 27: 1317–1328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16: 67–74, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science 332: 1436–1439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci USA 109: 5541–5546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim Biophys Acta 1799: 622–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK, Minze LJ, Hsueh W, Ma K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J 26: 3453–3463, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennig S, Strauss HM, Vanselow K, Yildiz O, Schulze S, Arens J, Kramer A, Wolf E. Structural and functional analyses of PAS domain interactions of the clock proteins Drosophila PERIOD and mouse PERIOD2. PLoS Biol 7: e94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hida A, Koike N, Hirose M, Hattori M, Sakaki Y, Tei H. The human and mouse Period1 genes: five well-conserved E-boxes additively contribute to the enhancement of mPer1 transcription. Genomics 65: 224–233, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419: 841–844, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ikeda H, Yong Q, Kurose T, Todo T, Mizunoya W, Fushiki T, Seino Y, Yamada Y. Clock gene defect disrupts light-dependency of autonomic nerve activity. Biochem Biophys Res Commun 364: 457–463, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349–354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kucera N, Schmalen I, Hennig S, Ollinger R, Strauss HM, Grudziecki A, Wieczorek C, Kramer A, Wolf E. Unwinding the differences of the mammalian PERIOD clock proteins from crystal structure to cellular function. Proc Natl Acad Sci USA 109: 3311–3316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107: 855–867, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci USA 108: 16451–16456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol 349: 82–90, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem 280: 37717–37724, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maywood ES, Drynan L, Chesham JE, Edwards MD, Dardente H, Fustin JM, Hazlerigg DG, O'Neill JS, Codner GF, Smyllie NJ, Brancaccio M, Hastings MH. Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1-luc transgenic reporter mouse. Proc Natl Acad Sci USA 110: 9547–9552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 23, 1019–1026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J 386: 575–581, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oster H, Baeriswyl S, Van Der Horst GT, Albrecht U. Loss of circadian rhythmicity in aging mPer1-/-mCry2-/- mutant mice. Genes Dev 17: 1366–1379, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oster H, van der Horst GT, Albrecht U. Daily variation of clock output gene activation in behaviorally arrhythmic mPer/mCry triple mutant mice. Chronobiol Int 20: 683–695, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Ozaki N, Noshiro M, Kawamoto T, Nakashima A, Honda K, Fukuzaki-Dohi U, Honma S, Fujimoto K, Tanimoto K, Tanne K, Kato Y. Regulation of basic helix-loop-helix transcription factors Dec1 and Dec2 by RORalpha and their roles in adipogenesis. Genes Cells 17: 109–121, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science 337: 599–602, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Prasai MJ, Pernicova I, Grant PJ, Scott EM. An endocrinologist's guide to the clock. J Clin Endocrinol Metab 96: 913–922, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal 8: e002, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of αENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1δ/ε. Am J Physiol Renal Physiol 303: F918–F927, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards J, Gumz ML. Advances in understanding the peripheral circadian clocks. FASEB J 26: 3602–3613, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards J, Gumz ML. Invited Review EB 2012: Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol 304: R1053–R1064, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossner MJ, Dorr J, Gass P, Schwab MH, Nave KA. SHARPs: mammalian enhancer-of-split- and hairy-related proteins coupled to neuronal stimulation. Mol Cell Neurosci 9: 460–475, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Sautin YY, Crawford JM, Svetlov SI. Enhancement of survival by LPA via Erk1/Erk2 and PI 3-kinase/Akt pathways in a murine hepatocyte cell line. Am J Physiol Cell Physiol 281: C2010–C2019, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Shen M, Yoshida E, Yan W, Kawamoto T, Suardita K, Koyano Y, Fujimoto K, Noshiro M, Kato Y. Basic helix-loop-helix protein DEC1 promotes chondrocyte differentiation at the early and terminal stages. J Biol Chem 277: 50112–50120, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol 22: 598–604, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59: 1151–1156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun H, Lu B, Li RQ, Flavell RA, Taneja R. Defective T cell activation and autoimmune disorder in Stra13-deficient mice. Nat Immunol 2: 1040–1047, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc Natl Acad Sci USA 97: 4058–4063, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet 37: 187–192, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Xu CX, Krager SL, Bottum KM, Liao DF, Tischkau SA. Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-alpha pathway activity in mice. Environ Health Perspect 119: 1739–1744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Xue J, Yang J, Xie M. Timed high-fat diet in the evening affects the hepatic circadian clock and PPARα-mediated lipogenic gene expressions in mice. Genes Nutr. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X, Liu Z, Shi G, Xing L, Wang X, Gu X, Qu Z, Dong Z, Xiong J, Gao X, Zhang C, Xu Y. The circadian clock influences heart performance. J Biol Rhythms 26: 402–411, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Xu CX, Krager SL, Liao DF, Tischkau SA. Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene. Toxicol Sci 115: 98–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]
- 60.Yessoufou A, Ategbo JM, Attakpa E, Hichami A, Moutairou K, Dramane KL, Khan NA. Peroxisome proliferator-activated receptor-alpha modulates insulin gene transcription factors and inflammation in adipose tissues in mice. Mol Cell Biochem 323: 101–111, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Yildiz O, Doi M, Yujnovsky I, Cardone L, Berndt A, Hennig S, Schulze S, Urbanke C, Sassone-Corsi P, Wolf E. Crystal structure and interactions of the PAS repeat region of the Drosophila clock protein PERIOD. Mol Cell 17: 69–82, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]