Abstract
Post-oral sugar actions enhance the intake of and preference for sugar-rich foods, a process referred to as appetition. Here, we investigated the role of intestinal sodium glucose cotransporters (SGLTs) in sugar appetition in C57BL/6J mice using sugars and nonmetabolizable sugar analogs that differ in their affinity for SGLT1 and SGLT3. In experiments 1 and 2, food-restricted mice were trained (1 h/day) to consume a flavored saccharin solution [conditioned stimulus (CS−)] paired with intragastric (IG) self-infusions of water and a different flavored solution (CS+) paired with infusions of 8 or 12% sugars (glucose, fructose, and galactose) or sugar analogs (α-methyl-d-glucopyranoside, MDG; 3-O-methyl-d-glucopyranoside, OMG). Subsequent two-bottle CS+ vs. CS− choice tests were conducted without coinfusions. Infusions of the SGLT1 ligands glucose, galactose, MDG, and OMG stimulated CS+ licking above CS− levels. However, only glucose, MDG, and galactose conditioned significant CS+ preferences, with the SGLT3 ligands (glucose, MDG) producing the strongest preferences. Fructose, which is not a ligand for SGLTs, failed to stimulate CS+ intake or preference. Experiment 3 revealed that IG infusion of MDG+phloridzin (an SGLT1/3 antagonist) blocked MDG appetition, whereas phloridzin had minimal effects on glucose-induced appetition. However, adding phloretin (a GLUT2 antagonist) to the glucose+phloridzin infusion blocked glucose appetition. Taken together, these findings suggest that humoral signals generated by intestinal SGLT1 and SGLT3, and to a lesser degree, GLUT2, mediate post-oral sugar appetition in mice. The MDG results indicate that sugar metabolism is not essential for the post-oral intake-stimulating and preference-conditioning actions of sugars in mice.
Keywords: post-oral sugar conditioning, glucose, fructose, galactose
food intake and preference are guided by oral sensations (taste, odor, and mouth feel) that contribute to the identification and hedonic evaluation of food flavor. Considerable progress has been made in identifying the taste receptors that respond to sugar, fat, and amino acids that provide attractive sweet, fatty, and umami flavors to foods (8). The appetite for such foods is further enhanced by the post-oral actions of ingested nutrients (50). This is demonstrated in laboratory rodents by the intake stimulation and learned preferences for arbitrary flavors (conditioned stimuli, CS) that are paired with gastric or intestinal infusions of sugar, fat, and proteins (or glutamate) (50). However, relatively little is known about the sites and identities of the sensors that mediate the post-oral appetite-stimulating actions of nutrients, a process we refer to as appetition (48). In the case of sugars, several findings implicate the upper small intestine as a primary site of action for glucose appetition. That is, preferences for flavored saccharin solutions are conditioned by gastric, duodenal, and jejunal glucose infusions, but not by gastric infusions with a closed pylorus, or by ileal, hepatic-portal, or intraperitoneal glucose infusions (2, 15, 67). Hepatic-portal glucose infusions produced preferences in other conditioning paradigms, however, which suggest that sugar sensors in both the upper intestinal and hepatic portal regions contribute to sugar conditioning (39, 58).
The presence of the oral sweet taste receptor (T1R2+T1R3) in the intestinal tract suggested the possibility that the same sensor that stimulates sugar appetite in the mouth also mediates sugar appetition in the gut (5). However, this notion is not supported by the findings that sweet ageusic mice missing critical sweet taste-signaling elements (T1R3, TRPM5, gustducin) demonstrate normal flavor-conditioning responses to IG sugar infusions (50). Furthermore, not all ligands of the sweet taste receptor are effective in conditioning flavor preferences by IG infusions. Whereas glucose and glucose-containing saccharides (sucrose, maltose, and maltodextrin) condition robust flavor preferences, IG fructose has weaker or no conditioning effects, whereas the nonnutritive sweetener sucralose conditioned a mild aversion (50). These findings indicate that gut sensors other than T1R2 and T1R3 mediate the appetition produced by gastric and intestinal glucose infusions. Two candidate sensors are sodium glucose cotransporters (SGLT1) and SGLT3. The sodium glucose cotransporter SGLT1 is a transporter of glucose in intestinal enterocytes and is also thought to serve as a glucose sensor in enteroendocrine cells (56, 63). The related protein SGLT3 has little or no transport function but may serve as a glucose sensor in the gut (56, 63). SGLT1 and SGLT3 are attractive candidates because they bind to glucose but not fructose. The two sensors differ in that glucose and galactose are substrates for SGLT1 but only glucose binds to SGLT3. In rats and mice, IG galactose failed to condition flavor preferences (49, 51), which would appear to implicate SGLT3 as the sensor responsible for post-oral glucose appetition. However, a recent study reported that IG intubation of glucose and galactose, but not fructose, conditioned place preferences in mice (30). In addition, we recently reported that sweet ageusic T1r3 and Trpm5 knockout (KO) mice expressed preferences for glucose and galactose, but not for fructose, in 24-h sugar vs. water tests, which we attributed to the differential post-oral actions of the sugars (68). Taken together, these findings suggest that galactose, like glucose, has post-oral reward actions in mice in some test situations.
The present study investigated the role of SGLT1 and SGLT3 in post-oral sugar appetition by using agonists and antagonists of these sugar sensors. This strategy has been used to characterize the sugar sensors involved in gut hormone release, sugar-induced satiety, and brain neural responses (12, 20, 43, 45, 54, 56). Experiment 1 compared the post-oral conditioning effects of glucose, galactose, and fructose using a newly developed IG self-infusion paradigm that reveals both rapid glucose stimulation of intake and flavor preference conditioning in C57BL/6J mice (67, 69). Experiment 2 extended this analysis by comparing the post-oral appetition effects of glucose with those of two nonmetabolizable glucose analogs: α-methyl-d-glucopyranoside (MDG) and 3-O-methyl-d-glucopyranoside (OMG). These analogs were of interest because they are both ligands for the SGLT1 transporter, and MDG is also a ligand for SGLT3 (56, 63). Furthermore, they both evoke physiological responses similar to glucose, such as inhibition of gastric emptying and release of GLP-1 and GIP hormones, although MDG is more effective than OMG in mimicking glucose actions in some responses (35, 44). Experiment 3A determined the effects of the SGLT1/3 antagonist phloridzin on the flavor-conditioning effects of glucose and MDG. Experiment 3B then determined whether blocking glucose actions on SGLT1 and SGLT3, as well as on the sugar transporter GLUT2, using a mixture of phloridzin and phloretin would more effectively prevent glucose-conditioned flavor preferences (see Ref. 29).
MATERIALS AND METHODS
Experiment 1—Glucose, Galactose, and Fructose
This experiment compared the post-oral appetition effects of IG self-infusions of glucose, galactose, and fructose, which have differential actions on SGLT1 and SGLT3. The test protocol was identical to that used in our recent study of 2–32% glucose self-infusions (67). In experiment 1A, the mice were infused with 8% sugar solutions based on our finding that 8% glucose was the threshold concentration to condition a CS+ preference (67). Experiment 1B determined whether a higher sugar concentration (12%) enhanced the conditioning response to galactose and fructose.
Animals.
Adult male C57BL/6J (B6) mice (10 wk old) born in the laboratory from Jackson Laboratories (Bar Harbor, ME) stock were singly housed in plastic tub cages kept in a test room maintained at 22°C with a 12:12-h light-dark cycle. The mice were maintained on chow (5001; PMI Nutrition International, Brentwood, MO) prior to food restriction. During testing, they were maintained at 85–90% of their ad libitum body weight by feeding them fixed-size chow pellets (0.5 or 1 g, Bio-Serv, Frenchtown, NJ), which allowed for precise adjustment of daily food rations. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Surgery.
Mice were fitted with IG catheters (0.84 mm OD × 0.36 mm ID, Micro-Renathane tubing, MRE-033; Braintree Scientific, Braintree, MA) while anesthetized with isoflurane (2%) inhalation, as previously described (52). About 10 days after surgery the mice were briefly (5 min) anesthetized with isoflurane, and tubing was attached to the gastric catheter and then passed through an infusion harness with a spring tether (CIH62, Instech Laboratories, Plymouth Meeting, PA). The tubing was then attached to an infusion swivel mounted on a counterbalanced lever (Instech Laboratories). The body weight of each mouse was measured before and after it was fitted with the infusion tether/swivel system; daily body weights were monitored by weighing the mouse with the attached infusion tether/swivel system. Each animal was then returned to a tub cage, and the swivel-counterbalanced lever was attached above the cage.
Apparatus.
IG infusion tests were conducted in plastic test cages (52). The sipper spouts were interfaced via electronic lickometers (Med Electronics, St. Albans, VT) to a computer, which operated a syringe pump (A-99; Razel Scientific, Stamford, CT) that infused liquid into the gastric catheters as the animals licked. The pump rate was nominally 0.5 ml/min, but the animal controlled the overall infusion rate and volume by its licking response. In particular, the computer accumulated licks during 3-s bins and activated the pump for 3 s when a criterion number of licks was recorded. The oral-to-infusion intake ratio was maintained at ∼1:1 by adjusting the lick criterion for each mouse. Daily oral fluid intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
Test solutions.
The CS solutions contained 0.025% sodium saccharin (Sigma Chemical, St. Louis, MO) flavored with 0.02% ethyl acetate or propyl acetate (Sigma). The CS− solution was paired with IG infusion of water, while the CS+ solution was paired with IG infusion of food-grade glucose, fructose (Honeyville Food Products, Rancho Cucamonga, CA), or galactose (Sigma). In experiment 1A, the 8% Fructose, 8% Galactose and 8% Glucose groups contained 14, 12, and 10 mice, respectively. In experiment 1B, the 12% Fructose, Galactose, and Glucose groups contained 12, 9, and 12 mice, respectively. For about half of the animals in each group, the CS− solution contained ethyl acetate, and the CS+ solution contained propyl acetate; the flavors were reversed for the remaining animals.
Procedure.
The mice were trained (1 h/day) in the test cages for two sessions with unflavored 0.025% sodium saccharin solution, while water-deprived and then for four sessions while food-restricted to 85–90% of their ad libitum body weight; saccharin intakes were paired with matched-volume infusions of water. The mice were then given three 1-h test sessions with the CS− saccharin solution paired with IG water infusions followed by three sessions with the CS+ saccharin solution paired with IG infusions of the appropriate sugar solution. The mice were then given two alternating (A) sessions, each with the CS−/IG water (days 1 and 3) and the CS+/IG sugar (days 2 and 4) to enhance their discrimination between the two solutions (67). In the last of each CS− and CS+ session, the mice were given a second sipper tube containing water not paired with IG infusions to familiarize them with the presence of two sipper tubes for the subsequent two-bottle test. The two-bottle test with the CS+ and CS− solutions, no longer paired with IG infusions, was conducted over four 1 h/day sessions.
Data analysis.
CS− licks and total intakes (oral + IG infusate) during the last two 1 h/day sessions were averaged. The data from these two sessions, referred to as test 0, and the licks and intakes during the three CS+ sessions (tests 1–3) were analyzed using a mixed-model ANOVA with a group factor (IG Sugar group) and repeated-measures factor (tests 0–3). Similarly, the mean CS− and CS+ licks during the two alternating one-bottle sessions and the four two-bottle tests were compared in separate ANOVAs. Additional analyses are described in the results.
RESULTS
Experiment 1A.
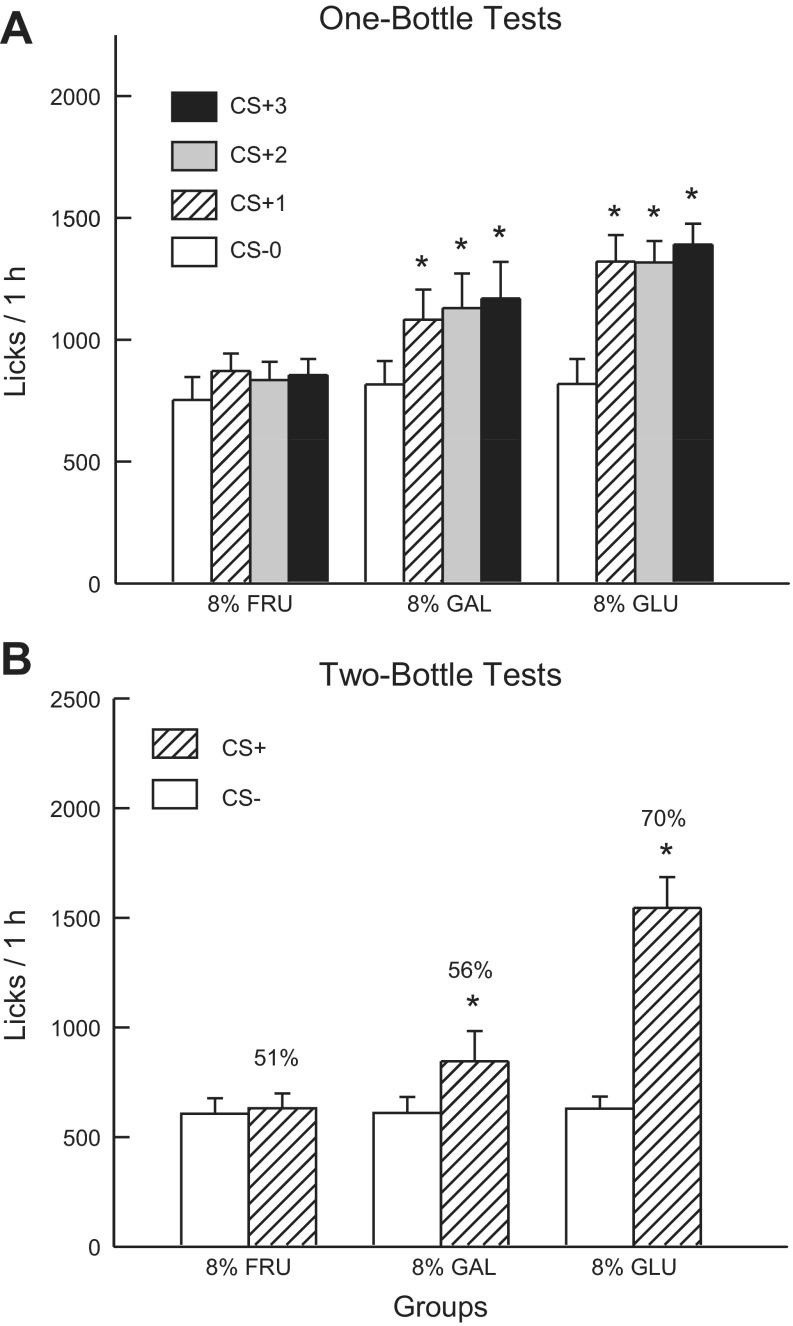
Fig. 1A shows the total 1-h lick data for CS− test 0 and CS+ tests 1–3. Analysis of these data revealed that the 8% sugar groups did not differ in their CS− licks paired with IG water self-infusion (test 0) but did differ in their CS+ licks paired with IG 8% sugar self-infusion [group × test interaction, F(6,99) = 4.32, P < 0.001]. The 8% Glucose and 8% Galactose groups, but not the 8% Fructose group, significantly increased 1-h licks when switched from the CS− to the CS+. A comparison of the CS+ tests 1–3 licks indicated that the 8% Glucose group licked more (P < 0.01) than the 8% Fructose group, while the licks of the 8% Galactose group were intermediate between the other two groups [F(2,32) = 6.2, P < 0.01]. Within-group analyses revealed that the 8% Galactose group [F(3,33) = 10.1, P < 0.001] and 8% Glucose group [F(3,27) = 14.5, P < 0.001] licked more in each of CS+ tests 1–3 than in CS− test 0 and that their licks in tests 1–3 did not differ. The 1-h intake data (CS solution + IG infusion) revealed a similar pattern of results [group × test interaction, F(6,99) = 4.77, P < 0.001]. Overall, intakes significantly increased from CS− test 0 to CS+ test 3 in the 8% Glucose (2.0 to 3.3 g/h) and the 8% Galactose (1.9 to 2.9 g/h) groups, but not in the 8% Fructose group (1.9 to 2.2 g/h). The CS− intakes of the groups did not differ in test 0, whereas their CS+ tests 1–3 intakes differed as follows: 8% Glucose ≥ 8% Galactose > 8% Fructose.
Fig. 1.
Experiment 1A: Values are expressed as means ± SE. A: 1-h total licks are plotted for one bottle tests 0–3. The mice drank (1 h/day) a conditioned stimuli (CS)-flavored saccharin solution paired with intragastric (IG) water infusions in test 0 before being switched to a CS+-flavored saccharin solution paired with IG sugar self-infusions in tests 1–3. The 8% GLU, 8% FRU, and 8% GAL groups were infused with 8% glucose, fructose, and galactose, respectively. B: 1-h licks are plotted for CS+- and CS−-flavored saccharin solutions during the two-bottle preference test for the 8% GLU, 8% FRU, and 8% GAL groups. CS+ and CS− intakes were not paired with IG infusions during test. Number atop bar represents mean percent preference for the CS+ solution. *Significant difference (P < 0.05) between test 0 vs. tests 1–3 licks and between CS+ vs. CS− licks.
In the alternating training sessions, the 8% Glucose and 8% Galactose groups licked more than the 8% Fructose group [F(2,33) = 7.9, P < 0.01]. In addition, the 8% Glucose and Galactose groups licked more (P < 0.001) in the CS+ than CS− sessions (1,467.0 vs. 1,152.1, 1,129.6 vs. 910.4, respectively), whereas the CS+ and CS− licks did not differ in the 8% Fructose group (769.8 vs. 833.9) [group × CS interaction, F(2,33) = 12.9; P < 0.001].
Figure 1B presents the lick data for the two-bottle CS+ vs. CS− choice test conducted without IG infusions. Analysis of these data revealed a group × CS interaction [F(2,33) = 22.6; P < 0.001]. In particular, the 8% Glucose and Galactose groups licked significantly more (P < 0.001 and P < 0.05, respectively) for the CS+ than CS−, while the CS licks of the 8% Fructose group did not differ. Furthermore, the CS+ licks differed (P < 0.001) among the groups: 8% Glucose > 8% Galactose = 8% Fructose groups, but CS− licks did not differ. A similar pattern of results was obtained in the analysis of two-bottle intake data except that the CS+ intakes of the 8% Glucose and Galactose groups only marginally differed (P = 0.052). Also, the Glucose group consumed less CS− than the Fructose and Galactose groups (data not shown). The groups also differed in their percent CS+ licks [F(2,33) = 27.9; P < 0.001]. The 8% Glucose group displayed a higher preference (70%, P < 0.01) than did 8% Galactose (56%) and Fructose (51%) groups, which did not differ from one another. Note that the 56% CS+ preference of the 8% Galactose group was very weak, but 10 of the 12 mice licked more for the CS+ than CS−.
Experiment 1B
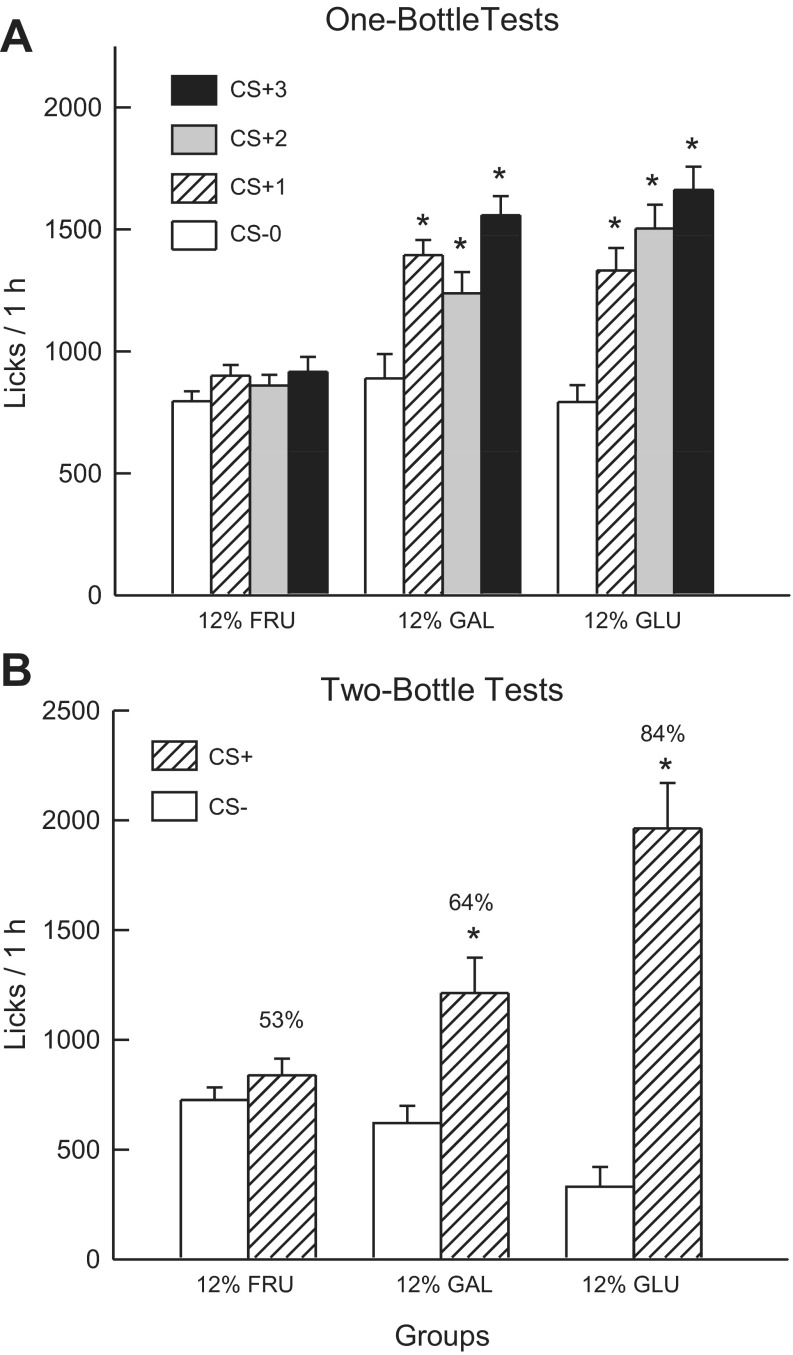
Fig. 2A shows the total 1-h lick data for CS− test 0 and CS+ tests 1–3. Analysis of these data revealed that the 12% sugar groups did not differ in their CS− licks paired with IG water self-infusion (test 0) but did differ in their CS+ licks paired with IG 12% sugar self-infusion in tests 1–3 [group × test interaction, F(6,99) = 14.80; P < 0.001]. The 12% Glucose and Galactose groups, but not the 12% Fructose group, significantly increased 1-h licks when switched from the CS− to the CS+. A comparison of the CS+ tests 1–3 licks indicated that the 12% Glucose and Galactose groups did not differ and both licked more (P < 0.01) than the 12% Fructose group [F(2,33) = 23.7; P < 0.001]. Within-group analyses revealed that the 12% Glucose group increased (P < 0.01) CS licks from test 0 to 1 and then in each successive CS+ test. The 12% Galactose group increased (P < 0.01) their licks from test 0 to 1 and then from test 2 to 3. The 1-h intake data revealed a similar pattern of results [group × test interaction, F(6,99) = 12.74, P < 0.001]. Overall, intakes significantly increased from CS− test 0 to CS+ test 3 in the 12% Glucose (2.1 to 3.5 g/h) and the 12% Galactose (2.0 to 3.2 g/h) groups, but not in the 12% Fructose group (2.1 to 2.2 g/h). The CS− intakes of the groups did not differ in test 0, whereas their CS+ test 1 and test 3 intakes differed as follows: 12% Glucose = 12% Galactose > 12% Fructose. Test 2 intakes differed as follows: 12% Glucose > 12% Galactose > 12% Fructose.
Fig. 2.
Experiment 1B: Values are expressed as means ± SE. A: 1-h total licks are plotted for one bottle tests 0–3. The mice drank (1 h/day) a CS−-flavored saccharin solution paired with IG water infusions in test 0 before being switched to a CS+-flavored saccharin solution paired with IG sugar self-infusions in tests 1–3. The 12% GLU, 12% FRU, and 12% GAL groups were infused with 12% glucose, fructose, and galactose, respectively. B: 1-h licks are plotted for CS+- and CS−-flavored saccharin solutions during the two-bottle preference test for the 12% GLU, 12% FRU, and 12% GAL groups. CS+ and CS− intakes were not paired with IG infusions during the test. Number atop bar represents mean percent preference for the CS+ solution. *Significant differences (P < 0.05) between test 0 vs. tests 1–3 licks and between CS+ vs. CS− licks.
In the alternating training sessions, the 12% Glucose and Galactose groups licked more than the 12% Fructose group [F(2,33) = 13.1; P < 0.01]. In addition, the 12% Glucose and Galactose groups licked more (P < 0.001) in the CS+ than CS− sessions (1,665.0 vs. 1,252.1, 1,375.8 vs. 1,092.5, respectively), whereas the CS+ and CS- licks did not differ in the 12% Fructose group (931.1 vs. 939.5) [group × CS interaction; F(2,33) = 9.7, P < 0.001].
Fig. 2B presents the lick data for the two-bottle CS+ vs. CS− choice test. Analysis of these data revealed a group × CS interaction [F(2,33) = 15.3, P < 0.001]. In particular, the 12% Glucose and Galactose groups licked significantly more (P < 0.01) for the CS+ than CS−, while the CS licks of the 12% Fructose group did not differ. Furthermore, the CS+ licks differed (P < 0.001) among the groups: 12% Glucose > 12% Galactose = 12% Fructose groups. CS− licks also differed (P < 0.01) among the groups: 12% Fructose = 12% Galactose > 12% Glucose. A similar pattern of results was obtained in the analysis of two-bottle intake data (data not shown). The groups also differed in their percent CS+ licks [F(2,33) = 34.0; P < 0.001]. The 12% Glucose group displayed a higher (P < 0.01) preference (84%) than the 12% Galactose group (64%), which displayed a higher preference than the 12% Fructose (53%) group.
DISCUSSION
Glucose.
As previously reported, the 8% Glucose mice licked significantly more for the CS+ than CS− beginning in CS+ test 1 and displayed a significant CS+ preference in the two-bottle choice test without IG infusions (67). Experiment 1B revealed an even greater stimulation of CS+ licking and CS+ preference in the 12% Glucose group. This confirms our prior concentration-response findings obtained with mice tested with 2, 4, 8, 16, and 32% glucose infusions. As detailed in our prior report, an analysis of within-session lick rates revealed that the IG glucose groups increased their CS+ licking as early as 4–6 min in CS+ test 1 and showed substantial increases in CS+ licking in minutes 1–3 of tests 2 and 3. Similar changes in lick rates were observed in the 12% Glucose group in the present study (data not shown).
Fructose.
In contrast to the Glucose groups, the 8% and 12% Fructose groups failed to increase their 1-h licks in CS+ tests 1–3 or display a significant preference for the CS+ over the CS−. This is consistent with prior 1-h lick data obtained with B6 mice drinking 0.8% sucralose and less “sweet” 8% glucose and 8% fructose solutions. That is, the mice that switched from sucralose to glucose licked much more for the sugar, whereas the mice that switched from sucralose to fructose licked much less sugar than sucralose (66). The present 1-h IG results are also in agreement with our 24-h IG study showing that IG infusions of 8% or 16% fructose, unlike glucose infusions, failed to stimulate intake or condition CS+ preferences in B6 mice (49). The 1- and 24-h IG conditioning results indicate that the post-oral actions of fructose have no reinforcing effects in B6 mice. This conclusion is compatible with the failure of naïve sweet ageusic T1r3 KO and Trpm5 KO mice to develop preferences for orally consumed fructose solutions, which contrasts with their robust experience-induced preferences for glucose solutions (68).
Galactose.
The present results provide the first evidence that IG infusions of galactose can stimulate intake and condition flavor preferences in rodents. The 8% and 12% galactose infusions increased lick rates in the very first CS+ test session and further stimulated licking in subsequent test sessions. Overall, the 8% and 12% galactose infusions stimulated CS+ licking as much as did the IG glucose infusions. However, galactose conditioned weaker CS+ preferences than did glucose. In particular, the preference of the 8% Galactose group, while statistically significant, was quite modest (56%) and substantially less than the 70% CS+ preference of the 8% Glucose group. The 12% galactose IG infusions increased the CS+ preference to 64%, but it was significantly lower than the 84% preference produced by the 12% glucose infusions.
To determine whether a higher galactose concentration would condition a still stronger CS+ preference, another group of mice (n = 12) was tested as in experiment 1 but with IG infusions of 16% galactose. These mice displayed only a marginal (P = 0.079) increase in CS+ licks in tests 1–3. The 16% galactose mice showed a weak (54%), albeit significant (P < 0.05), CS+ preference in the two-bottle tests, which contrasts with the 80% CS+ preference displayed by rats infused with 16% glucose infusions (67). Thus, while IG galactose can stimulate intake and condition a flavor preference, it is effective only at a limited concentration range and much less so than glucose. Postabsorptive metabolic factors may limit the appetition effects of galactose (see general discussion).
Experiment 2: Glucose Analogs
The present experiment further explored the role of intestinal SGLT proteins in sugar appetition by comparing the conditioning effects of two nonmetabolizable glucose analogs: MDG, a substrate for both SGLT1 and SGLT3, and OMG, a substrate for SGLT1 only. Prior studies have used these two glucose analogs to reveal the sugar-sensing mechanisms involved in gastrointestinal hormone release and satiety effects (7, 12, 18, 19, 21, 33, 37), but their abilities to stimulate intake and condition flavor preferences have not been previously investigated. One study reported, however, that OMG gastric intubation failed to condition a place preference in mice (30).
At the end of behavioral testing, the effects of the IG glucose and glucose analog infusions on blood glucose levels were measured. This was of interest because of the potential role of postabsorptive glucose in preference conditioning (39, 58). A prior study indicated that gastric intubation of MDG, unlike glucose, did not increase plasma glucose levels in B6 mice (37).
MATERIALS AND METHODS
Adult male B6 mice were housed and tested as in experiment 1. Experiment 2A included 8% MDG (n = 14) and 8% OMG (n = 15) groups and experiment 2B included 12% MDG (n = 14) and 12% OMG (n = 9) groups. Their licking responses were compared with those of the 8% and 12% Glucose groups of experiment 1.
Following the two-bottle tests, blood glucose levels were measured. Each mouse was infused with 0.6 ml of its respective sugar solution (8% or 12% glucose, MDG, or OMG) over a 10-min period (see Ref. 67). Tail blood samples were measured using a FreeStyle Freedom Lite (Abbott Diabetes Care, Alameda, CA) blood glucose meter just before (0 min), and 15, 30, and 60 min following the start of the IG infusion. Data were collected from an additional group of mice (n = 12) that were infused IG with 0.6 ml of water instead of glucose (67). The blood glucose data were analyzed using an ANOVA with a group factor and a repeated-measure factor (sample time points). In addition, incremental areas under the curve (IAUC) were calculated and compared across groups with a one-way ANOVA.
RESULTS
Experiment 2A
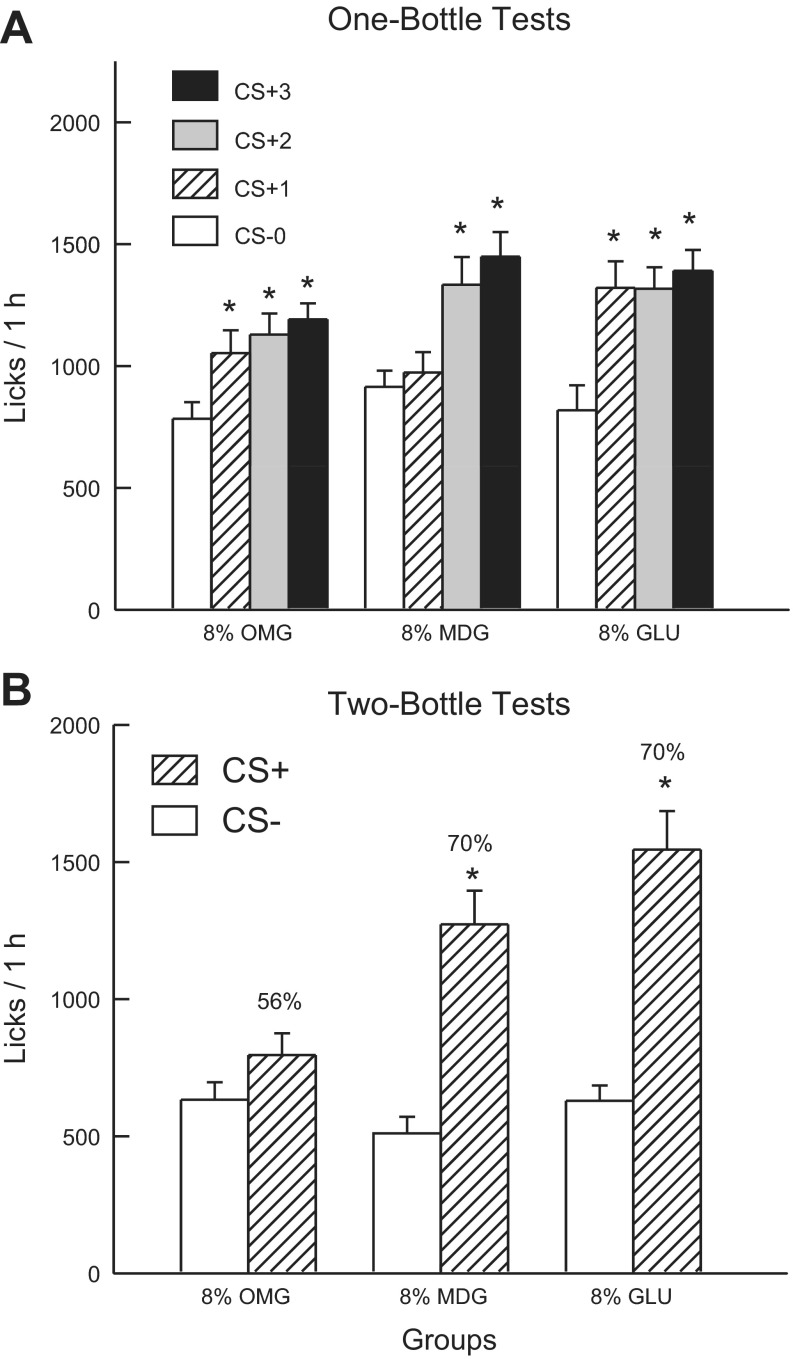
Fig. 3A shows the total 1-h lick data for CS− test 0 and CS+ tests 1–3. Analysis of these data revealed that the 8% sugar groups did not differ in their CS- licks paired with IG water self-infusion (test 0), and all three groups increased their licking response in the CS+ tests, although to different degrees [group × test interaction, F(6,108) = 4.4; P < 0.001]. A comparison of the CS+ tests 1–3 revealed that the Glucose group licked more than the 8% MDG group in test 1; there were no other group differences [F(4,72) = 4.9; P < 0.01]. In addition, within-group analyses revealed that the 8% Glucose and 8% OMG groups licked (P < 0.01) more in CS+ tests 1–3 than in CS− test 0, whereas the 8% MDG group licked more only in CS+ tests 2 and 3 than in CS− test 0. The 1-h intake data revealed a similar pattern of results [group × test interaction, F(6,108) = 4.46; P < 0.001]. Overall, all three groups significantly increased their solution intakes from CS− test 0 to CS+ test 3 (2.0 to 3.3 g/h for 8% Glucose, 2.4 to 3.2 g/h for 8% MDG, and 1.9 to 2.7 g/h for 8% OMG). The CS− intakes of the groups did not differ in test 0, whereas their CS+ test 1 intakes differed as follows: 8% Glucose > 8% OMG = 8% MDG; CS+ intakes did not differ in tests 2 and 3.
Fig. 3.
Experiment 2A: Values are expressed as means ± SE. A: 1-h total licks are plotted for one bottle tests 0–3. The mice drank (1 h/day) a CS−-flavored saccharin solution paired with IG water infusions in test 0 before being switched to a CS+-flavored saccharin solution paired with IG sugar self-infusions in tests 1–3. The 8% GLU, 8% MDG, and 8% OMG groups were infused with 8% glucose, MDG and OMG, respectively. B: 1-h licks are plotted for CS+- and CS−-flavored saccharin solutions during the two-bottle preference test for the 8% GLU, MDG, and OMG groups. CS+ and CS− intakes were not paired with IG infusions during the test. Number atop bar represents mean percent preference for the CS+ solution. *Significant differences (P < 0.05) between test 0 vs. tests 1–3 licks and between CS+ vs. CS− licks.
In the alternating training sessions, the groups differed in their CS+ and CS− licks [group × CS interaction, F(2,36) = 4.4; P < 0.05]. Only the 8% Glucose and 8% MDG groups licked more (P < 0.001) in the CS+ than CS− sessions (1,467.0 vs. 1,152.1, 1,391.4 vs. 1,102.2, respectively); CS+ and CS− licks did not significantly differ in the 8% OMG group (1,102.9 vs. 1,006.4).
Fig. 3B presents the lick data for the two-bottle CS+ vs. CS− choice test. Analysis of these data revealed a group × CS interaction [F(2,36) = 9.75; P < 0.001]. In particular, the 8% Glucose and MDG groups licked significantly more (P < 0.001) for the CS+ than CS−, while the CS licks of the 8% OMG group did not differ. Furthermore, the CS+ licks differed (P < 0.001) among the groups: 8% Glucose > 8% MDG > 8% OMG groups, but CS− licks did not differ. A similar pattern of results was obtained in the analysis of the two-bottle intake data, except that the CS+ intakes of the 8% Glucose and 8% MDG groups did not significantly differ (data not shown). The groups also differed in their percent CS+ licks [F(2,36) = 10.22; P < 0.001]. The 8% Glucose and MDG groups displayed similar preferences (70%) that exceeded (P < 0.01) that of the 8% OMG group (56%).
Experiment 2B
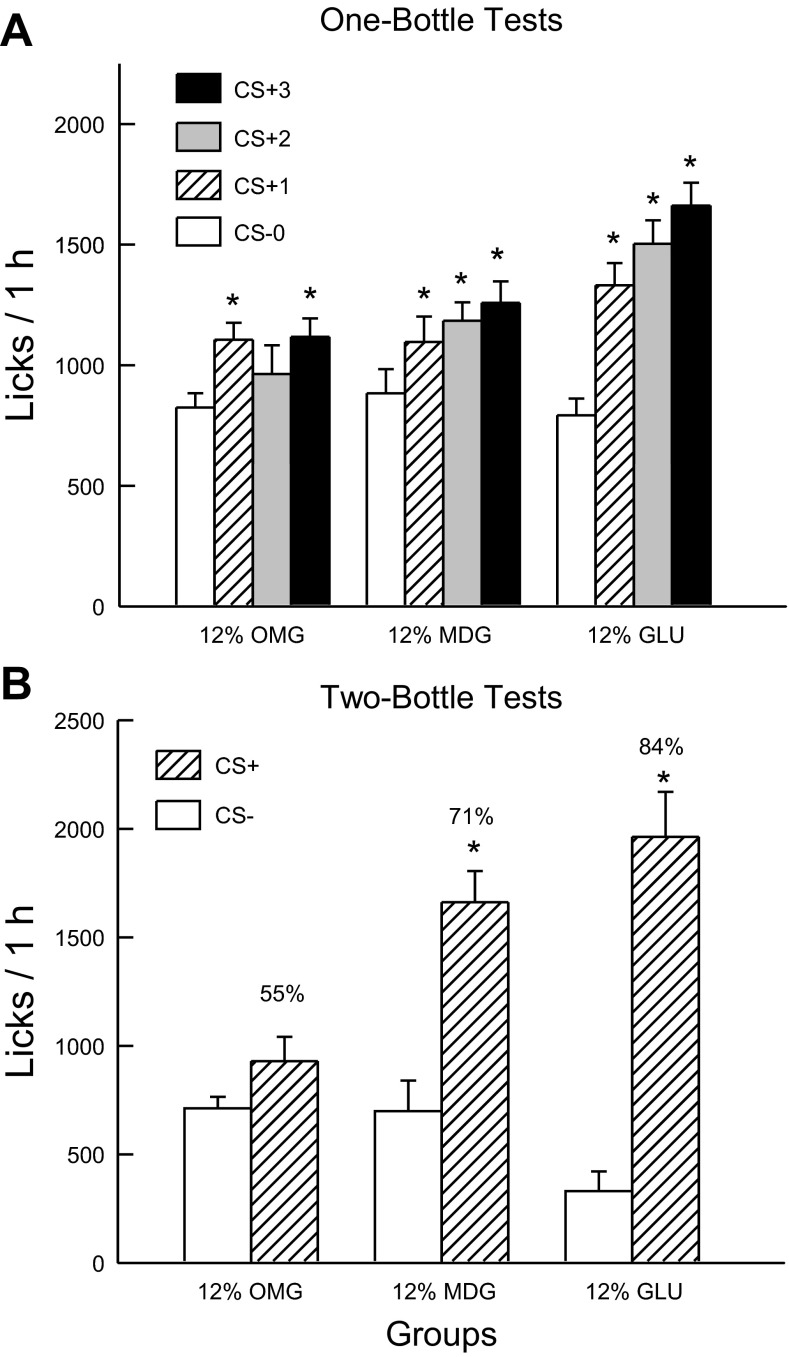
Fig. 4A shows the total 1-h licks for CS− test 0 and CS+ tests 1–3. Analysis of these data revealed that the 12% sugar groups did not differ in their CS− licks paired with IG water self-infusion (test 0), and all three groups increased their licking response in the CS+ tests although to different degrees [group × test interaction, F(6,96) = 7.65; P < 0.001]. A comparison of the CS+ tests 1–3 revealed that overall the CS+ licks of the 12% Glucose group exceeded that of the MDG and OMG groups, which did not differ from one another [F(2,32) = 6.74; P < 0.001]. Within-group analyses indicated that the 12% Glucose and MDG groups licked more in CS+ tests 1–3 than in CS− test 0, whereas the 12% OMG group licked more only in CS+ test 3 compared with CS− test 0. The 1-h intake data revealed a similar pattern of results [group × test interaction, F(6,96) = 4.8; P < 0.01]. All three groups significantly increased their solution intakes from CS− test 0 to CS+ test 3 (12% glucose: 2.1 to 3.5 g/h; 12% MDG: 2.1 to 2.8 g/h; 12% OMG: 1.9 to 2.4 g/h).
Fig. 4.
Experiment 2B: Values are expressed as means ± SE. A: 1-h total licks are plotted for one bottle tests 0–3. The mice drank (1 h/day) a CS−-flavored saccharin solution paired with IG water infusions in test 0 before being switched to a CS+-flavored saccharin solution paired with IG sugar self-infusions in tests 1–3. The 12% GLU, 12% MDG, and 12% OMG groups were infused with 12% glucose, MDG, and OMG, respectively. B: 1-h licks are plotted for CS+- and CS−-flavored saccharin solutions during the two-bottle preference test for the 12% GLU, MDG, and OMG groups. CS+ and CS− intakes were not paired with IG infusions during the test. Number atop bar represents mean percent preference for the CS+ solution. *Significant differences (P < 0.05) between test 0 vs. tests 1–3 licks and between CS+ vs. CS− licks.
In the alternating training sessions, the groups differed in their CS+ and CS− licks [group × CS interaction, F(2,32) = 7.9; P < 0.01]. Only the 12% Glucose and MDG groups licked more (P < 0.05) in the CS+ than CS− sessions (1,665.0 vs. 1,252.1, 1,368.8 vs. 1,230.3, respectively); CS+ and CS− licks were similar for the 12% OMG group (1,037.6 vs. 992.4).
Fig. 4B presents the lick data for the two-bottle CS+ vs. CS− choice test. Analysis of these data revealed a group × CS interaction [F(2,32) = 8.6; P < 0.001]. In particular, the 12% Glucose and MDG groups licked significantly more (P < 0.001) for the CS+ than CS−, while the CS+ and CS− licks of the 12% OMG group did not differ. Furthermore, the number of CS+ licks was greater in the 12% Glucose and MDG groups than in the 12% OMG group (P < 0.01), while the CS− licks did not differ. Similar results were obtained in the analysis of CS+ and CS− intakes during the two-bottle tests (data not shown). The groups also differed in their percent CS+ licks [F(2,32) = 9.6; P < 0.001] and the CS+ preference was greater in the 12% glucose group (84%) than the MDG group (71%), which, in turn, was greater than that of the OMG group (56%).
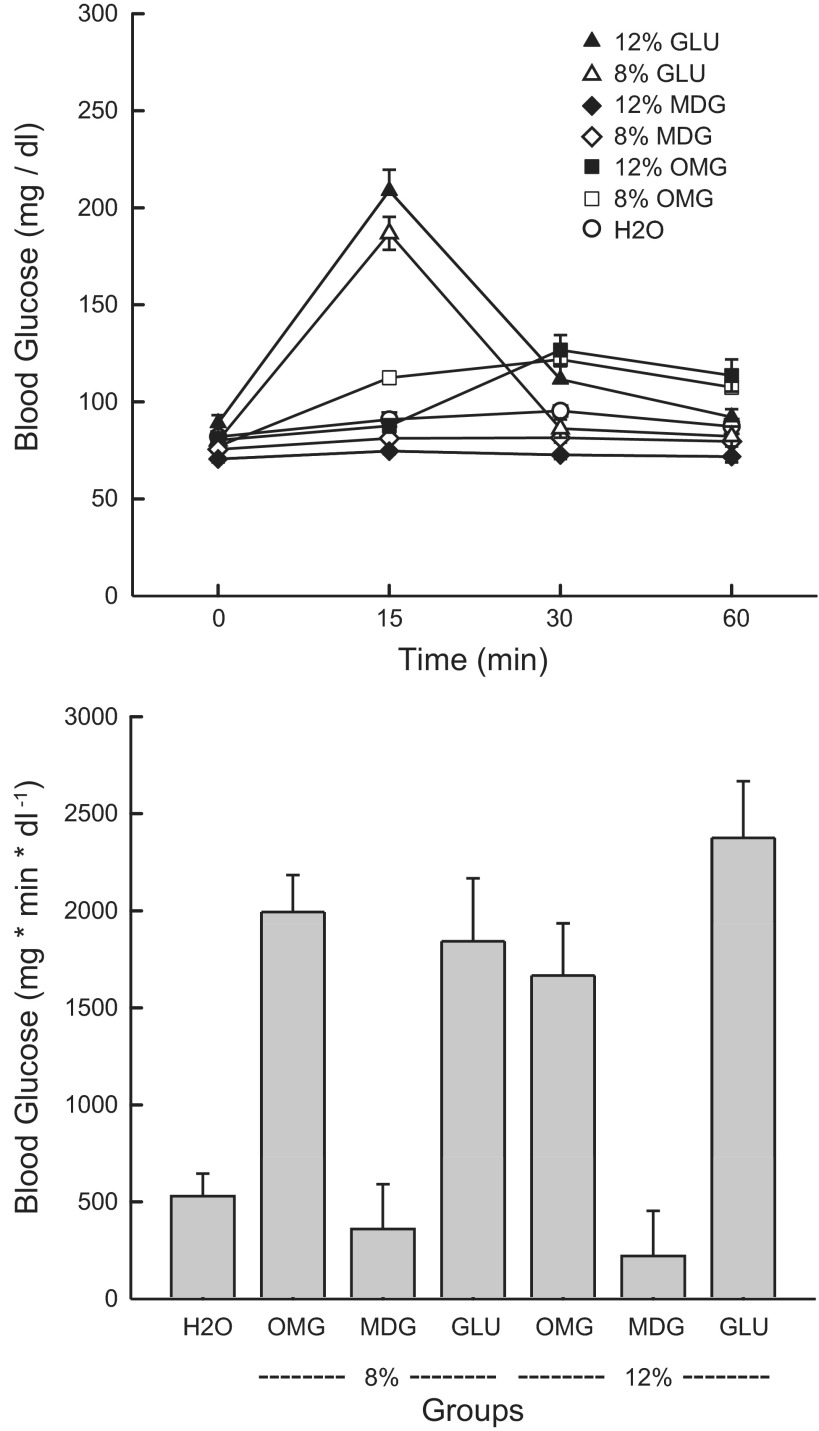
Blood glucose measures.
Fig. 5 presents the blood glucose (BG) data following IG infusions of water, glucose, MDG, or OMG in the different groups. Analysis of the absolute BG data revealed that overall, the three 8% sugar groups differed in their BG response [F(3,47) = 30.3, P < 0.001], and the effect varied as a function of time [group × time interaction, F(9,141) = 66.4; P < 0.001]. The groups did not differ at 0 min. At 15 min, they differed (P < 0.05) as follows: 8% Glucose > 8% OMG > H2O = 8% MDG. At 30 and 60 min, they differed (P < 0.05) as follows: 8% OMG > H2O = 8% Glucose = 8% MDG. Analysis of the IAUC data revealed the following group differences: 8% OMG = 8% Glucose > H2O = 8% MDG [F(3,47) = 26.49; P < 0.001] (Fig. 5).
Fig. 5.
Experiment 2: Values are expressed as means ± SE. A: blood glucose at 0, 15, 30, and 60 min after a 0.6-ml infusion of glucose, MDG, or OMG in 8% and 12% groups or water in the H2O group. B: incremental area under the curve (IAUC) for blood glucose after a 0.6-ml infusion of glucose, MDG, or OMG in 8% and 12% groups or water in the H2O group.
Analysis of the absolute BG data revealed that overall, the three 12% sugar groups differed in their BG response [F(3,43) = 53.76; P < 0.001], and the effect varied as a function of time point [group × time interaction, F(9,129) = 50.23; P < 0.001]. The groups differed (P < 0.001) at the 0-min time point in that the starting value in the 12% MDG group was lower than that of the other groups. At the 15-min point, the groups differed (P < 0.001) in that the 12% Glucose displayed higher BG levels than the other three groups, which did not differ from one another. At the 30-min time point, they differed (P < 0.05) as follows: 12% OMG = 12% Glucose > H2O > 12% MDG. At the 60-min time point, they differed (P < 0.001) as follows: 12% OMG > 12% Glucose = H2O > 12% MDG. Analysis of the IAUC also revealed the following group differences: 12% Glucose > 12% OMG > H2O = 12% MDG [F(3,43) = 27.89; P < 0.001].
DISCUSSION
MDG.
This experiment reports for the first time that a nonmetabolizable glucose analog can stimulate CS+ licking and condition a flavor preference; these responses occurred in the absence of changes in blood glucose levels. The 8% MDG infusions were nearly as effective in stimulating CS+ intake as 8% glucose and conditioned an identical preference (70%). The 8% infusions differed only in that MDG, unlike glucose, did not stimulate CS+ licking in test 1 (but see experiment 3). In contrast, the 12% MDG infusions were less effective than 12% glucose in stimulating intake in CS+ tests 1–3 and conditioned a weaker CS+ preference (71% vs. 84%). To determine whether a stronger preference could be obtained with a more concentrated MDG infusion, another group of mice (n = 12) was tested as in this experiment but with IG infusions of 16% MDG. These mice showed only a marginal increase (P = 0.075) in 1-h CS+ licks in tests 1–3. In the two-bottle choice test, the 16% MDG group preferred (P < 0.05) the CS+ to the CS−, although their preference (62%) was weaker than those of the 8% and 12% MDG groups (70–71%), as well as that of a 16% Glucose group (80%) (67). It may be that at higher concentrations, the accumulation of the nonmetabolizable MDG in the body generates satiating or other inhibitory signals that counteract the appetition signals generated by this sugar analog.
OMG.
The 8% and 12% OMG infusions stimulated CS+ licking about as much as did the MDG infusions but, unlike MDG (and glucose), did not condition significant CS+ preferences. The differential effectiveness of OMG and MDG may be due to the fact that OMG, unlike MDG, is not a substrate for SGLT3 (3). OMG results are consistent with the findings that galactose, which also only binds to SGLT1, conditioned weaker CS+ preferences than did glucose. However, it is also possible that, like galactose, OMG may have postabsorptive actions that interfere with CS+ conditioning. In particular, the IG OMG infusion produced a small but sustained increased BG level. Himsworth (23) previously reported a sustained increase in BG following an intravenous infusion of OMG, which he attributed to a glucoprivation response due to OMG competing with glucose for cellular uptake. Thus, OMG-induced glucoprivation in the B6 mice may have prevented CS+ flavor preference conditioning and perhaps place preference conditioning, as previously reported (30).
Experiment 3: Phlorizdin and Phloretin Effects on Glucose and MDG Appetition
The findings of the first two experiments that ligands of SGLT1 and/or SGLT3 (glucose, galactose, MDG, OMG) stimulate CS+ intake and, except for OMG, condition CS+ preferences suggest that the SGLT sensors mediate post-oral sugar appetition. If this is the case, then IG sugar conditioning should be blocked by the SGLT1/SGLT3 antagonist, phloridzin (17, 33). Experiment 3A tested this possibility by determining the effects of adding phloridzin to 8% glucose and 8% MDG infusions on stimulation of CS+ licking and preference conditioning. Galactose and OMG were not included in this experiment because of their weaker conditioning effects.
Experiment 3B determined whether combining the GLUT2 inhibitor phloretin with phloridzin would more completely block the flavor-conditioning actions of IG glucose self-infusions. This manipulation was based on a recent report that phloridzin alone only partially blocked glucose stimulation of gut hormones [GLP-1, GIP, peptide YY (PYY)] in an acute intestinal infusion preparation, whereas a phloretin+phloridzin cocktail was fully effective (29).
MATERIALS AND METHODS
Experiment 3A
Three groups of adult male B6 mice were housed and tested as in the prior experiments: an 8% Glucose+0.4% phloridzin (GLU+Pz, n = 12), an 8% MDG+0.4% phloridzin group (MDG+Pz; n = 12), and an 8% MDG group (8% MDG, n = 9). The 0.4% (8.4 mM) phloridzin (Sigma) concentration was based on the report of Savastano et al. (46), which investigated the drug's effect on the feeding inhibition produced by duodenal maltodextrin infusions in rats. This concentration was near the limit of solubility of phloridzin in the 8% sugar solutions. The data from the 8% GLU+Pz group were compared with the 8% Glucose group data from experiment 1A. A new 8% MDG group was included in this experiment to replicate the novel finding of experiment 2 that the nonmetabolizable glucose analog stimulated CS+ intake and conditioned a preference. Following behavioral testing, the effect of phloridzin on the blood glucose response to IG 8% glucose infusion was measured. No blood glucose analysis was performed on the 8% MDG+Pz and 8% MDG groups because 8% MDG did not alter blood glucose in experiment 2.
Experiment 3B
Two groups (n = 10 each) of adult male B6 mice were housed and tested as in the prior experiments. One group (GLU+Pz+Pt) was tested with 8% glucose self-infusions containing 0.4% (8.4 mM) phloridzin and 0.23% (8.4 mM) phloretin. Isomolar drug doses were used based on a prior study (29). [This study used a much lower dose (0.5 mM), but the inhibitors were added to a more dilute glucose solution (1.8%) than that used here.] Because of the limited solubility of phloretin, the glucose+phloridzin+phloretin mixture contained 3.25% 1 N NaOH to dissolve the drugs (38). A second group (GLU+Pz) was tested with 8% glucose self-infusions containing 0.4% phloridzin and 3.25% NaOH. This group was included to determine whether the addition of NaOH altered the conditioning response to 8% glucose+phloridzin infusions. The pH of glucose+phloretin+phloridzin and glucose+phloridzin solutions were 10.1 and 10.8, respectively. Following behavioral testing, the effects of phloridzin and phloretin on the blood glucose response to IG 8% glucose infusion were measured as in experiment 2 in the GLU+Pz and GLU+Pt+Pz groups. Two days after the BG test, a second BG test was conducted in some mice (5 GLU+Pz and 4 GLU+Pz+Pt) during which they were infused intragastrically with water containing 3.25% of 1 N NaOH.
RESULTS
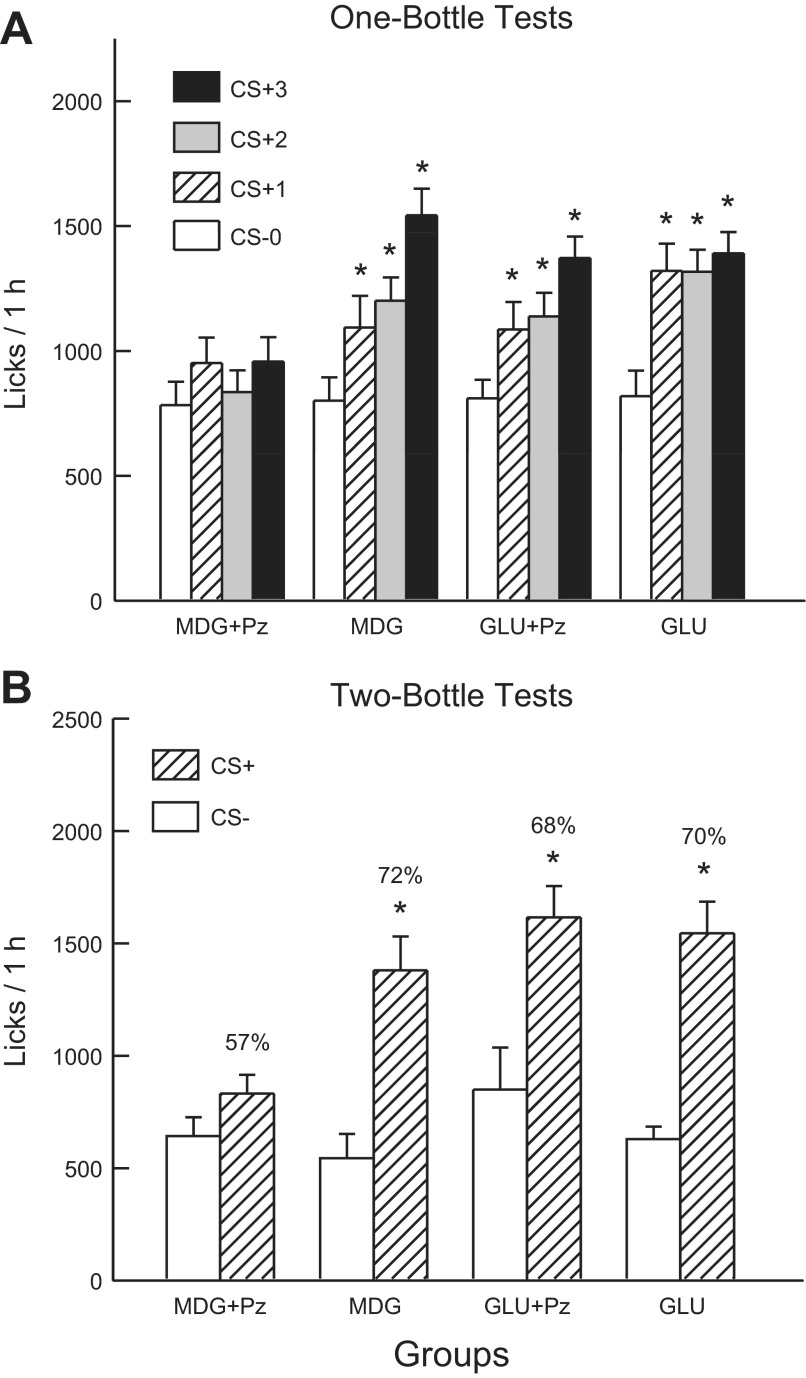
Experiment 3A
Analysis of 8% MDG and MDG+Pz groups revealed that the two groups did not differ in their CS− licks paired with IG water self-infusion (test 0) but only the 8% MDG group increased licks in CS+ tests 1–3 [group × test interaction, F(3,57) = 7.0, P < 0.001] (Fig. 6A). A comparison of the CS+ tests 1–3 licks indicated that the groups did not differ in CS+ test 1 but that the 8% MDG group licked more in CS+ tests 2 and 3 than did the MDG+Pz group. A within-group analysis revealed that the 8% MDG group licked more in CS+ tests 1 and 2 than in CS− test 0, and more in CS+ test 3 than in tests 0–2. The 1-h solution intake data revealed a similar pattern of results [group × test interaction, F(3,57) = 8.4, P < 0.001]. The 8% MDG Group increased (P < 0.01) its intakes from CS− test 0 to CS+ test 3 (2.1 to 3.6 g/h), whereas the 8% MDG+Pz group intakes did not significantly change from test 0 to test 3 (2.0 vs. 2.2 g/h).
Fig. 6.
Experiment 3A: Values are expressed as means ± SE. A: 1-h total licks are plotted for one bottle tests 0–3. The mice drank (1 h/day) a CS-flavored saccharin solution paired with IG water infusions in test 0 before being switched to a CS+-flavored saccharin solution paired with IG sugar infusions. The MDG+phloridzin (Pz), MDG, GLU+Pz, and GLU groups were infused with 8% MDG, 8% MDG+0.4% Pz, 8% glucose, and 8% glucose+ 0.4% Pz, respectively. B: 1-h licks are plotted for CS+ and CS−-flavored saccharin solutions during the two-bottle preference test for the MDG, MDG+Pz, GLU, and GLU+Pz groups. CS+ and CS− intakes were not paired with IG infusions during the test. Number atop bar represents mean percent preference for the CS+ solution. *Significant differences (P < 0.05) between test 0 vs. tests 1–3 licks and between CS+ vs. CS− licks.
In the alternating training sessions, the 8% MDG group licked more in the CS+ than in the CS− sessions (1,586.8 vs. 1,107.4), whereas the CS+ and CS− licks did not differ in the 8% MDG+Pz group [975.2 vs. 870.7; group × CS interaction, F(1,19) = 22.5, P < 0.01]. In addition, the MDG group licked more overall than did the MDG+Pz group [F(1,19) = 15.6; P < 0.001].
In the two-bottle choice test, the 8% MDG group licked significantly more (P < 0.001) for the CS+ than CS−, while the CS licks of the 8% MDG+Pz group did not differ [F(1,19) = 11.1, P < 0.01, Fig. 6B]. Furthermore, the number of CS+ licks was greater in the 8% MDG group than in the 8% MDG+Pz group (P < 0.001), while CS− licks did not differ. Similar results were obtained in the analysis of CS+ and CS− intakes during the two-bottle tests (data not shown). The CS+ preference was also greater in the 8% MDG than in the 8% MDG+Pz group [72% vs. 57%, t(19) = 3.1, P < 0.01].
Analysis of the 1-h lick data for the GLU+Pz and GLU groups indicated that overall, the groups did not differ in their CS licks, and both groups increased their licks from CS− test 0 to CS+ tests 1–3 [F(3,63) = 29.21; P < 0.001] (Fig. 6A). The groups differed, however, in the distribution of their within-session CS+ licks in tests 1–3, although not in their CS− licks in test 0. That is, the GLU+Pz group licked less (P < 0.01) during the first 20 min of the 1-h tests but more during the last 20 min compared with the GLU group [group × period interaction, F(2,42) = 95.6; P < 0.001]. The 1-h intake data revealed that both groups significantly increased their intakes from CS− test 0 to CS+ test 3 (2.0 to 3.3 g/h for 8% GLU and 2.0 to 2.9 g/h for 8% GLU+Pz), although intakes for the 8% GLU group exceeded (P < 0.05) that of the 8% GLU+Pz group in CS+ tests 1 and 2 [group × test interaction, F(3,63) = 2.8, P = 0.047].
In the alternating training sessions, the groups differed in their CS+ and CS− licks [group × CS interaction, F(1,21) = 13.2; P = 0.01]. Whereas the 8% GLU group licked more (P < 0.001) in CS+ than CS− sessions (1,467.0 vs. 1,152.1), the CS licks of the 8% GLU+Pz group did not differ (1,349.9 vs. 1,318.5).
The GLU+Pz mice, like the GLU mice, licked significantly more (P < 0.001) for the CS+ than CS in the two-bottle choice tests [F(1,21) = 22.85; P < 0.001], and there were no group differences (Fig. 6B). The GLU+Pz and GLU groups also displayed comparable CS+ preferences (68% and 70%, respectively). Similar results were obtained in the analysis of CS+ and CS− intakes during the two-bottle tests (data not shown).
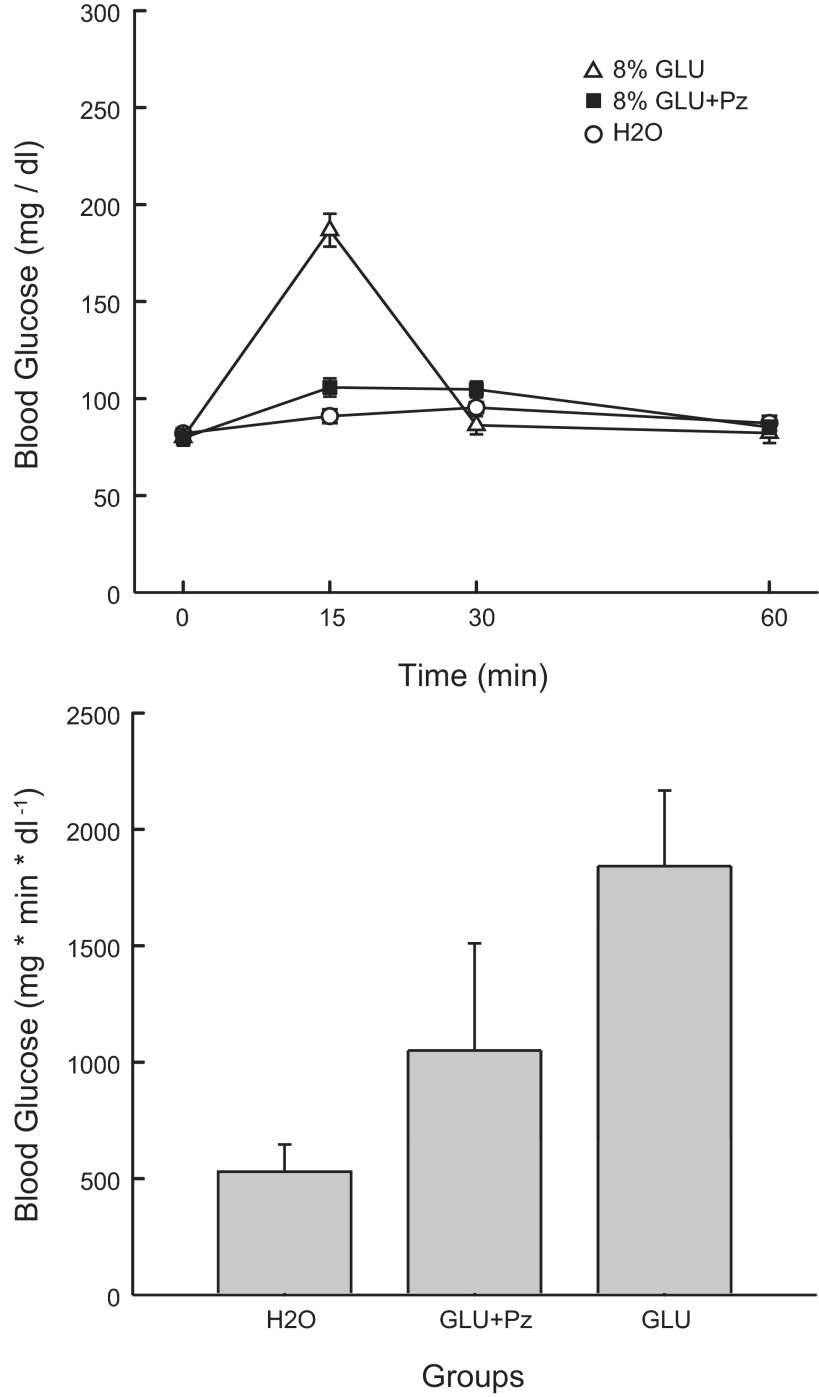
Analysis of the BG data for the GLU, GLU+Pz, and water groups indicated a group × concentration interaction [F(6,96) = 58.40, P < 0.001] (Fig. 7). Individual comparisons revealed higher (P < 0.05) BG levels in the GLU group compared with the GLU+Pz and water groups at the 15-min time point, whereas the GLU+Pz had a higher blood glucose than the GLU group at the 30-min time point. The IAUC analysis indicated that the 8% GLU group had a greater overall increase in BG than the 8% GLU+Pz group, which, in turn, had a greater increase than the Water group [F(2,32) = 13.90; P < 0.001] (Fig. 7).
Fig. 7.
Experiment 3A: Values are expressed as means ± SE. A: blood glucose at 0, 15, 30, and 60 min after a 0.6-ml infusion of 8% glucose, 8% glucose+0.4% phloridzin, or water in the GLU, GLU+Pz, and H2O groups. B: blood glucose IAUC in the GLU, GLU+Pz, and H2O groups.
Experiment 3B
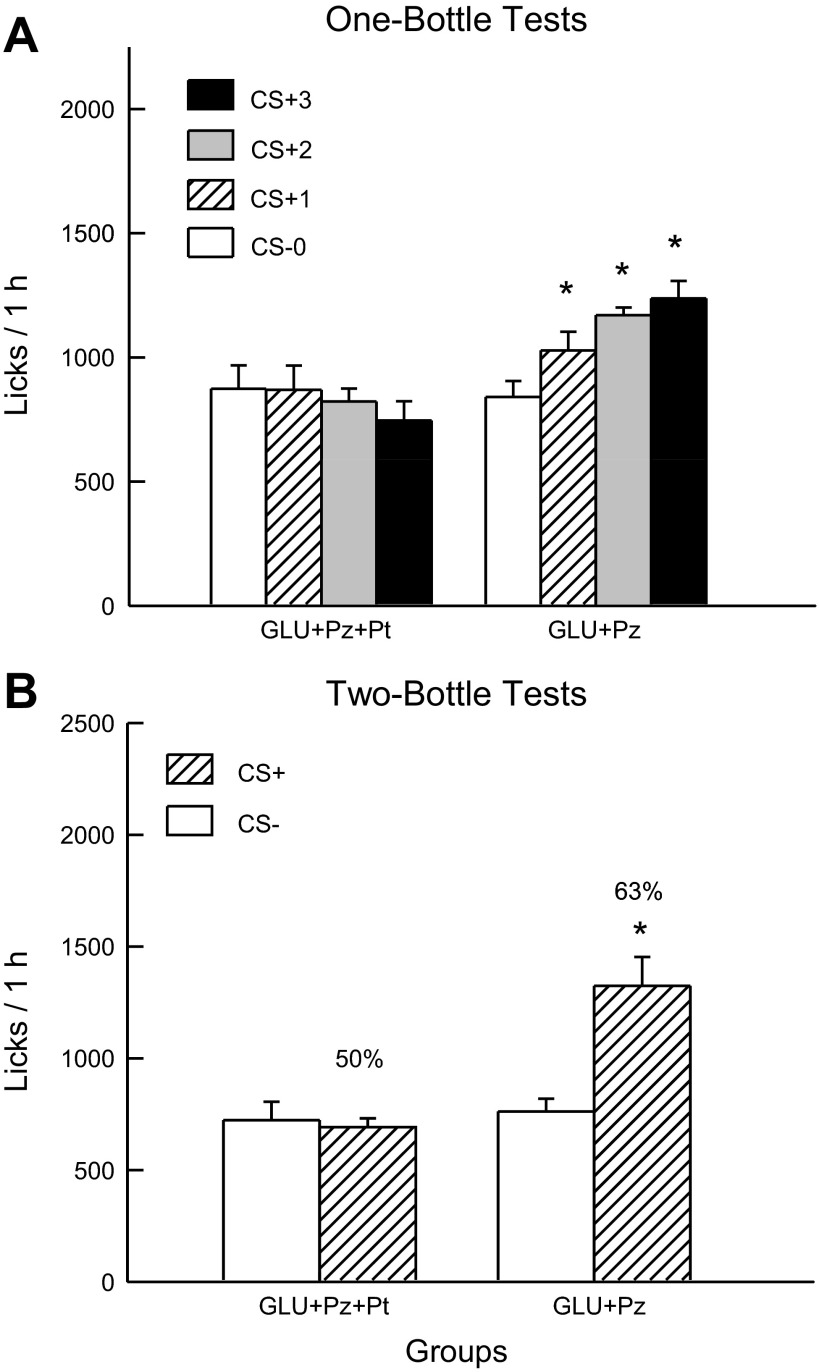
Analysis of the 1-h lick data indicated that the GLU+Pz and GLU+Pz+Pt groups did not differ in their CS− licks in test 0, but only the GLU+Pz group increased its licking response in the CS+ tests [group × test interaction, F(3,54) = 10.6, P < 0.001] (Fig. 8A). Within-group analyses indicated that the GLU+Pz group licked more in test 1 than in test 0 and more in tests 2 and 3 than in test 1 [F(3,27) = 22.5; P < 0.001]. The 1-h intake data revealed a similar pattern of results [group × test interaction, F(3,54) = 7.47; P < 0.001]: the GLU+Pz mice increased their intake from CS− test 0 to CS+ test 3 (2.1 to 2.6 g/h), while intake did not significantly change in the GLU+Pz+Pt (2.1 to 1.9 g/h).
Fig. 8.
Experiment 3B: Values are expressed as means ± SE. A: 1-h total licks are plotted for one bottle tests 0–3. The mice drank (1 h/day) a CS−-flavored saccharin solution paired with IG water infusions in test 0 before being switched to a CS+-flavored saccharin solution paired with IG sugar infusions. The GLU+Pz+phloretin (Pt) and GLU+Pz groups were infused with 8% glucose+0.4% Pz+0.23% Pt and 8% glucose+0.4% Pz containing 3.25% 1 N NaOH, respectively. B: 1-h licks are plotted for CS+- and CS−-flavored saccharin solutions during the two-bottle preference test with the GLU+Pz+Pt and GLU+Pz groups. CS+ and CS− intakes were not paired with IG infusions during the test. Number atop bar represents mean percent preference for the CS+ solution. *Significant differences (P < 0.05) between test 0 vs. tests 1–3 licks and between CS+ vs. CS− licks.
In the alternating training sessions, the GLU+Pz group licked more overall than the GLU+Pz+Pt group [F(1,18) = 26.8; P < 0.001]. The GLU+Pz group licked somewhat more in the CS+ than CS− sessions (1,226.4 vs. 1,172.0), whereas the GLU+Pz+Pt group showed the opposite pattern (863.9 vs. 909.2) but the group × CS interaction was not significant [P = 0.095].
The two-bottle test data revealed significant group differences (Fig. 8B): the GLU+Pz mice licked more (P < 0.01) for the CS+ than CS−, whereas the GLU+Pz+Pt mice did not differ in their CS licks [group × CS interaction, F(1,18) = 26.4, P < 0.001]. Similar results were obtained in the analysis of the two-bottle CS+ and CS− intake data (data not shown). The CS+ preference was also greater in the GLU+Pz group than in the GLU+Pz+Pt [63% vs. 50%, t(18) = 4.9; P < 0.001].
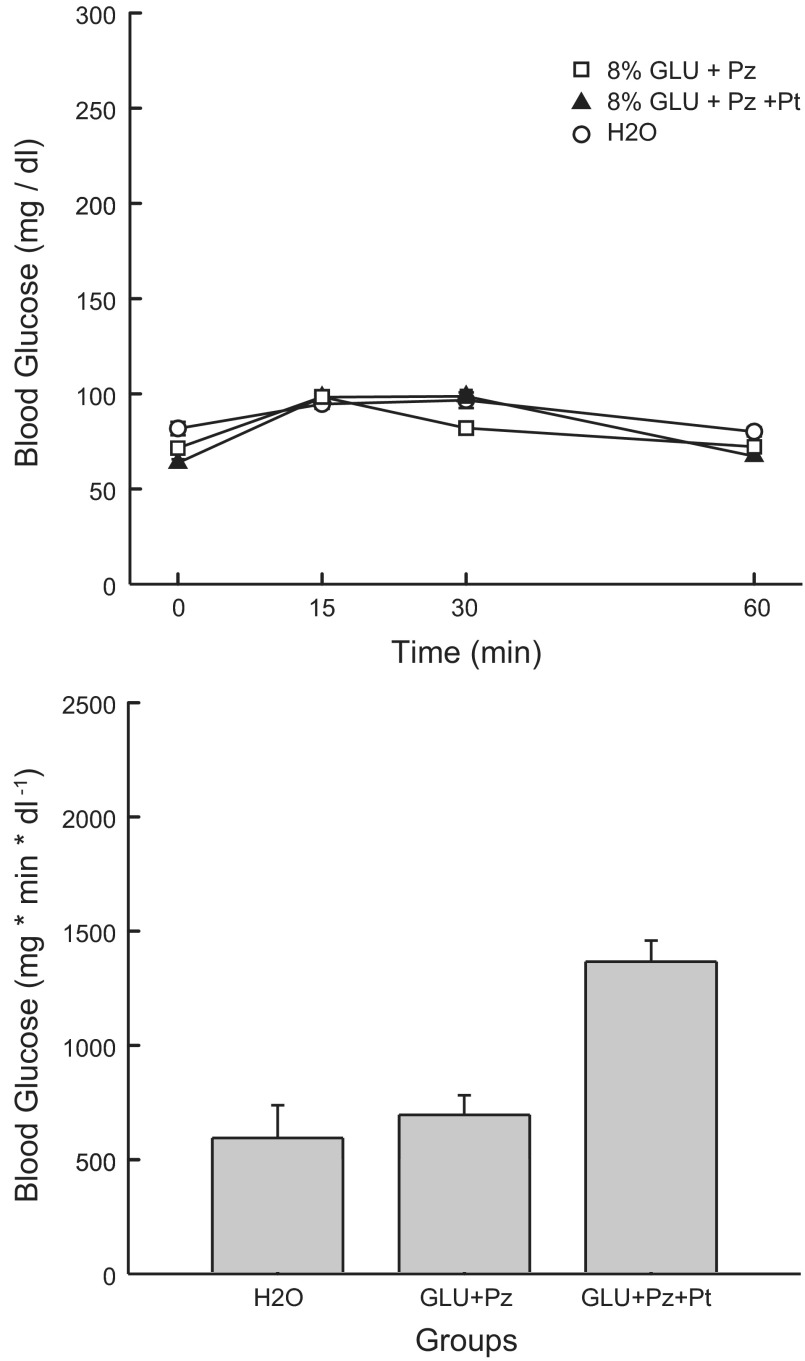
Analysis of the BG data for the GLU+Pz, GLU+Pz+Pt, and Water groups indicated small but significant differences [group × concentration interaction, F(6,75) = 8.32; P < 0.001] (Fig. 9). Individual comparisons revealed that groups did not differ at the 15-min time point, but they differed (P < 0.05) at the other time points as follows: time 0: Water > GLU+Pz > GLU+Pz+PT; time 30: GLU+Pt = GLU+Pz+Pt > Water; time 60: Water > GLU+Pz = GLU+Pz+Pt. The IAUC analysis indicated that the GLU+Pz+Pt group had a greater overall increase in BG than the GLU+Pz and Water groups [F(2,25) = 14.46, P < 0.001].
Fig. 9.
Experiment 3B: Values are expressed as means ± SE. A: blood glucose at 0, 15, 30, and 60 min after a 0.6-ml infusion of 8% glucose+0.4% Pz, 8% glucose+0.4% Pz+0.23% Pt, and water in the GLU+Pz, GLU+Pz+Pt, and H2O groups, respectively. All infusions contained 3.25% of 1 N NaOH. B: blood glucose IAUC in the GLU+Pz, GLU+Pz+Pt, and H2O groups.
DISCUSSION
The phloridzin findings of experiment 3A provide partial support for the involvement of SGLT1/SGLT3 in preference conditioning. In particular, phloridzin completely blocked MDG-induced stimulation of CS+ licking and preference conditioning. Yet, the same phloridzin dose had a relatively minor effect on glucose conditioning. Glucose+phloridzin stimulated licking nearly as much as glucose alone and produced a comparable CS+ preference. Phloridzin, however, reduced the stimulation of licking during the early part of the 1-h CS+ sessions and blocked the differential licking response in the alternating CS+ and CS− training sessions. Experiment 3B revealed that adding phloretin to the glucose + phloridzin infusion completely blocked the sugar-induced stimulation of CS+ licking and CS+ preference. The inclusion of NaOH in glucose infusion was not responsible for this effect because the Glu+Pz group, which also had NaOH in the glucose infusion, showed stimulation of CS+ licking in the 1-h sessions and a significant two-bottle CS+ preference. Unexpectedly, the GLU+Pz+Pt infusion increased blood glucose more than the GLU+Pz infusion, but this was due, in part, to the lower baseline blood glucose level of the former group.
GENERAL DISCUSSION
The present findings confirm reports that glucose and glucose-containing carbohydrates (sucrose, maltose, maltodextrin) have potent post-oral appetite-stimulating actions in mice and rats (50). Our prior findings identified the upper intestinal tract as a primary site for this glucose appetition effect and indicated that gut T1R2 and T1R3 sweet receptors are not essential for post-oral sugar conditioning (50). Instead, the present findings implicate SGLT1 and SGLT3 as the sugar sensors responsible for glucose appetition and demonstrate for the first time that sugar metabolism is not required for post-oral stimulation of intake and preference conditioning in the mouse.
Experiment 1 revealed that IG infusions of 8–12% glucose, and to a lesser extent, galactose, but not fructose, stimulated 1-h CS+ intake and conditioned significant CS+ preferences. The differential response to glucose and fructose extends our findings that only glucose self-infusions stimulated CS+ licking and preference in B6 mice tested with 24-h/day sessions (49). On the other hand, the galactose-conditioned flavor preference observed in experiment 1 contrasts with the failure of IG galactose to condition CS+ preferences in mice trained 24 h/day (49). Differences in the training procedures used in the present and prior study may explain the discrepant findings. In addition to test session length (1 vs. 24 h/day), the concentration of the saccharin used to sweeten the CS solutions differed substantially (0.025 vs. 0.2%). The use of the more palatable saccharin solution in the Sclafani and Ackroff (49) study may have driven galactose intake (via self-infusions) to a level that does not support flavor conditioning because of the limited ability of rodents to metabolize the sugar (4). In the 24-h IG study, the B6 mice consumed ∼23 g/day of a net 8% galactose solution when drinking the CS+ solution paired with IG infusions of 16% galactose, which is considerably more than the 9 g/day intake of B6 mice given the choice of 8% galactose and water in a 24-h two-bottle test (68). Furthermore, while the mice preferred 8% galactose to water, their galactose preference declined precipitously when the sugar concentration increased to 16 and 32%, which mirrors the decline in CS+ preference when the IG galactose infusion increased from 12% to 16% in the present study. Thus, impaired galactose metabolism may have contributed to the low CS+ preferences, relative to glucose, observed in the experiment 1.
The effectiveness of IG galactose but not fructose to condition a CS+ preference in B6 mice further discounts the involvement of gut T1R2 and T1R3 receptors in post-oral appetition given that fructose is more effective than galactose in stimulating sweet receptors (25, 27). Consistent with the present results, we recently reported that sweet ageusic T1r3 KO mice and Trpm5 KO mice developed preferences for galactose, but not fructose in 24-h sugar vs. water tests, which we attributed to the differential post-oral actions of the sugars. The present conditioning results with the monosaccharide sugars suggest the involvement of gut SGLT1 and SGLT3 sugar sensors, given that glucose binds to both SGLT1 and 3, galactose binds to SGLT1, and fructose is not a substrate for either sensor.
Further evidence for the involvement of gut SGLT sensors in sugar appetition is provided by the findings obtained with the nonmetabolizable glucose analogs in experiment 2. The IG self-infusions of MDG and OMG stimulated CS+ 1-h licking, and MDG conditioned significant CS+ preferences. Because both analogs bind to SGLT1 but only MDG binds to SGLT3, the findings would appear to implicate SGLT3 in the flavor-conditioning response. The significant, albeit weak CS+ preferences produced by the IG galactose infusions, however, indicate that SGLT1 stimulation alone can support flavor conditioning. At the 8% concentration, MDG was nearly identical to glucose in stimulating CS+ intake and preference. They differed only in that 8% MDG infusion failed to increase CS+ licking in test 1 of experiment 2, but it did so in experiment 3. At the higher concentrations (12–16%), however, MDG was significantly less effective than glucose. We previously reported that IG glucose-conditioned preferences increased from 52% to 91%, as glucose concentration increased from 2% to 32% (67). In the present study, MDG conditioned 70% preferences at 8% and 12% concentrations, whereas 16% MDG produced only a 62% CS+ preference. In addition, whereas 8% and 16% glucose infusions induced similar increases in CS+ intakes, CS+ intakes declined as MDG concentration increased from 8% to 16% MDG. As previously noted, the accumulation of the nonmetabolizable MDG at intestinal and/or postabsorptive sites may generate inhibitory signals that limit MDG-induced appetition.
In experiment 3, phloridzin completely blocked the CS+ intake-stimulating and preference-conditioning actions of the IG MDG infusions. This provides additional evidence that the appetite stimulation induced by the sugar analog is mediated by SGLT1 and/or SGLT3. On the other hand, phloridzin had minimal effects on glucose appetition. Conceivably, phloridzin may be more effective in inhibiting the binding of MDG than glucose to the SGLT1/3 sensors. Alternatively (or in addition), there may be another sensing process that contributes to glucose appetition. Consistent with this view, adding phloretin plus phloridzin to the IG glucose infusion completely blocked the ability of the sugar to stimulate CS+ licking and condition a preference. Phloretin is an inhibitor of GLUT2, a sugar transporter that may function as a glucose sensor in the intestinal tract (29). GLUT2 transports glucose and other sugars, but not MDG, from intestinal cells into the blood. Some evidence also indicates that, in the presence of high luminal sugar levels, GLUT2 is expressed in the luminal surface of the gut and contributes to glucose uptake and the release of GLP-1, GIP, and PYY hormones (29). Mace et al. (29) reported that glucose absorption and hormone release was reduced by only ∼50% by phloridzin, whereas a mixture of phloridzin+phloretin completely blocked the release of the three hormones (but see Ref. 37). If apical GLUT2 signaling contributes to the appetition response to glucose, this could explain why MDG is less effective than glucose at high concentrations in stimulating CS+ licking and preference. However, if this were the case, then fructose, which would be transported by apical GLUT2 (26), would be expected to have some appetition effect in B6 mice, which it did not. The role of GLUT2 in luminal sugar absorption remains a controversial issue (14, 28, 53), and its role in post-oral sugar appetition requires further investigation.
In addition to mimicking the post-oral appetition effect of glucose, MDG also appears to have a sweet taste, as evidenced by 1-min and 24-h preference tests with B6 mice (Sclafani A, unpublished data) and gustatory nerve recording and conditioned taste aversion tests in gerbils (24). Thus, MDG can be considered a nonnutritive sweetener to mice, although one quite different from the typical high-potency, nonnutritive sweeteners (e.g., saccharin, sucralose) that stimulate T1R2 and T1R3 receptors but not SGLT sensors. Interestingly, recent studies indicate that SGLT1, GLUT2, and other sugar transporters are colocalized with T1R3 in lingual taste cells, where they may contribute to the taste response to sugars (32, 59, 64). In contrast to B6 mice, preliminary findings indicate that MDG is minimally sweet to Sprague-Dawley rats and does not stimulate CS+ intake or preference when infused IG (Sclafani, A. and Ackroff, K., unpublished data). Rats and mice also differ in their oral and IG conditioning response to galactose [Sclafani, A., unpublished data (49, 51)]. It appears that MDG and galactose have different oral and postabsorptive actions in the two species.
While the present findings suggest that intestinal SGLT1 and SGLT3 and perhaps GLUT2 sugar sensors mediate post-oral sugar appetition, how the signals generated by these sensors reach the brain to alter intake and preference remains unclear. Vagal afferents are a potential pathway, but the available evidence argues against this neural route. In particular, total or selective (surgical, chemical) destruction of vagal afferents or vagal and sympathetic fibers did not prevent IG sugar conditioning in rats (50). Capsaicin-induced deafferentation also did not prevent glucose-induced appetition in B6 mice (66). These findings point to a humoral signaling pathway instead. Some studies suggest that sugar-stimulated insulin release might mediate CS+ flavor conditioning (60, 61), but this is challenged by the glucose-conditioned preferences displayed by insulin-deficient diabetic rats (1). The appetition effects produced by MDG in the present study further argue against a critical role for insulin given that gastric intubation of MDG did not increase insulin secretion in mice (37). MDG along with OMG, galactose, and glucose stimulate the release of various gut hormones, including GLP-1, GIP, and PYY via SGLT1, SGLT3, and/or GLUT2 sensors. Yet GLP-1 and PYY have suppressive effects on feeding and condition taste aversions rather than preferences (9, 13, 22, 55). There are no data to suggest that GIP stimulates sugar intake, and some findings indicate that fructose, which does not have a post-oral appetition effect in B6 mice, promotes GIP release in mice (18). Ghrelin is the one gut hormone known to stimulate eating and enhance food reward (31, 42), but glucose acts in the intestine to inhibit ghrelin release, which argues against its involvement in post-oral appetition (40, 62). Conceivably, intestinal glucose may promote the release of an as yet unidentified orexigenic hormone via SGLT1 and SGLT3 sensors that signals the brain to increase intake and condition a flavor preference.
The present study focused on the appetite-stimulating actions of sugars in the gut, which are distinct from the appetite-inhibitory or satiation actions of sugars, as reviewed elsewhere (48). Of particular interest here are studies related to SGLT involvement in sugar satiation and satiety. In an early study, duodenal infusions of glucose and fructose, but not galactose or OMG suppressed subsequent food intake, which argued against SGLT mediation in sugar-induced satiety (7). In contrast, later studies reported that intestinal infusions of glucose, galactose, OMG, and MDG, but not fructose, suppressed ongoing feeding in rats, which implicates SGLT signaling in the satiation process (33, 34). Most recently, Delaere et al. (12) observed that portal glucose and MDG, but not OMG, suppressed subsequent feeding in rats, which specifically implicates SGLT3 in this postabsorptive satiety response. The feeding-suppressive effect of portal glucose was blocked by phloridzin and capsaicin-induced visceral differentiation, which contrasts with the failure of phloridzin alone (experiment 3) or capsaicin treatment (66) to block glucose appetition. Thus, SGLT1 and SGLT3 appear to mediate at least some forms of sugar-induced satiety, as well as sugar-induced appetition. It may be that SGLT sensors generate several different signals depending upon their location in the viscera that stimulate intake when gut nutrient levels are low and suppress intake when levels are high.
Perspectives and Significance
Post-oral sugar-conditioned flavor preferences are often considered to be a form of “flavor-calorie” learning, in which the presumed unconditioned stimulus is generated by intracellular sugar metabolism (57). Previous results that isocaloric IG fructose and glucose infusions differ substantially in their flavor-conditioning actions argue against the idea that sugar-conditioned preference is based on the sugar's energy value per se (47, 50). The present findings that IG infusions of nonmetabolizable MDG, but not metabolizable fructose, stimulate CS+ intake and preference provides compelling new evidence that post-oral flavor conditioning does not require a signal related to sugar metabolism. The MDG findings also demonstrate that flavor conditioning can occur in the absence of changes in blood glucose. Other findings obtained with ileal, hepatic-portal, and intraperitoneal glucose infusions indicate that elevations in blood sugar are not sufficient to condition preferences when associated with a nonnutritive flavor source (e.g., saccharin solution) (2, 15, 67). These results, however, do not rule out a contributory role of postabsorptive sugar signaling and/or metabolism in enhancing food preferences when combined with preabsorptive signals (2, 10).
Post-oral sugar appetition is not unique to rodents but has been observed in species as diverse as flies, sheep, and humans (50). Interestingly, a recent report revealed that glucose preference in sweet ageusic Drosophila requires a sodium-glucose transporter analogous to the mammalian SGLT1 (16). Drosophila differs from the mouse, however, in that phloridzin blocked the fly glucose preference (16), whereas phloridzin plus phloretin were required to block glucose conditioning in the mouse. Also, unlike in the B6 mouse, fructose supported post-oral preferences in the fly (16, 36). Humans learn to prefer the flavor of glucose-rich drinks based on the sugar's post-oral actions (6, 11, 65). Recent fMRI data were interpreted as evidence that fructose activates the human brain reward system more than glucose (41), but behavioral evidence for fructose reward was not presented. Whether humans are like mice or flies in their food-conditioning response to fructose remains to be determined.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-31135.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.Z., K.A., and A.S. conception and design of research; S.Z. performed experiments; S.Z. analyzed data; S.Z., K.A., and A.S. interpreted results of experiments; S.Z. prepared figures; S.Z. and A.S. drafted manuscript; S.Z., K.A., and A.S. approved final version of manuscript; K.A. and A.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Kwame McCartney and Martin Zartarian for their expert technical assistance.
REFERENCES
- 1.Ackroff K, Sclafani A, Axen KV. Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite 28: 73–83, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99: 402–411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aljure O, Díez-Sampedro A. Functional characterization of mouse sodium/glucose transporter type 3b. Am J Physiol Cell Physiol 299: C58–C65, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Berman WF, Bautista JO, Rogers S, Segal S. Metabolism and transport of galactose by rat intestine. Biochim Biophys Acta 455: 90–101, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20: 64–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birch LL, McPhee L, Steinberg L, Sullivan S. Conditioned flavor preferences in young children. Physiol Behav 47: 501–505, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Booth DA, Jarman SP. Inhibition of food intake in the rat following complete absorption of glucose delivered into the stomach, intestine, or liver. J Physiol 259: 501–522, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 190: 285–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3–36) on conditioned taste aversion in rats. Peptides 27: 3193–3201, 2006 [DOI] [PubMed] [Google Scholar]
- 10.de Araujo IE, Ferreira JG, Tellez LA, Ren X, Yeckel CW. The gut-brain dopamine axis: A regulatory system for caloric intake. Physiol Behav 106: 394–399, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Curr Biol 23: 878–883, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaere F, Duchampt A, Mounien L, Seyer P, Duraffourd C, Zitoun C, Thorens B, Mithieux G. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Mol Metab 2: 47–53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, Exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drozdowski LA, Thomson AB. Intestinal sugar transport. World J Gastroenterol 12: 1657–1670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drucker DB, Sclafani A. The role of gastric and postgastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav 61: 351–358, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Dus M, Ai M, Suh GSB. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat Neurosci 16: 526–528, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev 21: 31–38, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Flatt PR, Kwasowski P, Bailey CJ. Stimulation of gastric inhibitory polypeptide release in ob/ob mice by oral administration of sugars and their analogues. J Nutr 119: 1300–1303, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Freeman S, Bohan DC, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose co-transporter. Am J Physiol Gastrointest Liver Physiol 291: G439–G445, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gonzàlez JA, Reimann F, Burdakov D. Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J Physiol 587: 41–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Halatchev IG, Cone RD. Peripheral administration of PYY3–36 produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Himsworth RL. Compensatory reactions to a lack of metabolizable glucose. J Physiol 198: 451–465, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakinovich W., Jr Taste aversion to sugars by the gerbil. Physiol Behav 28: 1065–1071, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Jakinovich W, Jr., Goldstein IJ. Stimulation of the gerbil's gustatory receptors by monosaccharides. Brain Res 110: 491–504, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: The role of GLUT2. Annu Rev Nutr 28: 35–54, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mace OJ, Marshall F. Gut chemosensing and the regulation of nutrient absorption and energy supply. J Anim Sci 91: 1932–1945, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and CasR in rat small intestine. J Physiol 590: 2917–2936, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura S, Yoneda T, Aki S, Eguchi A, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. Intragastric infusion of glucose enhances the rewarding effect of sorbitol fatty acid ester ingestion as measured by conditioned place preference in mice. Physiol Behav 99: 509–514, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Menzies JRW, Skibicka KP, Egecioglu E, Leng G, Dickson SL. Peripheral signals modifying food reward. In: Appetite Control, 4Handbook of Experimental Pharmacology, vol. 209, edited by Joost H-G. Berlin, Heidelberg: Springer, 2012, p. 131–158 [DOI] [PubMed] [Google Scholar]
- 32.Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat 219: 243–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer JH, Hlinka M, Tabrizi Y, Dimaso N, Raybould HE. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol Regul Integr Comp Physiol 275: R1293–R1307, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Meyer JH, Tabrizi Y, Dimaso N, Hlinka M, Raybould HE. Length of intestinal contact on nutrient-driven satiety. Am J Physiol Regul Integr Comp Physiol 275: R1308–R1319, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Mithieux G. Nutrient control of hunger by extrinsic gastrointestinal neurons. Trends Endocrinol Metab 24: 378–384, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS One 8: e56304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 297: E1358–E1365, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Oldendorf WH, Crane PD, Lawner PM, Braun LD. Rapid, transient drop in brain glucose after intravenous phloretin or 3-0-methyl-d-glucose. Stroke 14: 388–393, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PLoS One 6: e24992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146: 845–850, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 309: 63–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perello M, Zigman JM. The role of ghrelin in reward-based eating. Biol Psychol 72: 347–353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem 64: 349–356, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Raybould HE. Gut chemosensing: Interactions between gut endocrine cells and visceral afferents. Auton Neurosci 153: 41–46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritzel U, Fromme A, Ottleben M, Leonhardt U, Ramadori G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol 34: 18–21, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Savastano DM, Carelle M, Covasa M. Serotonin-type 3 receptors mediate intestinal Polycose- and glucose-induced suppression of intake. Am J Physiol Regul Integr Comp Physiol 288: R1499–R1508, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Sclafani A. How food preferences are learned—laboratory animal models. Proc Nutr Soc 54: 419–427, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav 106: 457–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol 302: R1119–R1133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav 67: 227–234, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol 299: R1643–R1650, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirazi-Beechey SP, Moran AW, Bravo D, Al Rammahi M. Intestinal glucose sensing and regulation of glucose absorption: implications for swine nutrition. J Anim Sci 89: 1854–1862, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Sykes S, Morgan LM, English J, Marks V. Evidence for preferential stimulation of gastric inhibitory polypeptide secretion in the rat by actively transported carbohydrates and their analogues. J Endocrinol 85: 201–207, 1980 [DOI] [PubMed] [Google Scholar]
- 55.Thiele TE, Vandijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. In: Appetite Control, Handbook of Experimental Pharmacology, vol. 209, edited by Joost H-G. Berlin Heidelberg. New York: Springer, 2012, p. 309–335 [DOI] [PubMed] [Google Scholar]
- 57.Tordoff MG. Metabolic basis of learned food preferences. In: Appetite and Nutrition, edited by Friedman MI, Tordoff MG, Kare MR. New York: Marcel Dekker, 1991, p. 239–260 [Google Scholar]
- 58.Tordoff MG, Friedman MI. Hepatic-portal glucose infusions decrease food intake and increase food preference. Am J Physiol Regul Integr Comp Physiol 251: R192–R196, 1986 [DOI] [PubMed] [Google Scholar]
- 59.Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res 345: 243–252, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Uneyama H, Torii K. Mechanisms of neural response to gastrointestinal nutritive stimuli: The gut-brain axis. Gastroenterology 137: 262–273, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Vanderweele DA, Oetting RL, Jones RE. Sham feeding, flavor associations and diet self-selection as indicators of feeding satiety or aversive effects of peptide hormones. Brain Res Bull 14: 529–535, 1985 [DOI] [PubMed] [Google Scholar]
- 62.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 144: 2765–2767, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA 108: 5431–5436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav 93: 798–806, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol Behav 109: 33–41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of deleting T1r3 or Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses 38: 421–437, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]