Abstract
We hypothesize that age-related skeletal muscle dysfunction and physical disability may be partially explained by alterations in the function of the myosin molecule. To test this hypothesis, skeletal muscle function at the whole muscle, single fiber, and molecular levels was measured in young (21–35 yr) and older (65–75 yr) male and female volunteers with similar physical activity levels. After adjusting for muscle size, older adults had similar knee extensor isometric torque values compared with young, but had lower isokinetic power, most notably in women. At the single-fiber and molecular levels, aging was associated with increased isometric tension, slowed myosin actin cross-bridge kinetics (longer myosin attachment times and reduced rates of myosin force production), greater myofilament lattice stiffness, and reduced phosphorylation of the fast myosin regulatory light chain; however, the age effect was driven primarily by women (i.e., age-by-sex interaction effects). In myosin heavy chain IIA fibers, single-fiber isometric tension and molecular level mechanical and kinetic indexes were correlated with whole muscle isokinetic power output. Collectively, considering that contractile dysfunction scales up through various anatomical levels, our results suggest a potential sex-specific molecular mechanism, reduced cross-bridge kinetics, contributes to the reduced physical capacity with aging in women. Thus these results support our hypothesis that age-related alterations in the myosin molecule contribute to skeletal muscle dysfunction and physical disability and indicate that this effect is stronger in women.
Keywords: aging, muscle fiber, mechanical properties
sarcopenia, defined as the loss of skeletal muscle mass and function with age, decreases the capacity for physical work, reduces ability to perform activities of daily living, contributes to physical disability, and increases the risk for falls and fractures (1, 20). Knowledge of the cellular and molecular mechanisms underlying age-related skeletal muscle contractile dysfunction is a necessary step toward developing more effective countermeasures to mitigate the development of physical disability.
Whole muscle power output decreases with age (5, 27) and, in elderly humans, is a strong predictor of physical disability (48). Since muscle power is the product of contractile force and velocity, aging must reduce force production, velocity, or both. There is disagreement, however, whether these contractile properties are altered with age or to what degree declining function can be attributable to muscle atrophy (4, 47). Results from studies of whole muscle function are complicated by the myriad of factors that impact voluntary joint torque production, including variation in motor unit recruitment, differential activation of agonist and antagonist muscles, altered structural properties of the tendons or extracellular matrix, and errors in the assessment of muscle size. Accordingly, a reductionist approach may be required to identify the specific mechanisms responsible for age-related contractile dysfunction.
Results of human studies examining the effect of aging on single-fiber and myosin function, however, have been equivocal. Single-fiber studies have shown reduced tension [force per fiber cross-sectional area (CSA)] or velocity with age (8, 9, 14, 24, 25, 28, 38, 63), while others show no differences or increases in tension or velocity (12, 47, 57). One reason for divergent results is that few studies have controlled for age-related reductions in physical activity (58), which can alter single-fiber contractile properties (8, 9). In vitro motility assays can directly assess isolated myosin function; however, studies in humans have yielded ambiguous results and may not reflect myosin behavior in the intact myofilament lattice (6, 8, 19). We are aware of one study that suggested myosin actin cross-bridge kinetics slow with age in human skeletal muscle fibers (37, 38); however, the experimental techniques used were insufficient to determine the specific steps of the cross-bridge cycle affected. Animal studies have shown age-related changes in cross-bridge kinetics, due primarily to alterations in the myosin molecule, which most likely underlie the decreased number of strongly bound cross bridges and reduced force generation found in older fibers (reviewed in Ref. 53). However, our understanding of how aging affects human skeletal muscle function at the level of the cross bridge is limited. Recently, our laboratory applied small-amplitude, sinusoidal length perturbation analysis to single human skeletal muscle fibers to assess myofilament mechanical properties, including specific steps of the cross-bridge cycle (33, 55). Because these measurements are performed in chemically skinned single fibers, the native, three-dimensional structure and regulatory protein content are maintained under physiological ionic conditions, such that cross-bridge function reflects the myriad of regulatory, structural, and mechanical forces present in vivo. Although this approach has been used to examine aging in insect flight muscle (32), it has never been used to examine age-related changes in human skeletal muscle.
We hypothesize that alterations in the function of the myosin molecule may partially explain age-related skeletal muscle dysfunction and physical disability. To test this hypothesis, voluntary knee extension performance and single-fiber measurements, including myosin actin cross-bridge kinetics, from the vastus lateralis of older (65–75 yr old) adults were compared with young (21–35 yr old). An important consideration in the recruitment of these cohorts was our attempt to match young and older adults for physical activity levels and to verify subjective assessments of activity status by accelerometry. These experimental considerations were included to minimize the confounding effects of muscle disuse that could develop in older volunteers because of decreases in volitional, weight-bearing activity.
MATERIALS AND METHODS
Ethical approval.
Written, informed consent was obtained from each volunteer before their participation. The protocol was approved by the Committees on Human Research at the University of Vermont and conformed to the Declaration of Helsinki.
Participants.
Twelve young (5 men, 7 women) and twelve older (5 men, 7 women) participants completed this study.
Self-reported physical activity was minimally active (no structured exercise program, and walking was their primary exercise) for the young volunteers. To match the walking activity of the young, older volunteers were recruited who were considered moderately active (3–5 sessions of ∼30 min of walking exercise/week). These recruitment criteria were used to obtain groups that were similar for habitual activity level, and this was verified using accelerometry measurements. All volunteers had no symptoms or signs of heart disease, hypertension, or diabetes (fasting blood glucose >112 mg/dl); normal resting electrocardiogram; normal electrocardiogram response to an exercise stress test; normal thyroid function; and normal blood cell counts and blood biochemistry values. Volunteers were excluded if they currently or had participated in a weight loss or exercise training program in the past year, a history of smoking (within 1 yr), unintentional weight loss of >2.5 kg during the last 3 mo, a body mass index > 30 kg/m2, a hospitalization longer than 3 days in the past 5 yr, an active neoplasm or history of one within the past 5 yr, or were taking/had taken hormone replacement therapy (combined estrogen/progestin for elderly women and testosterone for elderly men). Participants taking statins or oral corticosteroids were excluded. Young women taking oral contraceptives were included (n = 4), and all elderly women were postmenopausal (cessation of menses >1 yr).
Experimental protocol.
Eligibility was determined during screening visits, which also included whole muscle strength testing, a treadmill test, and resting and exercising electrocardiograms. At least 1 wk later, muscle tissue was obtained via percutaneous biopsy of the vastus lateralis under lidocaine anesthesia, and body composition was assessed (fasted). The majority of tissue was immediately placed into cold (4°C) dissecting solution, dissected into bundles, tied to glass rods at 4°C, and processed for single-fiber mechanical assessment, as described (33). The remainder was frozen in liquid nitrogen and stored at −80°C until analysis.
Whole body measurements.
Body mass was measured on a digital scale (ScaleTronix, Wheaton, IL), and total and regional body composition was assessed by dual-energy X-ray absorptiometry using a GE Lunar Prodigy densitometer (GE Lunar, Madison, WI). Leg fat-free mass was used as a proxy of muscle mass, as described (18). Peak oxygen consumption, or peak V̇o2 (the highest 30-s average V̇o2 during the final 2 min), was determined by treadmill test to volitional exhaustion using a Naughton protocol. Free-living physical activity was estimated using a single-plane accelerometer, as described (56).
Knee extensor muscle function.
Knee extensor peak torque production was measured under isometric and isokinetic conditions using a dynamometer (Humac Norm, CSMi, Stoughton, MA), as described (56). Briefly, after establishing range of motion and gravity correction, participants were asked to perform maximum voluntary contractions under isometric and isokinetic conditions. Isometric torque was measured at multiple knee angles (90°, 70°, 50°, and 30°) with the best of two attempts recorded as peak for each angle. Isokinetic torque was measured during four repeated maximum voluntary contractions at 60, 120, 180, 240, and 300°/s. Data were reviewed in post to ensure that the target velocity was obtained in at least three of the four repetitions, and that peak torque was assessed during the isovelocity phase of the contraction.
Myosin heavy chain isoform distribution and protein content.
Myofibrillar proteins were extracted from tissue homogenates, as described (34). Gels for measuring myosin heavy chain (MHC) isoform distribution, as well as MHC and actin protein content, were run, as described (34), with minor modifications to the protein content resolving gel (10%) and running conditions (70 V for 1 h, then 120 V for 4 h). MHC isoform distribution gels were silver stained using Silver Snap (Pierce, Rockford, IL) and scanned, and band intensity was determined by densitometry using Quantity One software (BioRad, Hercules, CA) with data expressed as a percentage of total MHC densitometric units. MHC and actin protein content gels were stained with Coomassie blue and scanned, and band intensity was determined by densitometry using Quantity One (BioRad) with data expressed as densitometric units per microgram of protein loaded. Recent reports have criticized prior studies measuring MHC protein content because of inadequate myosin solubilization (7). Our approach yielded solubilization of the majority of the myosin in the tissue sample (67% of high-salt extracted; Miller et al. unpublished observations). Moreover, our results are in keeping with prior studies using high-salt extraction buffer showing no age-related reduction in MHC protein content (54).
Phosphorylation of myofibrillar proteins.
Myofibrillar protein phosphorylation was determined by a combination of phosphoprotein-specific gel staining and liquid chromatography-mass spectrometry (LC-MS). Myofibrillar proteins were separated on 10% NuPage Novex Bis-Tris gels (Invitrogen, Grand Island, NY) at 160 V for 65 min. For phosphoprotein-specific gel staining analysis, gels containing duplicate samples were stained with Pro-Q Diamond phosphoprotein gel dye (Invitrogen), scanned using a Pharus FX Plus (BioRad), restained using GelCode Blue (Fisher Scientific, Pittsburgh, PA), and rescanned to quantify the protein abundance. The intensity of each band was quantified using the Quantity One program (BioRad), and phosphorylation level was determined as a ratio to the protein abundance, as recommended by the manufacturer. For site-specific identification and quantification of phosphorylation by LC-MS, additional 10% NuPage Novex Bis-Tris gels were run containing duplicate samples from each volunteer and stained with SimplyBlue SafeStain (Invitrogen). The individual protein bands corresponding to those that appeared on the phosphor-specific gel stain were excised and combined to produce two separate samples for each of the four groups (young men, young women, older men, and older women) using previously described techniques (45). The protein bands were destained in 50% acetonitrile and digested with 3 μg of trypsin (Promega, Madison, WI) in 200 μl of 50 mM ammonium bicarbonate (1 h on ice, then 18 h at 37°C). Trypsin was deactivated by adding 14 μl of 90% formic acid, and the peptides were extracted and dried in a speed vacuum device. For controls in the quantification scheme, two samples of resultant peptides were reconstituted with 0.16 units of alkaline phosphatase from E. coli (Sigma-Aldrich, St. Louis, MO) in 10 μl of 50 mM ammonium bicarbonate (18 h, 37°C), to remove endogenous phosphorylation, and dried in a speed vacuum device. The peptide samples were reconstituted in 0.04% triflouroacetic acid and 2% acetonitrile and analyzed by a minimum of duplicate LC-MS analysis.
LC was performed using a 0.1 mm inner diameter × 150 mm length P2000 laser pulled nanospray column (Sutter Instruments, Novato, CA) packed with 5 μm Magic C18AQ beads (Bruker-Michrom, Auburn, CA), coupled to a Shimadzu HPLC (Columbia, MD) and Thermo LTQ ion trap mass spectrometer (San Jose, CA), as previously described (26). Each sample was injected into water with 2% acetonitrile and 0.1% formic acid. The measured flow was 750 nl/min, and both the 0.1% formic acid and flow rate were kept constant for the duration of the run. After 25 min, the acetonitrile content was ramped linearly to 30%, at 53 min the gradient was increased to 40%, at 58 min the content was held isocratic, from 63–65 min the gradient was ramped to 80% acetonitrile, and at 70 min the gradient was returned to 2% acetonitrile, and the column was equilibrated for 18 min before the next injection.
In each experiment, data dependent LC-MS analyses (tandem mass spectrometry) were performed, spectra collected, and peptides identified as previously described (62). Initial SEQUEST searches used for quantification were performed using the IPI human protein sequence database (version 3.75), and the subsequent searches only contained the two isoforms of human myosin regulatory light chain (RLC) 2, the ventricle/cardiac (slow) isoform (P10916), and skeletal (fast) isoform (Q96A32), downloaded from UniProtKB. Measured ion currents corresponding to manually confirmed peptides of interest were extracted from the LC chromatogram, and the area under each LC peak was determined for use in the quantification of phosphorylation via a mass-balance approach (46, 62).
Single-fiber mechanical measurements.
Segments (∼2.5 mm) of chemically skinned single muscle fibers were isolated, and their ends fixed with glutaraldehyde, as described (33). Using a right-angled, mirrored prism and a digital filar eyepiece micrometer (Lasico, Los Angeles, CA), top and side fiber diameter measurements were made in relaxing solution (pCa 8) at three positions to calculate the fiber's average CSA, assuming an elliptical cross section. Fibers were subsequently processed for mechanical assessment and isometric tension, and dynamics stiffness measurements were performed under pCa 4.5 and rigor conditions at 15°C and 0.25 mM Pi, as described (34), and tension and sinusoidal analysis under pCa 4.5 conditions at 25°C and 5 mM Pi, as described (33). Measurements were performed at 15°C and 0.25 mM Pi to correspond with previous studies (8, 37, 38, 57, 63). Measurements at 25°C used 5 mM Pi to match with resting Pi levels in human skeletal muscle (42). Following mechanical measurements, single fibers were placed in gel loading buffer, heated for 2 min at 65°C, and stored at −80°C until determination of MHC isoform composition by SDS-PAGE, as described (33). We restricted analysis to MHC I and IIA fibers, as the number of IIX or hybrid fibers was insufficient to permit statistical analysis.
To relate sinusoidal analysis with specific steps in the cross-bridge cycle, the complex modulus (plotted as viscous vs. elastic modulus) at peak calcium activation was characterized by the following mathematical expression, as described in detail (33):
where ω = 2πf in s−1; A, B, and C are magnitudes expressed in kN/m2; 2πb and 2πc are characteristic rates expressed in s−1; i = −11/2; α = 1 s−1; and k = a unitless exponent. The A-process (characterized by parameters A and k) describes a linear relationship between the viscous and elastic moduli that has no kinetic or enzymatic dependence (35) and reflects the viscoelastic properties of the passive structural elements of the fiber across the oscillation frequency range. Under Ca2+-activated conditions, where myosin actin cross bridges are formed, the A-process represents the underlying stiffness of the lattice structure and the attached myosin heads in series (35, 39). The parameter A indicates the magnitude of a viscoelastic modulus, and k describes the degree to which measured viscoelastic mechanics represent purely elastic (k = 0) vs. purely viscous (k = 1) mechanical responses. We separated the parameter A into its real (A-elastic) and imaginary (A-viscous) parts using the following equation:
where A-elastic = A cos(k π/2) and A-viscous = A sin(k π/2).
The importance of deriving these components is that the physical meaning behind the elastic portion (A-elastic) and viscous portion (A-viscous) of the myofilament lattice structural elements is easier to understand than the characteristic parameters A and k. The B- and C-process magnitudes (B and C) are proportional to the number of myosin heads strongly bound to actin and the cross-bridge stiffness (22, 41). The frequency portion of the B-process (2πb) has been interpreted as the apparent (observed) rate of myosin force production or, in other words, the rate of myosin transition between the weakly and strongly bound states (64). The reciprocal of the frequency portion of the C-process, or (2πc)−1, represents the average myosin attachment time to actin, ton (41).
Statistics.
Data are means ± SE. Two-way analysis of variance was used to evaluate age and age-by-sex interaction effects. For variables with multiple observations within the same individual (e.g., single-fiber mechanical measurements), a linear mixed model was used, including a random effect to account for clustering of observations within individuals. For all analyses, if a main or interaction effect was noted (age or age by sex), post hoc contrasts were performed to identify pairwise differences. Relationships between variables were determined using Pearson correlation coefficients, with or without the inclusion of covariates. Any relationships with whole muscle isokinetic power were covaried with muscle mass using partial correlations. Statistical analyses were considered significant at P < 0.05, unless otherwise noted, and were performed using IBM SPSS Statistics (version 20.0, IBM, Armonk, NY) and SAS (version 9.3, SAS Institute, Cary, NC).
RESULTS
Participants.
Physical characteristics are shown in Table 1. Body mass index was greater in the older vs. young cohorts, although height and body mass showed no difference. Body composition measurements are expressed as a percentage of body mass to adjust for the variations in body size. Leg fat-free mass was lower in the older vs. young groups, while whole body fat and fat-free masses showed no differences between age groups. Peak V̇o2 was lower in the older vs. young cohorts. Daily physical activity level, measured by accelerometry over an average of 9.2 ± 0.3 days, was similar between age groups. For comparison, sedentary to minimally active older adults produce 200–300 kcal/day using identical measurement techniques (33, 55), showing that, as expected, the higher walking activity of moderately active older adults increased physical activity values from sedentary.
Table 1.
Clinical characteristics and physical activity levels of young and older groups
| Men |
Women |
||||
|---|---|---|---|---|---|
| Young | Older | Young | Older | Statistical Differences | |
| n | 5 | 5 | 7 | 7 | |
| Age, yr | 25.0 ± 1.4 | 69.5 ± 1.8 | 23.6 ± 1.5 | 68.4 ± 1.0 | Age† |
| Height, cm | 180.9 ± 2.0 | 178.4 ± 1.5 | 166.1 ± 2.9 | 158.0 ± 3.1 | |
| Body mass, kg | 72.8 ± 3.0 | 87.6 ± 4.6 | 60.9 ± 5.1 | 59.1 ± 3.9 | |
| BMI, kg/m2 | 22.4 ± 1.4 | 27.5 ± 1.3 | 21.9 ± 1.3 | 23.6 ± 1.1 | Age* |
| Fat mass, % | 21.7 ± 5.0 | 26.3 ± 2.7 | 29.9 ± 2.6 | 35.5 ± 2.9 | |
| Fat-free mass, % | 74.6 ± 5.2 | 69.6 ± 2.3 | 66.2 ± 2.6 | 60.6 ± 2.3 | |
| Leg fat-free mass, % | 27.6 ± 2.1 | 23.6 ± 1.0 | 23.9 ± 0.9 | 20.2 ± 1.2 | Age† |
| Peak oxygen consumption, ml·kg FFM−1·min−1 | 68.7 ± 2.7 | 47.2 ± 2.7 | 65.6 ± 3.2 | 47.4 ± 2.6 | Age† |
| Physical activity level, kcal/day | 385 ± 55 | 432 ± 91 | 448 ± 38 | 357 ± 45 | |
Values are means ± SE; n, no. of subjects. BMI, body mass index; FFM, fat-free mass. Fat and fat-free masses are presented as %body mass. Age = significant difference between young and older groups
P < 0.05,
P < 0.01.
Knee extensor muscle performance.
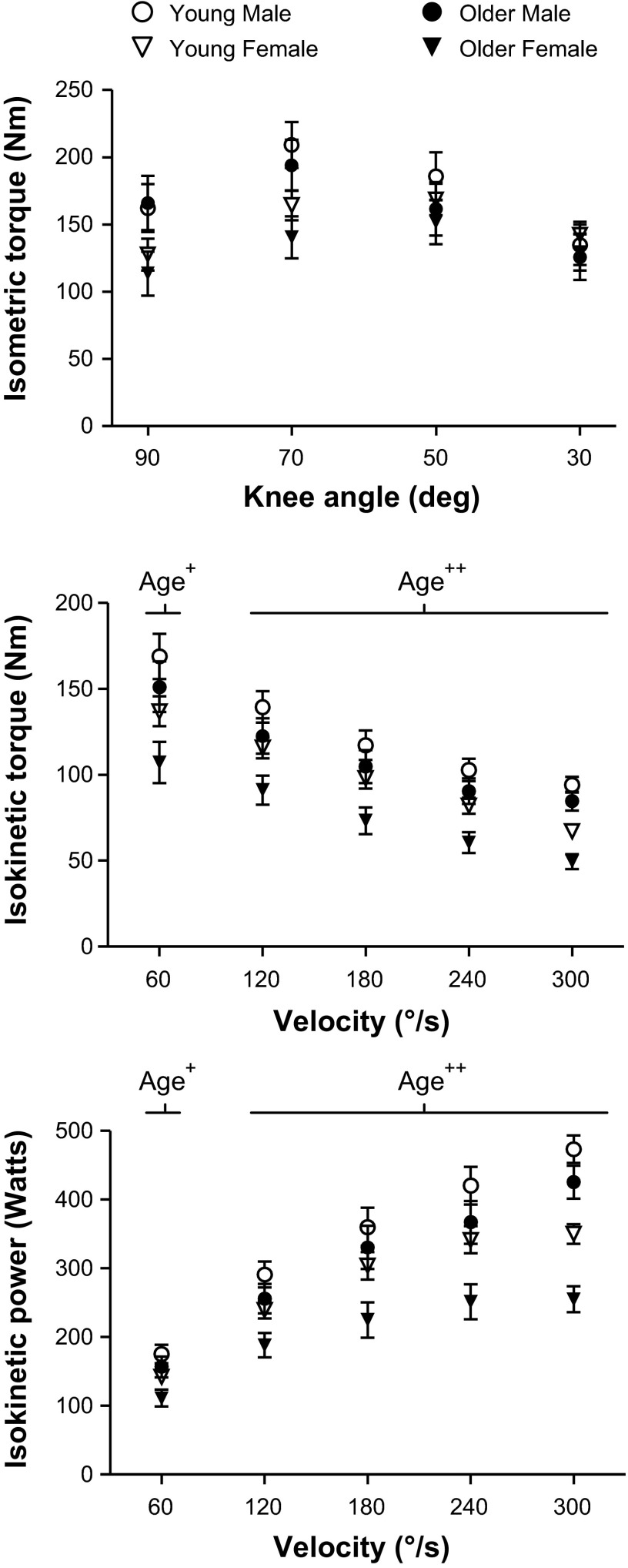
Isometric peak torque at various knee angles, adjusted for leg fat-free tissue mass, showed no difference between age groups (Fig. 1). In contrast, isokinetic knee extensor peak torque and power were lower in the older vs. young cohorts at all measured velocities. The lower isokinetic peak torque and power between the age groups were at least twice as large in women vs. men (21–27% in women and 8–13% in men) across all measured velocities, suggesting that dynamic contractile performance is more compromised in women than men with age, although age-by-sex interactions were not significant. Data from one older man was removed as he self-reported not producing maximal effort. Additionally, one young woman, one older woman, and one older man were removed from the 300°/s knee extension data, as they did not achieve the target velocity in at least 3 of their 4 repetitions.
Fig. 1.
Whole leg muscle contractile performance changes with age. Isometric knee extensor peak torque-knee angle, as well as isokinetic knee extensor peak torque-velocity and power-velocity curves, for young (5 men, 7 women) and older (4 men, 7 women) groups are shown. Most volunteers were able to achieve 300°/s (young: 5 men, 6 women; older: 3 men, 6 women). All data were statistically adjusted for leg fat-free tissue mass using analysis of covariance. Age = significant difference (+P < 0.05 or ++P < 0.01) between young and older groups.
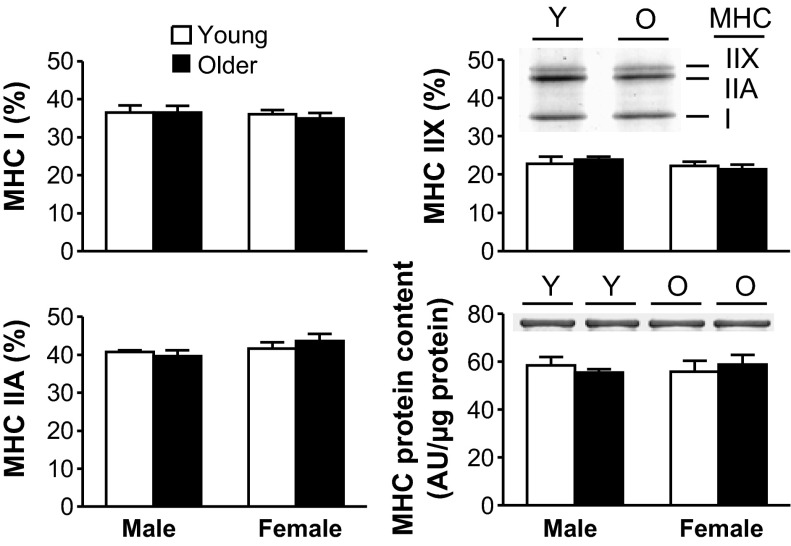
Tissue MHC and actin protein content and MHC isoform distribution.
We evaluated whether age-related alterations in whole muscle function are related to changes in the isoform distribution or amount of myosin. Neither the relative distributions of MHC isoforms, MHC content (Fig. 2), nor actin content (young man: 1,164 ± 81; older man: 1,083 ± 118; young woman: 1,128 ± 154; older woman: 1,140 ± 140 AU/μg protein) differed between age groups.
Fig. 2.
Skeletal muscle myosin heavy chain (MHC) isoform distributions and MHC content with age. MHC I, IIA, and IIX isoform distributions and MHC content from tissue homogenates (young male: n = 5; older male: n = 5; young female: n = 7; older female: n = 7), including representative gel sections from young (Y) and older (O) adults, are shown. AU, arbitrary units.
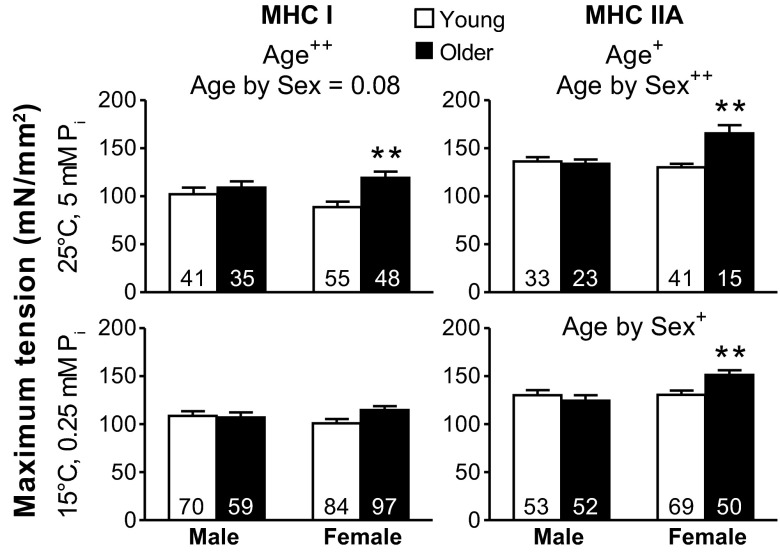
Maximum Ca2+-activated tensions.
Isometric tension measured before performing sinusoidal analysis (25°C and 5 mM Pi) was greater in the older vs. young cohorts in MHC I fibers and showed a trend (P = 0.077) toward an age-by-sex interaction (Fig. 3). Examination of pairwise differences showed that the greater isometric tension with age was significant in women, but not in men. In contrast, under conditions historically used for single muscle fibers studies (15°C and 0.25 mM Pi), no differences between age groups were evident for MHC I fibers. In MHC IIA fibers, isometric tension was greater in the older vs. young groups at 25°C and 5 mM Pi, whereas this age effect was not observed under 15°C and 0.25 mM Pi conditions. An age-by-sex interaction was found under both activating conditions in MHC IIA fibers, and pairwise comparisons showed that the greater tension with age was significant in women, but not in men. Altogether, these results show that the greater isometric tension with age was primarily driven by older women. Notably, isometric tensions are increased at higher temperatures and decreased at higher Pi levels (10), thereby similar values are produced at 25°C and 5 mM Pi compared with 15°C and 0.25 mM Pi. Although the tensions are similar between these two conditions, myosin kinetics at 25°C and 5 mM Pi should be increased (higher 2πb and shorter ton) in MHC I and IIA fibers (15, 60, 61), compared with 15°C and 0.25 mM Pi. Notably, the higher temperature and phosphate levels simulate conditions closer to in vivo as 5 mM Pi matches the resting levels in human skeletal muscle (42).
Fig. 3.
Maximum isometric tension differences for Ca2+-activated MHC I and IIA fibers with age. Single skeletal muscle fiber Ca2+-activated (pCa 4.5) tensions for MHC I and IIA fibers at 25°C and 5 mM Pi and at 15°C and 0.25 mM Pi in young and older groups are shown. The number of fibers is indicated at the base of each bar. Text indicates significant difference (+P < 0.05 or ++P < 0.01) between young and older groups (Age) and a significant interaction between age and sex (Age by Sex). Asterisks indicate significant pairwise differences (**P < 0.01) between young and older women.
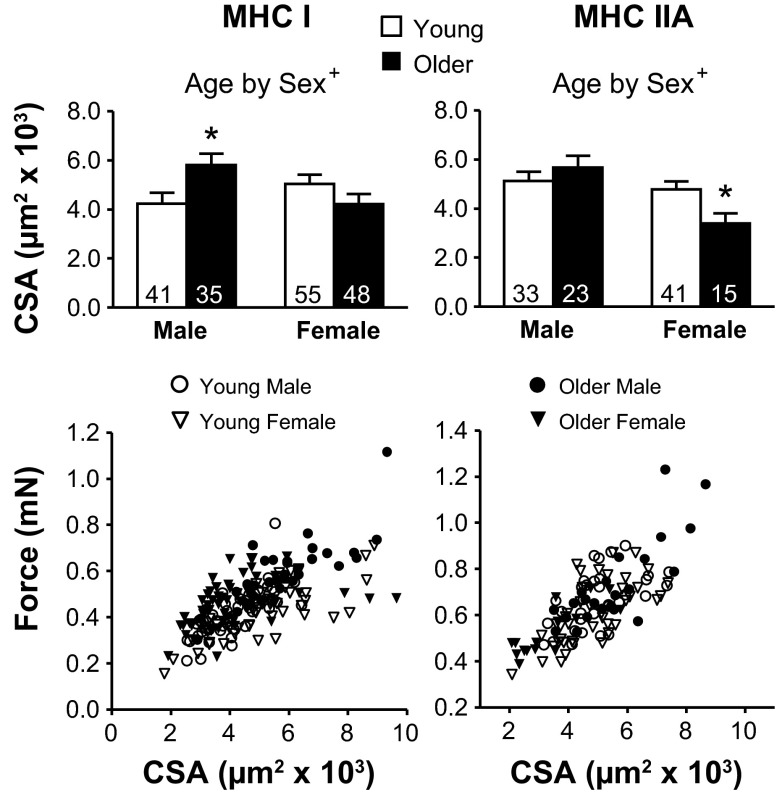
The CSAs in MHC I and IIA fibers showed an age-by-sex effect. Examination of pairwise differences showed this was due to older male MHC I fibers being larger than young male and to older female MHC IIA fibers being smaller than young female (Fig. 4). The reason for increased tension (force per CSA) in older women was examined by plotting the force vs. CSA for each fiber. At similar CSA values, older women produce more force than young in both fiber types (Fig. 4), indicating that their higher isometric tensions are due to their ability to produce more force.
Fig. 4.
Cross-sectional area (CSA) differences with age and force vs. CSA plots for MHC I and IIA fibers. The number of fibers is indicated at the base of each bar. Text indicates a significant interaction (+P < 0.05) between age and sex (Age by Sex). Asterisks indicate significant pairwise differences (*P < 0.05) between young and older men in MHC I fibers or between young and older women in MHC IIA fibers.
Single-fiber mechanics.
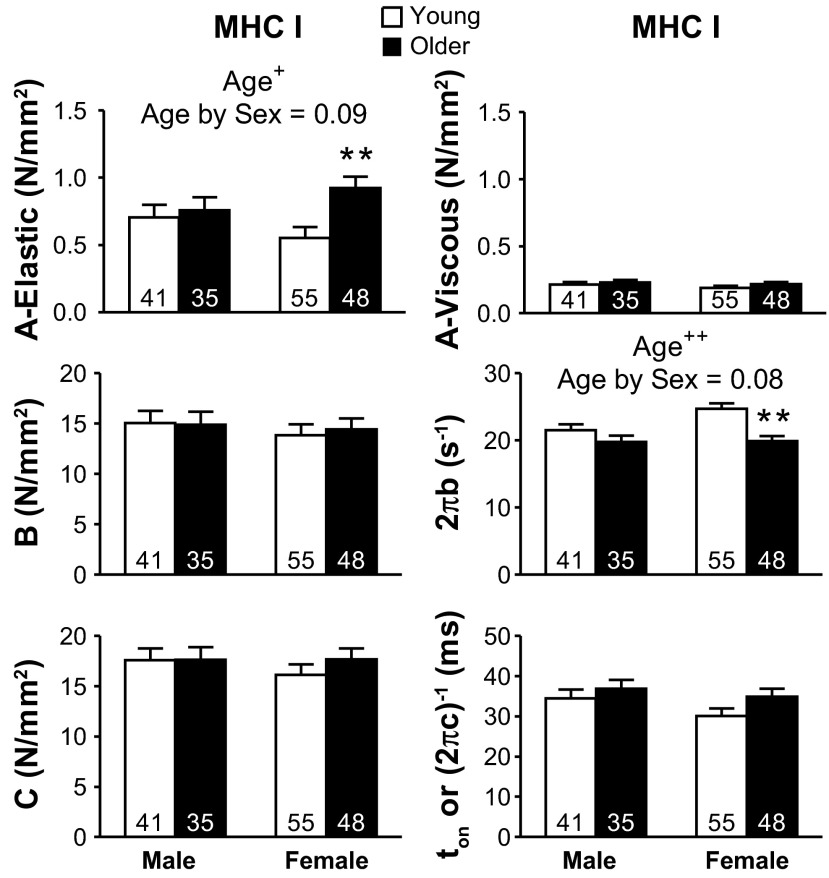
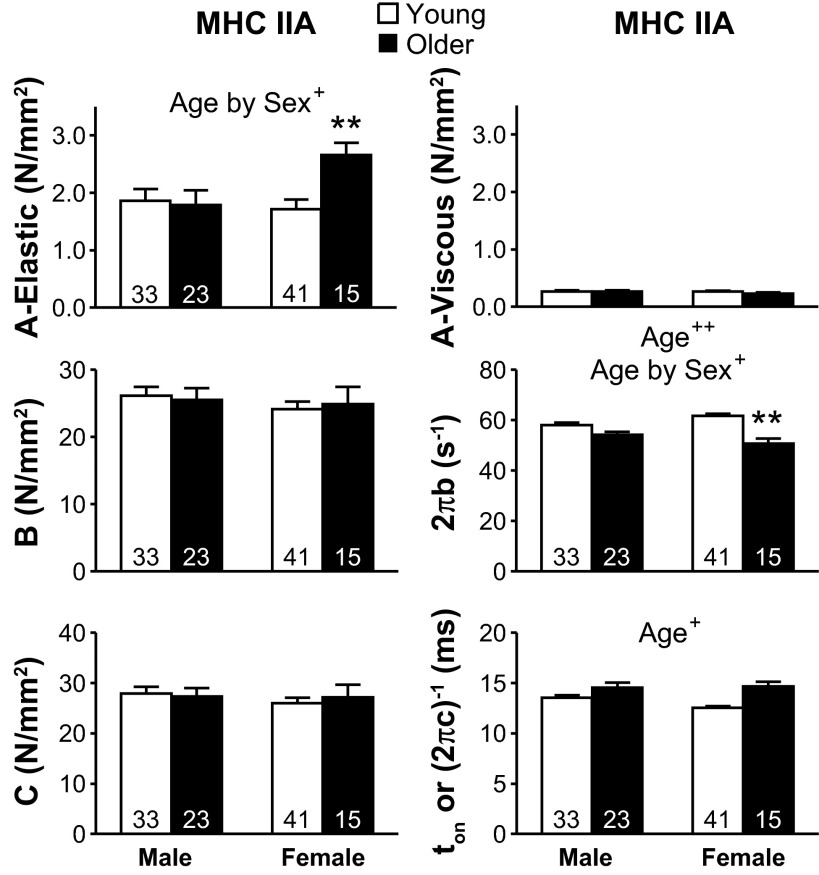
The six curve fit parameters estimated for MHC I and IIA are shown in Figs. 5 and 6, respectively. The A-elastic and A-viscous parameters reflect the elastic and viscous properties of the nonenzymatic, passive structural elements in the myofilaments. Parameters B and C are proportional to the number of myosin heads strongly bound to actin and the cross-bridge stiffness (22, 41). 2πb is interpreted as the apparent rate of myosin force production, or, in other words, the rate of myosin transition between the weakly and strongly bound states (64). Lastly, (2πc)−1 represents the ton to actin (41).
Fig. 5.
Sinusoidal analysis model parameters response for Ca2+-activated (pCa 4.5) MHC I fibers with age. A-elastic and A-viscous reflect the passive structural elements in the myofilaments. Parameters B and C are proportional to the number of myosin heads strongly bound to actin and cross-bridge stiffness. 2πb is the rate of myosin transition between the weakly and strongly bound states, and (2πc)−1 represents the average myosin attachment time (ton) to actin. Conditions: 25°C and 5 mM Pi. The number of fibers is indicated at the base of each bar. Text indicates significant difference (+P < 0.05 or ++P < 0.01) between young and older groups (Age) and a significant interaction between age and sex (Age by Sex). Asterisks indicate significant pairwise differences (**P < 0.01) between young and older women.
Fig. 6.
Sinusoidal analysis model parameters response for Ca2+-activated (pCa 4.5) MHC IIA fibers with age. Conditions: 25°C and 5 mM Pi. The number of fibers is indicated at the base of each bar. Text indicates significant difference (+P < 0.05 or ++P < 0.01) between young and older groups (Age) and a significant interaction between age and sex (Age by Sex). Asterisks indicate significant pairwise differences (**P < 0.01) between young and older women.
2πb showed the greatest decrease (P < 0.01) between young and older groups of the cross-bridge kinetic/mechanics parameters in both fiber types (Figs. 5 and 6). 2πb also showed an age-by-sex trend in MHC I (P = 0.08) and an interaction in IIA fibers, with pairwise comparisons showing that the lower 2πb with age was significant only in women. The ton was not different between age groups for MHC I fibers, but was greater in older adults for MHC IIA fibers. The age-related reduction of 2πb in older women in both fiber types and increase in ton in both sexes in MHC IIA fibers show that cross-bridge kinetics are slowed with age, especially in MHC IIA fibers from older women. A-elastic was greater in older individuals in MHC I fibers and showed an age-by-sex trend in MHC I (P = 0.09) and an interaction in IIA fibers. Pairwise comparisons showed that greater A-elastic with age was significant in women, but not men. A-viscous, B, and C showed no differences between age groups in MHC I or IIA fibers.
The number of MHC IIA fibers was the lowest in older women (n = 15), even though 90 total fibers were examined, similar to the other three groups (88 ± 11 fibers). The decrease in MHC IIA fibers in older women was due to an increase in hybrid fiber types, specifically MHC I/IIA and I/IIA/IIX, as MHC I percentages were similar to those of the other groups (older women vs. other three groups: MHC I: 53 vs. 50 ± 3%; MHC IIA: 17 vs. 36 ± 2%; remaining fiber types: 30 vs. 14 ± 4%). Fiber types are randomly selected, as fiber type is unknown until after experiments are completed, which suggests older women have more hybrid fibers than the other groups. Notably, MHC IIA fibers were studied in six of the seven volunteers, with five volunteers having multiple fibers (2.8 ± 0.2) and one volunteer having a single fiber. Contractile performance of the MHC IIA fibers in older women was similar within volunteers when multiple fibers were available, but was consistently different from the other three groups across volunteers. In other words, most, if not all, older women had values distinct from the other three groups, differences that provide an accurate assessment of age-related alterations within this cohort.
Single-fiber mechanics correlate to whole leg muscle contractile performance.
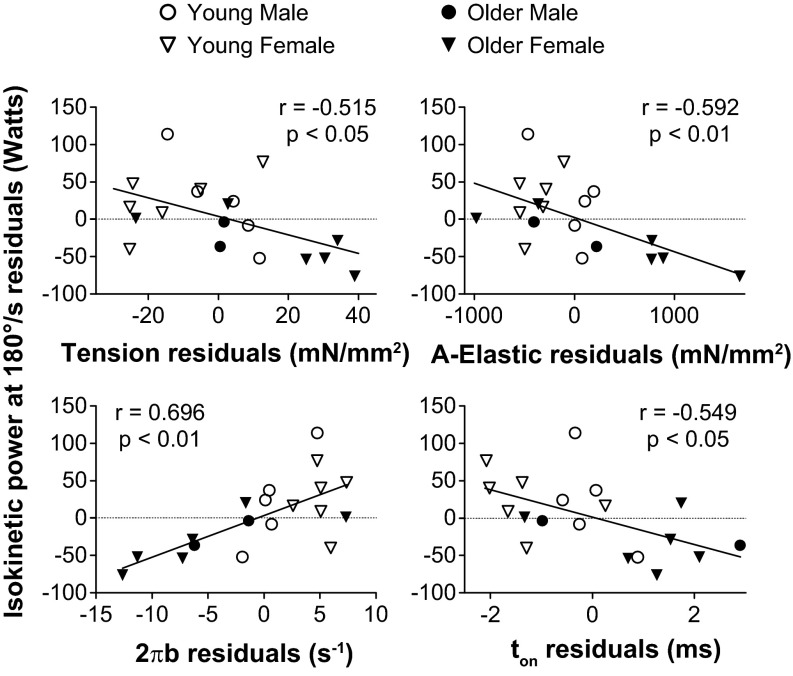
As changes at the molecular and cellular level may scale up to whole muscle performance, we examined whether the age-related alterations in molecular (A-elastic, ton, and 2πb) and cellular (isometric tension) function were related to the variations in whole muscle isokinetic power. To account for variations in muscle size, relationships with whole muscle isokinetic power were adjusted for leg fat-free mass using partial correlation analysis. While a few correlations were found in MHC I fibers, MHC IIA fibers showed that isokinetic power correlated at all contractile velocities with isometric tension, A-elastic, and 2πb and correlated at most velocities with ton (Table 2). By definition, a partial correlation can be visually depicted by plotting the residuals (observed minus predicted values) of the dependent and independent variables after both have been regressed on muscle mass. Representative correlations between whole muscle isokinetic power at 180°/s and single-fiber parameters in MHC IIA fibers are shown in Fig. 7.
Table 2.
Correlation coefficients for whole leg muscle isokinetic power and single-fiber Ca2+-activated isometric tension and sinusoidal analysis model parameters, including cross-bridge kinetics
| Isokinetic Velocity, °/s | Isometric Tension, mN/mm2 | A-Elastic, N/mm2 | 2πb, s−1 | ton, ms |
|---|---|---|---|---|
| MHC I | ||||
| 60 | −0.331 | −0.289 | 0.152 | 0.248 |
| 120 | −0.386 | −0.392 | 0.239 | 0.155 |
| 180 | −0.477* | −0.478* | 0.331 | 0.066 |
| 240 | −0.521* | −0.575* | 0.422 | −0.077 |
| 300 | −0.465 | −0.431 | 0.383 | 0.005 |
| MHC IIA | ||||
| 60 | −0.486* | −0.524* | 0.636† | −0.456* |
| 120 | −0.517* | −0.579* | 0.663† | −0.414 |
| 180 | −0.515* | −0.592† | 0.696† | −0.549* |
| 240 | −0.552* | −0.621† | 0.696† | −0.510* |
| 300 | −0.624† | −0.579* | 0.672† | −0.351 |
Isometric tension, single-fiber isometric tension at 25°C and 5 mM Pi. Correlation coefficients for isokinetic velocity data reflect partial correlations, where dependent and independent variables are controlled for leg fat-free mass from dual-energy X-ray absorptiometry. A-elastic, real part of parameter A; 2πb, rate of myosin transition between the weakly and strongly bound states; ton, myosin attachment time; MHC, myosin heavy chain
P < 0.05,
P < 0.01.
Fig. 7.
Relationship between whole leg muscle isokinetic power at 180°/s and single-fiber isometric tension and cross-bridge kinetics for MHC IIA fibers. Each variable was regressed on muscle mass, and residuals (observed minus predicted) were calculated. Plots of whole leg muscle isokinetic power at 180°/s residuals vs. other variables' residuals visually depicts the partial correlation of these variables adjusted for muscle mass.
We further examined the correlations between whole muscle isokinetic power and cellular/molecular functional indexes in MHC IIA fibers in men and women separately to investigate whether these relationships could be differentiated on the basis of sex. Men and women both showed significant correlations for whole muscle power vs. isometric tension, 2πb, and ton. Notably, the strength of the correlation coefficient was maintained, but the significance (P value) was decreased, as might be expected due to the reduced number of observations. These results indicate that both sexes exhibit correlations between whole muscle isokinetic power and single-fiber function.
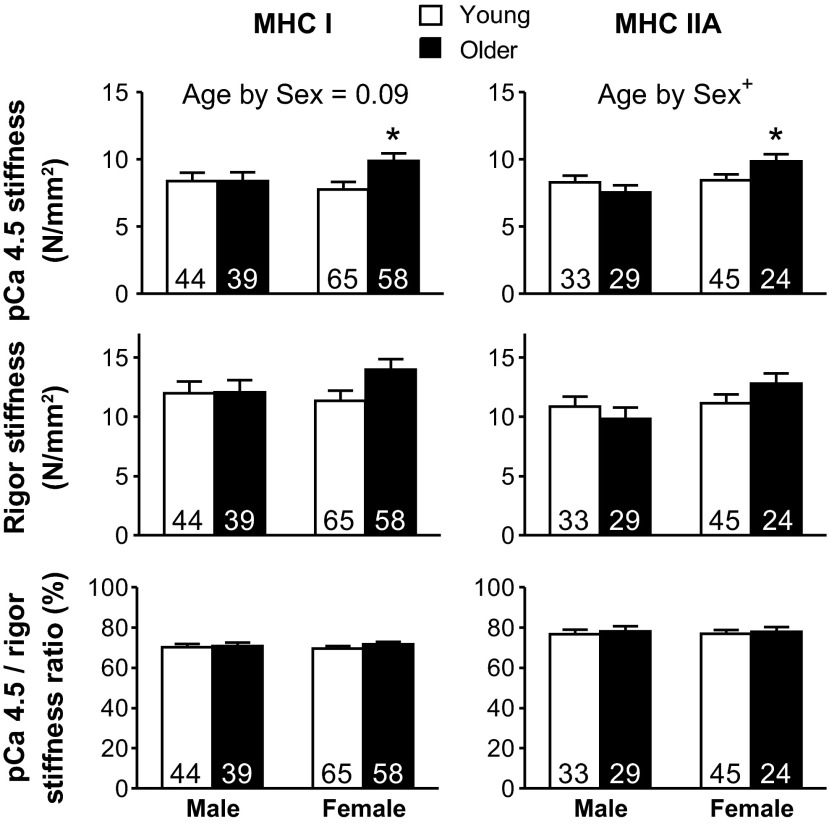
Maximum Ca2+-activated and rigor dynamic stiffness.
A trend toward an age-by-sex interaction effect in MHC I fibers (P = 0.09) and an interaction effect in MHC IIA fibers was found for pCa 4.5 stiffness (Fig. 8). Within each fiber type, the rigor stiffness, which is proportional to the total number of available myosin heads that can bind actin (i.e., total cross-bridge number), followed a similar pattern as the pCa stiffness (Fig. 8); however, no significant differences were found. Accordingly, the pCa 4.5/rigor stiffness ratio, which provides an estimate of the fraction of available myosin heads that bind actin during Ca2+ activation, was not different between young and older groups in MHC I and IIA fibers (Fig. 8).
Fig. 8.
Ca2+-activated and rigor dynamic stiffness changes for MHC I and IIA fibers with age. Single skeletal muscle fiber pCa 4.5 and rigor dynamic stiffness, as well as their stiffness ratio for MHC I and IIA fibers at 15°C and 0.25 mM Pi in young and older groups, are shown. The number of fibers is indicated at the base of each bar. Age by Sex, significant interaction (+P < 0.05) between age and sex. *Significant difference (P < 0.05) between young and older women.
Phosphorylation of myofibrillar proteins.
LC-MS was used to identify the four bands that were stained in the phosphoprotein-specific gels. These experiments identified the presence of multiple proteins in each band (1–4), including 1) fast and slow skeletal myosin binding protein-C isoforms; 2) α-tropomyosin and troponin T; and 3 and 4) myosin RLC-2, the ventricular/cardiac (slow) isoform [myosin light chain (MLC)-2s], and skeletal (fast) isoform (MLC-2f), with an enrichment of MLC-2s in band 3 and MLC-2f in band 4. Bands 1 and 2 contained the phosphorylated peptides 37EAPPEDQSpPTAEEPTGVFLK56 [mass-to-charge ratio (m/z) = 1,111.5] from fast skeletal myosin binding protein-C, and 269AISEELDHALNDMTSpI284 (m/z = 919.9) and 269AISEELDHALNDmTSpI284 (m/z = 927.9) from α-tropomyosin, as well as their nonphosphorylated analogs. The position of phosphate is indicated by (p), and methionine sulfoxide indicated by (m). Bands 3/4 contained the phosphorylated peptides 10AGGANSpNVFSMFEQTQIQEFK30 (m/z = 1,207.0) and 10AGGANSpNVFSmFEQTQIQEFK30 (m/z = 1,215.0) from MLC-2s, and 10TVEGG(SS)pSVFSMFDQTQIQEFK31 (m/z = 1,266.6) and 10TVEGG(SS)pSVFSmFDQTQIQEFK31 (m/z = 1,274.6) from MLC-2f, as well as their nonphosphorylated analogs. The position of phosphate was determined from the tandem mass spectrometry spectra at serine 15 in MLC-2s, but we could not discern whether serine 15 or 16 was phosphorylated in MLC-2f. However, the reduced and oxidized MLC-2f phosphopeptides eluted as two individual LC peaks, suggesting that the phosphorylation was on either serine 15 or 16.
LC-MS was also used to determine the absolute phosphorylation of MLC-2f and MLC-2s for all four groups combined. Due to the presence of both RLC isoforms in bands 3 and 4, the two bands were excised from the gel together and digested as single samples. Because the digestion and ionization efficiencies of each phosphopeptide are unknown, the percent phosphorylation at each site could not be directly determined from the abundance of the ion currents measured for the phosphopeptides, but was measured using a mass-balance approach (46, 62). This approach determines the degree of phosphorylation in a sample by comparing the abundance of peptides that were altered by phosphorylation to a control sample devoid of phosphorylation (details in supplemental information of Ref. 62). The MLC-2s peptides DTFAALGR (m/z = 425.7), EAFTImDQNR (m/z = 620.8), DGFIDKNDLR (m/z = 596.8), and GADPEETILNAFK (m/z = 702.9), and MLC-2f peptides DGIIDKEDLR (m/z = 587.3), LKGADPEDVITGAFK (m/z = 780.9), GADPEDVITGAFK (m/z = 660.3), and EAFTVIDQNR (m/z = 596.8) were used as reference peptides to account for digestion efficiency and the total abundance of the protein in each sample. For each sample, the degree of phosphorylation was calculated from the sum of the reduced and oxidized nonphosphorylated analogs of the phosphopeptides, as described (62).
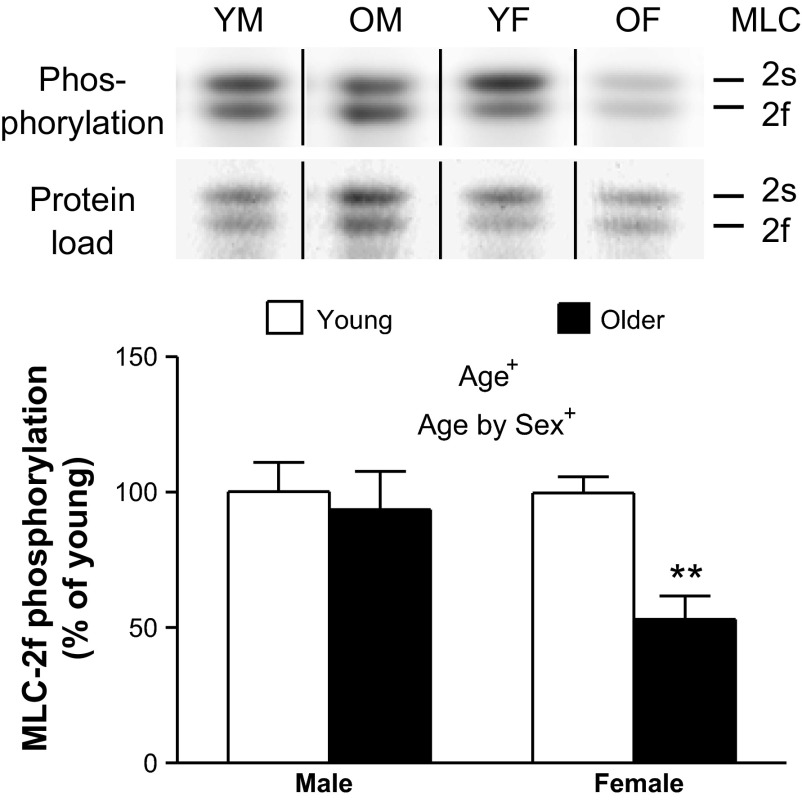
Using phosphostaining, only band 4 (primarily MLC-2f) showed an age-related change in relative phosphorylation level, with older women having a 1.8 ± 0.1-fold decrease in phosphorylation compared with the other three groups (Fig. 9). Using LC-MS, the absolute phosphorylation was 41 ± 4% for MLC-2f and 47 ± 5% for MLC-2s for all four groups combined, showing that the absolute phosphorylation levels are large enough to potentially be physiologically relevant. Using the relative abundances for each group from phosphostaining in combination with the absolute phosphorylation for all groups combined from the LC-MS data, we estimate the degree of phosphorylation for MLC-2f to be 47% for young men, 44% for older men, 47% for young women, and 25% for older women. This estimate is based on the assumption that the average degree of phosphorylation measured by LC-MS was underestimated due to the 1.8-fold reduction in phosphorylation in the older women, while the men and young women have nearly identical degrees of phosphorylation (Fig. 9).
Fig. 9.
The relative abundance of the fast isoform of the myosin regulatory light chain 2 (MLC-2f) phosphorylation per protein load with age. Representative gel lanes for MLC-2 slow isoform (MLC-2s) and MLC-2f stained for phosphorylation level (phosphoprotein dye) and protein load (GelCode Blue) are above, with vertical black lines demarcating where intervening lanes have been spliced out. Text indicates significant difference (+P < 0.05) between young and older groups (Age) and a significant interaction between age and sex (Age by Sex). Asterisks indicate significant pairwise differences (**P < 0.01) between young and older women. YM, young male (n = 5); OM, older male (n = 5); YF, young female (n = 7); OF, older female (n = 7).
DISCUSSION
This study examined skeletal muscle function from the whole muscle to the myosin actin cross-bridge level in young (21–35 yr) and older (65–75 yr) men and women who were matched for habitual physical activity levels. At the molecular level, older women demonstrated slower cross-bridge kinetics and greater myofilament lattice stiffness compared with young in both MHC I and IIA fibers, while older men had slower cross-bridge kinetics in MHC IIA fibers. Other human studies have suggested decreased cross-bridge kinetics and/or increased myofilament stiffness with age (8, 19, 37, 38), but ours is the first to perform measurements that permit the calculation of cross-bridge kinetic parameters and to identify specific steps in the cross-bridge cycle that are altered with age. Age-related alterations from the molecular to whole muscle level were more pronounced or, in some cases, completely driven by women, indicating the sexes respond differently to age. Importantly, because we experimentally matched young and older groups for physical activity level, the observed cellular/molecular level adaptations should more accurately reflect primary aging effects.
Age-related molecular alterations correlate with whole muscle performance.
What is the functional relevance of changes in cross-bridge kinetics and myofilament stiffness? We found that age-related reductions in cross-bridge kinetics and increases in myofilament lattice stiffness in MHC IIA fibers were related to decreases in voluntary knee extensor power output. We acknowledge that multiple physiological systems dictate whole muscle performance, and causation cannot be inferred from correlation analysis. However, given the primacy of myofilaments in determining muscle contractile function, we cautiously interpret our findings to suggest that age-related modifications in myofilament protein functional and/or structural properties contribute to diminished whole muscle performance. Our results suggest slower cross-bridge kinetics and increased myofilament stiffness as potential molecular mechanisms contributing to the whole muscle contractile phenotype that we and others have observed; namely, aging compromises whole muscle dynamic contractile performance to a greater extent than isometric function (4, 27). The fact that correlations between cellular/molecular functional indexes and whole muscle isokinetic power were only apparent in MHC IIA fibers may be explained by the fact that these fibers disproportionately determine whole muscle isokinetic function (16, 49), most likely because MHC IIA fibers produce three times the power output of MHC I fibers (3). Perhaps most notably, age effects in molecular contractile kinetics were largely driven by older women, who also demonstrated the largest age-related reductions in knee extensor power output, in keeping with prior studies that adjusted for muscle size or weight (21, 43). Considering that knee extensor power output is a strong predictor of physical disability (48), a greater reduction in molecular contractile function with age in women may contribute to their increased disability rate (17, 36).
The lower whole muscle isokinetic power output in older women may also be affected by their reduced MHC IIA CSA, which could result in a decreased whole muscle MHC IIA relative content. MHC IIA fibers have been found to incur age-related atrophy more than MHC I fibers (29), specifically in older women compared with older men (57). In addition, the increase in hybrid fiber types in older women suggests a potential reduction in pure MHC IIA fibers, as their MHC isoform distribution and content were unchanged compared with the other three groups. Thus MHC IIA molecular level alterations and atrophy may be coupled to ultimately lead to reduced whole muscle dynamic performance.
The lack of association between maximal Ca2+-activated tension and whole muscle isometric force production may be due to age-related changes in neural activation (50) or reduced in vivo Ca2+ release (11). An age-related reduction in Ca2+ levels would reduce in vivo isometric tension and even further slow cross-bridge kinetics (30) compared with our in vitro single-fiber measurements at maximal Ca2+ activation. Thus our measurements could be underestimating the effect of aging on cross-bridge kinetics.
Age-related molecular alterations effect single-fiber properties.
Our observation that single-fiber isometric tension increases in women and remains unchanged in men deserves comment, as several studies have found decreasing values with age (8, 14, 28, 38). As physical activity decreases with age (58) and reduced physical activity alters fiber contractile properties in older individuals (8, 9), our strict control for physical activity level may partially explain our findings. In addition, our isometric tension measurements are performed at 25°C with 5 mM Pi, conditions that are closer to in vivo compared with other single-fiber studies (typically 15°C with 0.25 mM Pi). Notably, our results are not unprecedented, as isometric tension with age has been shown to increase (57) and tends to be higher (19–28%), although not statistically significant (13, 24).
Our measurements of cross-bridge kinetics and myofilament stiffness allow us to propose a mechanism for the age-related increase in isometric tension found in women. Isometric tension is related to the number of strongly bound cross bridges, the unitary force generated per cross bridge (product of myosin step size and myosin stiffness), and myofilament stiffness (52). Because the number of strongly bound cross bridges and their stiffness (B and C, pCa 4.5/rigor stiffness) remained unchanged with age, we propose that the slower cross-bridge kinetics (reduced 2πb and longer ton) produce higher isometric tension by increasing the myofilament stiffness (A-elastic) and, in turn, force transmission, during Ca2+ activation. In support of our hypothesis, we have shown that slowing cross-bridge kinetics, by reducing MgATP concentration, increases myofilament stiffness with little to no changes in the number of strongly bound cross bridges (40).
If age-related differences in molecular function diminish whole muscle power output, single-fiber force production and/or velocity should be decreased. Our results do not support a decrease in single-fiber force production, as isometric tension either remained the same or increased with age. Although we did not measure velocity, we would predict a decrease in contractile velocity, owing to the longer ton with age [i.e., longer, strongly bound cross bridges decrease velocity (44, 59)] and slower 2πb [i.e., reduced myosin attachment rate decreases velocity (44, 59)]. Previous human single-fiber studies have found an age-related decrease in unloaded shortening velocity with no change in isometric tension (9, 24). Studies using isolated human myosin similarly found decreased in vitro motility with age (8, 19), suggesting that aging may alter the intrinsic function of myosin, in agreement with animal studies (reviewed in Ref. 53). Collectively, these studies indicate that aging decreases contractile velocity, most likely due to changes in the cross-bridge kinetic properties secondary to changes in the myosin molecule.
Age-related molecular alterations may be due to decreased MLC-2f phosphorylation.
The phosphorylation decrease in the fast isoform of myosin RLC-2 (MLC-2f) in older women provides a potential mechanism for their slower cross-bridge kinetics. In skeletal muscle, decreasing MLC-2f phosphorylation confines the myosin heads near the thick-filament backbone and reduces cross-bridge attachment rate (51), consistent with a slower 2πb. As the MLC-2f isoform is present in MHC I and IIA human skeletal fibers (2, 33), the phosphorylation decrease could explain the slower 2πb in both fiber types. In support of our hypothesis, we have shown that disrupting myosin RLC phosphorylation has significant effects on cross-bridge kinetics by greatly reducing 2πb as well as slightly reducing ton in transgenic Drosophila melanogaster (31). Animal studies have shown posttranslational modifications of myosin (53) and shifts in myosin essential light chain isoforms (23) with age, although these changes produce decreases in isometric tension, opposite our results in humans. As the aging phenotype most likely results from a number of molecular changes, our results do not exclude previously observed modifications, but suggest that MLC-2f phosphorylation may explain our most prominent age-related decrease in cross-bridge kinetics.
In summary, our results from young and older volunteers matched for habitual physical activity levels indicate that aging slows myosin actin cross-bridge kinetics in women, leading to decrements in whole body dynamic contractile performance. These results are especially relevant in light of previous studies indicating higher rates of disability and skeletal muscle contractile dysfunction in older women compared with men (17, 36). Rehabilitative interventions that increase cross-bridge kinetics, such as resistance training (6), may reduce or eliminate the age-related skeletal muscle dysfunction, but may need to be sex specific to address the unique cellular/molecular adaptations with age of men and women.
GRANTS
This study was supported by National Institutes of Health Grants AG-031303, AG-033547, HL-086902, HL-007647, RR-000109, and 8P20GM-103449.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.M., P.A.A., D.W.M., and M.J.T. conception and design of research; M.S.M., N.G.B., M.J.P., and M.E.J. performed experiments; M.S.M., N.G.B., D.M.C., M.J.P., and M.E.J. analyzed data; M.S.M., D.M.C., M.J.P., D.W.M., B.M.P., and M.J.T. interpreted results of experiments; M.S.M. and N.G.B. prepared figures; M.S.M. drafted manuscript; M.S.M., N.G.B., D.M.C., M.J.P., M.E.J., P.A.A., D.W.M., B.M.P., and M.J.T. edited and revised manuscript; M.S.M., N.G.B., D.M.C., M.J.P., M.E.J., P.A.A., D.W.M., B.M.P., and M.J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all of the volunteers who dedicated valuable time to these studies, and Alan Howard for statistical assistance.
REFERENCES
- 1.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Billeter R, Heizmann CW, Howald H, Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem 116: 389–395, 1981 [DOI] [PubMed] [Google Scholar]
- 3.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan DM, Kent-Braun JA. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol 111: 1345–1352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci 60: 148–156, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Canepari M, Rossi R, Pellegrino MA, Orrell RW, Cobbold M, Harridge S, Bottinelli R. Effects of resistance training on myosin function studied by the in vitro motility assay in young and older men. J Appl Physiol 98: 2390–2395, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cosper PF, Leinwand LA. Myosin heavy chain is not selectively decreased in murine cancer cachexia. Int J Cancer 130: 2722–2727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol 100: 603–611, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: implications for fatigue. Am J Physiol Cell Physiol 287: C673–C681, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Delbono O, O'Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol 148: 211–222, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve 28: 601–608, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol 105: 637–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Galler S, Wang BG, Kawai M. Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J 89: 3248–3260, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gur H, Gransberg L, vanDyke D, Knutsson E, Larsson L. Relationship between in vivo muscle force at different speeds of isokinetic movements and myosin isoform expression in men and women. Eur J Appl Physiol 88: 487–496, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hardy SE, Allore HG, Guo Z, Gill TM. Explaining the effect of gender on functional transitions in older persons. Gerontology 54: 79–86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson RN., Jr Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 52: 214–218, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Hook P, Sriramoju V, Larsson L. Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol Cell Physiol 280: C782–C788, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Katsiaras A, Newman AB, Kriska A, Brach J, Krishnaswami S, Feingold E, Kritchevsky SB, Li R, Harris TB, Schwartz A, Goodpaster BH. Skeletal muscle fatigue, strength, and quality in the elderly: the Health ABC Study. J Appl Physiol 99: 210–216, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kawai M, Saeki Y, Zhao Y. Crossbridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res 73: 35–50, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Torgerud WS, Mosser KH, Hirai H, Watanabe S, Asakura A, Thompson LV. Myosin light chain 3f attenuates age-induced decline in contractile velocity in MHC type II single muscle fibers. Aging Cell 11: 203–212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol 101: 906–917, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil 80: 447–455; quiz 456–447, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Krudysz-Amblo J, Jennings ME, 2nd, Mann KG, Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem 285: 3371–3382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol 95: 2361–2369, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272: C638–C649, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Metzger JM, Moss RL. Calcium-sensitive cross-bridge transitions in mammalian fast and slow skeletal muscle fibers. Science 247: 1088–1090, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Miller MS, Farman GP, Braddock JM, Soto-Adames FN, Irving TC, Vigoreaux JO, Maughan DW. Regulatory light chain phosphorylation and N-terminal extension increase cross-bridge binding and power output in Drosophila at in vivo myofilament lattice spacing. Biophys J 100: 1737–1746, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MS, Lekkas P, Braddock JM, Farman GP, Ballif BA, Irving TC, Maughan DW, Vigoreaux JO. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys J 95: 2391–2401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MS, VanBuren P, LeWinter MM, Braddock JM, Ades PA, Maughan DW, Palmer BM, Toth MJ. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol 588: 4039–4053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MS, VanBuren P, LeWinter MM, Lecker SH, Selby DE, Palmer BM, Maughan DW, Ades PA, Toth MJ. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail 2: 700–706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulieri LA, Barnes W, Leavitt BJ, Ittleman FP, LeWinter MM, Alpert NR, Maughan DW. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res 90: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Newman AB, Brach JS. Gender gap in longevity and disability in older persons. Epidemiol Rev 23: 343–350, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Ochala J, Dorer DJ, Frontera WR, Krivickas LS. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflügers Arch 452: 464–470, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62: 375–381, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Palmer BM, Fishbaugher DE, Schmitt JP, Wang Y, Alpert NR, Seidman CE, Seidman JG, VanBuren P, Maughan DW. Differential cross-bridge kinetics of FHC myosin mutations R403Q and R453C in heterozygous mouse myocardium. Am J Physiol Heart Circ Physiol 287: H91–H99, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Palmer BM, Schmitt JP, Seidman CE, Seidman JG, Wang Y, Bell SP, Lewinter MM, Maughan DW. Elevated rates of force development and MgATP binding in F764L and S532P myosin mutations causing dilated cardiomyopathy. J Mol Cell Cardiol 57: 23–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer BM, Suzuki T, Wang Y, Barnes WD, Miller MS, Maughan DW. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J 93: 760–769, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathare N, Walter GA, Stevens JE, Yang Z, Okerke E, Gibbs JD, Esterhai JL, Scarborough MT, Gibbs CP, Sweeney HL, Vandenborne K. Changes in inorganic phosphate and force production in human skeletal muscle after cast immobilization. J Appl Physiol 98: 307–314, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol 98: 211–220, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131: 784–795, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Previs MJ, Beck Previs S, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science 337: 1215–1218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Previs MJ, VanBuren P, Begin KJ, Vigoreaux JO, LeWinter MM, Matthews DE. Quantification of protein phosphorylation by liquid chromatography-mass spectrometry. Anal Chem 80: 5864–5872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid KF, Doros G, Clark DJ, Patten C, Carabello RJ, Cloutier GJ, Phillips EM, Krivickas LS, Frontera WR, Fielding RA. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol 112: 2289–2301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryushi T, Fukunaga T. Influence of subtypes of fast-twitch fibers on isokinetic strength in untrained men. Int J Sports Med 7: 250–253, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve 27: 99–101, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys 510: 120–128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanner BC, Daniel TL, Regnier M. Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLoS Comput Biol 3: e115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson LV. Age-related muscle dysfunction. Exp Gerontol 44: 106–111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab 288: E883–E891, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol 590: 1243–1259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan DW, Ades PA. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol 143: 276–282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Walcott S, Warshaw DM, Debold EP. Mechanical coupling between myosin molecules causes differences between ensemble and single-molecule measurements. Biophys J 103: 501–510, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang G, Kawai M. Effect of temperature on elementary steps of the cross-bridge cycle in rabbit soleus slow-twitch muscle fibres. J Physiol 531: 219–234, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G, Kawai M. Force generation and phosphate release steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys J 73: 878–894, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weith A, Sadayappan S, Gulick J, Previs MJ, Vanburen P, Robbins J, Warshaw DM. Unique single molecule binding of cardiac myosin binding protein-C to actin and phosphorylation-dependent inhibition of actomyosin motility requires 17 amino acids of the motif domain. J Mol Cell Cardiol 52: 219–227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 190: 229–241, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Kawai M. The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J 64: 197–210, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]