Abstract
Amygdala plasticity is an important contributor to the emotional-affective dimension of pain. Recently discovered neuropeptide S (NPS) has anxiolytic properties through actions in the amygdala. Behavioral data also suggest antinociceptive effects of centrally acting NPS, but site and mechanism of action remain to be determined. This is the first electrophysiological analysis of pain-related NPS effects in the brain. We combined whole cell patch-clamp recordings in brain slices and behavioral assays to test the hypothesis that NPS activates synaptic inhibition of amygdala output to suppress pain behavior in an arthritis pain model. Recordings of neurons in the laterocapsular division of the central nucleus (CeLC), which serves pain-related amygdala output functions, show that NPS inhibited the enhanced excitatory drive [monosynaptic excitatory postsynaptic currents (EPSCs)] from the basolateral amygdala (BLA) in the pain state. As shown by miniature EPSC analysis, the inhibitory effect of NPS did not involve direct postsynaptic action on CeLC neurons but rather a presynaptic, action potential-dependent network mechanism. Indeed, NPS increased external capsule (EC)-driven synaptic inhibition of CeLC neurons through PKA-dependent facilitatory postsynaptic action on a cluster of inhibitory intercalated (ITC) cells. NPS had no effect on BLA neurons. High-frequency stimulation (HFS) of excitatory EC inputs to ITC cells also inhibited synaptic activation of CeLC neurons, providing further evidence that ITC activation can control amygdala output. The cellular mechanisms by which EC-driven synaptic inhibition controls CeLC output remain to be determined. Administration of NPS into ITC, but not CeLC, also inhibited vocalizations and anxiety-like behavior in arthritic rats. A selective NPS receptor antagonist ([d-Cys(tBu)5]NPS) blocked electrophysiological and behavioral effects of NPS. Thus NPS is a novel tool to control amygdala output and pain-related affective behaviors through a direct action on inhibitory ITC cells.
Keywords: amygdala, pain behavior, neuropeptide S, synaptic transmission
neuroplasticity in the amygdala network of lateral-basolateral (LA-BLA) and central (CeA) nuclei has emerged as an important contributor to emotional-affective aspects of pain (Neugebauer et al. 2004, 2009). The laterocapsular CeA division (CeLC) receives nociceptive-specific information from spinal cord and brain stem (parabrachial area, PB), and most CeLC neurons respond exclusively or preferentially to noxious stimuli (Gauriau and Bernard 2004; Neugebauer et al. 2004, 2009). Synaptic plasticity of PB input to the CeLC has been observed in models of arthritic pain (Bird et al. 2005; Fu and Neugebauer 2008; Han et al. 2005b; Neugebauer et al. 2003), formalin-induced pain (Adedoyin et al. 2010), visceral pain (Han and Neugebauer 2004), and chronic neuropathic pain (Ikeda et al. 2007). The CeLC also receives highly processed multimodal, including nociceptive, information from thalamus and cortex through the LA-BLA network (Neugebauer et al. 2004, 2009). BLA input to the CeLC develops synaptic plasticity in pain models (Fu and Neugebauer 2008; Neugebauer et al. 2003; Ren et al. 2011; Ren and Neugebauer 2010). Pain-related synaptic plasticity is associated with increased responsiveness and output of CeLC neurons (Han et al. 2005b; Ji and Neugebauer 2007, 2009; Li and Neugebauer 2004a, 2004b; Neugebauer and Li 2003), which drives amygdala-dependent pain behaviors (Neugebauer et al. 2004, 2009).
Here we present evidence for a novel strategy to control CeLC output by targeting a cluster of GABAergic intercalated (ITC) cells at the border between LA-BLA and CeA (dorsomedial ITC cluster). Distinct from an ITC cluster on the lateral border of LA-BLA (lateral ITC) that controls cortical signals to BLA neurons (Marowsky et al. 2005), the dorsomedial ITC cells project strongly to the CeLC (for details see Amir et al. 2011; Busti et al. 2011) and serve as a feedforward inhibitory gate for signals to the CeA (Amano et al. 2010; Ehrlich et al. 2009; Likhtik et al. 2008; Pape and Pare 2010; Royer and Pare 2002). They receive strong excitatory input from the medial prefrontal cortex (mPFC) via the external capsule (EC) (Amir et al. 2011; McDonald 1998; Pinard et al. 2012) and are believed to mediate inhibitory influences of the mPFC on amygdala output as a mechanism for cognitive control of negative emotions during behavioral extinction (Jungling et al. 2008; Pape and Pare 2010). The role of ITC cells in pain remains to be determined, but in a previous study we speculated that ITC cells may be involved in the feedforward inhibition of CeLC neurons (Ren and Neugebauer 2010). We use the term “feedforward inhibition” to refer to glutamate-driven synaptic inhibition.
Importantly, neuropeptide S (NPS), a newly discovered 20-amino acid peptide, selectively enhances dorsomedial, but not lateral, ITC-dependent feedforward inhibition of CeA neurons to produce powerful anxiolytic effects (Jungling et al. 2008). NPS binds with high affinity to a Gq/Gs-coupled receptor (NPSR) to increase intracellular calcium and cAMP-PKA signaling (Guerrini et al. 2010; Reinscheid 2008). NPSR is expressed in discrete brain areas including the rat amygdala (Leonard and Ring 2011), where the highest level of NPSR mRNA is found in and around ITC cells, while NPSR is absent in other nuclei (LA, BLA, and CeA) relevant for pain processing (Xu et al. 2007).
Intracerebroventricular administration of NPS was shown to have not only anxiolytic (Jungling et al. 2008; Ruzza et al. 2012; Xu et al. 2004) but also antinociceptive (Li et al. 2009; Peng et al. 2010) effects. However, a mechanistic analysis of pain-related NPS effects has been lacking. Using a selective NPSR agonist (NPS; Guerrini et al. 2010; Reinscheid 2008) and antagonist ([d-Cys(tBu)5]NPS; Camarda et al. 2009), we tested the hypothesis that the amygdala is an important site for pain-inhibiting effects of NPS and that NPS increases feedforward inhibition of CeA neurons through a PKA-dependent postsynaptic action on inhibitory ITC cells. To do so, we used the well-established kaolin/carrageenan-induced monoarthritis model, which generates nocifensive and emotional-affective pain behaviors and synaptic plasticity in the amygdala network within a few hours, reaching a plateau effect at 5–6 h (Neugebauer et al. 2004, 2007, 2009).
METHODS
Animals
Male Sprague-Dawley rats (120–250 g) were housed in a temperature-controlled room and maintained on a 12:12-h day-night cycle. Water and food were available ad libitum. On the day of the experiment, rats were transferred from the animal facility and allowed to acclimate to the laboratory for at least 1 h. All experimental procedures conform to the guidelines of the International Association for the Study of Pain (IASP) and of the National Institutes of Health (NIH) and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB).
Arthritis Pain Model
In the group of arthritic rats, arthritis was induced in the left knee joint as previously described (Neugebauer et al. 2007). A kaolin suspension (4%, 100 μl) was injected into the left knee joint cavity through the patellar ligament. After repetitive flexions and extensions of the knee for 15 min, a carrageenan solution (2%, 100 μl) was injected into the knee joint cavity, and the leg was flexed and extended for another 5 min. Brain slices for electrophysiology were obtained 5–6 h after arthritis induction. This time point was also selected for the behavioral studies. Another group of rats (“normal”) did not receive any injections but were kept under the same conditions as the arthritic rats and handled similarly by the experimenter (including flexing the knee joint) before brain slices were obtained for electrophysiological studies. We showed previously that a sham control injection of sterile saline into the knee joint does not result in synaptic plasticity in the amygdala (Neugebauer et al. 2003). Therefore, in the present study normal animals were used as controls.
Electrophysiology in Brain Slices
Amygdala slice preparation.
Coronal brain slices (300–500 μm) were obtained from the right hemisphere as described previously (Li et al. 2011; for recent references see Ren et al. 2011; Ren and Neugebauer 2010). A single brain slice was transferred to the recording chamber and submerged in artificial cerebrospinal fluid (ACSF; 31 ± 1°C), which superfused the slice at 2 ml/min. ACSF contained (in mM) 117 NaCl, 4.7 KCl, 1.2 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, and 11 glucose. ACSF was oxygenated and equilibrated to pH 7.4 with a mixture of 95% O2-5% CO2. Only one or two brain slices per animal were used, one neuron was recorded in each slice, and a fresh slice was used for each new experimental protocol.
Patch-clamp recording.
Whole cell current- and voltage-clamp recordings were made from visually identified CeLC, ITC, and BLA neurons in brain slices from normal and arthritic rats with DIC-IR videomicroscopy as described previously (Li et al. 2011; for recent references see Ren et al. 2011; Ren and Neugebauer 2010). The boundaries of the different amygdalar nuclei are easily discerned under light microscopy (see Fig. 1 in Fu and Neugebauer 2008, Fig. 5 in Sah et al. 2003, and Fig. 1 in Watabe et al. 2013). Patch electrodes (4- to 6-MΩ tip resistance) made from borosilicate glass capillaries (1.5-mm outer diameter, 1.12-mm inner diameter) were filled with internal solution containing (in mM) 122 K-gluconate, 5 NaCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, and 0.4 Na3-GTP; pH was adjusted to 7.2–7.3 with KOH and osmolarity to 280 mosM with sucrose. A dual four-pole Bessel filter (Warner Instruments), a low-noise Digidata 1322 interface (Molecular Devices), Axoclamp-2B amplifier (Molecular Devices), a Pentium PC, and pCLAMP 10 software (Molecular Devices) were used for data acquisition and analysis. Head-stage voltage was monitored continuously on an oscilloscope to ensure precise performance of the amplifier. Neurons were voltage-clamped at −70 (near the chloride reversal potential) or 0 mV [reversal potential of excitatory postsynaptic currents (EPSCs)] for the study of excitatory and inhibitory transmission, respectively. The calculated equilibrium potential for chloride in this system was −68.99 mV (Nernst equation, pCLAMP 10). High-gigaohm seal and low series resistances (<20 MΩ) were checked throughout the experiment (using pCLAMP 10 membrane test function) to ensure high-quality recordings.
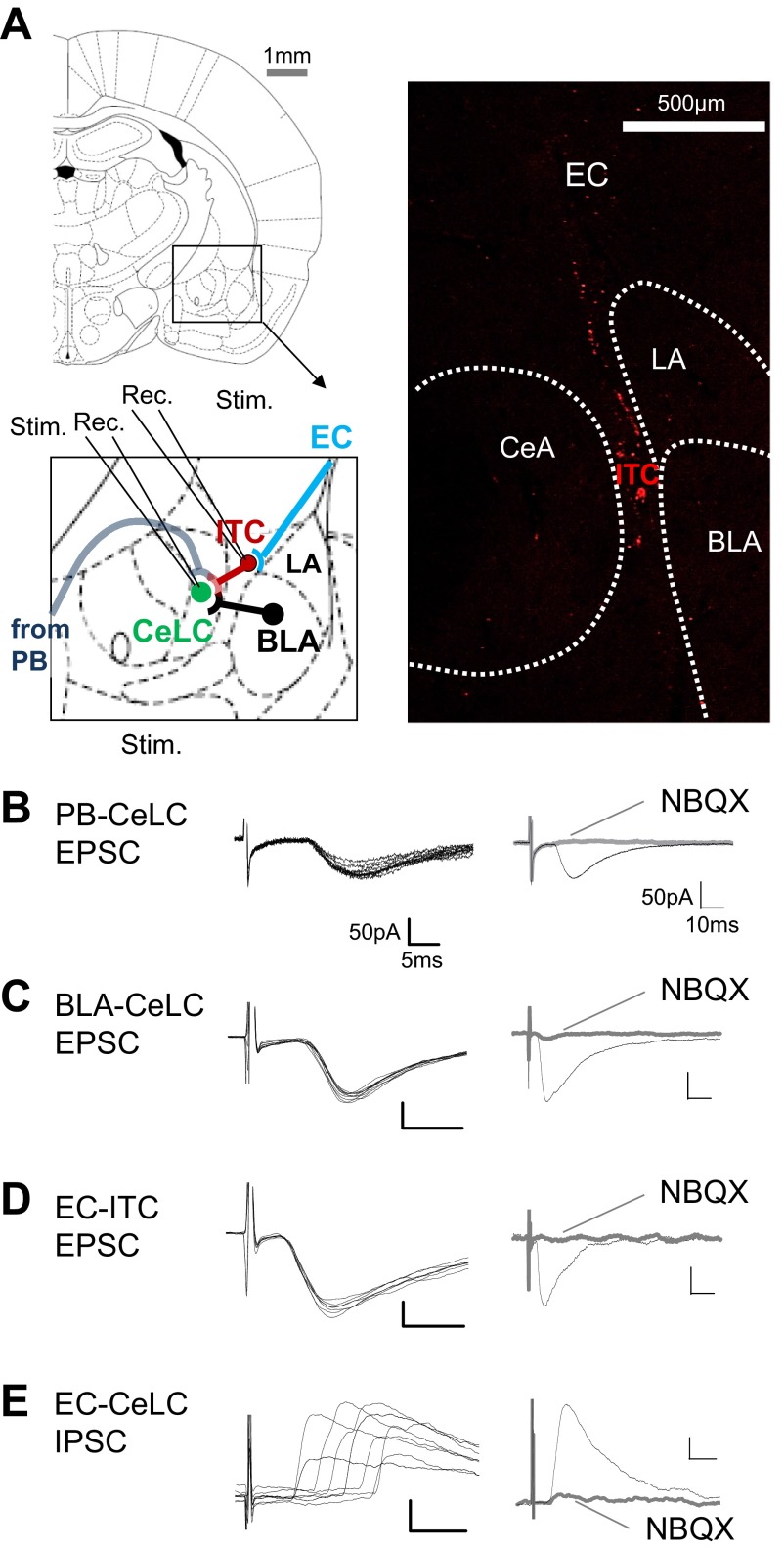
Fig. 1.
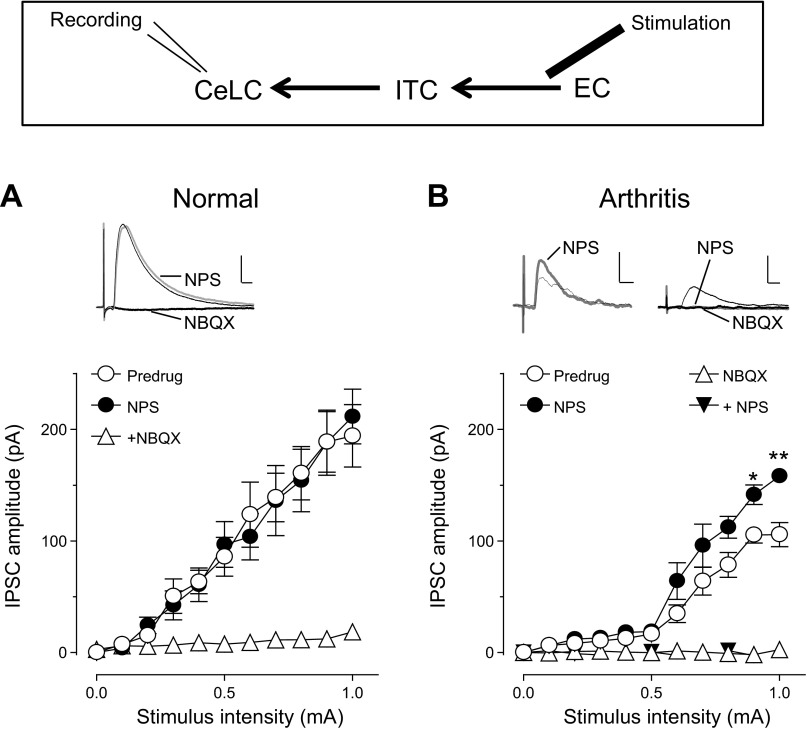
Synaptic transmission in amygdala brain slices. A: stimulation (Stim.) and recording (Rec.) sites in coronal brain slices containing the right amygdala. Different amygdala nuclei are shown. CeLC, laterocapsular division of the central nucleus (CeA); ITC, intercalated cell mass (medial paracapsular cluster); LA and BLA, lateral and basolateral nuclei, respectively. Synaptic responses were evoked in CeLC neurons by stimulation of parabrachial area (PB) input or in the BLA or external capsule (EC) and in ITC cells by stimulation of EC. Image on right shows orthogradely [from infralimbic medial prefrontal cortex (mPFC), see methods] labeled fibers travelling in the EC to ITC. Adapted from Paxinos and Watson (1998) with permission. B–D: monosynaptic excitatory postsynaptic currents (EPSCs) recorded at PB-CeLC synapse (B), BLA-CeLC synapse (C), and EC-ITC synapse (D) at −70 mV showed constant latency (left; traces show 10 sweeps) and were blocked by NBQX (10 μM; right). E: polysynaptic inhibitory postsynaptic currents (IPSCs) evoked by EC stimulation (EC-CeLC synapse) were recorded at 0 mV, showed variable latencies, and were inhibited by NBQX, indicating feedforward inhibition of CeLC neurons.
Fig. 5.
NPS increased excitatory synaptic responses of ITC neurons in the pain model: whole cell patch-clamp recordings of ITC cells. Diagram shows stimulation and recording sites. A: in brain slices from normal rats NPS (1 μM) had no effect on EPSCs evoked by EC stimulation (n = 5 neurons; P > 0.05, F1,88 = 3.55, main effect of drug, 2-way ANOVA). B: in slices from arthritic rats NPS (1 μM) increased EPSCs significantly (n = 5; P < 0.0001, F1,88 = 57.05, main effect of drug, 2-way ANOVA). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni posttests (compared with Predrug). The NPS receptor antagonist [d-Cys(tBu)5]NPS (antag., 10 μM) reversed the effect of NPS so that the I/O function was not different from Predrug (n = 5; P > 0.05, F1,88 = 3.83, main effect of drug, 2-way ANOVA). C: in brain slices from normal rats NPS had no effect on synaptically evoked spiking (E-S coupling) in ITC cells (n = 5; P > 0.05, paired t-test). D: in brain slices from arthritic rats NPS increased E-S coupling significantly (n = 5 neurons). The antagonist [d-Cys(tBu)5]NPS (10 μM) inhibited the effect of NPS (n = 5 neurons). C and D: bar histograms show means ± SE. Stimulus intensity was adjusted to evoke spikes in ∼50% of the trials before drug application. **P < 0.01, ANOVA with Bonferroni posttests. E and F: in brain slices from arthritic rats [d-Cys(tBu)5]NPS (10 μM) alone had no effect on I/O function (E; n = 5; P > 0.05, F1,88 = 2.01, main effect of drug, 2-way ANOVA) or on E-S coupling (F; n = 5; P > 0.05, paired t-test). A, B, and E: insets show individual EPSCs (averages of 8–10) evoked with a stimulus intensity of 0.9 mA. Scale bars, 50 pA, 10 ms. C, D, and F: voltage traces show synaptically evoked spikes. Scale bars, 30 mV, 5 ms.
Synaptic transmission.
With concentric bipolar stimulating electrodes (SNE-100, David Kopf Instruments), EPSCs and/or inhibitory postsynaptic currents (IPSCs) were evoked by focal electrical stimulation (150-μs square-wave pulses) of axons from the BLA and lateral PB and of axons in the EC that included afferents from the infralimbic prefrontal cortex identified by the fluorescent signal originating from anterogradely labeled fibers after stereotaxic injections of a fluorescent tracer [1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), Invitrogen] 10–12 days before brain tissue was taken (see Fig. 1). For stimulation of presumed PB inputs (PB-CeLC synapse), the stimulating electrode was positioned under microscopic control on the fiber tract that runs dorsomedial to the CeA and ventral to but outside of the caudate-putamen (Fu and Neugebauer 2008; Nakao et al. 2012; Neugebauer et al. 2003; Watabe et al. 2013). The trajectory of fibers in this pathway and their terminal arborizations in the CeLC have been described previously in great detail (see Fig. 9 in Sarhan et al. 2005). Although in the vicinity of this tract no afferents to the CeA other than from lateral PB have been described (Harrigan et al. 1994; Schwaber et al. 1988), we cannot exclude the possibility that this fiber tract contains fibers from other sources. Neurons were voltage-clamped at −70 mV (for EPSCs) or 0 mV (for IPSCs). Input-output (I/O) relationships of evoked EPSCs and IPSCs were obtained by increasing the stimulus intensity in 100-μA steps. For evaluation of a drug effect on synaptically evoked responses, the stimulus intensity was set to 50% of the intensity required for maximum responses. Peak amplitudes of individual PSCs were measured and averaged. Synaptically evoked spiking (E-S coupling) was measured in current-clamp mode at the EC-ITC, BLA-CeLC, and PB-CeLC synapses with a stimulus intensity that evoked action potentials (spikes) in ∼50% of the trials. The number of spikes evoked by 10 subsequent stimuli was counted (Kiritoshi et al. 2013; Ren and Neugebauer 2010; Sun and Neugebauer 2011). Evoked synaptic transmission (PSCs and E-S coupling) was measured every 3–5 min before and during drug application. Spontaneous and miniature (in 1 μM TTX) EPSCs (sEPSCs, mEPSCs) and IPSCs (sIPSCs, mIPSCs) were recorded at −70 mV and 0 mV, respectively, as described previously (Kiritoshi et al. 2013; Ren and Neugebauer 2010; Sun and Neugebauer 2011). A fixed length of traces (5 min) was analyzed for frequency and amplitude distributions with Mini Analysis program 5.3 (Synaptosoft). The root mean square (RMS) of background noise was computed for each set of data. Detection threshold was set to 3–4 times the RMS value. Peaks were detected automatically, but each detected event was then visually inspected to prevent the inclusion of false data. Spontaneous and miniature PSCs were measured 5–10 min before and 10–15 min during drug application.
Fig. 9.
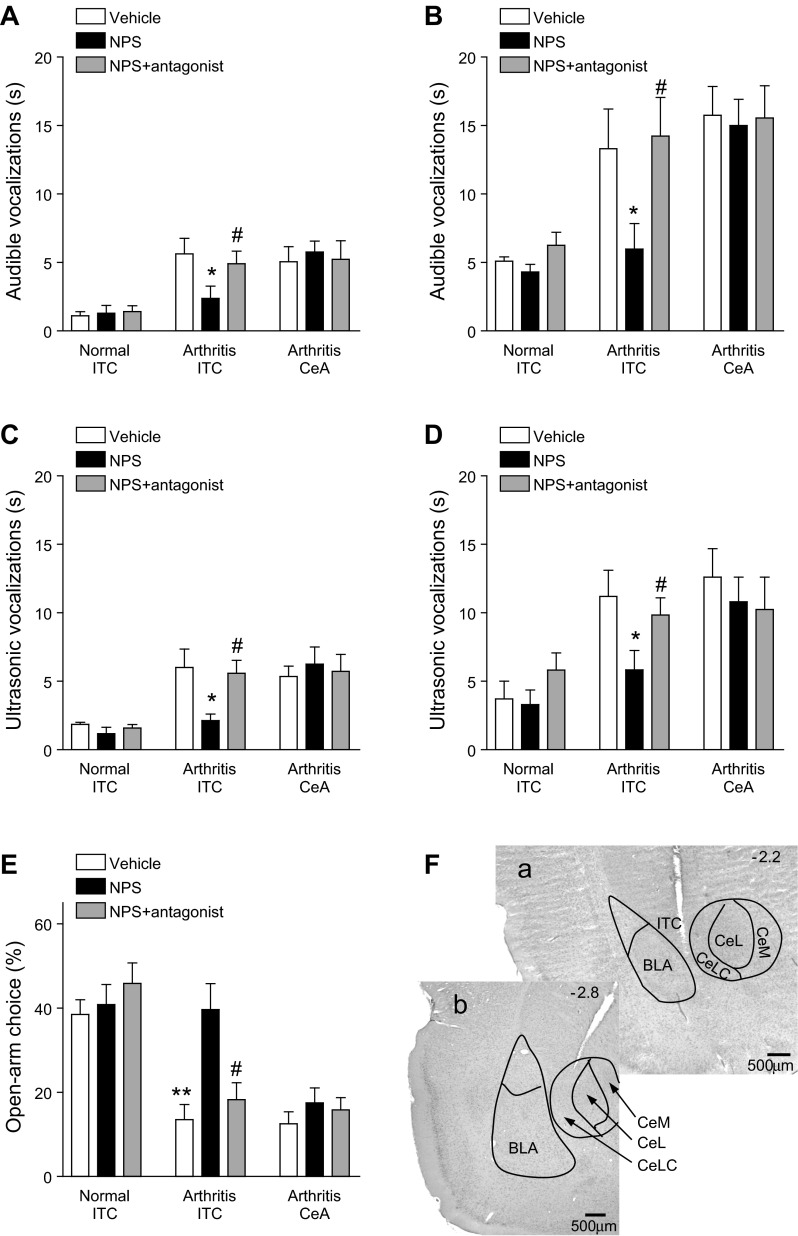
Administration of NPS into ITC, but not CeA, inhibited pain-related behaviors. Vehicle (ACSF), NPS (100 μM, concentration in microdialysis fiber) or NPS together with an NPSR antagonist ([d-Cys(tBu)5]NPS; 1 mM, concentration in microdialysis probe) were administered into ITC of normal or arthritic rats or into the CeA of arthritic rats (n = 5 in each of the 9 experimental groups). A and B: effect of these interventions on audible vocalizations evoked by innocuous (500 g/30 mm2; A) or noxious (2,000 g/30 mm2; B) mechanical compression of the knee (see methods). C and D: effect of interventions on ultrasonic vocalizations evoked by innocuous (C) or noxious (D) mechanical compression of the knee (see methods). E: effect of interventions on open-arm choice in the elevated plus maze test (open-arm entries expressed as % of total number of open-arm and closed-arm entries; see methods). Bar histograms show means ± SE. *P < 0.05, **P < 0.01, ANOVA with Bonferroni posttests (compared with vehicle); #P < 0.05, ANOVA with Bonferroni posttests (compared with NPS). F: histological analysis shows examples of microdialysis probes positioned in the ITC (a) and CeLC (b) in coronal brain slices (2.2 and 2.8 mm caudal to bregma; see methods). CeL and CeM, lateral and medial division of CeA, respectively. The data show that NPS had inhibitory NPS receptor-mediated effects when administered into the ITC of arthritic rats.
Drug application.
Drugs (see Drugs, below) were applied by gravity-driven superfusion of the brain slice in the ACSF (2 ml/min). Solution flow into the recording chamber (1-ml volume) was controlled with a three-way stopcock. Drugs were applied for at least 15 min to establish equilibrium in the tissue. Electrophysiological parameters were measured repeatedly to determine a plateau effect. ACSF served as vehicle control in all experiments.
Behavioral Tests
Audible and ultrasonic vocalizations.
Vocalizations were recorded and analyzed as described in detail previously (Han et al. 2005a; Ji et al. 2010; Li et al. 2011; Palazzo et al. 2008). The experimental setup (U.S. Patent 7,213,538) included a custom-designed recording chamber, a condenser microphone (audible range: 20 Hz–16 kHz) connected to a preamplifier, an ultrasound detector (set to record ultrasonic vocalizations in the 25 ± 4-kHz range), and filter and amplifier (UltraVox 4-channel system; Noldus Information Technology). Data acquisition software (UltraVox 2.0; Noldus Information Technology) monitored the occurrence of vocalizations within user-defined frequencies and recorded the number and duration of digitized events (audible and ultrasonic vocalizations). Animals were placed in the recording chamber for acclimation 1 h before the vocalization measurements. The recording chamber ensured the stable positioning of the animal at a fixed distance from the sound detectors and allowed the reproducible stimulation of the knee joint through openings for the hindlimbs. Brief (15 s) innocuous (500 g/30 mm2) and noxious (2,000 g/30 mm2) mechanical stimuli were applied to the knee with a calibrated forceps equipped with a force transducer. The total duration of vocalizations was recorded for 1 min, starting with the onset of the mechanical stimulus.
Elevated plus maze test.
Anxiety-like behavior was measured in the elevated plus maze as a decrease of open-arm choices (ratio of open-arm entries to total number of entries expressed as percentage) with a computerized recording and analysis system (Multi-Varimex version 1.00; Columbus Instruments) as described previously (Ji et al. 2007). Entries were measured every 5 min for a total observation period of 45 min. Open-arm ratio during the first 5 min period was used for the assessment of anxiety-like behavior (Walf and Frye 2007).
Drug application by microdialysis in awake behaving animals.
With a stereotaxic apparatus (David Kopf Instruments), a guide cannula was implanted stereotaxically the day before behavioral measurements as described in detail previously (Ji et al. 2010; Li et al. 2011). The animal was anesthetized with pentobarbital sodium (Nembutal, 50 mg/kg ip), and a small unilateral craniotomy was performed at the sutura frontoparietalis level. The guide cannula was implanted on the dorsal margin of the ITC or CeA in the right hemisphere, where pain-related changes have been shown (Ji and Neugebauer 2009), with the following coordinates (in mm): ITC: 2.2 caudal to bregma, 4.5 lateral to midline, depth 6.5; CeA: 2.8 caudal to bregma, 4.0 lateral to midline, depth 7.0. The cannula was fixed to the skull with dental acrylic (Plastics One). Antibiotic ointment was applied to the exposed tissue to prevent infection. On the day of the experiment, a microdialysis probe (CMA/11) was inserted through the guide cannula so that the probe protruded by 1 mm. The probe was connected to an infusion pump (Harvard Apparatus) and perfused with ACSF (oxygenated and equilibrated to pH 7.4).
Drugs (see Drugs, below) were dissolved in ACSF on the day of the experiment at a concentration 100-fold that predicted to be needed on the basis of our in vitro patch-clamp data because of the concentration gradient across the dialysis membrane and diffusion in the tissue (Ji et al. 2010; Li et al. 2011). Numbers in the text refer to drug concentrations in the microdialysis fiber. Drugs were applied by microdialysis at a rate of 5 μl/min for at least 20 min to establish equilibrium in the tissue. Before each drug application, ACSF was pumped through the fiber for at least 1 h to establish equilibrium in the tissue.
Histology
The position of the microdialysis probe in the ITC or CeA (placement control) was confirmed histologically. At the end of each behavioral experiment, the animal was euthanized and the brain was removed and submerged in 10% formalin. Tissues were stored in 20% sucrose before they were frozen-sectioned at 50 μm. Sections were mounted on gel-coated slides, stained with hematoxylin and eosin (H & E), and coverslipped. Positions of the microdialysis fibers were identified under the microscope and plotted on standard diagrams adapted from Paxinos and Watson (1998).
Drugs
The following drugs were used: 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX, non-NMDA receptor antagonist; Tocris Bioscience), NPS (Sigma-Aldrich), [d-Cys(tBu)5]NPS (selective NPSR antagonist, synthesized as described previously; Guerrini et al. 2009), (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester (KT5720, PKA inhibitor; Tocris Bioscience), and 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X, PKC inhibitor; Tocris Bioscience). Selectivity and target concentrations were established in our previous studies (Fu et al. 2008; Han et al. 2010; Li et al. 2011) and the literature (Camarda et al. 2009; Guerrini et al. 2009, 2010; Jungling et al. 2008; Ruzza et al. 2012). ACSF served as vehicle control in all experiments.
Statistical Analysis
All averaged values are given as means ± SE. Statistical significance was accepted at the level of P < 0.05. GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA) was used for all statistical analyses. For multiple comparisons, one-way ANOVA or two-way ANOVA was used with appropriate post hoc tests as indicated in the text and figure legends. Student's t-test (paired or unpaired when appropriate) was used to compare two sets of data. Kolmogorov-Smirnov test was used for cumulative distribution analysis of spontaneous and miniature synaptic events (Mini Analysis program 5.3; Synaptosoft).
RESULTS
Whole cell patch-clamp recordings were made from visually identified neurons in the CeLC (for details see Fu and Neugebauer 2008; Neugebauer et al. 2003) and in the dorsomedial cluster of ITC cells near the dorsolateral edge of the CeLC; these dorsomedial ITC cells project strongly to the lateral division of the central nucleus (for details see Amir et al. 2011) (Fig. 1A). Compared with CeLC neurons (n = 62), ITC cells (n = 50) had a more negative resting membrane potential (ITC, −78.0 ± 1.3 mV; CeLC, −60.4 ± 1.2 mV) and higher input resistance (ITC, 387 ± 22 MΩ; CeLC, 229 ± 15 MΩ); both types were “regular spiking,” but ITC cells showed a faster firing rate in response to depolarizing current pulses (ITC, 20.2 ± 2.2 spikes; CeLC, 9.2 ± 1.9 spikes; 300 pA, 500 ms). These characteristics are consistent with previous studies on rat CeLC neurons from our group (Fu and Neugebauer 2008; Neugebauer et al. 2003) and from others (Ikeda et al. 2007; Watabe et al. 2013) and with published data on rat ITC cells (Amir et al. 2011; Busti et al. 2011).
CeLC neurons recorded in this study are type A projection neurons (Neugebauer et al. 2004; Sah et al. 2003; Schiess et al. 1999) showing characteristics of regular-spiking PKCδ-negative “on” cells (Haubensak et al. 2010; Watabe et al. 2013). ITC cells, including those projecting to CeLC, have a very negative membrane potential, high input resistance, and limited spike frequency adaptation during prolonged depolarizing current pulses (Amir et al. 2011; Busti et al. 2011). A few cells (n = 5) were recorded in the BLA as controls. These neurons were pyramid shaped and had a low input resistance (64.2 ± 3.2 MΩ) and a resting membrane potential of −68.1 ± 2.5 mV, which is in agreement with published data (Rainnie 1999; Rainnie et al. 1993). The boundaries of the different amygdala nuclei are easily discerned under light microscopy (Fu and Neugebauer 2008; Sah et al. 2003; Watabe et al. 2013).
Monosynaptic EPSCs were evoked in CeLC neurons by stimulating afferent input from PB (PB-CeLC synapse; Fig. 1, A and B) and from BLA (BLA-CeLC synapse; Fig. 1, A and C). EPSCs showed constant latency calculated from stimulus artifact to onset of synaptic current; they were mediated by non-NMDA receptors (blocked by 10 μM NBQX). Stimulation of the EC, including anterogradely labeled fibers from the infralimbic mPFC (see methods), evoked non-NMDA receptor-mediated monosynaptic EPSCs in ITC neurons (EC-ITC synapse; Fig. 1, A and D) and polysynaptic IPSCs in CeLC neurons (EC-CeLC synapse; Fig. 1, A and E). Monosynaptic EPSCs followed high-frequency (20 Hz) synaptic stimulation reliably and had a fixed latency. In contrast, polysynaptic IPSCs did not follow high-frequency stimulation and their latencies showed larger variability; they were glutamate driven (inhibited by 10 μM NBQX), which is consistent with feedforward inhibition of CeLC neurons. Latencies and jitter values (SD of intraneuronal latency) at the different synapses were as follows: BLA-CeLC EPSCs, 6.1 ± 0.27 ms and 186 ± 27 μs, respectively (n = 15); PB-CeLC EPSCs, 9.1 ± 0.34 ms and 199 ± 21 μs (n = 10); EC-ITC EPSCs, 3.7 ± 18 ms and 150 ± 18 μs (n = 12); EC-CeLC IPSCs, 11.3 ± 47 ms and 1,208 ± 193 μs (n = 11). EPSCs and IPSCs were recorded at −70 mV and 0 mV, respectively (see methods).
In this study we tested the hypothesis that NPS activates ITC cells to inhibit CeLC neurons in a model of arthritis pain (Neugebauer et al. 2007), thus decreasing amygdala output and inhibiting pain-related behaviors. The first set of data shows the effect of NPS on synaptic transmission onto CeLC neurons (Figs. 2–4). Next, the site of action of NPS on ITC cells is described (Figs. 5–7). Analysis of the effect of EC-ITC stimulation on CeLC output addresses the functional significance of this circuitry (Fig. 8). Finally, behavioral consequences of NPS actions in the amygdala are shown in the awake animal (Fig. 9).
Fig. 2.
NPS inhibited the pain-related increase of excitatory synaptic responses in CeLC neurons: whole cell patch-clamp recordings of CeLC neurons. Diagram shows stimulation and recording sites. A: in brain slices from normal rats NPS (1 μM and 10 μM) had no effect on evoked EPSCs (1 μM NPS: n = 5 neurons, F1,88 = 0.28, P > 0.05; 10 μM NPS: n = 5 neurons, F1,88 = 1.17, P > 0.05, main effect of drug, 2-way ANOVA). B: in slices from arthritic rats NPS (1 μM) inhibited EPSCs significantly (n = 5; P < 0.0001, F1,88 = 35.14, main effect of drug, 2-way ANOVA). *P < 0.05, Bonferroni posttests. A and B: input-output (I/O) relationships (means ± SE) were obtained by measuring peak amplitudes as a function of afferent fiber stimulus intensity. Insets: individual EPSCs (averages of 8–10) evoked with a stimulus intensity of 0.9 mA. Scale bars, 50 pA, 10 ms. C: NPS (1 μM) had no effect on synaptically evoked spiking (E-S coupling) in CeLC neurons under normal conditions. D: in slices from arthritic rats NPS (1 μM) significantly decreased the number of synaptically evoked action potentials (spikes). Bar histograms show means ± SE. Stimulus intensity was adjusted to evoke spikes in ∼50% of the trials before drug application. *P < 0.05, paired t-test. Voltage traces show spikes evoked in individual CeLC neurons before (Predrug) and during NPS. Scale bars, 30 mV, 5 ms.
Fig. 4.
NPS increased feedforward inhibition of CeLC neurons in the pain model: whole cell patch-clamp recordings of CeLC neurons. Diagram shows stimulation and recording sites. A: in brain slices from normal rats NPS (1 μM) had no effect on IPSCs evoked by EC stimulation (n = 5 neurons; P > 0.05, F1,88 = 0.01, main effect of drug, 2-way ANOVA). NBQX (10 μM) inhibited IPSCs, suggesting that they were glutamate driven. B: in slices from arthritic rats NPS (1 μM) increased IPSCs significantly (n = 5; P < 0.0001, F1,88 = 24.76, main effect of drug, 2-way ANOVA). *P < 0.05, **P < 0.01, Bonferroni posttests. In the presence of NBQX (10 μM), NPS had no significant effect (n = 6 neurons; P > 0.05, F1,110 = 2.71, main effect of drug, 2-way ANOVA). I/O relationships (means ± SE) were obtained by averaging peak amplitudes as a function of afferent fiber stimulus intensity. Insets: individual IPSCs (averages of 8–10) evoked with a stimulus intensity of 0.9 mA. Scale bars, 100 pA, 10 ms.
Fig. 7.
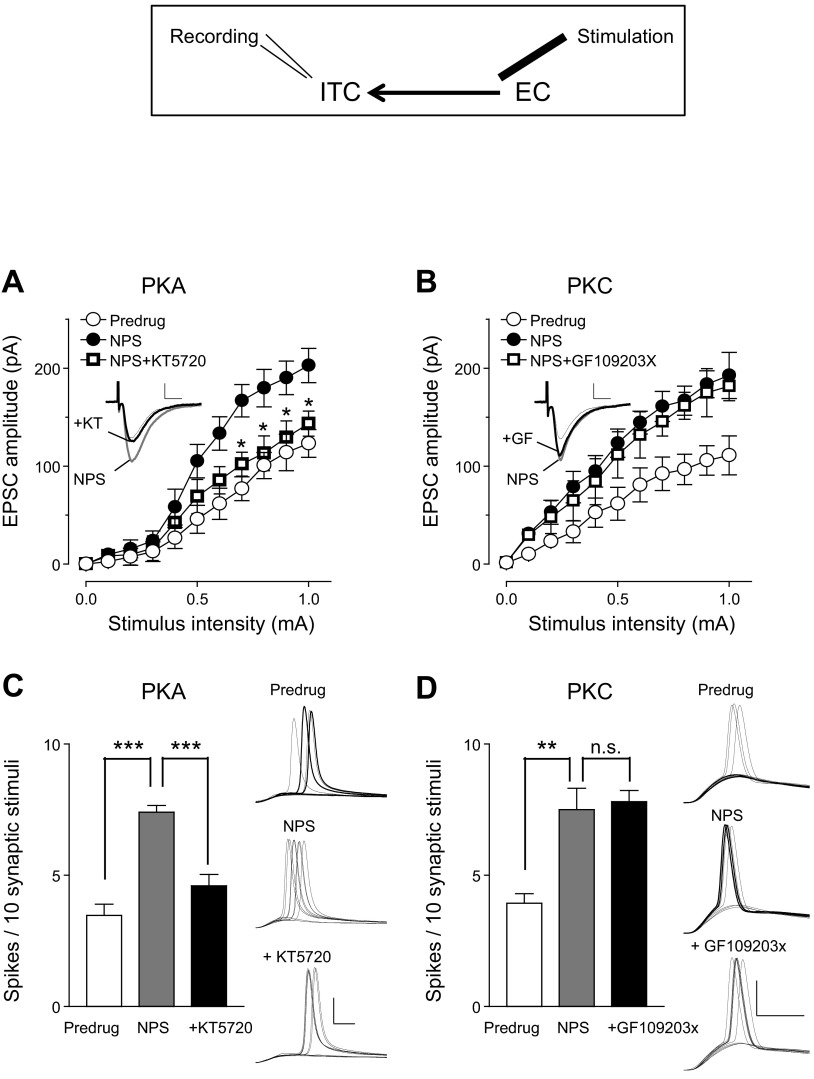
Inhibition of PKA, but not PKC, blocked the facilitatory effect of NPS on ITC cells in the pain model: whole cell patch-clamp recordings of ITC cells. Diagram shows stimulation and recording sites. A: I/O function of excitatory synaptic transmission (EPSCs) at the EC-ITC synapse before (Predrug) and during NPS (1 μM) and during coapplication of NPS and a PKA inhibitor [KT5720 (KT), 1 μM]. KT5720 reversed the effect of NPS (n = 5 neurons; P < 0.0001, F1,88 = 31.29, main effect of drug, 2-way ANOVA). *P < 0.01, ANOVA with Bonferroni posttests. B: a PKC inhibitor [GF109203X (GF), 1 μM] had no significant effect on the facilitation of EPSCs by NPS, i.e., I/O functions were not significantly different (n = 5 neurons; P > 0.05, F1,88 = 1.57, main effect of drug, 2-way ANOVA). A and B: Insets show individual EPSCs (averages of 8–10) evoked with a stimulus intensity of 0.9 mA. Scale bars, 50 pA, 10 ms. C: KT5720 inhibited the facilitatory effect of NPS on synaptically evoked spiking in ITC neurons (n = 5). D: GF109203X had no significant effect (n = 5 neurons). C and D: bar histograms show means ± SE. Stimulus intensity was adjusted to evoke spikes in ∼50% of the trials before drug application. **P < 0.01, ***P < 0.001, ANOVA with Bonferroni posttests; n.s., not significant. Voltage traces show spikes evoked in individual ITC cells before (Predrug) and during NPS and during coapplication of NPS and kinase inhibitors. Scale bars, 30 mV, 5 ms.
Fig. 8.
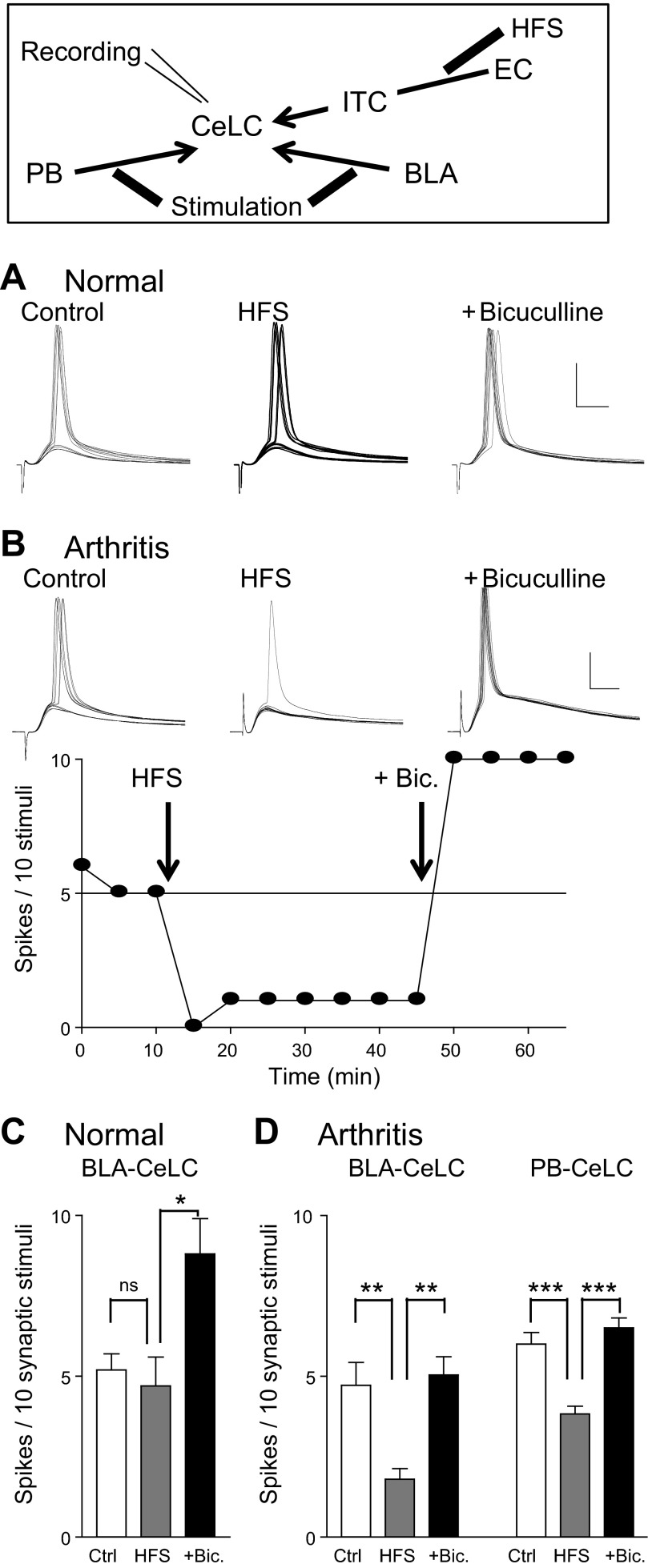
High-frequency synaptic stimulation (HFS) of EC input to ITC cells inhibited output of CeLC neurons in the pain model but not under normal conditions. Diagram shows stimulation and recording sites. Current-clamp recordings of CeLC neurons were made before [control (Ctrl)] and after HFS of EC fibers and in the presence of bicuculline (Bic., 10 μM) added to the ACSF. A: voltage traces show synaptically evoked spikes (E-S coupling; stimulation in BLA) of an individual CeLC neuron in a slice from a normal animal. B: synaptically evoked spikes (stimulation in BLA) of an individual CeLC neuron in a slice from an arthritic animal. Graph shows time course data. Each symbol represents the result (number of evoked spikes) of 10 stimuli. Test stimuli were applied every 5 min. A and B: scale bars, 30 mV, 10 ms. C: HFS at the EC-ITC synapse had no significant effect (ns) on E-S coupling at the BLA-CeLC synapse under normal conditions (n = 5 neurons). Bicuculline enhanced evoked spiking in these neurons significantly. D: HFS at the EC-ITC synapse inhibited E-S coupling significantly at the BLA-CeLC synapse (n = 5 neurons) and the PB-CeLC synapse (n = 6 neurons) in slices from arthritic rats. HFS-induced inhibition was reversed by addition of bicuculline (10 μM) in these neurons. *P < 0.05, **P < 0.01, ***P < 0.001, ANOVA with Bonferroni posttests.
NPS Inhibits Excitatory Drive and Output of CeLC Neurons in Arthritis Pain Model Through Presynaptic Network Action
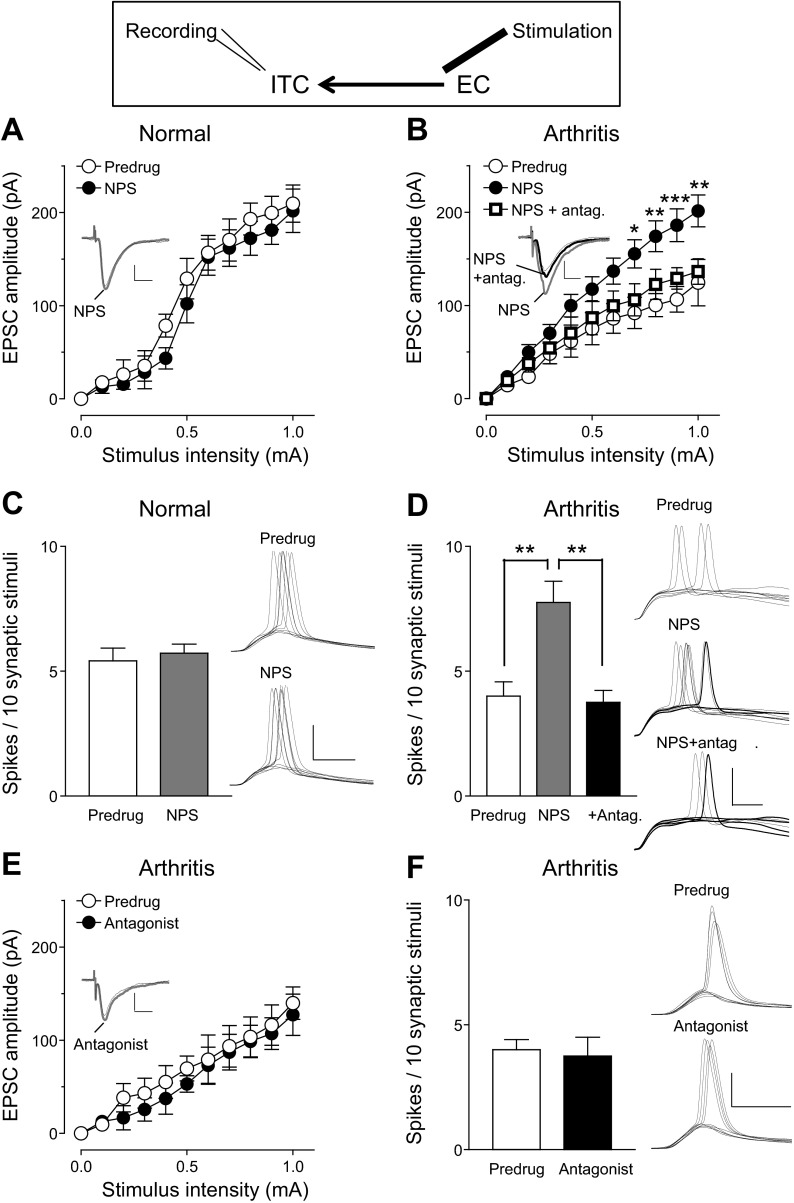
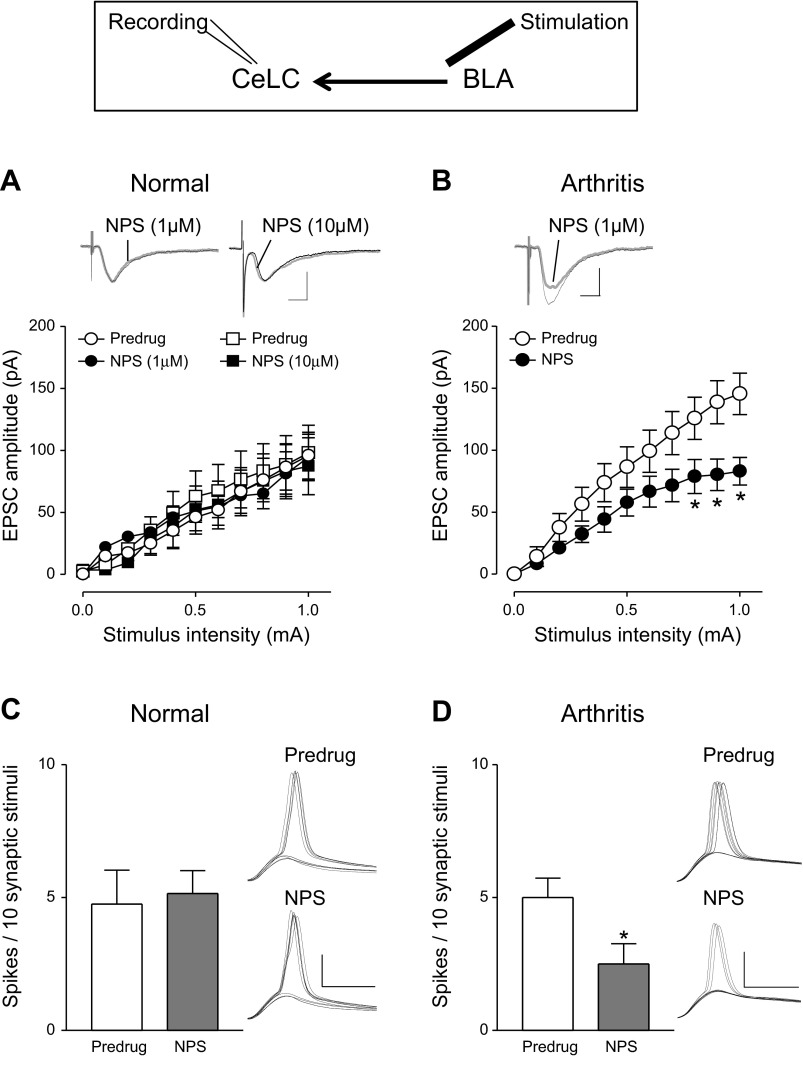
Pain-related synaptic plasticity of excitatory inputs to the CeLC is significantly associated with pain behaviors (Neugebauer et al. 2004, 2009). Therefore we tested the effect of NPS on excitatory transmission (evoked EPSCs) and output of CeLC neurons that is determined by the integration of synaptic inputs (excitatory postsynaptic potential-spike coupling, E-S coupling; Fig. 2). In agreement with our previous studies (Fu and Neugebauer 2008; Neugebauer et al. 2003; Ren et al. 2011; Ren and Neugebauer 2010) the I/O function of CeLC neurons for monosynaptic EPSCs evoked at the BLA-CeLC synapse was increased in slices from rats with arthritis (5–6 h after induction) compared with normal controls [Predrug in Fig. 2A (n = 10 neurons) compared with Predrug in Fig. 2B (n = 5); P < 0.0001, F1,143 = 17.55, main effect of intervention, 2-way ANOVA].
NPS (1 μM) inhibited monosynaptic EPSCs evoked at the BLA-CeLC synapse in slices from arthritic rats (Fig. 2B; n = 5 neurons, P < 0.0001, F1,88 = 35.14, main effect of drug, 2-way ANOVA). The inhibitory effect of NPS was observed after ∼3 min of drug application, reached stable levels by 10 min, and was largely reversible upon washout for 5–10 min. NPS had no significant effect on synaptic transmission under normal conditions, even at a higher concentration (Fig. 2A; 1 μM, n = 5, P > 0.05, F1,88 = 0.28; 10 μM, n = 5, P > 0.05, F1,88 = 1.17; main effect of drug, 2-way ANOVA). NPS (1 μM) also inhibited the output of CeLC neurons in slices from arthritic rats, which is evident from the decreased number of synaptically evoked action potentials (E-S coupling; Fig. 2D; n = 5, P < 0.05, paired t-test). NPS had no significant effect on E-S coupling under normal conditions (Fig. 2C; n = 5). E-S coupling was measured as the average number of evoked spikes per synaptic stimulation. Resting membrane potential was kept at −60 mV by injecting appropriate current in current-clamp mode. Stimulus intensity was adjusted to evoke spikes in 50% of the trials before drug application. Spike thresholds were similar in slices from normal and arthritic rats (0.9 ± 0.1 mA), but higher stimulus intensities evoked more spikes in the arthritis model than under normal conditions. We used spike threshold stimulation for the analysis of drug effects to provide a similar baseline in both conditions.
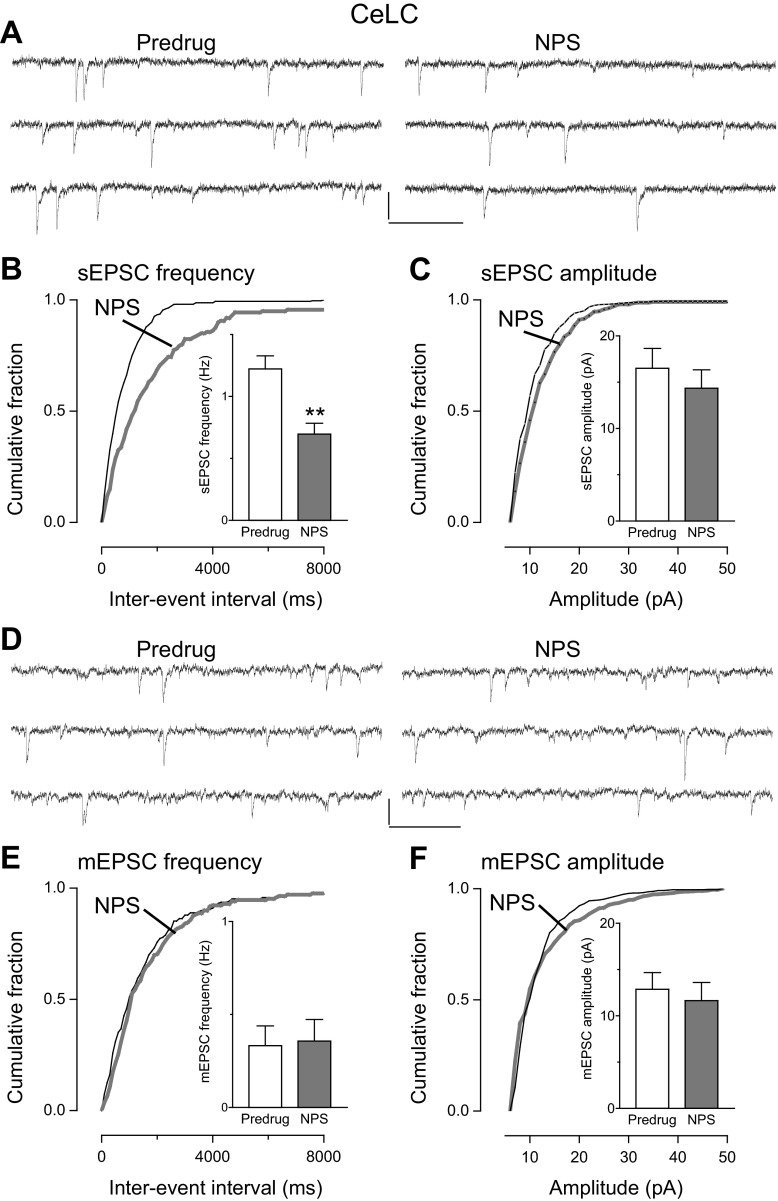
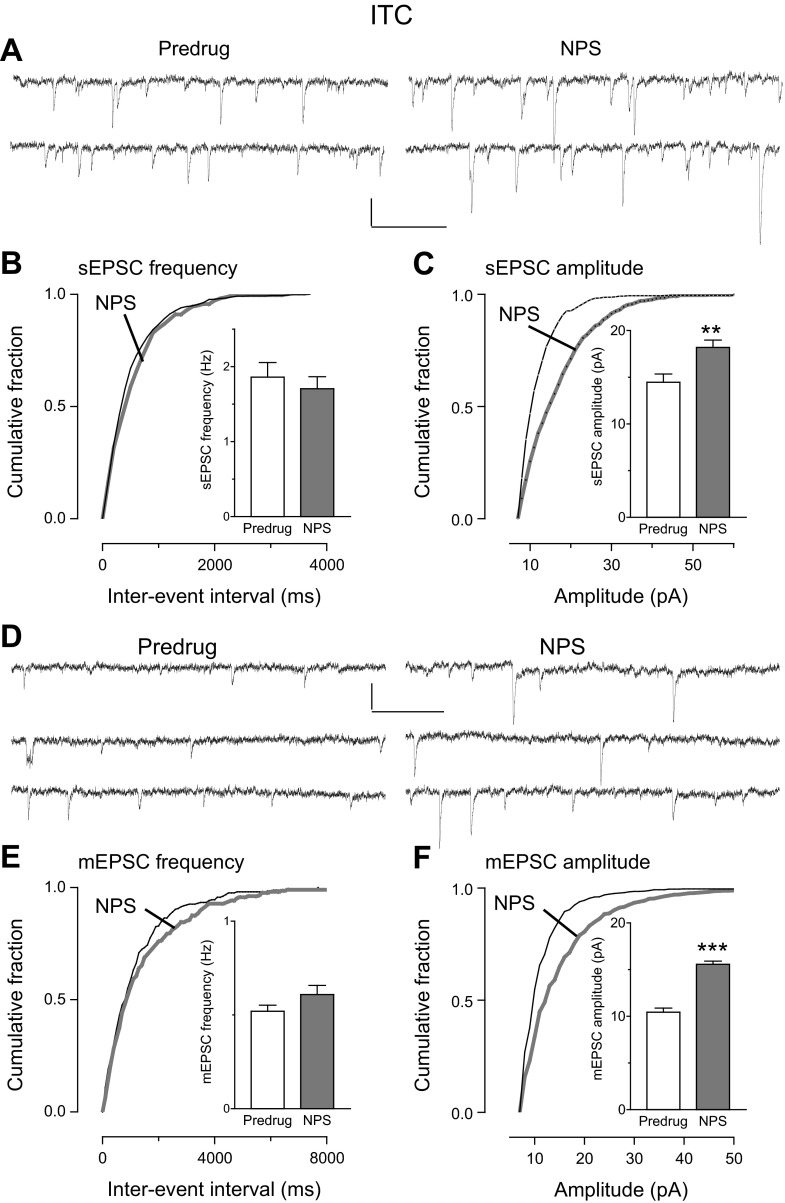
The inhibitory effect of NPS was due to a presynaptic, action potential-dependent mechanism consistent with a network action. This finding is based on the analysis of sEPSCs and mEPSCs, which is a well-established electrophysiological approach to determine pre- or postsynaptic mechanisms and has been used successfully in our previous studies (for recent references see Kiritoshi et al. 2013; Ren and Neugebauer 2010; Sun and Neugebauer 2011). In slices from arthritic rats, NPS (1 μM) decreased frequency (Fig. 3B), but not amplitude (Fig. 3C), of sEPSCs significantly (cumulative frequency distribution, P < 0.05, Kolmogorov-Smirnov test, individual neuron; mean frequency in the sample of neurons, n = 5, P < 0.01, paired t-test). NPS had no significant effect on frequency (Fig. 3E) and amplitude (Fig. 3F) of mEPSCs recorded in TTX (1 μM) (cumulative distribution, P > 0.05, Kolmogorov-Smirnov test, individual neuron; mean frequency and amplitude in the sample of neurons, n = 5, P > 0.05, paired t-test). These experiments were done only in slices from arthritic rats because NPS had no effect under normal conditions (see Fig. 2).
Fig. 3.
NPS inhibited frequency but not amplitude of spontaneous (s)EPSCs (A–C) but not miniature (m)EPSCs (D–F) in CeLC neurons in the pain model: whole cell voltage-clamp recordings of CeLC neurons in brain slices from arthritic rats. A–C: cumulative distribution analysis of sEPSC frequency (B) and amplitude (C) measured over periods of 5 min. NPS (1 μM) caused a significant shift toward larger interevent intervals (P < 0.05, Kolmogorov-Smirnov test, individual example), decreasing mean sEPSC frequency in the sample of neurons significantly (n = 5 neurons). NPS had no effect on sEPSC amplitude. A: sEPSCs in an individual CeLC neuron before (Predrug, left) and during (right) NPS. D–F: NPS had no significant effect on frequency (E) and amplitude (F) of mEPSCs measured over periods of 5 min (P > 0.05, Kolmogorov-Smirnov test for cumulative distribution in an individual neuron; P > 0.05, paired t-test for mean frequency and amplitude in the sample of neurons, n = 5). D: current traces of mEPSCs in an individual CeLC neuron before (Predrug) and during NPS. Bar histograms show means ± SE. Scale bars, 15 pA, 200 ms. **P < 0.01, paired t-test.
NPS Increases ITC-Mediated Feedforward Inhibition of CeLC Neurons in Arthritis Pain Model
Next we tested the hypothesis that the network action of NPS involved ITC cells to increase inhibition of CeLC neurons. Output from the central nucleus can be inhibited by prefrontal cortex stimulation through a mechanism that is generally believed to involve ITC cells (Amir et al. 2011; Pare et al. 2004; Quirk et al. 2003). Electrical stimulation of the EC, which contains glutamatergic projections from prefrontal cortex to the amygdala, including ITC (McDonald 1998; Pinard et al. 2012), evoked polysynaptic inhibitory synaptic currents (IPSCs) in CeLC neurons (see Fig. 1, A and E). Inhibitory transmission was decreased in slices from arthritic rats (n = 5 neurons) compared with controls (n = 5 neurons; Predrug in Fig. 4, A and B; P < 0.0001, F1,88 = 73.07, main effect of intervention, 2-way ANOVA). NPS (1 μM) increased evoked IPSCs in brain slices from arthritic rats significantly (n = 5; P < 0.0001, F1,88 = 24.76, main effect of drug, 2-way ANOVA; Fig. 4B) but had no effect under normal conditions (n = 5 neurons; P > 0.05, F1,88 = 0.01, main effect of drug, 2-way ANOVA; Fig. 4A). NBQX (10 μM) blocked EC-evoked IPSCs, indicating that they were glutamate driven, and prevented the effect of NPS (n = 6 neurons; Fig. 4B), confirming that the facilitation of IPSCs by NPS was due to an action on feedforward inhibition. The facilitatory effect of NPS was observed after ∼3 min of drug application, reached stable levels by 10 min, and was largely reversible upon washout for 5–10 min.
Recordings from ITC cells showed that excitatory synaptic transmission (EPSCs) at the EC-ITC synapse was significantly decreased in slices from arthritic rats compared with normal controls (Predrug in Fig. 5, A and B; n = 5 neurons each, P < 0.0001, F1,88 = 44.22, main effect of intervention, 2-way ANOVA). NPS (1 μM) increased I/O function of EPSCs at the EC-ITC synapse in brain slices from arthritic rats significantly (n = 5 neurons; P < 0.0001, F1,88 = 57.05, main effect of drug, 2-way ANOVA; Fig. 5B) but had no effect under normal conditions (n = 5; P > 0.05, F1,88 = 3.55, main effect of drug, 2-way ANOVA; Fig. 5A). NPS also increased synaptically evoked spiking (E-S coupling) in ITC cells in the arthritis model (n = 5 neurons; P < 0.01, Bonferroni posttest; Fig. 5D) but not under normal conditions (n = 5; P > 0.05, paired t-test; Fig. 5C). NPS had some effect on the intrinsic membrane properties of ITC cells, causing a small, but significant, depolarization (7.6 ± 0.6 mV; n = 13; P < 0.05, paired t-test) and concomitant increase in membrane input resistance (16.1 ± 3.5 MΩ, n = 10; P < 0.05, paired t-test). NPS also increased depolarization-induced spiking (from 8.0 ± 1.1 Hz to 12 ± 0.8 Hz, depolarizing current of 300 pA, 500 ms; P < 0.05, paired t-test). The effects of NPS were observed after ∼3 min of drug application and reached stable levels by 10 min.
The effects of NPS on excitatory synaptic transmission (Fig. 5B) and E-S coupling (Fig. 5D) were reversed by a selective NPS receptor antagonist ([d-Cys(tBu)5]NPS, 10 μM; n = 5 neurons each). The antagonist alone had no significant effect on EPSCs at the EC-ITC synapse (n = 5 neurons; P > 0.05, F1,88 = 2.01, main effect of drug, 2-way ANOVA; Fig. 5E) or on spiking evoked in ITC cells by EC stimulation (n = 5; P > 0.05, paired t-test; Fig. 5F). The data suggest that NPS can increase the synaptic drive and output of ITC cells, resulting in enhanced inhibition of CeLC. However, NPS receptors in the EC-ITC-CeLC circuitry are not activated endogenously in this model.
NPS Activates ITC Cells Through Postsynaptic Mechanism That Depends on PKA but Not PKC
To determine the synaptic site of action of NPS in the arthritis pain model, we measured sEPSCs and mEPSCs in ITC neurons. These experiments were done only in slices from arthritic rats because NPS had no effect under normal conditions (see Figs. 2, 4, and 5). NPS (1 μM) increased the amplitude, but not frequency, of sEPSCs (Fig. 6, A–C; n = 7 neurons) and mEPSCs (Fig. 6, D–F; n = 5 neurons). The facilitatory effect of NPS was evident from the cumulative distribution of sEPSC and mEPSC amplitudes (P < 0.05, Kolmogorov-Smirnov test, individual neurons shown in Fig. 6, A and D) and mean sEPSC and mEPSC amplitudes (P < 0.01 and P < 0.001, respectively, paired t-test).
Fig. 6.
NPS increased amplitude but not frequency of sEPSCs (A–C) and mEPSCs (D–F) in ITC cells in the pain model: whole cell voltage-clamp recordings of ITC cells. A–C: cumulative distribution analysis of sEPSC frequency (B) and amplitude (C) measured over periods of 5 min. NPS (1 μM) had no significant effect on cumulative interevent interval distribution and mean frequency of sEPSCs (n = 7 neurons) but caused a significant shift toward larger sEPSC amplitudes (cumulative distribution, P < 0.05, Kolmogorov-Smirnov test, individual neuron) and significant increase of mean amplitude in the sample of neurons (n = 7, P < 0.01, paired t-test). A: current traces show sEPSCs in an individual ITC cell before (Predrug, left) and during (right) NPS. D–F: NPS (1 μM) had no significant effect on cumulative interevent interval distribution and mean frequency of mEPSCs (n = 5 neurons) but caused a significant shift toward larger mEPSC amplitudes (cumulative distribution, P < 0.05, Kolmogorov-Smirnov test, individual neuron) and increased mean amplitude in the sample of neurons (n = 5, P < 0.001, paired t-test). D: mEPSCs in an individual ITC cell before (left) and during (right) NPS. Bar histograms show means ± SE. **P < 0.01, ***P < 0.001. Scale bars for current traces, 15 pA, 200 ms. posttests.
Importantly, NPS (1 μM) had no significant effect on mEPSCs recorded in pyramidal-shaped BLA neurons (n = 5). Mean frequency was 0.31 ± 0.07 Hz and 0.33 ± 0.07 Hz before and during NPS, respectively. Mean amplitude was 12.2 ± 2.1 pA and 11.4 ± 2.4 pA, respectively. The data suggest that NPS increased the excitatory drive of ITC cells through a postsynaptic action on ITC cells but not on BLA projection neurons.
Since NPS receptors can couple to Gq or Gs (Guerrini et al. 2010; Reinscheid 2008), we examined whether the facilitatory effect of NPS on ITC cells involved PKA and/or PKC. A PKA inhibitor (KT5720, 1 μM) reversed the facilitatory effect of NPS (1 μM) on EPSCs evoked at the EC-ITC synapse (Fig. 7A; n = 5 neurons, F1,88 = 31.29, P < 0.001, main effect of drug, 2-way ANOVA) and on synaptically evoked spiking of ITC cells (Fig. 7C; n = 5 neurons, P < 0.001, ANOVA with Bonferroni posttests). In contrast, a PKC inhibitor (GF109203X, 1 μM) had no significant effect on NPS-induced facilitation of evoked EPSCs (Fig. 7B; n = 5 neurons, F1,88 = 1.57, P > 0.05, main effect of drug, 2-way ANOVA) or on E-S coupling (Fig. 7D; n = 5 neurons, P > 0.05, ANOVA with Bonferroni posttest). These experiments were conducted only in slices from arthritic rats because NPS had no effect under normal conditions. The data suggest an important contribution of PKA, but not PKC, to the postsynaptic facilitatory effect of NPS on ITC cells.
High-Frequency Stimulation of EC Input to ITC Neurons Inhibits Synaptically Driven CeLC Output in Pain State but Not Under Normal Conditions
The results so far show that synaptic activation of ITC neurons is decreased in the arthritis pain model but can be rescued with NPS. NPS also increased feedforward inhibition of CeLC neurons. To link synaptic activation of ITC cells to the control of CeLC output, we measured the effect of high-frequency stimulation (HFS; 4 trains, 5 pulses/train, 100 Hz, 20-s interval; see Li et al. 2011) of the EC-ITC pathway on synaptically evoked spiking (E-S coupling) in CeLC neurons (Fig. 8). E-S coupling was measured by stimulating synapses that provide direct (PB-CeLC) or indirect (BLA-CeLC) nociceptive input to the CeLC and undergo pain-related plasticity (Ikeda et al. 2007; Nakao et al. 2012; Neugebauer et al. 2004, 2009). In all CeLC neurons a monosynaptic EPSC could be evoked by stimulation of inputs from PB or BLA, whereas EC stimulation evoked a polysynaptic IPSC. After voltage-clamp recordings verified convergent synaptic inputs, test stimulation of the PB-CeLC or BLA-CeLC synapse was repeated every 5 min before and after HFS of the EC-ITC synapse in current clamp. HFS inhibited spiking evoked by stimulation of inputs from the BLA-CeLC synapse (n = 5 neurons) and the PB-CeLC synapse (n = 6 neurons) in slices from arthritic rats (P < 0.01 and 0.001, ANOVA with Bonferroni posttests; Fig. 8, B and D). HFS-driven inhibition of CeLC output (E-S coupling) involved a GABAergic mechanism because it was reversed by bicuculline (10 μM), which is consistent with the role of ITC-mediated feedforward inhibition. In some experiments we recorded the effect of HFS on ITC cells (n = 3) and found increased E-S coupling in these cells (from 5 to 10 spikes/10 stimuli) and increased depolarization-induced spiking (from 15 ± 0.6 Hz to 22 ± 1.2 Hz, depolarizing current of 300 pA, 500 ms; P < 0.05, paired t-test), which persisted for >30 min. Under normal conditions (Fig. 8, A and C), HFS had no significant effect on E-S coupling in CeLC neurons (n = 5, P > 0.05), suggesting a pain-related change in the effectiveness of EC-ITC-driven control of CeLC processing similar to the change in NPS function.
Administration of NPS into ITC, but Not CeA, Inhibits Pain-Related Behaviors
Next we determined the behavioral significance of the electrophysiological effects of NPS in the amygdala (Fig. 9). Audible and ultrasonic vocalizations were evoked by brief (15 s) innocuous (Fig. 9, A and C) and noxious (Fig. 9, B and D) stimulation of the knee with a calibrated forceps (see methods). In agreement with our previous studies (Fu and Neugebauer 2008; Han et al. 2005a; Han and Neugebauer 2005; Ji et al. 2010; Neugebauer et al. 2007; Palazzo et al. 2008), the duration of vocalizations increased in the arthritis pain state (5–6 h after induction). Administration of NPS (100 μM, concentration in microdialysis fiber) into the ITC inhibited audible (Fig. 9, A and B) and ultrasonic (Fig. 9, C and D; see methods) vocalizations of arthritic rats (5–6 h after induction) significantly (n = 5 in each group; P < 0.05, ANOVA with Bonferroni posttests). The NPSR antagonist [d-Cys(tBu)5]NPS (1 mM, concentration in microdialysis probe) blocked the inhibitory effects of NPS. NPS administered into ITC alone or together with [d-Cys(tBu)5]NPS had no significant effect on vocalizations of normal rats (no arthritis; n = 5 in each group; P > 0.05, ANOVA with Bonferroni posttests; Fig. 9, A–D). Administration of NPS into CeA alone or together with [d-Cys(tBu)5]NPS had no significant effect on vocalizations of arthritic rats (n = 5 in each group; P > 0.05, ANOVA with Bonferroni posttests; Fig. 9, A–D), which is consistent with the absence of NPSR mRNA (Xu et al. 2007) and protein (Leonard and Ring 2011) in the CeA.
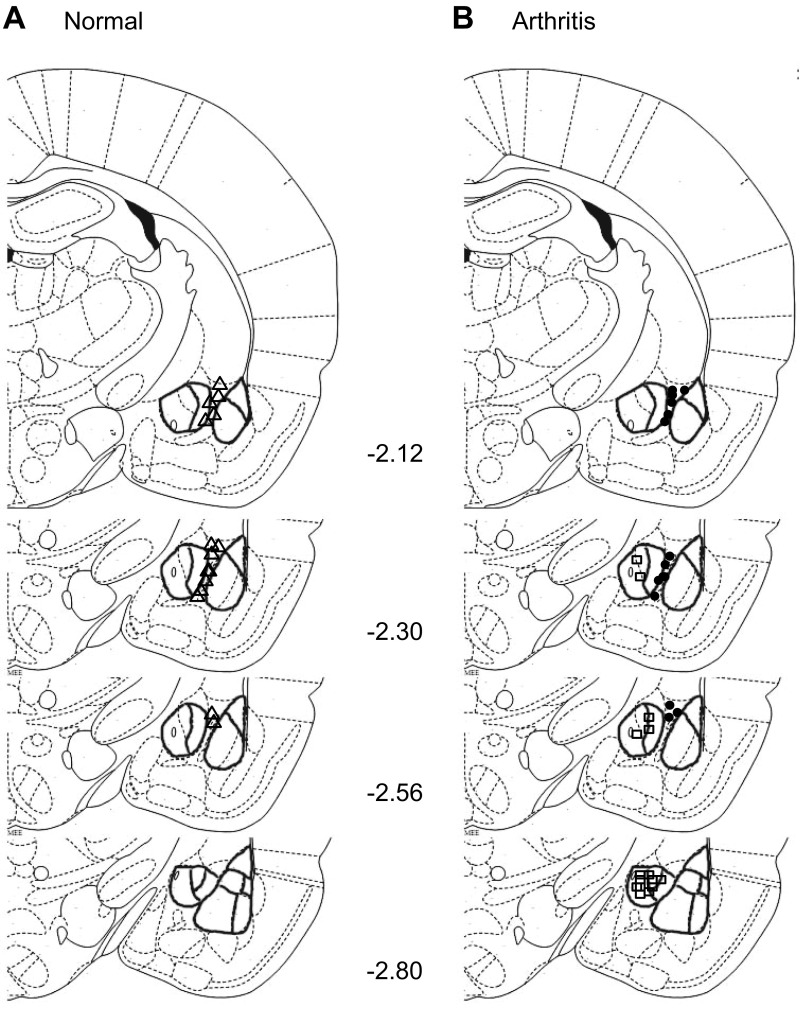
Arthritic rats showed increased anxiety-like behavior (Fig. 9E) measured as decreased open-arm preference over a period of 5 min in the elevated plus maze test (see methods), confirming results of our previous studies (Ji et al. 2007, 2010; Neugebauer et al. 2007; Palazzo et al. 2008). Administration of NPS into ITC inhibited anxiety-like behavior of arthritic rats (n = 5 in each group; P < 0.05, ANOVA with Bonferroni posttests). The anxiolytic effect of NPS was blocked by coapplication of [d-Cys(tBu)5]NPS. NPS administered into ITC had no significant in normal rats. NPS also had no effect when administered into the CeA of arthritic rats. The total number of arm entries over 45 min was significantly lower in arthritic rats (53 ± 5.1, n = 5) compared with normal rats (164 ± 15.9, n = 5; P < 0.001, ANOVA with Bonferroni posttests), but NPS did not significantly affect the activity level (60.4 ± 5.3, n = 5; P > 0.05, ANOVA with Bonferroni posttests). Figure 10 shows the position of the tips of the microdialysis fibers in ITC or CeA based on histological analysis as illustrated in Fig. 9F. The results suggest that NPS acts in the ITC, but not the CeA, to inhibit emotional-affective and anxiety-like behaviors in the arthritis pain model.
Fig. 10.
Position of microdialysis probes for drug application. A: microdialysis fiber in ITC (△; n = 15) of normal animals. B: microdialysis fiber in ITC (●; n = 15) or CeA (□; n = 15) of arthritic animals. Standard diagrams [adapted from Paxinos and Watson (1998) with permission] show coronal brain slices. Numbers indicate distance from bregma.
DISCUSSION
This study used an integrative approach of electrophysiology (patch clamp in brain slices) and behavioral assays to determine the role of NPS in pain-related amygdala processing and behaviors. NPS, a recently discovered neuromodulator, has anxiolytic properties through actions in the amygdala (Guerrini et al. 2010; Pape et al. 2010; Reinscheid 2008). Intracerebroventricular administration of NPS also produces antinociceptive effects (Li et al. 2009; Peng et al. 2010), but underlying mechanisms and site of action remain to be determined. Filling this knowledge gap is important because NPS may be a useful tool to control amygdala output, and amygdala plasticity correlates positively with pain-related behaviors (Neugebauer et al. 2004, 2009) and cognitive deficits (Ji et al. 2010).
The key novelties of this study concern NPS and ITC function in pain-related amygdala processing. NPS activated feedforward inhibition of amygdala (central nucleus) output by engaging the dorsomedial cluster of GABAergic ITC neurons positioned between BLA and CeLC (Amir et al. 2011; Pinard et al. 2012). Feedforward inhibition was measured in CeLC neurons as inhibitory synaptic currents that were blocked by a non-NMDA receptor antagonist, confirming glutamate-driven synaptic inhibition. The effect of NPS involved a postsynaptic NPS receptor-mediated mechanism that depended on PKA, but not PKC. NPS decreased excitatory synaptic drive and E-S coupling of CeLC neurons that reflects the integration of synaptic excitation determining neuronal output. Analysis of sEPSCs and mEPSCs showed that the inhibitory effect of NPS was not due to a direct postsynaptic action on CeLC neurons but involved a presynaptic network mechanism because NPS had no effect in the presence of TTX. We focused on ITC cells as a target for NPS because the dorsomedial cluster of GABAergic ITC cells mediates feedforward inhibition of CeA neurons, serving as an inhibitory gate (Amano et al. 2010; Ehrlich et al. 2009; Likhtik et al. 2008; Pape and Pare 2010; Royer and Pare 2002). NPS increased feedforward inhibition of CeLC neurons and excitatory drive and synaptically evoked output of ITC cells.
Feedforward inhibition of CeLC neurons and synaptic excitation of ITC cells were evoked by stimulating fibers in the EC, which is known to provide cortical input (including from mPFC) to the amygdala (Amir et al. 2011; McDonald 1998; Pinard et al. 2012). The infralimbic mPFC inhibits amygdala output to “extinguish” aversive behavior (Akirav and Maroun 2007; Herry et al. 2010; Kim and Richardson 2010; Likhtik et al. 2005; Maren and Quirk 2004; Pape and Pare 2010; Sah and Westbrook 2008; Sotres-Bayon and Quirk 2010). Increased infralimbic activity correlates with successful extinction of negative emotions (Chang et al. 2010; Kim et al. 2010; Knapska and Maren 2009; Mickley et al. 2005) and decreased activity with cognitive control deficits in behavioral extinction models (Chang and Maren 2010; Hefner et al. 2008; Kim et al. 2010; Mickley et al. 2007; Sierra-Mercado et al. 2011) and behavioral disinhibition (Dalley et al. 2011). We found decreased feedforward inhibition in the arthritis pain model (Fig. 4), which is consistent with abnormal inhibition of prefrontal cortical activity observed in the pain model in our previous studies (Ji et al. 2010; Ji and Neugebauer 2011). Although infralimbic projections to the ITC appear to be relatively moderate (Pinard et al. 2012), ITC cells show robust activation by chemical (Berretta et al. 2005) or electrical (Amir et al. 2011) stimulation of the infralimbic mPFC. We stimulated the EC including anterogradely labeled fibers from the infralimbic cortex (see methods). A limitation of this approach is that selective activation of infralimbic fibers is not possible with electrical stimulation but would require a different method such as optogenetics. Still, activation of ITC cells and feedforward inhibition of CeLC neurons by EC stimulation is consistent with the concept of cortical (infralimbic) control of amygdala processing.
EC stimulation inhibited neuronal output of CeLC neurons in response to stimulation of two synapses that carry nociceptive information to the CeLC, the PB-CeLC and BLA-CeA synapses (Neugebauer et al. 2004, 2009). It should be noted that synaptic inputs from PB and BLA converge on the same CeLC neuron; BLA afferents synapse on dendritic spines, whereas PB afferents form synapses on dendritic shafts (Dong et al. 2010). Heterosynaptic interaction between these distinct lines of input has been suggested to be required for synaptic plasticity related to fear conditioning and perhaps for pain-related plasticity as well (Watabe et al. 2013). ITC-mediated feedforward inhibition could decrease CeLC output observed in our study by disrupting this heterosynaptic interaction. The mechanism by which activation of ITC cells with NPS or HFS inhibits CeLC output remains to be determined (see discussion of limitations of the study, below). Our data suggest that feedforward inhibition involving NPS-driven ITC activation can regulate the processing of nociceptive information in CeLC neurons and CeLC output. CeLC neurons in this and our previous studies showed characteristics of regular-spiking type A projection neurons (Neugebauer et al. 2004; Sah et al. 2003; Schiess et al. 1999) and may correspond to PKCδ-negative “on” cells (Haubensak et al. 2010) that develop enhanced responses and synaptic plasticity after fear conditioning (Ciocchi et al. 2010; Watabe et al. 2013). CeLC neurons can form direct or indirect (via medial CeA or substantia innominata) connections with brain stem and forebrain areas to regulate behavioral responses (Bourgeais et al. 2001; Gray and Magnuson 1992; Neugebauer et al. 2004; Schiess et al. 1999).
NPS acted directly on ITC cells because it increased amplitude but not frequency distribution of mEPSCs. Changes at the postsynaptic membrane are known to alter quantal size (mEPSC amplitude distribution), whereas presynaptic changes at the transmitter release site affect frequency (Wyllie et al. 1994). This well-established electrophysiological approach has been used successfully in our previous studies to distinguish pre- and postsynaptic mechanisms in the amygdala (Fu and Neugebauer 2008; Han et al. 2005b, 2006; Ren et al. 2011; Ren and Neugebauer 2010; Sun and Neugebauer 2011). The direct action of NPS on ITC cells in the rat amygdala in our study is in contrast with findings in mice where NPS increased feedforward inhibition of CeA neurons through a presynaptic action on excitatory inputs to ITC cells, because it increased frequency, but not amplitude, of mEPSCs and reduced paired-pulse facilitation (Jungling et al. 2008). The lack of a direct effect on ITC cells in mice can be explained by the fact that in mice NPSR mRNA is not detected in ITC cells but in LA-BLA areas (Clark et al. 2011).
In our study, NPS was only effective in the arthritis pain model but not under normal conditions. Normal rats in our study exhibited little anxiety-like behavior (open-arm choice of 40%, see Fig. 9). In contrast, arthritic rats showed anxiety-like behavior and NPS had anxiolytic-like effects in this pain model, which is consistent with previous observations that NPS inhibited fear-potentiated startle but not startle after tone alone (Fendt et al. 2010) and had anxiolytic-like effects in mice exposed to immobilization stress but not in nonstressed animals (Chauveau et al. 2012). The absence of NPS effects under normal conditions may be explained by a lack of NPSR protein expression in the ITC, LA, BLA, and CeA of rats (Leonard and Ring 2011), although high levels of NPSR mRNA are found in the ITC but not LA, BLA, and CeA of rats (Xu et al. 2007). NPSR protein expression in the arthritis pain model remains to be determined.
The NPSR antagonist alone had no effect in the amygdala in our study and after intracerebroventricular administration in nociceptive assays (Li et al. 2009; Peng et al. 2010), suggesting that the NPS-NPSR system may not be endogenously activated under these conditions. This conclusion is supported by studies showing a lack of phenotype of NPSR(−/−) mice in tests for anxiety (elevated plus maze, open field, stress-induced hyperthermia), depressive-like behaviors (forced swimming), memory (novel object recognition), and nociception (formalin) (reviewed by Guerrini et al. 2010; Ruzza et al. 2012). However, others reported an amnesic (Okamura et al. 2011) and anxiogenic-like (Duangdao et al. 2009) phenotype for NPSR(−/−) mice. Strain differences may explain this discrepancy, suggesting that the endogenous NPS/NPSR system is activated under conditions of high anxiety (i.e., 129S6/SvEv mice and icv injected CD-1 mice) but not low anxiety (i.e., C57BL/6 mice and noninjected CD-1 mice; for discussion see Ruzza et al. 2012). An intriguing hypothesis is that the lack of endogenous NPSR activation and hence lack of ITC activation may play a key role in pain-related amygdala plasticity, permitting the uncontrolled access of nociceptive inputs to the amygdala output nucleus (CeLC).
Some limitations of our study need to be considered. The mechanism that renders NPS functional in pain-related plasticity compared with normal conditions remains to be determined. One possibility is a change in NPSR protein expression that appears to be absent under normal conditions despite the presence of mRNA as mentioned previously (Leonard and Ring 2011). Interestingly, HFS of EC inputs to ITC cells inhibited CeLC output in the pain model but not under normal conditions, suggesting a dramatic functional change of the NPS/NPSR system in the amygdala, which remains to be explored mechanistically. While our data show that ITC activation inhibits presumed nociceptive inputs via the PB-CeLC and BLA-CeLC synapses, the underlying mechanism is not yet clear. Inhibition of excitatory transmission through a presynaptic mechanism is one possibility, because NPS inhibited EPSCs recorded in CeLC neurons at a holding potential of −70 mV (near the calculated equilibrium potential for chloride, −68.99 mV; see methods) and mEPSC/sEPSC analysis showed a presynaptic network action for NPS. ITC cells are an important source of inhibitory control of CeLC neurons as evidenced by data in this study, and axons of ITC cells show extensive ramifications within the CeLC (Busti et al. 2011). NPS also increased inhibitory synaptic transmission onto CeLC neurons. ITC cells form synaptic contacts with the somata of CeLC neurons (Busti et al. 2011), whereas afferents from PB and BLA target the dendritic regions (Dong et al. 2010). Therefore, ITC cells are well positioned to exert efficient inhibition on CeLC neuronal processing and synaptic integration. The site of action of GABA released from ITC fiber terminals and the relative contributions of direct and presynaptic inhibition to the effects of NPS (and HFS) on CeLC processing remain to be determined. The present study shows that PKA but not PKC is a downstream effector of NPS effects on ITC cells. Our previous work showed an important role for PKA in pain-related synaptic plasticity of CeLC neurons (Fu et al. 2008; Fu and Neugebauer 2008; Han et al. 2005b) through a mechanism that involved NMDA receptor phosphorylation (Bird et al. 2005). However, the effects of PKA in ITC cells remain to be determined.
As a technical consideration regarding microdialysis drug application in the behavioral studies, the ITC represents a small brain area that may be difficult to target selectively even though our previous work showed that the spread of drugs applied by microdialysis does not exceed 0.5–1 mm around the tip of the microdialysis probe (Ji and Neugebauer 2008; Li et al. 2011). Importantly, NPS application into the central nucleus as a placement control had no effect, and our electrophysiological data showed that NPS did not act directly on CeLC neurons (Fig. 2) or BLA neurons (see NPS Activates ITC Cells Through Postsynaptic Mechanism That Depends on PKA but Not PKC) but on ITC cells (Fig. 6). These findings are consistent with the absence of NPSR mRNA (Xu et al. 2007) and protein (Leonard and Ring 2011) in LA, BLA, and CeA. Drug concentrations used in this study were derived from data in the literature. At 10 μM NPS has been shown to have near-maximum effects (Jungling et al. 2008). Using 1 and 10 μM we found no significant effect of NPS on baseline transmission under normal condition, but in the arthritis pain model 1 μM NPS clearly inhibited synaptic plasticity. Furthermore, the effects of NPS were blocked by a selective NPSR antagonist, suggesting that appropriate concentrations were used that were sensitive to detect functional changes. Finally, supraspinal administration of NPS has been shown to stimulate locomotor activity (Guerrini et al. 2010), which could confound behavioral results. This is unlikely, however, because NPS had no effect on spinal reflexes, decreased vocalizations that are not affected by locomotion, and increased open-arm but not closed-arm choices selectively. Although NPS produced a slight, but nonsignificant, increase of total arm entries in the plus maze test, the selective increase in open-arm preference and the differential effects on behavioral outcome measures are inconsistent with confounding effects of locomotor facilitation by NPS.
In conclusion, the data suggest that NPS can increase the synaptic drive and output of ITC cells, resulting in enhanced feedforward inhibition of pain-related processing in the CeLC and decreased pain-related behaviors. However, NPSR in the EC-ITC-CeLC circuitry are not activated endogenously in this model. Lack of endogenous NPSR activation may contribute to the persistence of pain-related amygdala plasticity, whereas NPS may be a useful tool to enhance cortical control of amygdala processing to inhibit amygdala output and pain-related behaviors.
GRANTS
Research related to this study was supported by National Institute of Neurological Disorders and Stroke Grants NS-38261, NS-081121, and NS-11255.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.R., T.K., S.G., and G.J. performed experiments; W.R., T.K., S.G., and G.J. analyzed data; W.R., T.K., S.G., G.J., R.G., G.C., and V.N. interpreted results of experiments; W.R., T.K., S.G., G.J., and V.N. prepared figures; W.R. drafted manuscript; W.R., T.K., S.G., G.J., R.G., G.C., and V.N. approved final version of manuscript; R.G., G.C., and V.N. conception and design of research; G.C. and V.N. edited and revised manuscript.
REFERENCES
- Adedoyin MO, Vicini S, Neale JH. Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model. Mol Pain 6: 60–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast 2007: 30873, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13: 489–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105: 3054–3066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132: 943–953, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol 564: 907–921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeais L, Gauriau C, Bernard JF. Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci 14: 229–255, 2001 [DOI] [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Satzler K, Singewald N, Capogna M, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci 31: 5131–5144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda V, Rizzi A, Ruzza C, Zucchini S, Marzola G, Marzola E, Guerrini R, Salvadori S, Reinscheid RK, Regoli D, Calo G. In vitro and in vivo pharmacological characterization of the neuropeptide S receptor antagonist [d-Cys(tBu)5]neuropeptide S. J Pharmacol Exp Ther 328: 549–555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One 5: e11971, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci 124: 391–397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape HC. Prevention of stress-impaired fear extinction through neuropeptide S action in the lateral amygdala. Neuropsychopharmacology 37: 1588–1599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468: 277–282, 2010 [DOI] [PubMed] [Google Scholar]
- Clark SD, Duangdao DM, Schulz S, Zhang L, Liu X, Xu YL, Reinscheid RK. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J Comp Neurol 519: 1867–1893, 2011 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69: 680–694, 2011 [DOI] [PubMed] [Google Scholar]
- Dong YL, Fukazawa Y, Wang W, Kamasawa N, Shigemoto R. Differential postsynaptic compartments in the laterocapsular division of the central nucleus of amygdala for afferents from the parabrachial nucleus and the basolateral nucleus in the rat. J Comp Neurol 518: 4771–4791, 2010 [DOI] [PubMed] [Google Scholar]
- Duangdao DM, Clark SD, Okamura N, Reinscheid RK. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav Brain Res 205: 1–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771, 2009 [DOI] [PubMed] [Google Scholar]
- Fendt M, Imobersteg S, Burki H, McAllister KH, Sailer AW. Intra-amygdala injections of neuropeptide S block fear-potentiated startle. Neurosci Lett 474: 154–157, 2010 [DOI] [PubMed] [Google Scholar]
- Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain 4: 26–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28: 3861–3876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468: 24–56, 2004 [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides 13: 451–460, 1992 [DOI] [PubMed] [Google Scholar]
- Guerrini R, Camarda V, Trapella C, Calò G, Rizzi A, Ruzza C, Fiorini S, Marzola E, Reinscheid RK, Regoli D, Salvadori S. Synthesis and biological activity of human neuropeptide S analogues modified in position 5: identification of potent and pure neuropeptide S receptor antagonists. J Med Chem 52: 524–529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Salvadori S, Rizzi A, Regoli D, Calò G. Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med Res Rev 30: 751–777, 2010 [DOI] [PubMed] [Google Scholar]
- Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 6: 10–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods 141: 261–269, 2005a [DOI] [PubMed] [Google Scholar]
- Han JS, Fu Y, Bird GC, Neugebauer V. Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Mol Pain 2: 18–29, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Li W, Neugebauer V. Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci 25: 10717–10728, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci Lett 361: 254–257, 2004 [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 113: 211–222, 2005 [DOI] [PubMed] [Google Scholar]
- Harrigan EA, Magnuson DJ, Thunstedt GM, Gray TS. Corticotropin releasing factor neurons are innervated by calcitonin gene-related peptide terminals in the rat central amygdaloid nucleus. Brain Res Bull 33: 529–534, 1994 [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci 28: 8074–8085, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci 31: 599–612, 2010 [DOI] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127: 161–172, 2007 [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 3: 13–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol 97: 3893–3904, 2007 [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol 99: 1201–1212, 2008 [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 1102: 2253–2264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABAA receptors. J Neurophysiol 106: 2642–2652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30: 5451–5464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59: 298–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry 67: 297–303, 2010 [DOI] [PubMed] [Google Scholar]
- Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. J Neurosci 30: 832–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritoshi T, Sun H, Ren W, Stauffer SR, Lindsley CW, Conn PJ, Neugebauer V. Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacology 66: 170–178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 16: 486–493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SK, Ring RH. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience 172: 153–163, 2011 [DOI] [PubMed] [Google Scholar]
- Li W, Chang M, Peng YL, Gao YH, Zhang JN, Han RW, Wang R. Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul Pept 156: 90–95, 2009 [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain 110: 112–122, 2004a [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol 91: 13–24, 2004b [DOI] [PubMed] [Google Scholar]
- Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci 31: 1114–1127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454: 642–645, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci 25: 7429–7437, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852, 2004 [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48: 1025–1037, 2005 [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol 55: 257–332, 1998 [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman JA, Biada JM, Disorbo A. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res 1152: 139–157, 2007 [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res 1051: 176–182, 2005 [DOI] [PubMed] [Google Scholar]
- Nakao A, Takahashi Y, Nagase M, Ikeda R, Kato F. Role of capsaicin-sensitive C-fiber afferents in neuropathic pain-induced synaptic potentiation in the nociceptive amygdala. Mol Pain 8: 51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 60: 226–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain 3: 8–20, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 89: 716–727, 2003 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23: 52–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 10: 221–234, 2004 [DOI] [PubMed] [Google Scholar]
- Okamura N, Garau C, Duangdao DM, Clark SD, Jungling K, Pape HC, Reinscheid RK. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology 36: 744–752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology 55: 537–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Jungling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology 58: 29–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol 92: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1998 [DOI] [PubMed] [Google Scholar]
- Peng YL, Zhang JN, Chang M, Li W, Han RW, Wang R. Effects of central neuropeptide S in the mouse formalin test. Peptides 31: 1878–1883, 2010 [DOI] [PubMed] [Google Scholar]
- Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205: 112–124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol 82: 69–85, 1999 [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol 69: 1350–1362, 1993 [DOI] [PubMed] [Google Scholar]
- Reinscheid RK. Neuropeptide S: anatomy, pharmacology, genetics and physiological functions. Results Probl Cell Differ 46: 145–158, 2008 [DOI] [PubMed] [Google Scholar]
- Ren W, Neugebauer V. Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol Pain 6: 93–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Palazzo E, Maione S, Neugebauer V. Differential effects of mGluR7 and mGluR8 activation on pain-related synaptic activity in the amygdala. Neuropharmacology 61: 1334–1344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 115: 455–462, 2002 [DOI] [PubMed] [Google Scholar]
- Ruzza C, Pulga A, Rizzi A, Marzola G, Guerrini R, Calò G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 62: 1999–2009, 2012 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural neuroscience: the circuit of fear. Nature 454: 589–590, 2008 [DOI] [PubMed] [Google Scholar]
- Sarhan M, Freund-Mercier MJ, Veinante P. Branching patterns of parabrachial neurons projecting to the central extended amgydala: single axonal reconstructions. J Comp Neurol 491: 418–442, 2005 [DOI] [PubMed] [Google Scholar]
- Schiess MC, Callahan PM, Zheng H. Characterization of the electrophysiological and morphological properties of rat central amygdala neurons in vitro. J Neurosci Res 58: 663–673, 1999 [PubMed] [Google Scholar]
- Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol 270: 416–426, 1988 [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36: 529–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20: 231–235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol 106: 960–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322–328, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain 6: 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Manabe T, Nicoll RA. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron 12: 127–138, 1994 [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol 500: 84–102, 2007 [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43: 487–497, 2004 [DOI] [PubMed] [Google Scholar]