Abstract
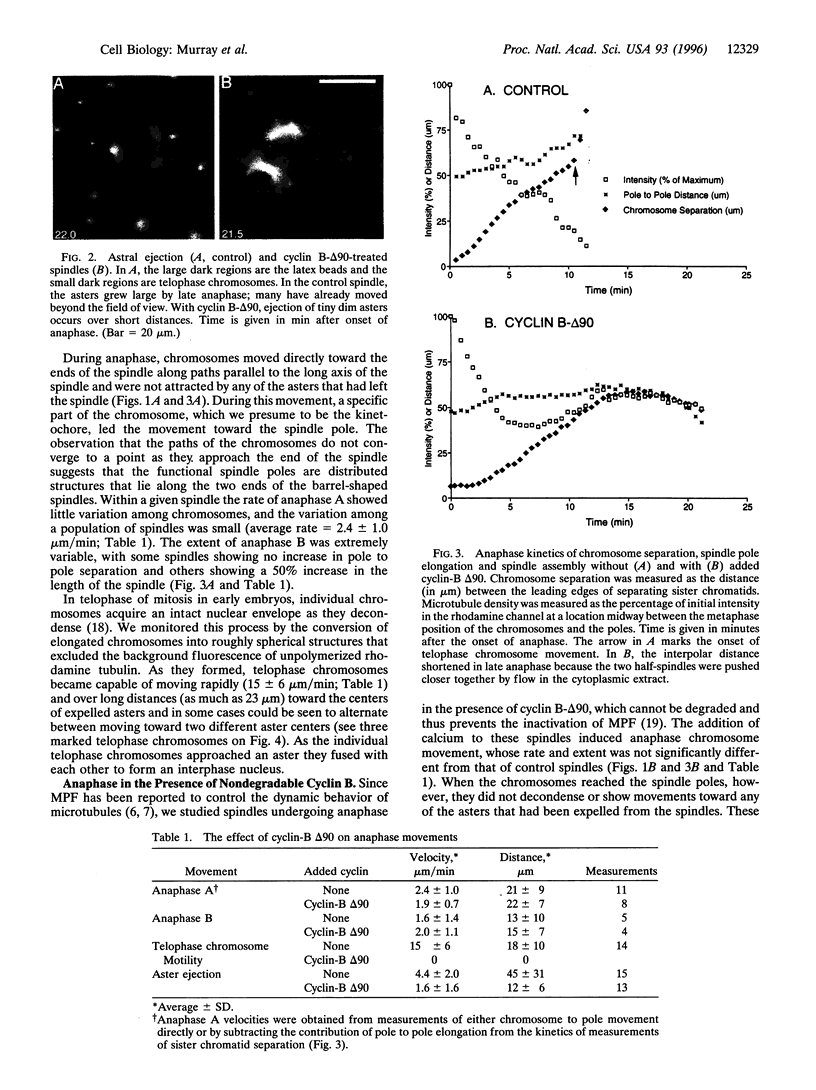
We used digital fluorescence microscopy to make real-time observations of anaphase chromosome movement and changes in microtubule organization in spindles assembled in Xenopus egg extracts. Anaphase chromosome movement in these extracts resembled that seen in living vertebrate cells. During anaphase chromosomes moved toward the spindle poles (anaphase A) and the majority reached positions very close to the spindle poles. The average rate of chromosome to pole movement (2.4 microns/min) was similar to earlier measurements of poleward microtubule flux during metaphase. An increase in pole-to-pole distance (anaphase B) occurred in some spindles. The polyploidy of the spindles we examined allowed us to observe two novel features of mitosis. First, during anaphase, multiple microtubule organizing centers migrated 40 microns or more away from the spindle poles. Second, in telophase, decondensing chromosomes often moved rapidly (7-23 microns/min) away from the spindle poles toward the centers of these asters. This telophase chromosome movement suggests that the surface of decondensing chromosomes, and by extension those of intact nuclei, bear minus-end-directed microtubule motors. Preventing the inactivation of Cdc2/cyclin B complexes by adding nondegradable cyclin B allowed anaphase A to occur at normal velocities, but reduced the ejection of asters from the spindles, blocked chromosome decondensation, and inhibited telophase chromosome movement. In the presence of nondegradable cyclin B, chromosome movement to the poles converted bipolar spindles into pairs of independent monopolar spindles, demonstrating the role of sister chromatid linkage in maintaining spindle bipolarity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson J. F. Demonstration of a colcemid-sensitive attractive force acting between the nucleus and a center. J Cell Biol. 1971 Nov;51(21):579–583. doi: 10.1083/jcb.51.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Blangy A., Lane H. A., d'Hérin P., Harper M., Kress M., Nigg E. A. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995 Dec 29;83(7):1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Kang S. J., Satterwhite L. L., Rose M. D., Skeen V. P., Salmon E. D. Yeast Kar3 is a minus-end microtubule motor protein that destabilizes microtubules preferentially at the minus ends. EMBO J. 1994 Jun 1;13(11):2708–2713. doi: 10.1002/j.1460-2075.1994.tb06561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y., Nakano Y. Micromanipulation studies of the mitotic apparatus in sand dollar eggs. Cell Motil Cytoskeleton. 1988;10(1-2):172–184. doi: 10.1002/cm.970100122. [DOI] [PubMed] [Google Scholar]
- Holloway S. L., Glotzer M., King R. W., Murray A. W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993 Jul 2;73(7):1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Inoué S., Salmon E. D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995 Dec;6(12):1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Dan K., Goodenough D. Ultrastructure and 3H-thymidine incorporation by chromosome vesicles in sea urchin embryos. Chromosoma. 1981;83(4):441–453. doi: 10.1007/BF00328271. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Kobayashi S., Mitchison T., Kirschner M. Role of the centrosome in organizing the interphase microtubule array: properties of cytoplasts containing or lacking centrosomes. J Cell Biol. 1984 May;98(5):1763–1776. doi: 10.1083/jcb.98.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Newport J., Hubble R., Kirschner M. Interconversion of metaphase and interphase microtubule arrays, as studied by the injection of centrosomes and nuclei into Xenopus eggs. J Cell Biol. 1984 May;98(5):1730–1745. doi: 10.1083/jcb.98.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J Cell Biol. 1981 Dec;91(3 Pt 1):822–826. doi: 10.1083/jcb.91.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989 Aug;109(2):637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. J., Salmon E. D. Poleward kinetochore fiber movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J Cell Biol. 1992 Nov;119(3):569–582. doi: 10.1083/jcb.119.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Paschal B. M., Shpetner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987 Sep;105(3):1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Inoué T., Desai A., Murray A. W. High resolution multimode digital imaging system for mitosis studies in vivo and in vitro. Biol Bull. 1994 Oct;187(2):231–232. doi: 10.1086/BBLv187n2p231. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., Mitchison T. J. Mitotic spindle assembly by two different pathways in vitro. J Cell Biol. 1991 Mar;112(5):925–940. doi: 10.1083/jcb.112.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. E., Mitchison T. J. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. E., Mitchison T. J. Poleward microtubule flux mitotic spindles assembled in vitro. J Cell Biol. 1991 Mar;112(5):941–954. doi: 10.1083/jcb.112.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu C. E., Murray A. W. Sister chromatid separation in frog egg extracts requires DNA topoisomerase II activity during anaphase. J Cell Biol. 1992 Jun;117(5):921–934. doi: 10.1083/jcb.117.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. M., Busa W. B., Wilson K. L. Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell. 1993 Jul 2;73(7):1411–1422. doi: 10.1016/0092-8674(93)90366-x. [DOI] [PubMed] [Google Scholar]
- Surana U., Amon A., Dowzer C., McGrew J., Byers B., Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993 May;12(5):1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Berrez J. M., Antony C., Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs: requirement for phosphorylated factors and cytoplasmic dynein. J Cell Biol. 1991 Mar;112(6):1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Dogterom M., Stelzer E., Karsenti E., Leibler S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol. 1992 Sep;118(5):1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernos I., Karsenti E. Motors involved in spindle assembly and chromosome segregation. Curr Opin Cell Biol. 1996 Feb;8(1):4–9. doi: 10.1016/s0955-0674(96)80041-x. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Hunt T., Ikawa Y., Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991 Jul 18;352(6332):247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- Waters J. C., Salmon E. D. Chromosomes take an active role in spindle assembly. Bioessays. 1995 Nov;17(11):911–914. doi: 10.1002/bies.950171102. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Kronebusch P. J., Borisy G. G. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J Cell Biol. 1995 Nov;131(3):721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]