Abstract
Health economics has been an established feature of the research, policymaking, practice and management in the delivery of healthcare. However its role is increasing as the cost of healthcare begins to drive changes in most healthcare systems. Thus the output from cost effectiveness studies is now being taken into account when making reimbursement decisions, e.g. in Australia and the United Kingdom. Against this background it is also recognised that the health economic tools employed in healthcare, and particularly the output from the use of these tools however, are not always employed in the routine delivery of services. One of the notable consequences of this situation is the poor record of innovation in healthcare with respect to the adoption of new technologies, and the realisation of their benefits.
The evidence base for the effectiveness of diagnostic services is well known to be limited, and one consequence of this has been a very limited literature on cost effectiveness. One reason for this situation is undoubtedly the reimbursement strategies employed in laboratory medicine for many years, simplistically based on the complexity of the test procedure, and the delivery as a cost-per-test service. This has proved a disincentive to generate the required evidence, and little effort to generate an integrated investment and disinvestment business case, associated with care pathway changes.
Point-of-care testing creates a particularly challenging scenario because, on the one hand, the unit cost-per-test is larger through the loss of the economy of scale offered by automation, whilst it offers the potential of substantial savings through enabling rapid delivery of results, and reduction of facility costs. This is important when many health systems are planning for complete system redesign. We review the literature on economic assessment of point-of-care testing in the context of these developments.
Introduction
There is a growing realisation around the world, particularly in developed nations, that healthcare budgets cannot continue to grow at the rate that they have done so over the last half century and resources are now limited. In addition there are also concerns about the quality and efficiency of care delivery systems.1 These issues pose considerable challenges which have been exacerbated by the recent global financial crisis (GFC) and indeed, the post-GFC reality for some countries is that healthcare budgets are now being reduced. We are thus entering an era where health economics and the allocation of resources are going to play an even more important role.
Pathology or laboratory medicine is not immune from these challenges despite the fact that a relatively small portion of healthcare costs (<5%) is spent on medical testing. Some countries are already experiencing reductions in laboratory budgets such as in Australia where the government has recently made changes to the Medicare Benefits Schedule to reduce the amount of reimbursement that laboratories receive for testing;2 similar trends are seen in other countries.3,4 This represents a major change from the situation of the last several decades where laboratories have gone through an enormous expansion, both in terms of the range of tests they perform, the volume of testing, and the investment in technology. The advances in technology and software have certainly delivered significant efficiency and productivity dividend.5
However in parallel with the concern over health spending is the realisation that investments in technology have been a significant factor in the escalating cost of healthcare.6 Furthermore innovation in healthcare has not necessarily delivered all that was promised, in part because there are considerable barriers to adoption of new technologies.7–9 While until now laboratories have delivered undoubted efficiencies and significantly improved testing quality, the economic challenges are likely to force them, as well as healthcare policymakers and service providers, to give more consideration to the value that their services offer across the whole health system through a more evidence based approach to practice. Policymakers attempting to curb the rising cost of healthcare delivery are looking at improving quality, system redesign and innovation. Quality improvement and innovation are built on the consideration of three aspects of healthcare delivery (i) structure, (ii) processes, and (iii) outcomes.10 Structure comprises resources and organisational characteristics. The analysis of healthcare resource utilisation, through economic analysis is perhaps one of the least understood and least utilised areas of evidence-based laboratory medicine. In good economic times it has not been necessary to be overly concerned about what tests might cost or perhaps more importantly, whether they deliver economic as well as clinical benefits; the business model for most laboratories has evolved on the basis of being a profit centre. The consequence of this in times of fiscal constraint is that efforts are made to drive down the cost of delivering tests. That situation is changing and many countries already have organisations such as the Medical Services Advisory Committee in Australia11 and the National Institute for Health and Care Excellence in the UK12 which review applications for adoption of new tests and consider a wide range of evidence including economic outcomes, such as cost effectiveness and “value-for-money”.
As laboratory budgets decrease in real terms, there will be increasing focus on existing testing as well as possible adoption of new tests. Much of this testing is associated with a very limited evidence base and what evidence does exist, virtually none relates to the normal metrics of economic evaluation of health technology, that is cost per quality adjusted life year. A 1999 systematic review of economic studies of testing which included both laboratory and radiology testing identified 28 studies of laboratory testing that included economic outcomes.13 Yet an encouraging sign of the growing interest in this area is the publication in 2008 of a systematic review of data from the Tufts Medical Centre Cost-Effectiveness Analysis Registry which showed a substantial increase in recent years of studies of the cost-effectiveness of laboratory testing.14
Point-of-care testing (POCT) is one of the innovations that can potentially impact on the quality of care, as well as on system redesign and a more patient centred approach to care. However it is a technology that is perceived as “expensive” when considered in terms of the common approach to reimbursement, namely the complexity of the test, or cost-per-test. We therefore chose to review the economic evidence for POCT as it offers the perfect case for assessing the impact of such testing on outcomes.
It is also important to understand the impact of analytical performance on outcomes – as well as the cost of ensuring the necessary quality of performance. This may be particularly important as in order to simplify the operation of a POCT system it may provide results in a semi-quantitative or analogue manner. The system must then be judged on its diagnostic performance as well as its technical performance. The majority of studies employed to establish the use of a test have been based on analytical methods performed in the clinical laboratory, this includes assessments of biological variation. This data is therefore associated with stated levels of accuracy and precision. It is important, therefore, to be aware of this when considering the use of POCT, both at the point of adoption of the technology as well as in routine implementation. Training, quality control and external quality assurance are important tools for assessing and maintaining the required level of analytical performance of POCT devices. Typically these functions will have been built into the laboratory overhead costs and reimbursement tariffs – as well as being a cost that benefits from the economies of scale associated with a central laboratory. It is important to consider these costs in relation to the adoption of POCT, and the associated economic analysis.
Below we briefly review the types of economic analysis used in healthcare and testing, and then show how some of these techniques have been applied to point of care tests and the resulting outcomes.
Types of Economic Assessment
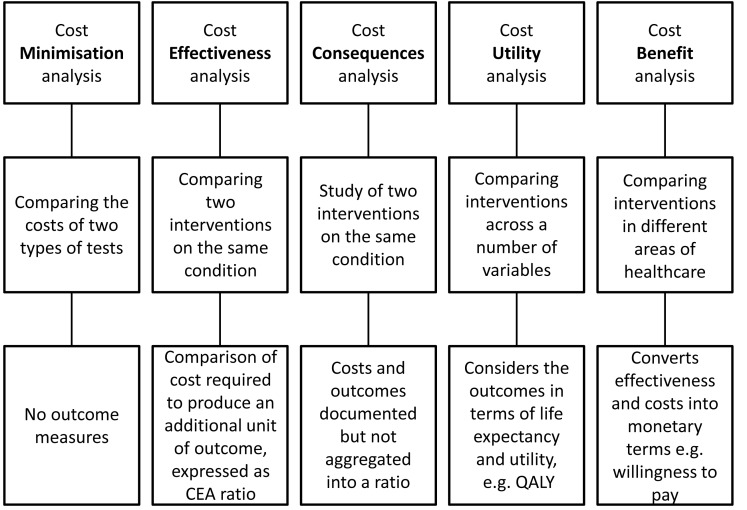
Our description of the various type of economic analysis will be brief as sources of more detailed information are readily available.15–17 Figure 1 shows the major types of economic analyses used in healthcare applications including laboratory-based testing. On the far left is so-called cost minimisation analysis. There are many reports of this type in the literature but since they only compare the costs of two or more types of testing, and include no outcomes of the testing, they are of very limited use. That notwithstanding it is an approach commonly used by funding agencies when making decisions about adopting new tests, as many reimbursement systems are based, primarily, on the test complexity.
Figure 1.
Summary of the different types of health economic analyses that have been employed with POCT, indicating the inputs to, and products of, the analyses.
CEA: Cost effectiveness analysis, QALY: quality adjusted life year
In a cost minimisation analysis the assumption is made that the health outcome from the two technologies being compared is equal, the objective being to determine whether the cost of the new technology is equal to or less than the old technology. Therefore, outcome measures are compared, but need to demonstrate that they are equivalent or non-inferior. Thus cost minimisation analysis is analogous to the way that tests are funded or reimbursed in most health systems, namely on the basis of the complexity of the test, and delivered on a fee-for-service basis. This approach is somewhat at odds with the development of evidence on the use of diagnostic tests advocated by Sackett and Haynes who stated that the key question was whether “…. patients undergoing the diagnostic test fare better than similar untested patients?”18 More recently Ferrante di Ruffano et al. have stated that “the value of a diagnostic test is not simply measured by its accuracy, but depends on how it affects patient health”. Both of these groups have set the evidence-based agenda for diagnostics – including that for economic assessment – with the implicit statement that a diagnostic test is one part of a test-and-treat (act) intervention, and therefore needs to be assessed, reimbursed – and applied – as such.19
Cost effectiveness analysis (CEA) compares two interventions, where the costs are identified in monetary terms and the outcomes in non-monetary terms. The CEA can compare across a number of possible interventions and outcomes for the same condition. The CEA compares the incremental cost divided by the incremental effect: this can be described in an incremental cost effectiveness ratio (ICER), and can be expressed in an equation:
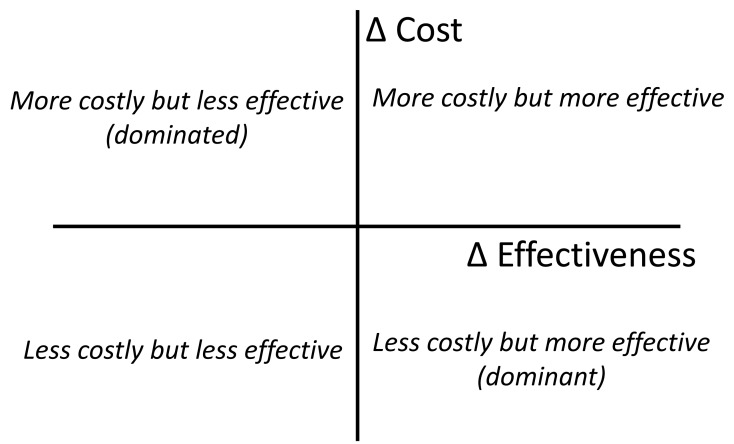
where C1 and E1 are the cost and effect in the intervention group, and C2 and E2 are the cost and effects in the control group. This can be presented graphically in the Cost Effectiveness Plane: see Figure 2.20 This illustrates the decisions that can be informed by the analysis. Thus in the case of POCT one might compare the use of POCT with a laboratory-based delivery of the result, e.g. in the use of natriuretic peptide in primary care to aid the diagnosis of heart failure. The outcomes that could be considered include the cost per number of hospital admissions avoided or cost per life years gained. This highlights the complexity of cost effectiveness studies of diagnostic tests – because the test may be used for diagnosis and monitoring (a “test-and-treat” intervention).
Figure 2.
The four quadrant cost effectiveness plane.
In recent times another technique called costs consequences analysis (CCA) has been advocated as an easier alternative to CEA. In the NICE (National Institute for Clinical Health and Excellence) glossary it is described as comparing “the costs (such as treatment and hospital care) and the consequences (such as health outcomes) of a test or treatment with a suitable alternative. Unlike cost-benefit analysis or cost-effectiveness analysis, it does not attempt to summarise outcomes in a single measure (like the quality-adjusted life year) or in financial terms. Instead, outcomes are shown in their natural units (some of which may be monetary) and it is left to decision-makers to determine whether, overall, the treatment is worth carrying out”.21 The major difference to CEA is that incremental costs and outcomes are presented separately and not aggregated into a ratio. This approach was first suggested to make the economic effects more transparent as it was suggested that policymakers rarely made decisions based on cost effectiveness ratios.22 This approach was subsequently challenged on the basis that the approach was very similar to cost benefit analysis.23 In the case of the use of POCT for natriuretic peptides referred to earlier, a CCA approach would consider outcomes such as the number of heart failure cases diagnosed in the two testing modalities, whilst the costs would be calculated from the process and consumable (test) costs. Thus it can be used where the decision makers need to make an assessment of the value of the health outcome measured (e.g. the number of extra cases of heart failure diagnosed) without explicitly going through the step of trying to place a value on this. Cost-Benefit Analysis (CBA) is similar to CEA in that the monetary costs of two or more interventions are measured but effectiveness or the outcomes are also measured in monetary values; the latter can be challenging to achieve.
Cost-utility analysis (CUA) is similar to CEA but converts the change in health outcomes into a measure of the quality and quantity of life and the ICER is measured in the cost per quality adjusted life year (QALY). A unit of QALY combines both length and quality of life into a single outcome where 1 is perfect health and 0 is death. All of the studies considered in this review will be of the Cost Consequences, Cost Effectiveness or of the Cost Utility type.
Experimental Design, Evidence and Outcomes
Tests may be employed to inform a range of clinical decisions, broadly described in terms of screening, diagnosis, treatment selection and monitoring. Formal screening programmes are somewhat unique in that they comprise diagnosis and monitoring, as a fully integrated “test-and-treat intervention” with the expectation of trial evidence demonstrating a difference in health outcomes. Adoption of a formal screening programme also involves a cost effectiveness analysis.24 In the case of a diagnostic test, it is acceptable to use a study examining the diagnostic accuracy of the test and to model from this to the expected gain in health outcomes. The approach to treatment is considered to be the same. This approach is considered to be valid if the new test (e.g. POCT) is safer or more specific, but of similar sensitivity. However if the POCT is significantly more or less sensitive this may influence the number of cases detected, i.e. the diagnostic accuracy of the test. In the case of POCT it may be possible to initiate treatment sooner when compared with a laboratory-based process, and this in effect represents a different treatment regimen.25 If the earlier test information generated by POCT influences the choice of treatment then it can be considered as a monitoring test, where it is effectively part of a new “test-and-treat intervention” and then it is important to consider a randomised controlled trial in order to demonstrate a difference in health outcomes.26
Outcome measures can be considered as clinical, operational or economic; operational impact is often incorporated into consideration of the economic impact of a test, but it is useful to consider separately as it can identify the change in process and practice, which may be helpful when considering adoption and implementation of the test. Whilst clinical outcomes are expressed in terms of morbidity and mortality, intermediate or surrogate outcome measures are often used in studies of test performance; this approach is also taken when looking at operational and economic outcomes. Some examples of surrogate outcomes are given in the Table. These outcome measures are often used in performance improvement initiatives.27 There are limitations in the use of these surrogate measures in outcomes studies, one of the main ones being (for obvious practical reasons) the measures are used over a relatively short period of time, e.g. one to two years. Thus HbA1c is a surrogate measure of glycaemic control in the treatment of diabetes, and would need to be modelled to the clinical outcome of interest, e.g. rate of development of diabetic nephropathy. In the case of warfarin management time in INR therapeutic range as a surrogate measure would be modelled to give the impact on hospital admissions.
Table.
Some examples of intermediate or surrogate outcome measures.
Adapted from The Physician Consortium for Performance Improvement, American Medical Association.79
| Types of outcome measure | Examples of outcome measure |
|---|---|
| Clinical | |
|
| |
| Mortality | Death rate |
| Morbidity | Reduced rate of diabetic nephropathy |
| Intermediate outcomes | Reduction in HbA1c |
| Time INR in therapeutic range | |
| Complication rate | |
| Recurrence rate | |
|
| |
| Operational | |
|
| |
| Intermediate outcomes | Emergency department triage time |
| Length of stay in Emergency Department | |
| Number of clinic visits per year | |
| Operation time | |
| Recovery time | |
|
| |
| Economic | |
|
| |
| Resource utilisation | Cost per QALY |
| Intermediate outcomes | Emergency admission rate |
| Length of stay in Emergency Department | |
| Blood product utilisation | |
| Clinic costs | |
| Cost per episode | |
Quality of life measures used in a cost utility analysis might be measured directly in a trial (as was done in the DiGEM trial28) or the utilities might be applied in using a quality of life measured in people with a particular health state (e.g. a measure of the average quality of life of people with stroke).
Analytical Performance of POCT Systems
The majority of studies employed to establish the use of a test have been based on analytical methods performed in the clinical laboratory, this includes assessments of biological variation. It is important to be aware of this when considering the use of POCT, both at the point of considering adoption of the technology as well as in routine implementation. Quality control and external quality assurance are important tools for maintaining the required level of analytical performance.
Ideally economic data will be generated from controlled trials of clinical effectiveness or if that is not possible, through observational studies. A theoretical alternative to performing such studies is to use modelling or decision analysis tools. The latter are basically decision trees through which all the possible consequences or outcomes that can flow from all the alternative options are mapped out. Each of the consequences has a mathematical probability of occurrence and costs. There are various types of models employed in the evaluation of diagnostic tests.29 Some studies measure health outcomes and quality of life directly within a trial, as in the case of the DIGEM study.30 These can be particularly valuable, but economic evaluations invariably require some kind of modelling, whether they are based on the results of a trial which is unusual, or using data in the literature from a variety of reported sources. The probabilities in each arm of the decision tree must sum to 1.0. There will also be a payoff or cost associated with each consequence and the expected value of a particular strategy can be calculated from the probabilities possible to measure all the changes in health outcomes over the course of a trial, and because health outcomes are likely to occur after the duration of a trial. Therefore, even in trials that include economic evaluations, this is unlikely to be sufficient to make a determination regarding the cost-effectiveness of a technology. This emphasises one of the benefits of ongoing market surveillance or performance management to monitor the long term benefit from adopting a new technology. Examples of using such models to compare the effectiveness and costs of POCT versus central laboratory tests as well as other economic evaluation techniques will be described below.
Economic Analysis of POCT
We sought to illustrate the experience of economic analysis of POCT using papers that reported on studies that compared POCT to another type of testing and contained details of the outcomes achieved from using the different tests and the resulting costs. We do not claim that this search found all available papers but of the ones we found, they were classified as follows:
Randomised Controlled Trials (RCTs) including CEA or CUA
RCTs with cost consequence studies
Systematic Reviews including modelling to generate data for CEA or CUA
Cost consequence studies
Decision analysis modelling to generate CEA or CUA
Randomised Controlled Trials Including Economic Evidence
From our search of the literature we identified seven RCTs of POCT which included economic evidence. Five of these included a formal cost effectiveness analysis28,31–34 while in two there was a cost consequence type of analysis.35,36
There have been many observational studies but a limited number of RCTs of self-monitoring of blood glucose (SMBG) in type 2 diabetes and most of the economic evidence has come from decision analysis or modelling studies which will be reviewed below. The RCT of SMBG by Farmer et al., the DiGEM Trial,30 included a formal cost utility analysis, the results of which were subsequently published by Simon et al.28 The main outcome measures in this study of 453 patients with non-insulin treated type 2 diabetes were QALY and the direct healthcare costs of the intervention compared to normal care. The study showed that both the less and more intensive monitoring interventions were more expensive than usual care and furthermore there was an initial decrease in QALY for those subjects using intensive monitoring. Given that the original paper by Farmer et al. had shown only modest improvements in HbA1c levels which are unlikely to be of significant health benefit,30 it is perhaps not surprising that the economic evidence also does not overwhelmingly support SMBG in non-insulin treated diabetics.
The number of patients self-monitoring for INR levels is also growing but, unlike SMBG, the evidence for both self-monitoring and self-management (monitoring plus self-dosing) does support this practice in certain patients.37 The review showed a significant reduction in thromboembolic events, all-cause mortality and major haemorrhage in the case of patients self-monitoring, whilst in those self-managing there were significant reductions in thromboembolic events and death, but not in major haemorrhage. Of the many RCTs of self-monitoring that have been performed none have included direct cost effectiveness analysis although the data has been used to model outcomes (see below). Several studies have addressed an alternative POCT model for INR where the testing is performed by the general practitioner. Thus Claes et al. measured the cost effectiveness of various interventions related to INR testing.31 These included multifaceted education, with or without feedback, testing performed in the GP practice and computer-directed dosing. Activity-based costing (i.e. assigning costs to each activity) was used to determine global costs in these various intervention groups with the cost effectiveness being defined as the cost per additional day of the patient’s INR level being within 0.5 of the target INR level. The results showed that the intervention using INR levels measured in the GP surgery combined with multifaceted education was dominant over usual care because of increased quality (more patients with INR values closer to the target value) and less cost. Sensitivity analysis showed that this cost-effectiveness could be improved over time and with more practices adopting the intervention.
INR testing by general practitioners was also included in the trial of POCT in general practice carried out in Australia by Lawrence et al.32 together with diabetes related tests for HbA1c, urinary albumin/creatinine ratio (ACR) and lipids. The main outcome measures were “the proportion of patients and of tests with results within the target range, and change in test results from baseline”, The trial, in 4968 patients across 53 practices, showed no improvements in INR levels in those patients receiving POCT compared to central laboratory testing but significant improvements in POCT patients for HbA1c, ACR and all lipids except HDL.38 However the point estimate of direct costs to the health care system for POCT was greater for all tests except ACR but none of the differences were significant.32 While the trial identified that POCT provided significantly less cost for the patients as might be expected in patients living in locations some distance from a pathology laboratory, this cost did not have significant influence on the overall results.
Another RCT of INR testing in general practice has been reported by Parry et al. but only in the form of the costs associated with the two arms of the trial.35 Like the GP trial in Australia, effectiveness was measured through the proportion of people with INR values in the therapeutic range. The INR test values in the 122 patients in the GP intervention arm also had their dosing provided via a decision support system. In this study POCT patients did achieve better levels of control as judged by time in the therapeutic range as compared to usual care but the costs were higher - £170 versus £69 per year and this raises the question as to whether the healthcare system or society is prepared to pay this extra cost for reducing the risk of serious events.
Testing for CRP in general practice has been advocated as a means to combat over-prescribing of antibiotics in patients with lower respiratory tract infections. An economic analysis of this practice using a cluster RCT comparing usual care and both POCT and POCT for CRP in combination with communication skills, has been described by Cals et al.33 The authors chose the antibiotic prescribing at the index consultation as the outcome measure, with a 28 day study period for each patient. The results showed that both improved communications skills and POCT for CRP, both separately and together, were cost effective but communications skills alone was dominant meaning that it was both more effective and less costly than normal care. One of the challenges associated with this study is that reducing the prescription of antibiotics in order to reduce antibiotic resistance is a very long-term goal that is difficult to measure and it is not known whether society is willing to pay the increased costs of POCT that, in combination with communications skills, might reduce antibiotic resistance even more than the use of communication skills alone. The authors chose not to perform a cost utility analysis with QALYs as they considered it unreasonable to expect any difference for this short term illness. The authors suggested a different model would be required that linked “antibiotic resistance in society and the potential lives lost or saved”.
The management of dyspepsia or patients with Helicobacter pylori is a major task for GPs with many patients being referred for endoscopy. Delaney et al. investigated whether a strategy of POCT for Helicobacter and only sending positive patients for endoscopy could result in cost savings and patient improvements compared to usual care.36 The 478 patients were randomised to the two arms and at the end of 12 months effectiveness was assessed by symptoms and a quality of life questionnaire. The results of the trial showed no difference in effectiveness of the two arms with both groups of patients having improvements over time but the costs were higher in the intervention arm compared to normal care (£368 versus £253).
The role of POCT within hospitals is more established than in community care but good quality studies are relatively scarce, and economic evidence even rarer. One of the few RCTs in this area examines the potential economic impact of being able to provide a test more quickly and enable quicker clinical decisions such as patient discharge, thereby achieving potential operational savings. Fitzgerald et al. have provided an economic analysis from the Randomised Assessment of Treatment Using Panel Assay of Cardiac Markers or RATPAC Trial which showed that POCT did reduce hospital admissions compared to central laboratory testing.39 The outcomes measured in the economic analysis were QALYs and the costs associated with each patient’s stay in the hospital.34 The determination of the QALY was based on the combination of responses to a questionnaire examining five dimensions of health (EQ-5D) to give an individual tariff for each patient and survival data. Interestingly while admissions were reduced in the POCT group the economic costs were higher, and the probability that usual care dominated POCT (more effective and less expensive) was 0.888. This result is believed to be due to the fact that admissions in the POCT group resulted in more expensive items such as cardiac care and intensive care; whether this resulted in unmeasured patient benefits is unknown. A possible cause of these differences may be due to the POCT troponin assay being of a different analytical performance to that used in the central laboratory. This can only be resolved by carrying out a study using assays at POCT and in the laboratory with identical protocols including timing, cut-off values and analytical performance. Thokola et al. undertook a decision analysis model on a hypothetical population to estimate the cost effectiveness of delaying cardiac marker measurement, i.e. the consequence of not testing at presentation (effectively POCT) and using a similar approach to assessment of the QALY.40 They examined the use of a high sensitivity troponin assay and found that testing at presentation was the most cost effective. It might be considered unusual to employ the QALY as the outcome measure when it is the impact of a change in process that is being examined, and the use of survival data assumes that the post diagnostic care is the same as that for patients where testing is delayed by use of the central laboratory.
Economic Evidence from Systematic Reviews
Systematic reviews of economic analysis are not advisable because the cost structures and resource implications are not generalisable across jurisdictions. However, a systematic review of the literature on effectiveness is an important part of an economic evaluation. We located three such studies in the literature, two of which are devoted to INR testing41,42 and one to tests for deep vein thrombosis including D-Dimer.43 The latter includes tests by both laboratory and point of care and will not be considered further here, due to the predominance of the former and limited differentiation of the POCT studies.
The UK health technology assessment by Connock et al. reviewed all controlled trials of self-monitoring and self-management of INR compared to clinic care.41 This included seven studies of cost effectiveness but only the study done in the UK was considered applicable to that setting. However five of the identified studies favoured patient self-management compared to usual care. The report also developed a Markov model to evaluate cost-effectiveness similar to models in other studies and using input data from a previous trial of INR testing in the UK.44 Using a cost perspective from the UK NHS, the incremental cost of self-monitoring per QALY in 2007 was £122,365 over five years and £63,655 over 10 years. The estimated probability that self-monitoring is cost effective using a threshold of £30,000 /QALY is 44% over 10 years.
A similar health care technology assessment has been conducted in Belgium by Gailly et al.42 They also conducted a systematic review of the literature and identified three reviews and six primary economic evaluations. Their conclusion was that the cost effectiveness of POCT for INR is uncertain with many variables. For their own determination of cost-effectiveness based on Belgian cost data and data on the clinical effectiveness from their own meta-analysis, they limited their analysis to only the patient self-management model since they determined that this was the only POCT model that impacted on patient mortality. On the basis of a 26 tests per year protocol, patients who are self-managing are expected to gain 0.6 life-years compared to usual care with an incremental saving of €2964 over a 10 year period. For self-monitoring the authors showed that it can be cost saving depending on the number of tests and visits to GPs.
Cost Consequence Studies
Less robust but possibly informative evidence is provided by a number of observational studies of POCT that have included measurement of costs associated with the outcomes achieved in the studies but without determining cost effectiveness; we have categorised these as cost consequence studies. The settings of the papers highlighted below include self-monitoring, community testing and acute testing in hospitals.45–51
The first cited study examines the costs consequences of telemedicine being applied to support self-monitoring of blood glucose (SMBG). Kesavadev et al. demonstrated in a retrospective study of 1000 patients that following the telemedicine and SMBG intervention there was a significant fall in mean HbA1c over six months from levels at the start of the intervention.45 The deficiencies in this study, aside from the observational design, include the short time period of the study and limited cost analysis. The latter showed that the cost of the intervention including testing was estimated to be US$9.66 per month compared to the estimated cost of a visit to the Medical Centre of between US$5–15, although any change in the frequency of hospital clinic visits was not reported.
The number of patients on warfarin or coumadin therapy is growing as is the need for easy access to testing, particularly for patients who are not able to self-monitor or self-manage their therapy. Hospitals and clinics are playing a role here to speed up the testing process by providing POCT devices in clinics rather than sending specimens to central laboratories with consequent delays. Kong et al. compared the costs and outcomes from using POCT in an INR clinic in Singapore.46 They showed in a before-and-after study that there was no significant increase in complications such as thrombosis or bleeding following introduction of POCT. POCT enabled a reduction in the time of the average clinic visit of 35 minutes and the cost of testing was similar for both laboratory and POCT.
Several cost consequence type of studies are on the use of POCT in acute medicine areas, four within the emergency department (ED) and one in operating theatres. In the latter case, Spalding et al. examined the potential for cost savings through reduction in usage of blood products.47 In a before and after study of 1422 patients undergoing cardiac surgery the authors measured the effect of using a point of care device to measure various coagulation related parameters using the ROTEM® device in the theatre during surgery. The device enabled a substantial reduction in the use of blood products – from €125,828 to €55,925 – and this cost saving far outweighed the cost of the ROTEM® testing.
Another related application is the study of Mahieu et al. which looked at the impact of using at point of care, a multi-parameter critical care testing analyser, to reduce iatrogenic blood loss, anaemia and the consequent need for blood transfusion in neonatal patients.48 While the deployment of these instruments is commonplace, there are few studies which quantitate the potential benefits. In a retrospective cohort study the authors showed that after deployment of the analyser, there were significant decreases in central laboratory testing, as well as a significant reduction in babies requiring transfusion. The latter outcome led to a cost saving overall of 8.3% per neonate.
The need for quick results to enable quicker clinical decisions is one of the major reasons to use POCT in the ED and other acute areas. Like the study by Fitzgerald et al. cited previously Apple et al. looked at the cost consequences of providing rapid troponin testing in a short-stay cardiology unit.49 The study used an observational retrospective design with 271 patients being consecutively enrolled in the pre-POCT period where troponins were measured in the central laboratory and then in 274 patients following POCT adoption. The mean turnaround time of POCT troponins was significantly less than that from the central laboratory as was the mean length of stay of POCT patients. There was no overall difference in the clinical effectiveness of the two troponin strategies but there were substantial reductions in hospital charges in patients in the post POCT testing period. While this study showed the cost effectiveness of the POCT strategy, its observational design and small numbers are major limitations on its applicability.
Operational savings like those described above were also the target of a study by Mills et al. who used a POCT device to measure respiratory syncytial virus (RSV) on children presenting to the ED.50 Infection control is a key goal of such patients, primarily by isolating them in separate areas on admission. However this requires rapid transmission of test results to determine their infection status which is difficult to achieve with central laboratory testing. Using the POCT device for RSV, the authors showed in a prospective study over four months, that they saved 568 cubicle days in the ED by being able to immediately move patients after testing to a designated area. Thus significant extra capacity within the ED was generated through the rapid testing. The clinical performance of the assay was satisfactory for the purpose of separating infected patients. The additional cost of testing was relatively small when compared to costs such as the savings achieved through less cleaning of cubicles within the ED.
Another application of rapid testing in the ED for an infectious agent is described by Hatamabadi et al.51 They used the Tetanus Quick Stick to determine the vaccination status of patients and to guide therapy in those patients with certain tetanus-prone wounds. This therapy is relatively expensive and by only giving it to patients who do not have adequate immunisation as determined by the rapid test, the therapy savings amounted to a reduction in cost per patient from €12.1 to €9.48.
Use of Modelling Studies to Generate Economic Outcomes
As was discussed in the introduction, modelling or decision analysis tools offer a theoretical analysis of possible economic outcomes based on cost-effectiveness and cost-utility analysis. The strength of their overall validity is dependent on several factors, one of which is the quality or robustness of the data that is input into the model. This data includes test performance e.g. sensitivity and specificity, the prevalence of the disease and its complications in the population on whom the model is being used and accurate treatment costs.
We identified 23 papers that used models of various kinds to generate outcomes. They included: six papers on SMBG,52–57 13 on various infectious diseases papers including a systematic review58–71 and one each on INR,72 D-Dimer73 and fibronectin testing.74 Not all these studies will be reviewed in detail, but salient points from a selection of them will be highlighted.
The significant numbers of papers in the literature on modelling outcomes from SMBG is indicative of the controversial nature of this type of testing. The provision of testing strips to an increasing number of type 2 diabetics represents a significant cost in many countries and yet the evidence for their clinical effectiveness remains unclear as we saw in the RCT of Simon et al. described previously.28 The six modelling studies of SMBG that we cite in this review generate data for healthcare systems based in five different countries - US, UK, Canada, Czech Republic and Switzerland. However the inputs such as diabetes outcomes and reductions in HbA1c from SMBG are often from different countries such as the UK PDS, Kaiser Permanente in the US or the ROSSO study from Germany. Most papers use a Markov model and in two papers this was used in conjunction with the same CORE outcomes projection model while the study of Weber et al. used a so-called matched-pair analysis technique to project outcome costs.
A study from Canada by Cameron et al. used the UKPDS to forecast complications and cost consequences while a reduction in HbA1c by 0.25% from SMBG was factored into the model.52 Their findings indicated that SMBG of more than seven times per week was not an efficient use of resources while less frequent testing might be more cost effective. In contrast Palmer et al. in their Markov model used a higher figure for the decrease in HbA1c due to SMBG, as shown by data from a large observational model, and consequently showed that the cost-effectiveness ratios supported using SMBG in the UK.53 Papers documenting modelling or similar studies are all supportive of the cost effectiveness of SMBG in United States,54 Switzerland,55 and the Czech Republic.56 However in a review of modelling studies of SMBG in Canada Tunis et al. highlights how dependent the models are on inputs and how the assumptions used can affect the resulting cost-effectiveness findings.57
Another specialty area where modelling studies have often been used is for infectious disease testing in developing nations where for various reasons, access to effective laboratory based testing is limited and the mortality and morbidity due to diseases such as syphilis and HIV remain high. By definition such countries have extremely limited healthcare budgets and so a key question is whether the use of relatively expensive POCT devices (compared to laboratory tests) is an efficient use of resources. Performing RCTs in such countries is difficult so modelling using decision analysis can provide useful information and possibly guide future trials and/or future testing strategies.
A typical example of such a study is that of Rydzak et al. who investigated the cost effectiveness of prenatal syphilis screening in sub-Saharan Africa using POCT.58 Their model was populated with data from the literature on the acquisition and natural history of syphilis in pregnant women over their lifetime. They compared testing strategies that involved no screening, testing by both an initial rapid test followed by confirmatory Treponema pallidum test and testing also by a POCT immunochromatographic strip. The outcomes from the model included adverse pregnancy outcomes, lifetime costs and incremental cost effectiveness ratios. The results from the model showed that the most cost effective strategy was using POCT during the initial visit which saved $170,020 per 1000 women over their lifetime compared to no screening and substantially decreased negative pregnancy outcomes.
Another similar modelling study of antenatal syphilis screening in Tanzania identified that the cost effectiveness of POCT tests is dependent on their costs and sensitivity for high titre active syphilis.59 Vickerman et al. showed while POCT had the potential to save more disability-adjusted life years (DALYs), the tests needed to be less costly than the existing laboratory test in order to be cost-effective. However the existing test requires a well-equipped laboratory and since few of these exist in rural locations, POCT has the potential to enable more screening for maternal syphilis to take place, thereby improving mortality and morbidity.
Modelling has been used to determine the potential benefits of POCT in a wide variety of areas including malaria,60 HIV,61,62, syphilis,63 gonorrhea,64 chlamydia,65,66 Helicobacter pylori,67 adenoviral conjunctivitis,68 pharyngitis,69 influenza,70,71 INR,72 D-dimer73 and fetal fibronectin.74 The majority of such studies have shown results which are either supportive of POCT or at least indicate that it has potential which should be further investigated through trials or other observational studies.
One of the benefits of modelling is that it includes sensitivity analysis, whereby the effects of changes in both probabilities (e.g. of health states) and costs and can be examined. This can then be used to examine the robustness of the evaluation of the technology, as well as proving a more critical dimension to the appraisal of the process of care when considering the adoption of new technology. Sensitivity analysis was utilised in several of the studies mentioned in this review (e.g. Cals et al.,33 Fitgerald et al.,34 Thokola et al.40).
Conclusions
The findings of this review illustrate a very confused picture regarding the economic analysis of POCT, as well as the overall picture regarding the value of diagnostics and the reimbursement strategies used across the world. In broad terms reimbursement strategies are based on the complexity of the test, which can be summarised as the cost of the reagents and the investment in resources to perform the test. It could be argued that this approach has spawned the business model that is prevalent in laboratory medicine, namely one that rewards on the basis of cost per test, and thence a drive to perform more tests to sustain the business. This has led to an explosion in automation of testing to increase the productivity of the testing service. However this development has been at the expense of the immediacy in the relationship between clinician and patient. The value of tests are then seen in the context of their cost rather than their impact on health outcomes. This is now being challenged in laboratory medicine with the emergence of “genetic tests” and the emergence of so-called personalised medicine, and companion diagnostics, although these are not in any way new concepts.75,76 The talk is now of a more value-based approach to reimbursement with the advent of new genetic markers, whilst the concept has also found advocates in other diagnostic modalities, e.g. imaging.77 The relevance of this is that value only accrues when the test is used and there is an improvement in the patient’s health, as observed by Ferrante di Ruffano et al.19 i.e. test results in themselves have no value. This is therefore the way in which the value of POCT has to be assessed, as by definition the cost of the POCT consumable is likely to be greater than laboratory testing, as a result of the loss of the economy of scale that can be achieved through the use of automation and centralised facilities. However a simple observation of the saving by reducing the need for a clinic visit illustrates the potential economic benefit of POCT.
When investigating the impact of new technology in healthcare there are three primary considerations (i) the impact on patient outcomes, (ii) the impact on the process of care, and (iii) the impact on resource utilisation. The first is concerned with the raison d’etre of the technology, and the innovation, whilst the other two are concerned with the adoption of the technology. All innovation involves change by definition, and in the case of POCT, this is more apparent. The primary outcomes relate to the patient outcomes, and their impact (including economic) on the individual patient and society. The impact on process and resource utilisation is more focussed on healthcare provision. POCT is, in itself, a change of the (delivery) process, although it may also impact on other parts of the care pathway process, as well as the impact of rapid decision making.
Such considerations require some basic information at the outset if assessing the potential use of POCT. Fundamental to this is a clear understanding of the care process, the resource utilisation associated with that pathway and the performance metrics associated with that pathway. The second requirement is to determine the unmet need that has led to consideration of POCT as a solution; this enables the appropriate evidence to be sought, and it is this evidence that underpins the assessment of cost effectiveness. It has been recognised on a number of occasions that the quality of evidence is poor. This has been in part due to a failure to account for the points made earlier, as well as poor quality of study design. As a consequence the evidence of clinical effectiveness is poor. This, together with a poor understanding of the financial resources involved in delivering the diagnostic elements of the care pathway, and the downstream consequences contribute to a weak literature on the cost effectiveness of POCT. It is clear from this review that there are only a small number of cost effectiveness studies of POCT that have been undertaken, despite the fact that it would appear that POCT has the potential to radically change the process of care, and make big savings in the utilisation of resources, e.g. by reducing emergency admissions, reducing hospitalisations, reducing the length of stay, as well as enabling more care to be delivered closer to home.
It has been acknowledged, however, that if a process does not change with the introduction of POCT you cannot expect there to be any saving.78 Another problem may be an inability to apportion specific costs to individual elements of a care process, and therefore to then be able to quantify the potential disinvestment. In many health systems resources are bundled into a single payment e.g. the diagnostic related group (DRG) or health related group (HRG) payment. Whilst this may mean that information on actual costs incurred may be difficult to obtain, it also means that the translation into practice is complicated by a need to change a payment group.
This discussion is moving from the assessment of the utility of a new technology to adoption, and this is where the application of cost effectiveness data is important. Assessment of cost effectiveness is primarily of use to the policymaker and the purchaser, as against the healthcare provider charged with adoption of the technology in order to meet a recognised unmet need. However cost effectiveness also should be informing the reimbursement strategy; this can be tested during the cost effectiveness analysis using sensitivity analysis. However, whilst it might be possible to identify an appropriate level of reimbursement, the adoption strategy has to go back to the initial evidence relating to resource utilisation in order to plan the implementation programme, and in particular the disinvestment requirement. Whilst translating potential savings on utilisation of drugs or blood products into practice may be straightforward, leveraging the reduction in length of stay, or hospitalisation episodes requires more careful consideration. However it is these savings that are often identified in the evidence generated on the use of POCT. This is where cost consequence analysis may have more practical value in promoting innovation and adoption of new technologies.
We conclude from this review that the evidence on cost effectiveness of POCT is limited. This is in part due to the limited quality of evidence of clinical effectiveness. There is a need for better understanding of the care pathway and the processes embedded in those pathways, and how they will change with the introduction of POCT. There is also a need to have better information on the resource utilisation across all elements of the care pathway, and how that can be leveraged for economic benefit to the stakeholders with the introduction of POCT. In order for this to be of value to both policymakers and providers there needs to be an understanding of the financial flows with all of the provider organisations delivering the full care pathway, and a willingness to modify those flows in the interest of all stakeholders.
Footnotes
Competing Interests: None declared (CPP). AStJ has received consultant fees from Abbott Diagnostics.
References
- 1.Kohn LT, Corrigan JM, Donaldson MS, editors. To Err Is Human: Building a Safer Health System. Washington, DC: Institute of Medicine, National Academies Press; 2000. pp. 1–287. [PubMed] [Google Scholar]
- 2.Australian Government Department of Health and Ageing Pathology services to be better managed and funded, delivering $550 million saving to taxpayer. http://www.health.gov.au/internet/ministers/publishing.nsf/Content/mr-yr11-nr-nr065.htm?OpenDocument&yr=2011&mth=04 (Accessed 4 July 2013).
- 3.Birenbaum MS. Medical Laboratory Observer; Feb, 2011. Healthcare reform: dumping on Medicare Part B. http://www.mlo-online.com/articles/201102/healthcare-reform-rn-dumping-on-medicare-part-b.php (Accessed 4 July 2013). [PubMed] [Google Scholar]
- 4.Beastall GH. The modernisation of pathology and laboratory medicine in the UK: networking into the future. Clin Biochem Rev. 2008;29:3–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkozi L, Simson E, Ramanathan L. The effects of total laboratory automation on the management of a clinical chemistry laboratory. Retrospective analysis of 36 years. Clin Chim Acta. 2003;329:89–94. doi: 10.1016/s0009-8981(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 6.Callahan D. Health care costs and medical technology. In: Crowley M, editor. From Birth to Death and Bench to Clinic: The Hastings Center Bioethics Briefing Book for Journalists, Policymakers, and Campaigns. Garrison, NY: The Hastings Center; 2008. pp. 79–82. [Google Scholar]
- 7.Robert G, Greenhalgh T, MacFarlane F, Peacock R. Organisational factors influencing technology adoption and assimilation in the NHS: a systematic literature review. 2009. www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1819-223_V01.pdf (Accessed 4 July 2013).
- 8.Cutler DM. Where are the health care entrepreneurs? The failure of organizational innovation in health care. http://ideas.repec.org/p/nbr/nberwo/16030.html (Accessed 4 July 2013).
- 9.Council of the European Union Council conclusions on innovation in the medical device sector. http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/lsa/122397.pdf (Accessed 4 July 2013).
- 10.Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83:691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medical Services Advisory Committee Department of Health and Ageing, Australian Government. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/guidelines-2012 (Accessed 4 July 2013).
- 12.Medical Technologies Evaluation Programme National Institute for Health and Care Excellence, UK. http://www.nice.org.uk/aboutnice/whatwedo/aboutmedicaltechnologies/medicaltechnologiesprogramme.jsp (Accessed 4 July 2013).
- 13.Severens JL, van der Wilt G-J. Economic evaluation of diagnostic tests. A review of published studies. Int J Technol Assess Health Care. 1999;15:480–96. doi: 10.1017/s0266462399153169. [DOI] [PubMed] [Google Scholar]
- 14.Fang C, Otero HJ, Greenberg D, Neumann PJ. Cost-utility analyses of diagnostic laboratory tests: a systematic review. Value Health. 2011;14:1010–8. doi: 10.1016/j.jval.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 15.Drummond MF, Sculphur MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford, UK: Oxford University Press; 2004. pp. 1–396. [Google Scholar]
- 16.Scott MA, Raftery JP. Evidence-based laboratory medicine: health economics, reimbursement, and the value proposition. In: Price CP, Christenson RH, editors. Evidence-Based Laboratory Medicine. Second Edition. Washington DC: AACC Press; 2007. pp. 321–45. [Google Scholar]
- 17.St John A, Price CP. Health economics and point of care testing. In: Price CP, St John A, Kricka LL, editors. Point of Care Testing. Third Edition. Washington DC: AACC Press; 2010. pp. 267–78. [Google Scholar]
- 18.Sackett DL, Haynes RB. The architecture of diagnostic research. BMJ. 2002;324:539–41. doi: 10.1136/bmj.324.7336.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, Bossuyt PM, Deeks JJ. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ. 2012;344:e686. doi: 10.1136/bmj.e686. [DOI] [PubMed] [Google Scholar]
- 20.Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making. 1990;10:212–4. doi: 10.1177/0272989X9001000308. [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence Glossary. http://www.nice.org.uk/website/glossary/glossary.jsp?alpha=C (Accessed 4 July 2013).
- 22.Kaufman R, Watkins R. Cost consequences analysis; a case study. Human Resources Development Quarterly. 1996;7:87–100. [Google Scholar]
- 23.Wilkinson D. Cost-benefit analysis versus cost-consequences analysis. Performance Improvement Quarterly. 1999;12:71–81. [Google Scholar]
- 24.Wilson JM, Junger G. Principles and practice of screening for disease. 1968. World Health Organization Public Health Papers No. 34. http://whqlibdoc.who.int/php/WHO_PHP_34.pdf (Accessed 4 July 2013).
- 25.Lord SJ, Irwig L, Simes RJ. When is measuring sensitivity and specificity sufficient to evaluate a diagnostic test, and when do we need randomized trials? Ann Intern Med. 2006;144:850–5. doi: 10.7326/0003-4819-144-11-200606060-00011. [DOI] [PubMed] [Google Scholar]
- 26.Lord SJ, Irwig L, Bossuyt PM. Using the principles of randomized controlled trial design to guide test evaluation. Med Decis Making. 2009;29:E1–12. doi: 10.1177/0272989X09340584. [DOI] [PubMed] [Google Scholar]
- 27.Price CP. Evidence-based laboratory medicine: is it working in practice? Clin Biochem Rev. 2012;33:13–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A, Diabetes Glycaemic Education and Monitoring Trial Group Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336:1177–80. doi: 10.1136/bmj.39526.674873.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton AJ, Cooper NJ, Goodacre S, Stevenson M. Integration of meta-analysis and economic decision modeling for evaluating diagnostic tests. Med Decis Making. 2008;28:650–67. doi: 10.1177/0272989X08324036. [DOI] [PubMed] [Google Scholar]
- 30.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claes N, Moeremans K, Frank B, Jef A, Jos V, Herman VL, et al. Estimating the cost-effectiveness of quality-improving interventions in oral anticoagulation management within general practice. Value Health. 2006;9:369–76. doi: 10.1111/j.1524-4733.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 32.Laurence CO, Moss JR, Briggs NE, Beilby JJ, PoCT Trial Management Group The cost-effectiveness of point of care testing in a general practice setting: results from a randomised controlled trial. BMC Health Serv Res. 2010;10:165. doi: 10.1186/1472-6963-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cals JW, Ament AJ, Hood K, Butler CC, Hopstaken RM, Wassink GF, et al. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. J Eval Clin Pract. 2011;17:1059–69. doi: 10.1111/j.1365-2753.2010.01472.x. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald P, Goodacre SW, Cross E, Dixon S. Cost-effectiveness of point-of-care biomarker assessment for suspected myocardial infarction: the randomized assessment of treatment using panel assay of cardiac markers (RATPAC) trial. Acad Emerg Med. 2011;18:488–95. doi: 10.1111/j.1553-2712.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- 35.Parry D, Fitzmaurice D, Raftery J. Anticoagulation management in primary care: a trial-based economic evaluation. Br J Haematol. 2000;111:530–3. doi: 10.1046/j.1365-2141.2000.02360.x. [DOI] [PubMed] [Google Scholar]
- 36.Delaney BC, Wilson S, Roalfe A, Roberts L, Redman V, Wearn A, et al. Randomised controlled trial of Helicobacter pylori testing and endoscopy for dyspepsia in primary care. BMJ. 2001;322:898–901. doi: 10.1136/bmj.322.7291.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heneghan C, Alonso-Coello P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet. 2006;367:404–11. doi: 10.1016/S0140-6736(06)68139-7. [DOI] [PubMed] [Google Scholar]
- 38.Bubner TK, Laurence CO, Gialamas A, Yelland LN, Ryan P, Willson KJ, et al. Effectiveness of point-of-care testing for therapeutic control of chronic conditions: results from the PoCT in General Practice Trial. Med J Aust. 2009;190:624–6. doi: 10.5694/j.1326-5377.2009.tb02590.x. [DOI] [PubMed] [Google Scholar]
- 39.Goodacre SW, Bradburn M, Cross E, Collinson P, Gray A, Hall AS, RATPAC Research Team The Randomised Assessment of Treatment using Panel Assay of Cardiac Markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart. 2011;97:190–6. doi: 10.1136/hrt.2010.203166. [DOI] [PubMed] [Google Scholar]
- 40.Thokala P, Goodacre SW, Collinson PO, Stevens JW, Mills NL, Newby DE, et al. Cost-effectiveness of presentation versus delayed troponin testing for acute myocardial infarction. Heart. 2012;98:1498–503. doi: 10.1136/heartjnl-2012-302188. [DOI] [PubMed] [Google Scholar]
- 41.Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess. 2007;11:iii–iv. ix–66. doi: 10.3310/hta11380. [DOI] [PubMed] [Google Scholar]
- 42.Gailly J, Gerkens S, Van Den Bruel A, Devriese S, Obyn C, Cleemput I. Use of point-of-care devices in patients with oral anticoagulation : a health technology assessment. Brussels: Belgian Health Care Knowledge Centre (KCE); 2009. KCE Reports 117C. [Google Scholar]
- 43.Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al. Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis. (iii–iv) Health Technol Assess. 2006;10:1–168. iii–iv. doi: 10.3310/hta10150. [DOI] [PubMed] [Google Scholar]
- 44.Jowett S, Bryan S, Murray E, McCahon D, Raftery J, Hobbs FD, et al. Patient self-management of anticoagulation therapy: a trial-based cost-effectiveness analysis. Br J Haematol. 2006;134:632–9. doi: 10.1111/j.1365-2141.2006.06243.x. [DOI] [PubMed] [Google Scholar]
- 45.Kesavadev J, Shankar A, Pillai PB, Krishnan G, Jothydev S. Cost-effective use of telemedicine and self-monitoring of blood glucose via Diabetes Tele Management System (DTMS) to achieve target glycosylated hemoglobin values without serious symptomatic hypoglycemia in 1,000 subjects with type 2 diabetes mellitus—a retrospective study. Diabetes Technol Ther. 2012;14:772–6. doi: 10.1089/dia.2012.0088. [DOI] [PubMed] [Google Scholar]
- 46.Kong MC, Lim TG, Ng HJ, Chan YH, Lee LH. Feasibility, cost-effectiveness and patients’ acceptance of point-of-care INR testing in a hospital-based anticoagulation clinic. Ann Hematol. 2008;87:905–10. doi: 10.1007/s00277-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 47.Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of “bedside” thrombelastography (ROTEM) Eur J Cardiothorac Surg. 2007;31:1052–7. doi: 10.1016/j.ejcts.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Mahieu L, Marien A, De Dooy J, Mahieu M, Mahieu H, Van Hoof V. Implementation of a multi-parameter Point-of-Care-blood test analyzer reduces central laboratory testing and need for blood transfusions in very low birth weight infants. Clin Chim Acta. 2012;413:325–30. doi: 10.1016/j.cca.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 49.Apple FS, Chung AY, Kogut ME, Bubany S, Murakami MM. Decreased patient charges following implementation of point-of-care cardiac troponin monitoring in acute coronary syndrome patients in a community hospital cardiology unit. Clin Chim Acta. 2006;370:191–5. doi: 10.1016/j.cca.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Mills JM, Harper J, Broomfield D, Templeton KE. Rapid testing for respiratory syncytial virus in a paediatric emergency department: benefits for infection control and bed management. J Hosp Infect. 2011;77:248–51. doi: 10.1016/j.jhin.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Hatamabadi HR, Abdalvand A, Safari S, Kariman H, Dolatabadi AA, Shahrami A, et al. Tetanus Quick Stick as an applicable and cost-effective test in assessment of immunity status. Am J Emerg Med. 2011;29:717–20. doi: 10.1016/j.ajem.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 52.Cameron C, Coyle D, Ur E, Klarenbach S. Cost-effectiveness of self-monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin. CMAJ. 2010;182:28–34. doi: 10.1503/cmaj.090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer AJ, Dinneen S, Gavin JR, 3rd, Gray A, Herman WH, Karter AJ. Cost-utility analysis in a UK setting of self-monitoring of blood glucose in patients with type 2 diabetes. Curr Med Res Opin. 2006;22:861–72. doi: 10.1185/030079906X104669. [DOI] [PubMed] [Google Scholar]
- 54.Tunis SL, Minshall ME. Self-monitoring of blood glucose in type 2 diabetes: cost-effectiveness in the United States. Am J Manag Care. 2008;14:131–40. [PubMed] [Google Scholar]
- 55.Pollock RF, Valentine WJ, Goodall G, Brändle M. Evaluating the cost-effectiveness of self-monitoring of blood glucose in type 2 diabetes patients on oral anti-diabetic agents. Swiss Med Wkly. 2010;140:w13103. doi: 10.4414/smw.2010.13103. [DOI] [PubMed] [Google Scholar]
- 56.Weber C, Kocher S, Neeser K, Bartaskova D. Impact of self-measurement of blood glucose on complications of type 2 diabetes: economic analysis from a Czech perspective. Curr Med Res Opin. 2010;26:289–96. doi: 10.1185/03007990903479224. [DOI] [PubMed] [Google Scholar]
- 57.Tunis SL. Cost effectiveness of self-monitoring of blood glucose (SMBG) for patients with type 2 diabetes and not on insulin: impact of modelling assumptions on recent Canadian findings. Appl Health Econ Health Policy. 2011;9:351–65. doi: 10.2165/11594270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Rydzak CE, Goldie SJ. Cost-effectiveness of rapid point-of-care prenatal syphilis screening in sub-Saharan Africa. Sex Transm Dis. 2008;35:775–84. doi: 10.1097/OLQ.0b013e318176196d. [DOI] [PubMed] [Google Scholar]
- 59.Vickerman P, Peeling RW, Terris-Prestholt F, Changalucha J, Mabey D, Watson-Jones D, et al. Modelling the cost-effectiveness of introducing rapid syphilis tests into an antenatal syphilis screening programme in Mwanza, Tanzania. Sex Transm Infect. 2006;82(Suppl 5):v38–43. doi: 10.1136/sti.2006.021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, et al. Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull World Health Organ. 2008;86:101–10. doi: 10.2471/BLT.07.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vickerman P, Watts C, Peeling RW, Mabey D, Alary M. Modelling the cost effectiveness of rapid point of care diagnostic tests for the control of HIV and other sexually transmitted infections among female sex workers. Sex Transm Infect. 2006;82:403–12. doi: 10.1136/sti.2006.020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doyle NM, Levison JE, Gardner MO. Rapid HIV versus enzyme-linked immunosorbent assay screening in a low-risk Mexican American population presenting in labor: a cost-effectiveness analysis. Am J Obstet Gynecol. 2005;193:1280–5. doi: 10.1016/j.ajog.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Owusu-Edusei K, Jr, Gift TL, Ballard RC. Cost-effectiveness of a dual non-treponemal/treponemal syphilis point-of-care test to prevent adverse pregnancy outcomes in sub-Saharan Africa. Sex Transm Dis. 2011;38:997–1003. doi: 10.1097/OLQ.0b013e3182260987. [DOI] [PubMed] [Google Scholar]
- 64.Aledort JE, Hook EW, 3rd, Weinstein MC, Goldie SJ. The cost effectiveness of gonorrhea screening in urban emergency departments. Sex Transm Dis. 2005;32:425–36. doi: 10.1097/01.olq.0000154501.22566.fa. [DOI] [PubMed] [Google Scholar]
- 65.Hislop J, Quayyum Z, Flett G, Boachie C, Fraser C, Mowatt G. Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men. (iii–iv) Health Technol Assess. 2010;14:1–97. iii–iv. doi: 10.3310/hta14290. [DOI] [PubMed] [Google Scholar]
- 66.Swain GR, McDonald RA, Pfister JR, Gradus MS, Sedmak GV, Singh A. Decision analysis: point-of-care Chlamydia testing vs. laboratory-based methods. Clin Med Res. 2004;2:29–35. doi: 10.3121/cmr.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fauli S, Thue G. Economic consequences of near-patient test results: the case of tests for the Helicobacter Pylori bacterium in dyspepsia. Eur J Health Econ. 2008;9:221–8. doi: 10.1007/s10198-007-0064-3. [DOI] [PubMed] [Google Scholar]
- 68.Udeh BL, Schneider JE, Ohsfeldt RL. Cost effectiveness of a point-of-care test for adenoviral conjunctivitis. Am J Med Sci. 2008;336:254–64. doi: 10.1097/MAJ.0b013e3181637417. [DOI] [PubMed] [Google Scholar]
- 69.Neuner JM, Hamel MB, Phillips RS, Bona K, Aronson MD. Diagnosis and management of adults with pharyngitis. A cost-effectiveness analysis. Ann Intern Med. 2003;139:113–22. doi: 10.7326/0003-4819-139-2-200307150-00011. [DOI] [PubMed] [Google Scholar]
- 70.Siddiqui MR, Edmunds WJ. Cost-effectiveness of antiviral stockpiling and near-patient testing for potential influenza pandemic. Emerg Infect Dis. 2008;14:267–74. doi: 10.3201/eid1402.070478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sintchenko V, Gilbert GL, Coiera E, Dwyer D. Treat or test first? Decision analysis of empirical antiviral treatment of influenza virus infection versus treatment based on rapid test results. J Clin Virol. 2002;25:15–21. doi: 10.1016/s1386-6532(00)00182-7. [DOI] [PubMed] [Google Scholar]
- 72.Gerkens S, Gailly J, Obyn C, Devriese S, Cleemput I. Economic evaluation of the use of point-of-care devices in patients with long term oral anticoagulation. J Thromb Thrombolysis. 2012;34:300–9. doi: 10.1007/s11239-012-0715-9. [DOI] [PubMed] [Google Scholar]
- 73.Ten Cate-Hoek AJ, Toll DB, Büller HR, Hoes AW, Moons KG, Oudega R, et al. Cost-effectiveness of ruling out deep venous thrombosis in primary care versus care as usual. J Thromb Haemost. 2009;7:2042–9. doi: 10.1111/j.1538-7836.2009.03627.x. [DOI] [PubMed] [Google Scholar]
- 74.Sullivan A, Hueppchen NA, Satin AJ. Cost effectiveness of bedside fetal fibronectin testing varies according to treatment algorithm. J Matern Fetal Med. 2001;10:380–4. doi: 10.1080/714052778. [DOI] [PubMed] [Google Scholar]
- 75.Ramsey SD, Veenstra DL, Garrison LP, Jr, Carlson R, Billings P, Carlson J, et al. Toward evidence-based assessment for coverage and reimbursement of laboratory-based diagnostic and genetic tests. Am J Manag Care. 2006;12:197–202. [PubMed] [Google Scholar]
- 76.Lindor RA, Allocco SJ, Cheatham L, Cortese DA, Hall RF, Mangold WJ, Jr, et al. Regulatory and reimbursement innovation. Sci Transl Med. 2013;5:176cm3. doi: 10.1126/scitranslmed.3005437. [DOI] [PubMed] [Google Scholar]
- 77.Garrison LP, Jr, Bresnahan BW, Higashi MK, Hollingworth W, Jarvik JG. Innovation in diagnostic imaging services: assessing the potential for value-based reimbursement. Acad Radiol. 2011;18:1109–14. doi: 10.1016/j.acra.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Khunti K, Stone MA, Burden AC, Turner D, Raymond NT, Burden M, et al. Randomised controlled trial of near-patient testing for glycated haemoglobin in people with type 2 diabetes mellitus. Br J Gen Pract. 2006;56:511–7. [PMC free article] [PubMed] [Google Scholar]
- 79.The Physician Consortium for Performance Improvement, American Medical Association http://www.ama-assn.org/resources/doc/cqi/pcpi-outcome-measures-framework.pdf (Accessed 4 July 2013). [PubMed]