Significance
Marine organisms disperse mostly by ocean currents as larval propagules. Therefore, it is commonly thought that the duration of the larval stage is the fundamental determinant of geographic range size. Using a global compilation of reef fish traits, we test an alternative hypothesis: adult traits associated with population establishment and persistence in novel areas are better predictors of geographic range size than larval traits. We conclude that colonization success is as primary determinant of successful range extension and of geographic range size among tropical reef fishes.
Keywords: macroecology, marine dispersal, colonization
Abstract
Most marine organisms disperse via ocean currents as larvae, so it is often assumed that larval-stage duration is the primary determinant of geographic range size. However, empirical tests of this relationship have yielded mixed results, and alternative hypotheses have rarely been considered. Here we assess the relative influence of adult and larval-traits on geographic range size using a global dataset encompassing 590 species of tropical reef fishes in 47 families, the largest compilation of such data to date for any marine group. We analyze this database using linear mixed-effect models to control for phylogeny and geographical limits on range size. Our analysis indicates that three adult traits likely to affect the capacity of new colonizers to survive and establish reproductive populations (body size, schooling behavior, and nocturnal activity) are equal or better predictors of geographic range size than pelagic larval duration. We conclude that adult life-history traits that affect the postdispersal persistence of new populations are primary determinants of successful range extension and, consequently, of geographic range size among tropical reef fishes.
Geographic range size is a fundamental biogeographic variable that, among other effects (1, 2), strongly influences a species susceptibility to extinction (3, 4). Because most marine organisms disperse as larval propagules transported by ocean currents, it is often assumed that the duration of the larval stage is the fundamental determinant of their dispersal ability, and hence their range size (5, 6). Tropical reef fishes have geographic ranges that vary greatly in size, from a few square kilometers around tiny isolated islands to entire ocean basins (7–9). Given that pelagic larval duration (PLD) also varies greatly among such fishes, from only a few days to many months, the effects of PLD on dispersal potential became an early focus of investigation on general determinants of range size among those fishes and other near-shore marine species (10–12). However, although it has become evident that PLD is unlikely to be a primary determinant of geographic range size (13–16), alternative hypotheses have only recently begun to be considered (9).
To expand its geographic range, a species must successfully colonize new areas following the dispersal of its propagules (17). Consequently, attributes other than pelagic dispersal capacity may largely determine how widely reef fishes are distributed geographically (9). Here we assess the relative importance of seven adult and larval traits in influencing geographic range sizes of tropical reef fishes at the global scale. We do so using data from 590 species of tropical reef fishes in 47 families, the largest compilation of such data currently available for any marine group (Dataset S1). Traits directly linked to larval dispersal potential include PLD and spawning mode. Adult traits include maximum body size, schooling behavior, nocturnal activity, use of multiple habitat types, and adult depth range. The adult-biology traits chosen are not directly related to larval dispersal potential, but may influence the propensity for range expansion by affecting the establishment and persistence of new populations, as suggested by a recent study on Atlantic reef fishes (9). For example, schooling (18, 19) and nocturnal activity (20) reduce predation risk and thereby increase the chance of postsettlement survival. Broad habitat use and depth range indicate ecological generality, which is thought to influence establishment success in new environments (21). Finally, body size is linked to both predation risk and ecological generality (22).
Evaluation of these hypotheses is challenging because species traits are phylogenetically nonindependent (23) and unevenly distributed among families. Previous studies of dispersal–range-size relationships have controlled for effects of phylogeny, and limits on range-size imposed by ocean-basin size, by separately analyzing subsets of data (7, 16). However, this approach reduces statistical power (23, 24) and the ability to assess the generality of the effects of different factors. Our analysis controls for the nonindependence of shared traits among related species by using linear mixed-effects modeling (LMM) treating family and genus as nested random effects (9, 23). Our analysis includes species from three different regions that vary greatly in maximum (longitudinal or latitudinal) extent: the Indo-Central Pacific (ICP; ∼22,000 km), the tropical Atlantic (TA; ∼12,000 km), and the tropical eastern Pacific (TEP; ∼5,000 km). To control for this variation, we include region and its interactions with other variables as fixed effects in our models. Modeling the data in this way, we are unique in being able to assess the relative importance of various adult and larval traits as determinants of range size among tropical reef-fish, as a group, at the global scale.
Results and Discussion
Our analysis shows that region is the most significant predictor of geographic range size (Table 1). This result could be because of constraints of regional extent on range size within a region, or to differences in the underlying structure of the range-size frequency distribution within each region. To assess the importance of regional extent, we repeated the analysis after expressing the range size of each species as the ratio between the range size of species and the extent of the region. When range sizes were standardized in this way, all effects of species traits were maintained, and the region effect declined from the most important to the least important predictor (Table 1).
Table 1.
Significant variables ranked according to their independent effects
| All species (n = 590) |
Trans-Pacific species removed (n = 564) |
||||||||
| Variable | df | F value | P value | IE (%) | Variable | df | F value | P value | IE (%) |
| Geographic range size | |||||||||
| Region | 2 | 117.83 | <0.001 | 53.3 | Region | 2 | 104.80 | <0.001 | 66.9 |
| PLD | 1 | 54.72 | <0.001 | 17.3 | Body size | 1 | 31.44 | <0.001 | 9.6 |
| Body size | 1 | 69.86 | <0.001 | 15.5 | Nocturnal | 1 | 16.69 | <0.001 | 8.4 |
| Nocturnal | 1 | 18.22 | <0.001 | 7.8 | Schooling | 1 | 15.45 | <0.001 | 8.0 |
| Schooling | 1 | 12.63 | <0.001 | 6.0 | PLD | 1 | 12.23 | <0.001 | 6.9 |
| Ratio between the species range size and region size | |||||||||
| Body size | 1 | 111.70 | <0.001 | 37.6 | Body size | 1 | 90.06 | <0.001 | 36.0 |
| PLD | 1 | 23.28 | <0.001 | 24.8 | Nocturnal | 1 | 38.13 | <0.001 | 25.9 |
| Nocturnal | 1 | 33.17 | <0.001 | 19.4 | Schooling | 1 | 12.94 | <0.001 | 16.2 |
| Schooling | 1 | 11.47 | <0.001 | 13.9 | PLD | 1 | 11.62 | 0.001 | 13.3 |
| Region | 2 | 2.09 | 0.123 | 4.2 | Region | 2 | 3.48 | 0.031 | 8.3 |
The independent effects (IE) value corresponds to the percentage of the explained variance accounted for by each explanatory variable as calculated using hierarchical partitioning. In Geographic range size, ranking is according to IE on geographic range size. In the ratio between the species range size and region size ranking is according to IE on the ratio between the species range size and region size. Degrees of freedom (df), test statistics (F value), and probabilities (P value) are listed for each coefficient in each model.
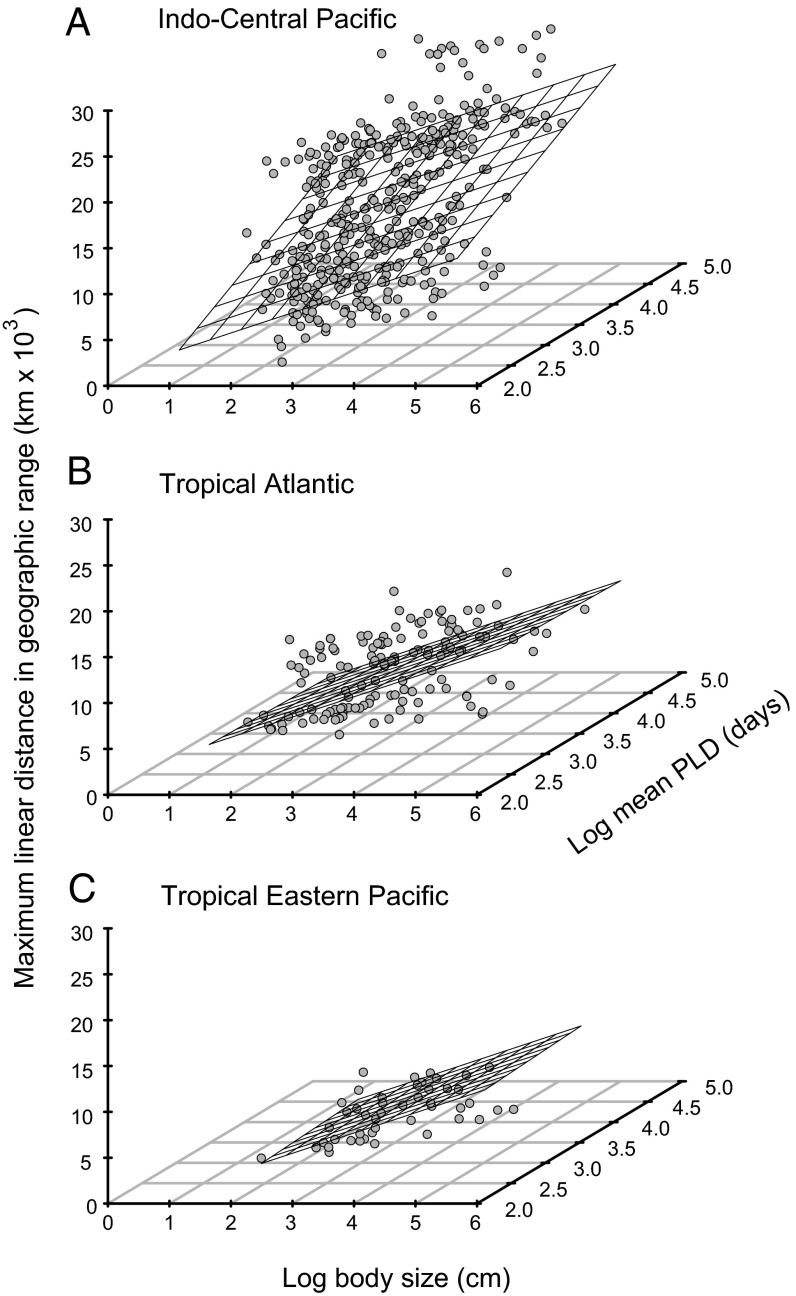
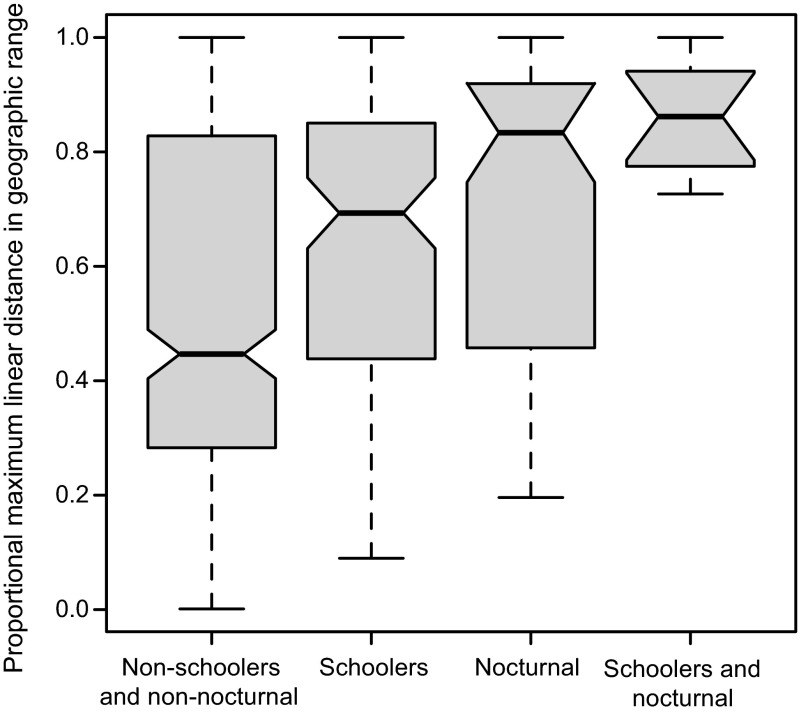
Our analysis also shows that three of five adult life-history traits—body size, schooling behavior, and nocturnal activity—are significant predictors of geographic range size (Figs. 1 and 2) for the global dataset (Table 2). Although PLD is also a significant predictor of range size at the global scale, only the largest of the three regions, the ICP, showed such a correlation (Table 2 and Table S1), confirming the finding of a previous analysis (7). The ICP correlation is largely driven by the few transpacific species that cross the world’s largest oceanic barrier, the 4,000+-km-wide Eastern Pacific Barrier (EPB) that separates the ICP from the TEP (7). After the removal of those trans-Pacific species, the effect of PLD dropped from the second-most influential factor, globally, to the least-important trait (Table 1). A recent analysis of global ocean circulation patterns and habitat distributions (16) indicates that larvae of most tropical-reef species have PLDs sufficient to reach most habitat patches. Our results are consistent with that analysis: they show that the PLD effect is evident only at the largest spatial scale and when habitat patches are most isolated.
Fig. 1.
Relationships among geographic range size, body size and mean PLD. (A) Indo-Central Pacific, (B) tropical Atlantic, (C) tropical eastern Pacific. The regression plane is the prediction from the LMM. Points represent the observed data.
Fig. 2.
Effects of schooling behavior and nocturnal activity on the ratio between the range size of species and the extent of the region. Dashed vertical lines, gray bars, and black horizontal lines represent, respectively, data range, interquartile range, and median. Nonoverlapping notches among the gray bars signify a difference at the 95% confidence level.
Table 2.
Parameters of the final predictive LMM
| Variable | Estimate | SE | t value | P value |
| Intercept | 987.76 | 4425.75 | 0.223 | 0.823 |
| Body size | 2210.34 | 335.41 | 6.589 | <0.001 |
| Schooling behavior | 2030.28 | 609.71 | 3.329 | 0.001 |
| Nocturnal activity | 2503.27 | 881.27 | 2.840 | 0.004 |
| PLD: Tropical Atlantic | −247.54 | 1295.63 | −0.191 | 0.848 |
| PLD: Indo-Central Pacific | 4935.42 | 1434.00 | 3.441 | <0.001 |
| PLD: Tropical Eastern Pacific | 501.14 | 2406.30 | 0.208 | 0.835 |
| Region: Indo-Central Pacific | -10483.26 | 4915.94 | −2.132 | 0.033 |
| Region: Tropical Eastern Pacific | −5102.71 | 8397.86 | −0.607 | 0.543 |
Parameters estimated in a LMM with MLD of geographical range size (in kilometers) as the response variable for larval and adult traits including an interaction between PLD and biogeographic region, and genus nested within family as a random variable. Estimate, coefficient estimate of explanatory variables; SE, test statistic (t value) and probability (P value). P values are significant (P < 0.05) for coefficients if in bold. Reference levels for this regression were set as “Tropical Atlantic” for region and as “no” for both schooling behavior and nocturnal activity.
Our analysis provides further evidence for the view that range extensions are strongly influenced by adult life-history traits (9), factors likely to affect the capacity of new colonizers to survive and establish reproductive populations. All three of the positive adult-biology correlates of range size we identified—maximum body size, schooling behavior, and nocturnal activity—may enhance the probability of population establishment after propagule arrival.
Predation is one of the main processes influencing the structure and species composition of ecological communities (25), especially assemblages of coral reef fishes (26). If these factors limit the number of locally coexisting species (27, 28), predators may inhibit nonnative species establishment (29), and thereby constrain the geographic ranges of prey (30). Piscivorous predators are ubiquitous in coral reefs. Mortality is disproportionally higher among new recruits (31, 32), although juveniles and adults are not immune to predation, as evidenced by morphological, chemical and behavioral antipredatory mechanisms they use (26, 33). Among reef fishes, predation may be particularly important for species at early-stage colonization if predators tend to target rarer species (27, 34). Immigrant species may have better chances of survivorship and establishment if they can rely on specific antipredator mechanisms.
Reduction in predation risk is considered one of the main benefits of schooling in fish (35), and per capita mortality rates in schools of reef fishes decrease as school size increases (36). Predators are less successful at singling out individual prey from large schools because of the “confusion effect” (34). Survivorship may increase in both single-species schools and mixed-species schools not only through the confusion effect but also through more effective foraging by social observation, better vigilance for predators, and greater economy of time budgeting (35). Mixed-species schools are common among recruiting juveniles of reef fishes, sometimes involving species with different diets, which reduces the competitive costs while retaining the benefits of social behavior (19). Schooling may also increase the chance of finding mates among a scarce cohort of colonizers, improve food detection, and enhance access to resources protected by territorial competitors (37, 38). Thus, schooling fishes may have greater relative potential for population establishment and persistence after reaching new areas.
Recently, direct evidence has emerged that diel activity patterns of reef fishes are influenced by ongoing predation intensity: day-active nocturnal fish were much more common at a predator-depleted atoll in the Central Pacific than at a neighboring atoll with a large population of predators (20). This finding supports the view that nocturnal activity allows prey to avoid interactions with day-active predators. Differences in the morphology and behavior of nocturnal and diurnal reef fishes also are consistent with reduced predation intensity at night (39). Nocturnal planktivores are relatively deep bodied and robust, in contrast to their more streamlined diurnal counterparts, and there is a general reduction of schooling at night (39). Whatever the ultimate reasons for the development of nocturnal activity among reef fishes, nocturnal species may be exposed to a smaller subset of predators than day-active fishes (26, 39).
Positive relationships between body size and range size have been documented for diverse taxonomic groups of both terrestrial and marine organisms (2). Larger fish species are generally less susceptible to predation than smaller species (22, 31). Mortality rate of reef fishes are high immediately following settlement of the larval stage, regardless of adult size (26, 31–33). However, juveniles of large species tend to grow faster than those of smaller species (31, 40, 41), allowing them to quickly reach a size refuge (31). Furthermore, large species can generally use a broader range of food types, and are more tolerant of environmental variability than small species (22, 42). Finally, in general, body size is positively correlated with longevity among marine fishes (43). This relationship may influence species establishment and persistence at new range outposts by reducing the probability of local extinction between sporadic long-distance recruitment (44) (i.e., the “storage effect”).
Broad habitat use and depth range, both assumed to indicate ecological generality, were not significant correlates of geographic range size in our analysis. Analyses of ecological generality as a predictor of establishment success, mainly among birds, have produced mixed outcomes (45). In a previous study of the likelihood of reef fish crossing dispersal barriers in the Atlantic Ocean (9), broad habitat use correlated with crossing a coastal barrier within which adults of generalist species likely can survive, but not with crossing the Atlantic Ocean, where only pelagic propagules can succeed. Therefore, habitat generality may be important for only a specific type of barrier, which in turn may account for its lack of global relevance as a predictor of range size. Depth range and spawning mode had no statistical effect in either this study or the study on dispersal barriers in the Atlantic (9).
There are differences in the geographic distribution of reef habitat in the three ocean regions that might be expected to produce differences in factors affecting range size. The ICP consists of a vast network of islands and continental coastlines separated by less than 900 km (46), except for the ∼2,000 km isolation of peripheral islands like Hawaii and Easter Island. The TEP and TA are dominated by relatively continuous continental coastlines, with patches of nonreef habitats separating biogeographically distinct provinces within each region (9, 47) and a few isolated oceanic islands separated by ocean gaps up to ∼1,000 km. The TA is the only region with a large central ocean gap, the 3,500+-km-wide Atlantic. Despite these interregional differences in habitat geography, the same three adult traits (schooling, nocturnal activity, and large size) influence range size in all three ocean regions (Figs. 1 and 2), emphasizing the importance of these traits and colonization ability globally.
We conclude that adult life-history traits that enhance the probability of population establishment and persistence are important determinants of the potential for successful range extension by tropical reef fishes in general. The implication of our results is that factors affecting species persistence in new range outposts are more important for determining the size of geographic ranges than larval dispersal potential (17). The exception relates to the relatively uncommon crossings of the world’s largest oceanic barrier, the EPB (48), where the PLD does have a prominent role. Our analysis has implications not only for biogeographic analyses but also for understanding the effects of climate change. Predictions of poleward range shifts by marine species in response to ocean warming have been based on their thermo-physiological tolerances (49). However, our results indicate other life-history traits that may constrain range extension and thereby influence extinction risks (4).
Materials and Methods
Species Traits.
We selected life-history traits that are thought to potentially influence range sizes (2, 9), and for which data are readily available for all species for which we also have PLD data. Body size is correlated with many other biological attributes of species, including range size (2). Schooling behavior and nocturnal activity are mechanisms that may diminish predation risk (18–20), and thereby enhance range expansion by assisting colonization. Environmental generalist species might be expected to have greater establishment success than specialists in new and different environments (21). Here, such generalists included species that have larger depth ranges and those that use other habitats in addition to structural reefs (soft bottoms, seagrass/macroalgae beds, mangroves, and estuaries).
PLD is linked to the time larvae are susceptible to pelagic dispersal. Spawning mode (production of pelagic or benthic eggs), besides acting as a proxy for PLD (9), influences the stage of development at which larvae enter the pelagic zone. The eggs of pelagic spawners are immediately subjected to transport by currents, whereas the eggs of benthic spawners are deposited on the substrate and the larvae enter the pelagic realm only after developing for some time in the egg before hatching. Existing data on tropical reef fish PLD were compiled from the primary literature for 446 species (see references in Dataset S1). Additional data on PLDs were obtained for 227 species, from which 144 are uniquely reported, by aging settlers through analyses of daily growth increments and settlement marks in otoliths (50). Because of a lack of sufficient data in one or more regions we did not include one other factor that we assessed in our study of range expansion across large barriers in the Atlantic Ocean (9): flotsam-rafting behavior by postlarval stages. Data on spawning mode, maximum total length (our metric for body size), depth range, schooling behavior, multihabitat use, and nocturnal activity were obtained from the primary literature and the global fish data aggregator FishBase (www.fishbase.org). We considered schooling species to be those that regularly form polarized, cohesive groups of 20 or more individuals. Diurnal and nocturnal were defined by the main period of day that each species actively forages.
Geographic Range.
For comparability with previous studies we used the linear distance between the farthest two range endpoints—maximum linear distance (MLD) in kilometers—as a metric of geographic range size (7, 16). Those studies found that the MLD of range size is strongly correlated with the combined maximum latitudinal and longitudinal extent of ranges, and they considered MLD to represent an adequate descriptor of a species’ geographic extent (7). Data on species range limits were obtained from guidebooks (51–55) and from the Ocean Biogeographic Information System (OBIS), a global aggregator of geo-referenced collection records (www.iobis.org). Data from both FishBase and OBIS were screened by us and complemented from our own records (56) (Dataset S1). Endpoint geographic coordinates were determined to the nearest degree using Google Earth (earth.google.com). The MLD was measured using the function “geodist” in the R package “gmt” (57). Species were grouped in terms of their residence in three well-defined biogeographic regions, the physical dimensions of which delineate the maximum attainable range size in each region: the Indo-central Pacific region (from the Red Sea to Easter Island), the tropical Atlantic (from the northwest Gulf of Mexico to the southern Gulf of Guinea), and the tropical eastern Pacific (from the northern Gulf of California to northern Peru). Primarily tropical species that extend their ranges to temperate zones had their full range considered in the analysis. Although it is in the Pacific, together with the ICP, the TEP is a well-recognized biogeographic entity that has a substantial fauna with a high level of endemism (∼75%) (47). Only TEP endemics were included in the TEP group in our analyses, which avoided possible confounding effects of recent trans-Pacific crossers not having realized their full range potential in that region. The few trans-Pacific species that occur in both the ICP and TEP were included as members of the ICP fauna because most of the range of each is in the ICP and most appear to have migrated from there to the TEP (48). We did not consider the western and eastern sides of the TA as separate regions because there are insufficient data on the PLDs of species endemic to the tropical eastern Atlantic, which has a substantially lower rate of endemism than the tropical western Atlantic (58).
Statistical Analysis.
The LMM was fitted using the function “lme” from the package “nlme” (59) in R. The response variable was the MLD between two points along the geographic range boundary. PLD, spawning mode, maximum body size, schooling behavior, nocturnal activity, depth range, multihabitat use, and region were included as fixed variables, and genus and family were included as nested random variables. Interactions between region and each fixed factor were considered in the full model. For model selection, we followed the procedure recommended by Zuur et al. (60) of a backward stepwise removal of nonsignificant fixed-effect terms (P > 0.05) from the full model based on log-likelihood ratio tests (Table S1). Partitioning of variance to determine the relative importance (percent of explained variance; independent effects in Table 1) accounted for by each explanatory variable in the model was calculated by hierarchical partitioning using the R package “hier.part” (61).
Supplementary Material
Acknowledgments
We thank R. Lecomte for her assistance with otholith work and for giving access to her laboratory facilities; C. Osenberg, C. St. Mary, T. Adam, B. Chockley, and many volunteers for their assistance in the field and at the laboratory; and Mark Hixon and two anonymous reviewers for comments in the manuscript. This study was supported in part by an International Macquarie University Research Excellence Scholarship (to O.J.L.); the Smithsonian Tropical Research Institute (D.R.R.); the SISBIOTA program by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (S.R.F.); and the Australian Research Council (J.S.M.). The Fondation de la Recherche pour la Biodiversité funded this research through the “Résilience de communautés: rôle du couplage entre diversités taxonomique et fonctionnelle, les poissons de récif pour modèle“ (RESICOD) program and the General Approach to Species-Abundance Relationships (GASPAR) program. Part of the data were collected through US National Sea Grant R/LR-B-52.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304074110/-/DCSupplemental.
References
- 1.Gaston KJ. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 2.Brown JH, Stevens GC, Kaufman DM. The geographic range: Size, shape, boundaries, and internal structure. Annu Rev Ecol Syst. 1996;27:597–623. [Google Scholar]
- 3.Gaston KJ, Blackburn TM. Macroecology and conservation biology. In: Blackburn TM, Gaston KJ, editors. Macroecology: Concepts and Consequences. Oxford: Blackwell; 2003. pp. 345–367. [Google Scholar]
- 4.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 5.Shanks AL, Grantham BA, Carr MH. Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl. 2003;13(S1):S159–S169. [Google Scholar]
- 6.Lindsay SM. Dispersal of marine organisms and the grand challenges in biology: An introduction to the symposium. Integr Comp Biol. 2012;52(4):443–446. doi: 10.1093/icb/ics106. [DOI] [PubMed] [Google Scholar]
- 7.Lester SE, Ruttenberg BI. The relationship between pelagic larval duration and range size in tropical reef fishes: A synthetic analysis. Proc Biol Sci. 2005;272(1563):585–591. doi: 10.1098/rspb.2004.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victor BC, Wellington GM. Endemism and the pelagic larval duration of reef fishes in the eastern Pacific Ocean. Mar Ecol Prog Ser. 2000;205:241–248. [Google Scholar]
- 9.Luiz OJ, et al. Ecological traits influencing range expansion across large oceanic dispersal barriers: Insights from tropical Atlantic reef fishes. Proc Biol Sci. 2012;279(1730):1033–1040. doi: 10.1098/rspb.2011.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brothers EB, Thresher RE. Pelagic duration, dispersal and the distribution of Indo-Pacific coral reef fishes. In: Reaka ML, editor. The Ecology of Coral Reefs. Washington, DC: US Depart of Commerce; 1985. pp. 53–69. [Google Scholar]
- 11.Thresher RE, Brothers EB. Reproductive ecology and biogeography of Indo-West Pacific angelfishes (Pisces: Pomacanthidae) Evolution. 1985;39(4):878–887. doi: 10.1111/j.1558-5646.1985.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 12.Thresher RE, Colin PL, Bell LJ. Planktonic duration, distribution and population structure of western and central Pacific damselfishes (Pomacentridae) Copeia. 1989;1989(2):420–434. [Google Scholar]
- 13.Lester SE, Ruttenberg BI, Gaines SD, Kinlan BP. The relationship between dispersal ability and geographic range size. Ecol Lett. 2007;10(8):745–758. doi: 10.1111/j.1461-0248.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 14.Paulay G, Meyer C. Dispersal and divergence across the greatest ocean region: Do larvae matter? Integr Comp Biol. 2006;46(3):269–281. doi: 10.1093/icb/icj027. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe K, Toonen RJ. Marine connectivity: A new look at pelagic larval duration and genetic metrics of dispersal. Mar Ecol Prog Ser. 2011;436:291–305. [Google Scholar]
- 16.Mora C, et al. High connectivity among habitats precludes the relationship between dispersal and range size in tropical reef fishes. Ecography. 2012;35(1):89–96. [Google Scholar]
- 17.Marshall DJ, Monro K, Bode M, Keough MJ, Swearer S. Phenotype-environment mismatches reduce connectivity in the sea. Ecol Lett. 2010;13(1):128–140. doi: 10.1111/j.1461-0248.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandin SA, Pacala SW. Fish aggregation results in inversely density-dependent predation on continuous coral reefs. Ecology. 2005;86(6):1520–1530. [Google Scholar]
- 19.DeMartini EE, Anderson TW, Friedlander AM, Beets JP. Predator biomass, prey density, and species composition effects on group size in recruit coral reef fishes. Mar Biol. 2011;158(11):2437–2447. [Google Scholar]
- 20.McCauley DJ, Hoffmann E, Young HS, Micheli F. Night shift: Expansion of temporal niche use following reductions in predator density. PLoS ONE. 2012;7(6):e38871. doi: 10.1371/journal.pone.0038871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassey P, Blackburn TM, Sol D, Duncan RP, Lockwood JL. Global patterns of introduction effort and establishment success in birds. Proc Biol Sci. 2004;271(Suppl 6):S405–S408. doi: 10.1098/rsbl.2004.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munday PL, Jones GP. The ecological implications of small body size among coral-reef fishes. Oceanogr Mar Biol Annu Rev. 1998;36:373–411. [Google Scholar]
- 23.Bunnefeld N, Phillimore AB. Island, archipelago and taxon effects: Mixed models as a means of dealing with the imperfect design of nature's experiments. Ecography. 2012;35(1):15–22. [Google Scholar]
- 24.Hortal J. Escaping the trap of low sample size in island biogeography. Frontiers of Biogeography. 2012;3(4):127–129. [Google Scholar]
- 25.Connell JH. Some mechanisms producing structure in natural communities: A model and evidence from field experiments. In: Cody ML, Diamond JA, editors. Ecology and Evolution of Communities. Cambridge, MA: Belknap-Harvard Univ Press; 1975. pp. 460–490. [Google Scholar]
- 26.Hixon MA. Predation as a process structuring coral reef fish communities. In: Sale PF, editor. The Ecology of Fishes on Coral Reefs. San Diego: Academic; 1991. pp. 475–508. [Google Scholar]
- 27.Almany GR, Webster MS. Odd species out as predators reduce diversity of coral-reef fishes. Ecology. 2004;85(11):2933–2937. [Google Scholar]
- 28.Heinlein J, Stier A, Steele M. Predators reduce abundance and species richness of coral reef fish recruits via non-selective predation. Coral Reefs. 2010;29(2):527–532. [Google Scholar]
- 29.Wanger TC, et al. Endemic predators, invasive prey and native diversity. Proc Biol Sci. 2011;278(1706):690–694. doi: 10.1098/rspb.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt RD, Barfield M, Filin I, Forde S. Predation and the evolutionary dynamics of species ranges. Am Nat. 2011;178(4):488–500. doi: 10.1086/661909. [DOI] [PubMed] [Google Scholar]
- 31.Sogard SM. Size-selective mortality in the juvenile stage of teleost fishes: A review. Bull Mar Sci. 1997;60(3):1129–1157. [Google Scholar]
- 32.Almany GR, Webster MS. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs. 2006;25(1):19–22. [Google Scholar]
- 33.Webster MS. Role of predators in the early post-settlement demography of coral-reef fishes. Oecologia. 2002;131(1):52–60. doi: 10.1007/s00442-001-0860-x. [DOI] [PubMed] [Google Scholar]
- 34.Almany GR, Peacock LF, Syms C, McCormick MI, Jones GP. Predators target rare prey in coral reef fish assemblages. Oecologia. 2007;152(4):751–761. doi: 10.1007/s00442-007-0693-3. [DOI] [PubMed] [Google Scholar]
- 35.Pitcher TJ, Parrish JK. Function of shoaling behaviour in teleosts. In: Pitcher TJ, editor. Behaviour of Teleost Fishes. London: Chapman & Hall; 1993. pp. 363–439. [Google Scholar]
- 36.White JW, et al. Synthesizing mechanisms of density dependence in reef fishes: Behavior, habitat configuration, and observational scale. Ecology. 2010;91(7):1949–1961. doi: 10.1890/09-0298.1. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher TJ, Magurran AE, Winfield IJ. Fish in larger shoals find food faster. Behav Ecol Sociobiol. 1982;10(2):149–151. [Google Scholar]
- 38.Robertson DR, Swetman HPA, Fletcher EA, Cleland MG. Schooling as a mechanism for circumventing the territoriality of competitors. Ecology. 1976;57(6):1208–1220. [Google Scholar]
- 39.Helfman GS. Fish behaviour by day, night and twilight. In: Pitcher TJ, editor. Behaviour of Teleost Fishes. London: Chapman & Hall; 1993. pp. 479–511. [Google Scholar]
- 40.Buesa RJ. Growth rate of tropical demersal fishes. Mar Ecol Prog Ser. 1987;36:191–199. [Google Scholar]
- 41.Legendre M, Albaret JJ. Maximum observed length as an indicator of growth rate in tropical fishes. Aquaculture. 1991;94(4):327–341. [Google Scholar]
- 42.Robertson DR. Implications of body size for interspecific interactions and assemblage organization among coral-reef fishes. Aust J Ecol. 1998;23(3):252–257. [Google Scholar]
- 43.Roff DA. The Evolution of Life Histories: Theory and Analysis. New York: Chapman & Hall; 1992. [Google Scholar]
- 44.Warner RR, Chesson PL. Coexistence mediated by recruitment fluctuations: A field guide to the storage effect. Am Nat. 1985;125(6):769–787. [Google Scholar]
- 45.Duncan RP, Blackburn TM, Sol D. The ecology of bird introductions. Annu Rev Ecol Syst. 2003;34:71–98. [Google Scholar]
- 46.Schultz JK, et al. Global phylogeography and seascape genetics of the lemon sharks (genus Negaprion) Mol Ecol. 2008;17(24):5336–5348. doi: 10.1111/j.1365-294X.2008.04000.x. [DOI] [PubMed] [Google Scholar]
- 47.Robertson DR, Cramer KL. Shore fishes and biogeographic subdivisions of the Tropical Eastern Pacific. Mar Ecol Prog Ser. 2009;380:1–17. [Google Scholar]
- 48.Lessios HA, Robertson DR. Crossing the impassable: Genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc Biol Sci. 2006;273(1598):2201–2208. doi: 10.1098/rspb.2006.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nature Climate Change. 2012;2:686–690. [Google Scholar]
- 50.Wilson DT, McCormick MI. Microstructure of settlement-marks in the otoliths of tropical reef fishes. Mar Biol. 1999;134(1):29–41. [Google Scholar]
- 51.Randall JE. Caribbean Reef Fishes. 3rd Ed. Neptune City, NJ: TFH Publications; 1996. [Google Scholar]
- 52. Robertson DR, Van Tassel J (2012) Fishes: Greater Caribbean. An identification Guide to the Shore-Fish Fauna of the Caribbean and Adjacent Areas. (iOS App. Copyright Smithsonian Institution, Left Coast R&C, Santa Cruz, California). Available at https://itunes.apple.com/us/app/fishes-greater-caribbean/id570048678?mt=8. Accessed September 2012.
- 53. Robertson DR, Allen G (2012) Fishes: East Pacific. An Identification Guide to the Shore-Fish Fauna of the Tropical Eastern Pacific. (iOS App. Copyright Smithsonian Institution, Left Coast R&C, Santa Cruz, California). Available at https://itunes.apple.com/us/app/fishes-east-pacific/id494644648?mt=8. Accessed September 2012.
- 54.Allen G, Steene R, Humann P, DeLoach N. Reef Fish Identification: Tropical Pacific. Jacksonville, FL: New World Publications; 2003. [Google Scholar]
- 55.Allen G, Erdmann MV. Reef Fishes of the East Indies. Honolulu: Univ of Hawaii Press; 2012. [Google Scholar]
- 56.Robertson DR. Global biogeographical data bases on marine fishes: Caveat emptor. Divers Distrib. 2008;14(6):891–892. [Google Scholar]
- 57. Magnusson A (2011) gmt: Interface Between GMT Map-Making Software and R. R package v1.1-9 Available at http://cran.r-project.org/web/packages/gmt/index.html. Accessed April 2012.
- 58.Floeter SR, et al. Atlantic reef fish biogeography and evolution. J Biogeogr. 2008;35(1):22–47. [Google Scholar]
- 59. Pinheiro J, Bates D, DebRoy S, Sarkar D (2012) nlme: Linear and Nonlinear Mixed Effects Models. R package v3.1-104. Available at http://cran.r-project.org/web/packages/nlme/index.html. Accessed November 2011.
- 60.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York, NY: Springer; 2009. [Google Scholar]
- 61. Walsh C, MacNally R (2013) hier.part: Hierarquical partitioning. R package v1.0-4. Available at http://cran.r-project.org/web/packages/hier.part/index.html. Accessed January 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.