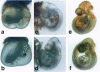
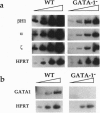
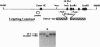Abstract
The X chromosome-linked transcription factor GATA-1 is expressed specifically in erythroid, mast, megakaryocyte, and eosinophil lineages, as well as in hematopoietic progenitors. Prior studies revealed that gene-disrupted GATA-1- embryonic stem cells give rise to adult (or definitive) erythroid precursors arrested at the proerythroblast stage in vitro and fail to contribute to adult red blood cells in chimeric mice but did not clarify a role in embryonic (or yolk sac derived) erythroid cells. To examine the consequences of GATA-1 loss on embryonic erythropoiesis in vivo, we inactivated the GATA-1 locus in embryonic stem cells by gene targeting and transmitted the mutated allele through the mouse germ line. Male GATA-1- embryos die between embryonic day 10.5 and 11.5 (E10.5-E11.5) of gestation. At E9.5, GATA-1- embryos exhibit extreme pallor yet contain embryonic erythroid cells arrested at an early proerythroblast-like stage of their development. Embryos stain weakly with benzidine reagent, and yolk sac cells express globin RNAs, indicating globin gene activation in the absence of GATA-1. Female heterozygotes (GATA-1+/-) are born pale due to random inactivation of the X chromosome bearing the normal allele. However, these mice recover during the neonatal period, presumably as a result of in vivo selection for progenitors able to express GATA-1. Our findings conclusively establish the essential role for GATA-1 in erythropoiesis within the context of the intact developing mouse and further demonstrate that the block to cellular maturation is similar in GATA-1- embryonic and definitive erythroid precursors. Moreover, the recovery of GATA-1+/- mice from anemia seen at birth provides evidence indicating a role for GATA-1 at the hematopoietic progenitor cell level.
Full text
PDF
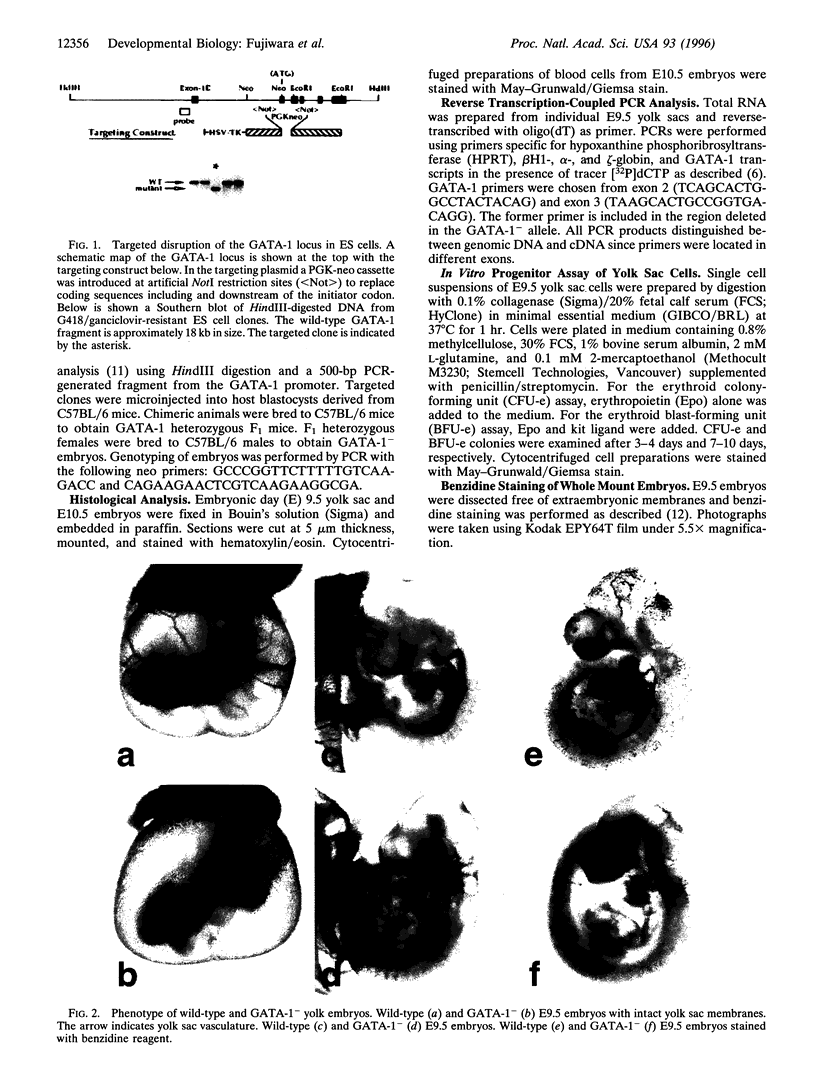


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Iuchi I., Yamamoto M. Erythropoiesis in the developing rainbow trout, Salmo gairdneri irideus: histochemical and immunochemical detection of erythropoietic organs. J Exp Zool. 1983 Jun;226(3):409–417. doi: 10.1002/jez.1402260311. [DOI] [PubMed] [Google Scholar]
- Keller G., Kennedy M., Papayannopoulou T., Wiles M. V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993 Jan;13(1):473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Regulation of globin gene expression in erythroid cells. Eur J Biochem. 1995 Jul 15;231(2):271–281. doi: 10.1111/j.1432-1033.1995.tb20697.x. [DOI] [PubMed] [Google Scholar]
- Pevny L., Lin C. S., D'Agati V., Simon M. C., Orkin S. H., Costantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995 Jan;121(1):163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Tsai F. Y., Keller G., Kuo F. C., Weiss M., Chen J., Rosenblatt M., Alt F. W., Orkin S. H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994 Sep 15;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Keller G., Orkin S. H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994 May 15;8(10):1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Orkin S. H. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9623–9627. doi: 10.1073/pnas.92.21.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Tsai S. F., Burgess S., Matsudaira P., Bruns G. A., Orkin S. H. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990 Jan;87(2):668–672. doi: 10.1073/pnas.87.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]