Abstract
A universal coagulation test that reliably detects prolonged coagulation time in patients, irrespective of the anticoagulant administered, has not been available to date. An easily miniaturised, novel μ-fluidic universal coagulation test employing surface acoustic waves (SAW) is presented here. SAW was employed to instantly mix and recalcify 6 μl citrated whole blood and image correlation analysis was used to quantify clot formation kinetics. The detection of clinically relevant anticoagulant dosing with old anticoagulants (unfractionated heparin, argatroban) and new anticoagulants (dabigatran, rivaroxaban) has been tested and compared to standard plasma coagulation assays. The applicability of this novel method has been confirmed in a small patient population. Coagulation was dose-proportionally prolonged with heparin, argatroban, dabigatran, and rivaroxaban, comparable to standard tests. Aspirin and clopidogrel did not interfere with the SAW-induced clotting time (SAW-CT), whereas the strong GPIIb/IIIa-inhibitor abciximab did interfere. Preliminary clinical data prove the suitability of the SAW-CT in patients being treated with warfarin, rivaroxaban, or dabigatran. The system principally allows assessment of whole blood coagulation in humans in a point-of-care setting. This method could be used in stroke units, emergency vehicles, general and intensive care wards, as well as for laboratory and home testing of coagulation.
INTRODUCTION
Coagulation under physiological conditions in flowing blood involves platelets and coagulation factors present in plasma. Usually, activated platelets trigger the coagulation cascade and cascade products, such as thrombin, reinforce platelet activation. A clot, therefore, is formed from plasma components (fibrin) and corpuscular components, with variable composition depending upon whether the clot is formed in a vein or an artery.
Many patients could profit from a simple coagulation test employing whole, unprocessed blood, carried out under flow conditions. Peri-procedural monitoring of haemostasis to direct pro- and anti-thrombotic intervention in patients undergoing emergency or elective surgery or catheter intervention would profit from a simple coagulation test. Rapid on site assessment in emergency situations to determine whether a patient has been pretreated with anticoagulatory drugs would be another application opportunity. Laboratory assessment to monitor anticoagulatory drugs before other anticoagulatory agents are administered is another setting where a simple coagulation test would be an asset. A portable device to allow self-testing of patients on oral anticoagulation at home is another possibility. Ideally, a point-of-care (POC) device to measure blood coagulation does not require skilled laboratory personnel, does not require blood sample processing after withdrawal (fresh whole blood can be used) and the test result is quickly available.
Most available test principles for plasma coagulation analysis in the laboratory employ the addition of substrates such as Factor Xa or an activator (e.g., anti-FXa-activity assays [a-FXa], prothrombin time [PT], activated partial thromboplastin time [aPTT], and the Activated Clotting Time [ACT]) such as thromboplastin or kaolin. These triggers are specific for the coagulation pathway inhibited by a single anticoagulant agent. The endpoint of the test reaction is the formation of a fibrin clot or chromogen generation. However, most available coagulation assays (except ACT) require separating blood components by centrifugation to determine plasma coagulation. Centrifugation is time consuming, requires laboratory equipment and trained personnel. Anti-FXa-tests are often only available in large hospitals. The value of these tests lies in diagnosing uncommon coagulation defects and also allows testing the influence of various anticoagulants, provided the specific coagulation pathway inhibited by the anticoagulant is known.
A variety of lab-on-chip (LOC) methods utilize the small sample requirement of microfluidic techniques. LOC techniques have been used to analyze microthrombus formation1 and to investigate how mixing influences coagulation.2 The technique has been used for on-chip aPTT measurements in an argatroban titrated plug-based microfluidic system3 to investigate coagulation-induced obstruction in micro-fluidic channels.4 The technique has also been applied to evaluate the influence of heparin on incorporation of fluorescent labelled fibrinogen in a growing thrombus in lateral flow assays.5, 6 Platelet activation7 and controlled agonist flux to platelets have also been investigated by microfluidics.8 Here, the first use of a device employing surface acoustic waves (SAWs) to mix a drop of blood and induce coagulation is described. The basic principle is continuous recording of translational motion of fluorescent microspheres in recalcified citrated blood, measuring the time required for the microspheres to cease movement due to clot formation. The SAW induced streaming of whole blood can easily be downscaled to fulfil the requirements for a point-of-care assay to determine clotting time. Contrary to conventional methods, in this assay blood coagulation is monitored in a small sample under flow conditions by optical displacement analysis without further biochemical or physical processing steps, except recalcification. The method detects direct and indirect thrombin inhibitors and also direct FXa-inhibitors. Since one advantage of this test is that only very small amounts of blood are required (6 μl in contrast to about 500 μl in a conventional coagulation test), this method might also be applied to the preclinical evaluation of anticoagulatory agents, without having to sacrifice small animals.
EXPERIMENTAL
Materials
Unfractionated heparin (UFH) was purchased from Ratiopharm (Ulm, Germany), argatroban from Mitsubishi (Düsseldorf, Germany), dabigatran (BIBR 953; Cat.No. S2196) and rivaroxaban (Cat.No. S3002) were purchased from Absource (Munich, Germany) and abciximab was from Centocor (Leiden, The Netherlands). Stock solutions of dabigatran and rivaroxaban were prepared in water-free DMSO and stored at −30 °C, desiccated until use and further diluted in 0.9% NaCl prior to use.
Fluorescent microspheres were purchased from Bangs Laboratories (Cat.No. FC04F-10529, Fishers, IN), elastomers were molded by Ibidi (Martinsried, Germany), the holder frame with the SAW-Chip and the rf generator (Generator 09) were manufactured by Beckman Coulter Biomedical (Munich, Germany).
The Device. To investigate the hydrodynamic response in whole blood, a self-developed planar microfluidic device (Fig. 1)9 was employed. Applying a radio frequency (rf) signal to an interdigital transducer (IDT) on the piezoelectric substrate lithium niobate initiates a surface acoustic wave (SAW). When the SAW encounters a solid/liquid interface, it is transferred into a sound wave that travels in the liquid at an angle deviating 23° from normal. The resulting pressure gradient induces liquid flow in the propagating direction of the sound wave and is called acoustic streaming.10, 11 The SAW technology can, therefore, be employed to instantly mix and recalcify very small amounts of liquid.
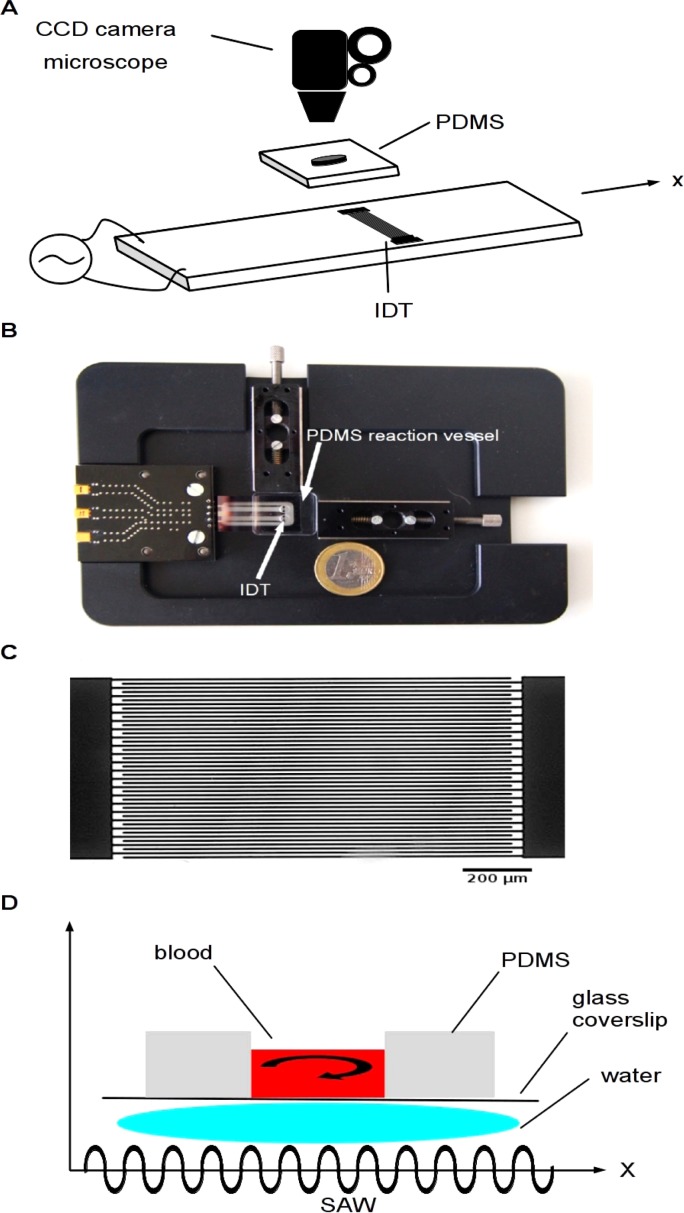
Figure 1.
(a) Schematic representation of the surface-acoustic wave-clotting time (SAW-CT) device. The polydimethylsiloxane (PDMS) reaction vessel contains a central hole, is mounted on the holder frame and aligned with the hole directly above an interdigital transducer (IDT) to permit SAW-induced mixing of blood pipetted into the hole. The device is placed under a fluorescence microscope equipped with a charge-coupled device (CCD) camera to record coagulation kinetics. The camera is computer controlled and images are stored on the hard disk drive. The radio frequency (rf) generator output (frequency and rf power) is directed by the computer and the IDT generates surface-acoustic waves to mix and instantly recalcify the blood. (b) Frame holder with the hole in the PDMS reaction vessel aligned with IDT. (c) Photomicrograph of IDT. The IDT consists of interdigitating gold electrodes with 22 pairs of fingers. The rf frequency is 140 MHz, which corresponds to a finger spacing of 25.2 μm. Scale bar represents 200 μm. D: Schematic illustration of SAW interaction with blood. The PDMS reaction vessel is mounted on a glass coverslip and placed on the holder frame. The vessel is placed on the SAW-chip so that the IDT transducer is centered below the vessel hole in the PDMS reaction vessel. Acoustic contact between the SAW-chip and the reaction vessel is achieved by a thin water film between the glass coverslip beneath the reaction vessel and the IDT on the holder frame. The SAW generated by the IDT on the chip is transferred into a sound wave in the water film and the sound wave is transmitted through the glass coverslip, inducing streaming and recirculation in the blood loaded PDMS reaction vessel.
The reaction vessel is made from polydimethylsiloxane (PDMS, Sylgard 148, Dow Corning), is casted in a milled brass mould and cured for 2 days at room temperature. The polymer vessel is 21 × 18 × 2.5 mm and has a central 3 mm hole in the bottom. To increase adhesion to glass, the PDMS is treated with 200 W oxygen plasma for 2 min. After mounting the vessel on a glass coverslip, it is placed on a holder frame and aligned on top of the SAW-chip so that the IDT is below the middle of the vessel hole. The acoustic contact between the SAW-chip and reaction vessel is achieved with a thin water film. The SAW generated by the IDT is transferred into a sound wave in the water film (Fig. 1d). The sound wave is transmitted through the glass coverslip bottom, inducing streaming in the blood loaded vessel and induces rapid recalcification in citrated blood (Fig. 2a). The rf power (2 W) for the IDT is controlled by a custom made rf generator with 2 independent output channels at 140–170 MHz. The generator is controlled by laptop via USB.
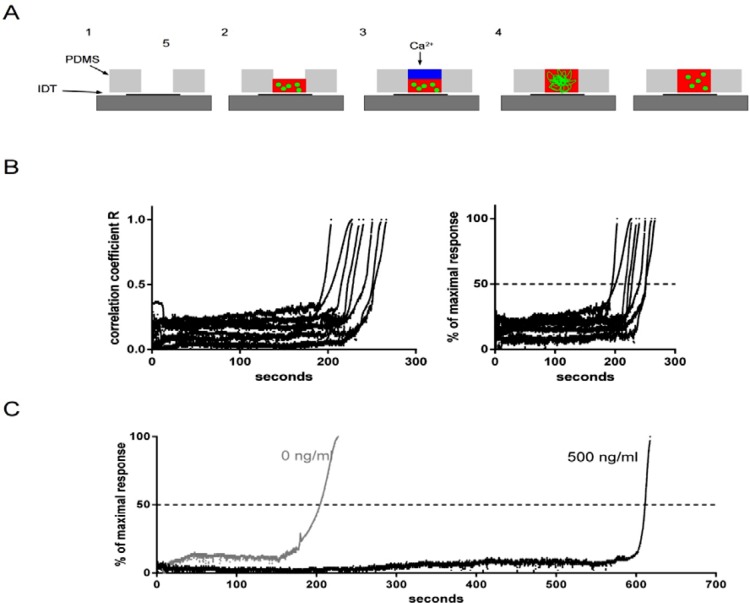
Figure 2.
(a) Assay steps. The PDMS reaction vessel is placed over the IDT so that the hole is directly above the IDT (1) and 6 μl citrated whole blood containing fluorescent microspheres and drug or buffer are placed into the well of the reaction vessel (2). 6 μl 25 mM calcium chloride solution is layered onto the blood (3) and after starting the rf-generator the blood is instantly mixed and recalcified (4). The process is recorded by the CCD camera and shown in the in the videos in the supplementary material.19 After approximately 3 minutes the coagulation process is complete, a clot is formed and microsphere movement approaches zero and stops (5). (b) Left panel: Correlation kinetics of clot formation in untreated blood from 8 healthy subjects. Blood coagulation time of 8 healthy subjects was evaluated by the SAW-CT assay. The curves show the results from the image processing. All subjects responded within a reasonable time frame and the slopes of the reaction curves were similar, not differing significantly between subjects. Right panel: transformation of the kinetics in the correlation coefficient R to the target variable in the SAW-CT assay. The values from the right panel are normalised between 0 and 100% and the time point at which 50% of the maximal response is reached is reported throughout this text as SAW-CT. The values of the SAW-CT in the 8 volunteers ranged from 195 to 251 s with a mean ± S.E.M. of 226 ± 7 s. (c) Coagulation kinetics by image correlation analysis. Images are subjected to a correlation analysis with the image processing software ImageJ and the correlation coefficient R is plotted versus time. A high correlation coefficient R denotes good similarity between the images, whereas a low coefficient denotes images with different spatial distribution of pixel intensity in the images. Identical images give a correlation coefficient of 1. Analysis of the upper images revealed that in untreated blood (0 ng/ml) coagulation begins at approximately 165 s. The correlation coefficient R begins to rise from background baseline levels 0.1 (grey line), rapidly increases and at about 220 s approaches 1, meaning the images show identical spatial distribution of fluorescent microspheres. The reaction is complete and the formed clot effectively restricts the motion of the microspheres in the blood. Adding factor Xa inhibitor rivaroxaban at a concentration of 500 ng/ml blood (black line) drastically delays the coagulation response. The coagulation reaction begins at 600 s and is complete at approximately 617 s after SAW initiation and recalcification.
The SAW-chip
The SAW-chip (Fig. 1a) employed here is 15 × 30 mm and is glued into an opening of a metal holder frame (Fig. 1b). It has two interdigitated transducers (IDT) with an aperture of 1 mm and a distance between the IDTs of 4 mm. Only one IDT per sample is necessary, but two IDTs enable two samples to be mixed simultaneously. The IDT consists of two comb-like interdigitated gold electrodes with 22 pairs of fingers, produced by vapour deposition and standard lithography. To protect the gold from mechanical damage, it is covered by 800 nm of sputtered silicon dioxide. Contact pads are opened by another lithography step followed by HF etching.
The anisotropic piezoelectric substrate is a Y-cut LiNbO3 with the crystal axis rotated around the X-axis by 128° (128° Y-Cut). The fingers of the IDT are perpendicular to the X-axis (Fig. 1a) and the alternating radio frequency (rf) excites a Rayleigh wave propagating in the direction of the X-axis. The rf is 140 MHz, corresponding to a finger spacing of 25.2 μm (Fig. 1c shows a photomicrograph of the IDT). Electrical connection to the rf power is through pogo pins and the impedance of the SAW chip is matched to the 50 Ohm of the rf generator by a matching circuit.
Sample processing
1.5 μl of a fluorescent microsphere suspension is added to 1 ml citrated whole blood together with 1 μl drug of the desired concentration (for in vitro tests of anticoagulants) or buffer (for control samples or samples obtained from anticoagulated patients). 6 μl of the blood containing the microspheres and drug is placed in the PDMS reaction vessel under a fluorescence microscope. The blood is recalcified by layering 6 μl of 25 mM CaCl2 solution onto the blood and mixing is immediately initiated by starting the generator, inducing SAW. The whole reaction is recorded in real time with a CCD camera (Lumenera, Ottawa, Canada) by fluorescence microscopy (Nikon, Amstelveen, The Netherlands).
Data analysis
Images are recorded with a temporal resolution of 26 frames per second and stored on a connected hard-drive for off-line analysis. When coagulation of the recalcified blood is initiated, the degree of freedom in translational motion of the fluorescent microspheres in the clotted blood is restricted. When coagulation is complete and a thrombus is formed, the majority of fluorescent tracer microspheres are trapped in the clot, and movement ceases. The quantitative monitoring of clot formation and coagulation kinetics is based on a freely available12 digital image correlation. This is an image processing technique for accurate 2D measurement of variation in successive images, used here to monitor displacement of the fluorescent tracer microspheres in the blood. (http://www.gcsca.net/IJ/ImageCorrelationJ.html).
The plugin computes statistical parameters (correlation coefficient R and the parameter C, interception with the ordinate) between consecutive video images. After approximately 200 s coagulation is complete, a clot has formed and the movement of the fluorescent microsphere tracer microspheres ceases. Successive images become increasingly identical, the correlation coefficient R increases and approaches the maximum value of 1 (identical images) at the time of final clot formation. The correlation coefficients are normalised between 0% and 100% and the time corresponding to 50% of maximal response is referred to as SAW-clotting time SAW-CT (Figures 2b, 2c).
Experiments
Concentration-response to anticoagulants in vitro
Blood from healthy subjects denying any drug intake within the last 2 weeks was collected by venipuncture with a 17-gauge butterfly needle in tubes containing 0.106 M tri-sodium-citrate. The citrated blood from 5–7 subjects was preincubated with clinically relevant concentrations of unfractionated heparin (0–1.0 IU/ml final concentration), the direct FXa-inhibitor rivaroxaban (0–500 ng/ml final concentration), with the direct thrombin inhibitor argatroban (0–4000 ng/ml final concentration) and dabigatran (0–1000 ng/ml final concentration). An aliquot was used for the SAW analysis within 30 min after preparation. The rest of the blood sample was centrifuged at 3500 × g for 15 min and the resulting plasma frozen at −25 °C until analysis with other coagulation assays (see below). In a subset of samples, the intraindividual variation of SAW-CT was measured by repeated analysis of the same sample or repeated analysis of blood from the same subject within several days to weeks.
In vivo test
The influence of dual antiplatelet therapy on SAW-CT was investigated by having 7 healthy volunteers ingest 100 mg aspirin and 300 mg clopidogrel. Coagulation kinetics were compared before and 1 day after administration.
In vivo–ex vitro test
Influence of aspirin on the SAW-CT response to dabigatran or rivaroxaban was investigated by having 2 healthy volunteers ingest 500 mg aspirin. The coagulation kinetics in dabigatran and rivaroxaban treated blood (in vitro) was compared before and 3 h after aspirin administration.
Practical test on patient blood
Blood samples from 7 patients under various anticoagulant regimens were analysed.
The protocols for blood donation and for experimental administration of antiplatelet agents were approved by the Institutional Review Board of the University Clinic of Frankfurt/Main, and written informed consent was obtained from all participants.
The following coagulation tests were carried out on the plasma samples.
Activated partial thromboplastin time (aPTT): activated partial thromboplastin time (aPTT-SP-Liquid, Instrumentation Laboratory, Munich, Germany, normal range 22–26 s) was determined on a coagulation analyzer (ACL® 6000, Instrumentation Laboratory; Milan, Italy) as has previously been described.13, 14, 15 This test was applied to plasma samples containing UFH and direct thrombin inhibitors.
Prothrombin time (PT): Prothrombin time (PT, Instrumentation Laboratory, Munich, Germany, normal range 10–12 s) was determined on a coagulation analyzer (ACL® 6000, Instrumentation Laboratory; Milan, Italy) as has previously been described.13, 16 This test was applied to plasma samples containing rivaroxaban since direct FXa inhibitors influence PT more than aPTT.17
Statistics
Data are presented as mean ± standard error of the mean (S.E.M.). Dose-dependency as trend analysis was computed by one-way analysis of variance and the Kruskal-Wallis test with Dunn's multiple comparison18 by comparing corresponding doses with untreated samples. p values were corrected for multiple comparisons in confidence intervals and significance by alpha adjustment. All statistical computations were performed with GraphPad Prism 6.0 (GraphPad software Inc., La Jolla, CA).
Results
Assay
6 μl citrated blood were recalcified by adding calcium (Figure 2a) and the reaction was initiated by starting the transducer and applying the SAW, resulting in instant mixing of the blood. Video 1 in the supplementary material19 shows a programmed cycle of 10 s 10 dBm output power followed by a 2 s pause, then 10 s 20 dBm, 2 s pause and finally 10 s 27 dBm (the maximum power delivered by the device). The blood sample is monitored by recording the fluorescent signal of the microspheres in the blood to trace microsphere motion. Microsphere tumbling is terminated as the blood changes from liquid to solid, reflecting coagulation progress and clot formation (Figure 2b and video 2 in the supplementary material19) and is prolonged upon anticoagulant drug addition (Figure 2c). In untreated blood, the clot is formed at approximately 200 s, whereas anticoagulation prolonged the coagulation time in the SAW assay to more than 600 s. Thus, the translational motion of the microspheres was used to measure coagulation kinetics.
Microsphere presence did not change the coagulation kinetics. PT measurements on an ACL600 did not change upon microsphere addition (98.4 ± 3.7% of untreated control, n = 5). PT times in untreated samples were 92.8 ± 5.7 s and 90.3 ± 4.9 s after microsphere addition. It was also possible to stain the platelets with the fluorescent dye DOIC6 in whole blood to eliminate the addition of microspheres and perform the SAW-CT assay. Monitoring the coagulation time with fluorescently labelled platelets gave results identical to microsphere treated blood, although the signal to noise ratio in microsphere supplemented blood was superior to DIOC6-labelled blood (data not shown).
Target variable and variation
Similar coagulation kinetics measured by SAW-CT in the untreated blood of 8 healthy individuals (Figure 2b) was observed with nearly identical slopes of the correlation coefficient curves and a coagulation time of 226 ± 7 s.
Intra-day variance on repeatedly measured SAW-CTs of a single blood sample from 4 individuals over an extended period of time (stored up to 10 h at room temperature) resulted in an 11.2 ± 1.4% coefficient of variation. Inter-day variance was calculated by measuring the SAW-CT in 4 individuals on different days over an extended time period (159–687 days) and resulted in a coefficient of variation of 14.1 ± 3.8%. The SAW-CT did not change markedly after the sample was left at room temperature for 24 h, compared to measurement directly after vein puncture (data not shown).
Influence of anticoagulants in vitro
Blood containing clinically relevant doses of UFH were subjected to analysis by SAW-CT and compared with aPTT. aPTT increased with ascending heparin concentrations up to 0.5 IU/ml, but it was not possible to measure aPTT at 1.0 IU/ml due to instrument restrictions, designated n.a. in Figure 3. Increasing concentrations of UFH also prolonged SAW-CT (see Figure 3a). Concentrations exceeding 0.25 IU/ml elicited a significant prolongation of SAW-CT and aPTT, and concentrations much in excess of 1.0 IU/ml elicited a SAW-CT response (dose-proportionality up to 5 IU/ml; data not shown). In contrast to UFH, no reaction in either aPTT or SAW-CT was elicited with the synthetic, FXa-selective pentasaccharide fondaparinux (data not shown).
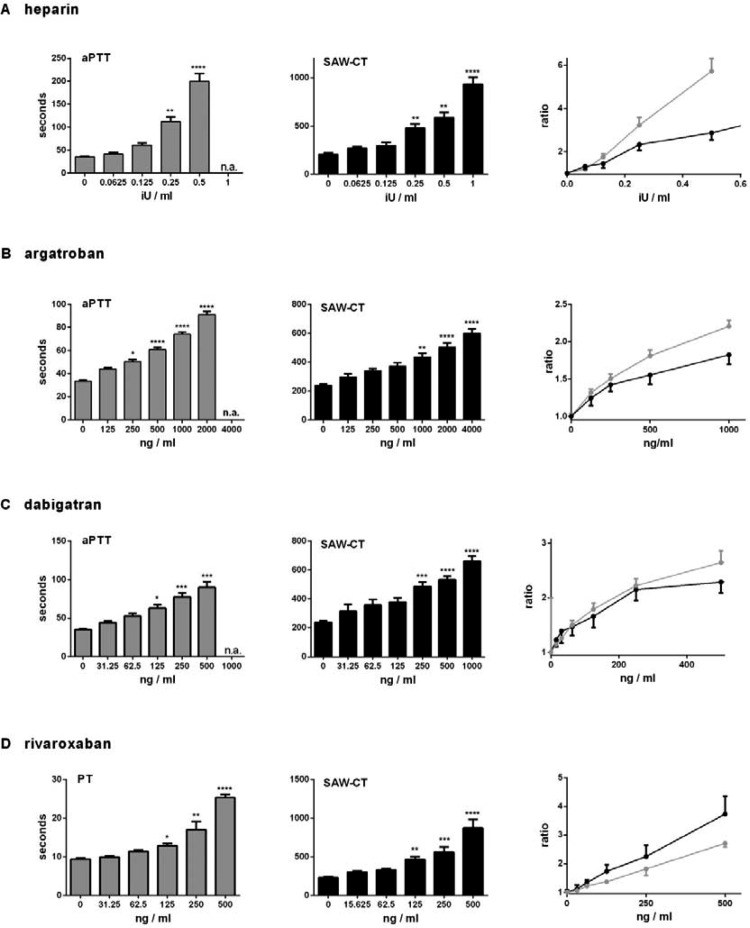
Figure 3.
Coagulation kinetics of blood containing clinically relevant doses of heparin (a), argatroban (b), dabigatran (c) and rivaroxaban (d). The drugs or buffer were added to 1 ml blood and aPTT, PT and SAW-CT were evaluated in 7 healthy volunteers. The ratio was derived by setting the coagulation times of untreated blood to 1. n.a. signifies that it was not possible to measure this concentration. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus buffer alone using the Kruskal-Wallis test with Dunn's multiple comparison test.
Increasing doses of the direct thrombin inhibitor argatroban prolonged aPTT times dose-proportionally up to 2000 ng/ml. The same was seen with the thrombin-inhibitor specific ecarin time test (ECAT) (data not shown). Likewise, argatroban dose-dependently prolonged the SAW-CT (Figure 3b). In contrast to the aPTT, SAW-CT dose-proportionality was still present at maximal doses (4000 ng/ml). Concentrations exceeding 250 ng/ml elicited a significant prolongation of aPTT, whereas 1000 ng/ml were needed in the SAW-CT assay to show significantly prolonged coagulation times, compared to untreated controls.
The direct thrombin inhibitor dabigatran dose-proportionally increased the aPTT up to 500 ng/ml and the SAW-CT up to the highest dose of 1000 ng/ml. aPTT measurement at 1000 ng/ml was not possible (see Figure 3c). Concentrations ≥125 ng/ml dabigatran elicited a significant prolongation of aPTT and ≥250 ng/ml in SAW-CT, compared to untreated control.
The direct FXa- inhibitor rivaroxaban dose-dependently increased the PT up to 500 ng/ml as in the SAW-CT (see Figure 3d). Rivaxoban was the only tested drug for which the slope of the ratio concentration curve was higher than the standard PT assay. Compared to untreated controls, concentrations ≥125 ng/ml rivaroxaban elicited a significant prolongation in SAW-CT compared to ≥250 ng/ml for significant prolongation in PT.
Influence of antiplatelet agents in vivo
Acetylsalicylic acid together with clopidogrel in clinical samples did not influence SAW-CT (data not shown), but the GPIIb/IIIa inhibitor abciximab in vitro prolonged coagulation times in the SAW-CT assay. Addition of 1:500 (v/v) abciximab to the blood of healthy donors prolonged coagulation times from 222 ± 7 s to 300 ± 19 s, n = 12, p = 0.001 in unpaired t-test). The impact of aspirin treatment on the SAW-response to dabigatran and rivaroxaban was negligible (Fig. 4).
Figure 4.
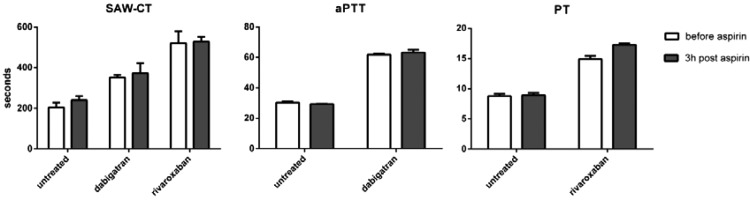
Blood from healthy donors containing 125 ng/ml dagibatran, 250 ng/ml rivaroxaban or buffer (untreated) was measured before and 3 h after ingestion of 500 mg aspirin in SAW-based assay, aPTT (for dabigatran) and PT (for rivaroxaban). Mean ± S.E.M.
DISCUSSION
Two main advantages of the SAW-CT assay, compared to other methods, are apparent. The first advantage is that the SAW-CT assay monitors all types of clinically used anticoagulants in a single test, including the new oral anticoagulants such as the FXa inhibitor, rivaroxaban, and the direct thrombin inhibitor dabigatran. Clinically relevant concentrations of heparin,20, 21 argatroban,22, 23 dabigatran24, 25, 26, 27, 28 and rivaroxaban17, 29, 30, 31, 32, 33, 34 were used in the SAW-CT assay to evaluate the usefulness of the new method to monitor anticoagulant therapy. The second advantage over other methods is that the SAW-CT assay can potentially be employed in point-of-care diagnostics.
The device also measured the anticoagulatory effect in the presence of aspirin without affecting the anticoagulant induced SAW-CT time, this would have complicated interpreting the read-out, given the widespread use of low dose aspirin.
The use of a global clotting test with a simple test principle without a specific biochemical trigger (except calcium-resupplementation) is advantageous. However, the test does not differentiate between agents, which should not be necessary when the anticoagulant or antiplatelet drug is identifiable, as is normally the case, or at least a considerable inhibition by any agent is detected (which, e.g., then enables or disables further therapeutic options). For example, a treating physician would be in the position to reliably adjust the necessary thrombolysis dose to minimize the risk of intracranial bleeding due to t-PA synergism with previously administered anticoagulants. It is essential that results be obtained quickly in such a situation to ensure that thrombolysis is initiated as soon as possible.
Construction of an ultraportable device with a single-use cartridge containing calcium chloride and microspheres and a possible coagulation accelerator, such as a negatively charged nanoclay is feasible. This prototype was designed to be as simple as possible, so no coagulation accelerators such as kaolin were added. The aPTT ratios obtained from commercial analyzers were always higher than the SAW-CT ratios, and it is reasonable to speculate that addition of negatively charged surfaces may accelerate the clot formation kinetics in the SAW-CT assay and that the SAW-CT ratios and sensitivity would thereby increase.
A pre-loaded cartridge could be inserted into the device and a drop of blood introduced into the reaction vessel by a fluid actuator system. A tiny image sensor with a resolution much smaller than 1 megapixel would be sufficient to perform correlation analysis if placed at a very small distance to the reaction well. Fluorescent light can easily be generated by an LED array35 suitable to incorporation into an ultraportable device. Recently, progress has been made in miniaturising fluorescence microscopes and integrating them into mass-produced μ-fluidic devices.36 Lens free, on-chip imaging has also been reported.37 Since a very small blood volume (6 μl) is needed, this method might also be applicable for investigating response to anticoagulation in small laboratory animals, such as mice, which would then not need to be sacrificed. To validate the suitability of this method, a small hand-held prototype that can be operated by untrained personnel and can be used to perform studies in a large patient population is currently under construction.
During clot formation coagulation kinetics are quantified by measuring biophysical properties of blood, such as increased resistance (impedance measurements), viscosity enhancement monitored with magnetic beads or optical evaluation of turbidity, which rises during blood solidification. Optical methods can be divided in nephelometric and turbidimetric methods that measure clot assembly and degradation at visible wavelengths.38 Nephelometry is the determination of scattered light in a specimen at certain angles. During the fibrin clot formation, the scattered light intensity increases. Turbidimetric methods are based on the change of light absorption in the specimen during clot formation. Initially, absorbance in the sample is weak and increases during clot formation. When the clot is fully formed, absorbance remains high. Advantages of optical methods are high sensitivity and easy automation. A disadvantage is the susceptibility to interference with specific plasma components and low sensitivity in certain disorders.38 Other clot detection methods include mechanical clot detection, whereby fibrin strands attach to a moving mechanical part that ultimately either completes or opens an electrical circuit. Viscosity-based detection relies on magnetic beads. During clotting plasma viscosity increases, decreasing translational freedom of the magnetic beads, which is recorded. In the double magnetic beads method, the magnetic signal generated by the attracting swing amplitude of a pair of magnetic beads during solidification in the sample is recorded. Advantages of non-optical methods are that interference with plasma from lipemic or icteric patients is absent and that fewer reagents are required.39, 40, 41
The advantage of fluorescence imaging as used in the SAW-CT over other optical methods, such as nephelometric and turbidimetric imaging, is that the presence of erythrocytes, which interfere with non-fluorescent imaging modes, do not interfere with fluorescence. Fluorescence imaging does not require trained personnel or centrifugation and the SAW-CT principle can be applied in a POC device by anyone, including the patient. Furthermore, it is expected that plasma variations that interfere with non-fluorescent imaging will not interfere with fluorescence imaging in SAW-CT. Compared to clot detection methods used in commercial coagulation analysers, SAW-CT might be most closely related to measuring the change in viscosity during solidification with the magnetic bead methods. The correlation analysis in SAW-CT detects a limitation of translational freedom in suspended microspheres, as do magnetic bead methods.
Microfluidic approaches to measure coagulation are numerous. The dependency of the coagulation response on spatial dynamics such as tissue factor distribution and shear rate42, 43 has been shown. Plug-based microfluidic systems have been developed to measure the aPTT3 in the presence and absence of argatroban by measuring erythrocyte movement under bright-field microscopy, when whole blood was used, and fluorescence microscopy when plasma was used. However, it remains unclear whether this system can suitably measure oral FXa inhibition and no automatic image processing technique has been reported. Lateral microflow assays6 utilizing fluorescently labelled fibrinogen5 have been used to precisely measure the onset of coagulation after the addition of thrombin.6 This method can be used to detect heparin concentrations ranging from 0.25 to 2.00 IU/ml, which is comparable to the SAW-CT measuring range. The results from this assay closely correlate to conventional aPTT. It would be of interest to know whether this assay is capable of detecting relevant doses of oral FXa inhibitors. Microchannels have been used to mimic microthrombus formation in the microcirculation and capillary occlusion.1 Membrane-based microfluidic devices have been used to control agonist flow into blood platelets8 and label-free platelet activation measurements7 by means of change in hydrodynamic size. However, all these systems are not intended to monitor anticoagulant therapy in a POC setting. Simultaneous quartz crystal microbalance with dissipation monitoring (QCM-D) and surface plasmon resonance (SPR) measurements have been used to analyze surface kinetics of coagulation44 and detect heparin in whole blood samples. However, as with the microfluidic ACT system iSTAT, this technique is not suitable for detecting heparin concentrations less than 1 IU/ml and thus will surely fail to detect new anticoagulants such as the oral FXa inhibitors. The QCM is a member of a wider class of sensors based on surface acoustic waves and the instruments share similar operating principles. In the SAW-CT, SAW is used to mix re-calcified blood, but SAW can also be used as a biosensor. SAW-based sensors have been applied to analyse prothrombin times45 and platelet aggregation.46 The results from these studies are also very promising and may pave the way for another microfluidic point-of-care assay to monitor coagulation.
The SAW method is well-suited to handling and manipulating small liquid volumes. Asymmetry in SAW radiation has successfully been applied to aggregate particles, concentrating them at the centre of a droplet. This SAW-induced particle concentration process operates much more quickly than conventional methods and opens the possibility of improving biosensor technology.47 Furthermore, SAW actuation has been employed to achieve highly efficient, rapid seeding of particles into porous cell scaffolds48 and quickly pump liquids through microchannels.49 SAW-induced microfluidic handling could significantly contribute to advancing vascular medicine. For example, investigating the von Willebrand factor50 could be facilitated by pumping whole blood51 through different types of blood vessel models52 with different cross sections, simulating arteriolar networks. This technique could be applied to investigating phenotypes and platelet adhesion mechanisms in diverse von Willebrand diseases53, 54 where a highly limited amount of blood is available for flow-based adhesion assays.55, 56 Microfluidic techniques could also be applied to individual platelets,57 white blood cells58 and exploited for other blood components such as plasma.59 These techniques could be useful in this context, since flow properties of blood cells in microchannels60, 61 diverge from bulk flow properties and platelet adhesion rates are very sensitive to hematocrit change.62, 63 Tight hematocrit control might be necessary to gain reproducible platelet adhesion rates in flow-based adhesion assays.56
CONCLUSIONS
The SAW-CT is a novel microfluidic approach to monitor anticoagulant pharmacotherapy, potentially in a point-of-care setting, and can quantitatively detect all clinically employed anticoagulants in a single test. Furthermore, at present, no other method monitors the new anticoagulants. Established coagulation tests in the clinic are not sensitive enough to reliably identify patients receiving new oral anticoagulants, such as rivaroxaban. SAW-CT is the only test combining SAW induced mixing with fluorescent imaging and automatic image processing. The method can principally be scaled down for an ultra-portable device, which can be employed bed-side or in ambulant care, facilitating decisions for appropriate diagnosis and therapy regarding anticoagulants.
ACKNOWLEDGMENTS
Funding was received from BMBF Germany, Federal Ministry of Education and Research Project “On-Chip Flusskammer,” FKZ: 01EZ0850.
The presented method is subject to German and international patents. DMPA patent number 10 2011 001 952 and international patent applications (WO 2012/139752).
References
- Kamada H., Hattori K., Hayashi T., and Suzuki K., Thromb. Res. 114, 195 (2004). 10.1016/j.thromres.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Pompano R. R., Liu W., Du W., and Ismagilov R. F., Annu. Rev. Anal. Chem. (Palo Alto Calif) 4, 59 (2011). 10.1146/annurev.anchem.012809.102303 [DOI] [PubMed] [Google Scholar]
- Song H., Li H. W., Munson M. S., Van Ha T. G., and Ismagilov R. F., Anal. Chem. 78, 4839 (2006). 10.1021/ac0601718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metref L. B., Vallet F., Blanc V., Goetschmann N., and Renaud P., Micro Nanosyst. 1, 41 (2009). 10.2174/1876402910901010041 [DOI] [Google Scholar]
- Dudek M. M., Kent N. J., Gu P., Fan Z. H., and Killard A. J., Analyst 136, 1816 (2011). 10.1039/c0an00907e [DOI] [PubMed] [Google Scholar]
- Dudek M. M., Kent N., Gustafsson K. M., Lindahl T. L., and Killard A. J., Anal. Chem. 83, 319 (2011). 10.1021/ac102436v [DOI] [PubMed] [Google Scholar]
- Inglis D. W., Morton K. J., Davis J. A., Zieziulewicz T. J., Lawrence D. A., Austin R. H., and Sturm J. C., Lab Chip 8, 925 (2008). 10.1039/b800721g [DOI] [PubMed] [Google Scholar]
- Neeves K. B. and Diamond S. L., Lab Chip 8, 701 (2008). 10.1039/b717824g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S. W., Nuschele S., Wixforth A., Gorzelanny C., Alexander-Katz A., Netz R. R., and Schneider M. F., Proc. Natl. Acad. Sci. U.S.A. 104, 7899 (2007). 10.1073/pnas.0608422104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenberg Z., Muller H., Habermuller H., Geisbauer A., Pipper J., Felbel J., Kielpinski M., Scriba J., and Wixforth A., Lab Chip 5, 308 (2005). 10.1039/b412712a [DOI] [PubMed] [Google Scholar]
- Frommelt T., Kostur M., Wenzel-Schafer M., Talkner P., Hanggi P., and Wixforth A., Phys. Rev. Lett. 100, 034502 (2008). 10.1103/PhysRevLett.100.034502 [DOI] [PubMed] [Google Scholar]
- Chinga G. and Syverud K., Nord. Pulp Pap. Res. J. 22, 441 (2007). 10.3183/NPPRJ-2007-22-04-p441-446 [DOI] [Google Scholar]
- Ng V. L., Clin. Lab Med. 29, 253 (2009). 10.1016/j.cll.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Ray M. J. and Hawson G. A., Clin. Lab Haematol. 11, 221 (1989). 10.1111/j.1365-2257.1989.tb00212.x [DOI] [PubMed] [Google Scholar]
- van den Besselaar A. M., Neuteboom J., and Bertina R. M., Blood Coagul Fibrinolysis 4, 895 (1993). [PubMed] [Google Scholar]
- Rossi E., Mondonico P., Lombardi A., and Preda L., Thromb. Res. 52, 453 (1988). 10.1016/0049-3848(88)90029-1 [DOI] [PubMed] [Google Scholar]
- Graff J., von Hentig N., Misselwitz F., Kubitza D., Becka M., Breddin H. K., and Harder S., J. Clin. Pharmacol. 47, 1398 (2007). 10.1177/0091270007302952 [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E., Comput. Methods Programs Biomed. 23, 57 (1986). 10.1016/0169-2607(86)90081-7 [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.4824043 for video sequences showing SAW-induced mixing and coagulation. Supplemental video 1 shows a programmed cycle of 10 seconds 10 dBm output power followed by 2 seconds stop, then 10 seconds 20 dBm, 2 seconds pause and finally 10 seconds 27 dBm, the maximum power delivered by the device. Supplemental video 2 shows the recorded events in the reaction vessel in untreated blood (left panel, 0 ng/ml) and blood treated with 500 ng/ml rivaroxaban (right panel, 500 ng/ml). The video runs at 4 times normal speed.
- Bendetowicz A. V., Beguin S., Caplain H., and Hemker H. C., Thromb. Haemostasis 71, 305 (1994). [PubMed] [Google Scholar]
- Bonar R. A., Favaloro E. J., and Marsden K., Semin Thromb Hemost 38, 632 (2012). 10.1055/s-0032-1321954 [DOI] [PubMed] [Google Scholar]
- Harder S., Graff J., Klinkhardt U., von Hentig N., Walenga J. M., Watanabe H., Osakabe M., and Breddin H. K., Thromb. Haemostasis 91, 1137 (2004). 10.1160/TH03-12-0794 [DOI] [PubMed] [Google Scholar]
- Akimoto K., Klinkhardt U., Zeiher A., Niethammer M., and Harder S., J. Clin. Pharmacol. 51, 805 (2011). 10.1177/0091270010372627 [DOI] [PubMed] [Google Scholar]
- Hariharan S. and Madabushi R., J. Clin. Pharmacol. 52(S1), 119S–125S (2012). 10.1177/0091270011415527 [DOI] [PubMed] [Google Scholar]
- Stangier J., Stahle H., Rathgen K., Roth W., Reseski K., and Kornicke T., J. Clin. Pharmacol. 52(2), 243–250 (2012). 10.1177/0091270010393342 [DOI] [PubMed] [Google Scholar]
- Stangier J. and Clemens A., Clin. Appl. Thromb. Hemost. 15(Suppl 1), 9S (2009). 10.1177/1076029609343004 [DOI] [PubMed] [Google Scholar]
- Stangier J., Stahle H., Rathgen K., Roth W., and Shakeri-Nejad K., J. Clin. Pharmacol. 48, 1411 (2008). 10.1177/0091270008324179 [DOI] [PubMed] [Google Scholar]
- Stangier J., Rathgen K., Stahle H., Gansser D., and Roth W., Br. J. Clin. Pharmacol. 64, 292 (2007). 10.1111/j.1365-2125.2007.02899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Roth A., and Mueck W., Curr. Med. Res. Opin. 24, 2757 (2008). 10.1185/03007990802361499 [DOI] [PubMed] [Google Scholar]
- Jiang J., Hu Y., Zhang J., Yang J., Mueck W., Kubitza D., Bauer R. J., Meng L., and Hu P., Thromb. Haemostasis 103, 234 (2010). 10.1160/TH09-03-0196 [DOI] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Zuehlsdorf M., and Mueck W., J. Clin. Pharmacol. 47, 218 (2007). 10.1177/0091270006296058 [DOI] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Mueck W., and Zuehlsdorf M., J. Clin. Pharmacol. 46, 981 (2006). 10.1177/0091270006292127 [DOI] [PubMed] [Google Scholar]
- Zhao X., Sun P., Zhou Y., Liu Y., Zhang H., Mueck W., Kubitza D., Bauer R. J., and Cui Y., Br. J. Clin. Pharmacol. 68, 77 (2009). 10.1111/j.1365-2125.2009.03390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Voith B., Zuehlsdorf M., and Wensing G., Clin. Pharmacol. Ther. 78, 412 (2005). 10.1016/j.clpt.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Prisco D. and Paniccia R., Thromb. J. 1, 1 (2003). 10.1186/1477-9560-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K. K., Burns L. D., Cocker E. D., Nimmerjahn A., Ziv Y., Gamal A. E., and Schnitzer M. J., Nat. Methods 8, 871 (2011). 10.1038/nmeth.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A., Luo W., Su T. W., Gorocs Z., Xue L., Isikman S. O., Coskun A. F., Mudanyali O., and Ozcan A., Nat. Methods 9, 889 (2012). 10.1038/nmeth.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geffen M. and van Heerde W. L., Throm. Res. 129, 681 (2012). 10.1016/j.thromres.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Heins M. and Reinauer H., J. Int. Fed. Clin. Chem. 8, 117 (1996). [PubMed] [Google Scholar]
- McCraw A., Hillarp A., and Echenagucia M., Haemophilia 16(Suppl 5), 74 (2010). 10.1111/j.1365-2516.2010.02302.x [DOI] [PubMed] [Google Scholar]
- McGlinchey K., Adv. Admin. Lab. 19, 26 (2010). [Google Scholar]
- Kastrup C. J., Runyon M. K., Shen F., and Ismagilov R. F., Proc. Natl. Acad. Sci. U.S.A. 103, 15747 (2006). 10.1073/pnas.0605560103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Kastrup C. J., and Ismagilov R. F., Thromb. Res. 122(Suppl 1), S27 (2008). 10.1016/S0049-3848(08)70015-X [DOI] [PubMed] [Google Scholar]
- Vikinge T. P., Hansson K. M., Sandstrom P., Liedberg B., Lindahl T. L., Lundstrom I., Tengvall P., and Hook F., Biosens. Bioelectron. 15, 605 (2000). 10.1016/S0956-5663(00)00125-1 [DOI] [PubMed] [Google Scholar]
- Muller L., Sinn S., Drechsel H., Ziegler C., Wendel H. P., Northoff H., and Gehring F. K., Anal. Chem. 82, 658 (2010). 10.1021/ac9021117 [DOI] [PubMed] [Google Scholar]
- Sinn S., Muller L., Drechsel H., Wandel M., Northoff H., Ziemer G., Wendel H. P., and Gehring F. K., Analyst 135, 2930 (2010). 10.1039/c0an00474j [DOI] [PubMed] [Google Scholar]
- Li H., Friend J. R., and Yeo L. Y., Biomed. Microdevices 9, 647 (2007). 10.1007/s10544-007-9058-2 [DOI] [PubMed] [Google Scholar]
- Li H., Friend J. R., and Yeo L. Y., Biomaterials 28, 4098 (2007). 10.1016/j.biomaterials.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Yeo L. Y. and Friend J. R., Biomicrofluidics 3, 12002 (2009). 10.1063/1.3056040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah M. A., Huck V., Niemeyer V., Desch A., Angerer J. I., McKinnon T. A. J., Wixforth A., Schneider S. W., and Schneider M. F., Biomicrofluidics 7, 044124 (2013). 10.1063/1.4819746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Johnston I. D., Tan C. K., and Tracey M. C., Biomicrofluidics 4, 44112 (2010). 10.1063/1.3528327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Itoyama T., Yoshida K., Sawada Y., Ikeda S., Fukuda T., Matsuda T., Negoro M., and Arai F., Biomicrofluidics 4, 46505 (2010). 10.1063/1.3523471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Annu. Rev. Med. 56, 173 (2005). 10.1146/annurev.med.56.082103.104713 [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Thromb. Haemost. 98, 55 (2007). 10.1160/TH07-04-0279 [DOI] [PubMed] [Google Scholar]
- Meyer dos Santos S., Klinkhardt U., Scholich K., Nelson K., Monsefi N., Deckmyn H., Kuczka K., Zorn A., and Harder S., Blood 117, 4999 (2011). 10.1182/blood-2011-02-335471 [DOI] [PubMed] [Google Scholar]
- Meyer Dos Santos S., Klinkhardt U., Schneppenheim R., and Harder S., Platelets 21, 60 (2010). 10.3109/09537100903410609 [DOI] [PubMed] [Google Scholar]
- Piacentini N., Mernier G., Tornay R., and Renaud P., Biomicrofluidics 5, 34122 (2011). 10.1063/1.3640045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Chen D., Yuan T., Xie Y., and Chen X., Biomicrofluidics 7, 034106 (2013). 10.1063/1.4808179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsy A. et al. , Biomicrofluidics 6, 12804 (2012). 10.1063/1.3672188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J. M., Dusting J., Kaliviotis E., and Balabani S., Biomicrofluidics 6, 24119 (2012). 10.1063/1.4717755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leble V., Lima R., Dias R., Fernandes C., Ishikawa T., Imai Y., and Yamaguchi T., Biomicrofluidics 5, 44120 (2011). 10.1063/1.3672689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Kameneva M. V., and Antaki J. F., Biorheology 44, 161 (2007). [PubMed] [Google Scholar]
- Turitto V. T. and Weiss H. J., Science 207, 541 (1980). 10.1126/science.7352265 [DOI] [PubMed] [Google Scholar]