Abstract
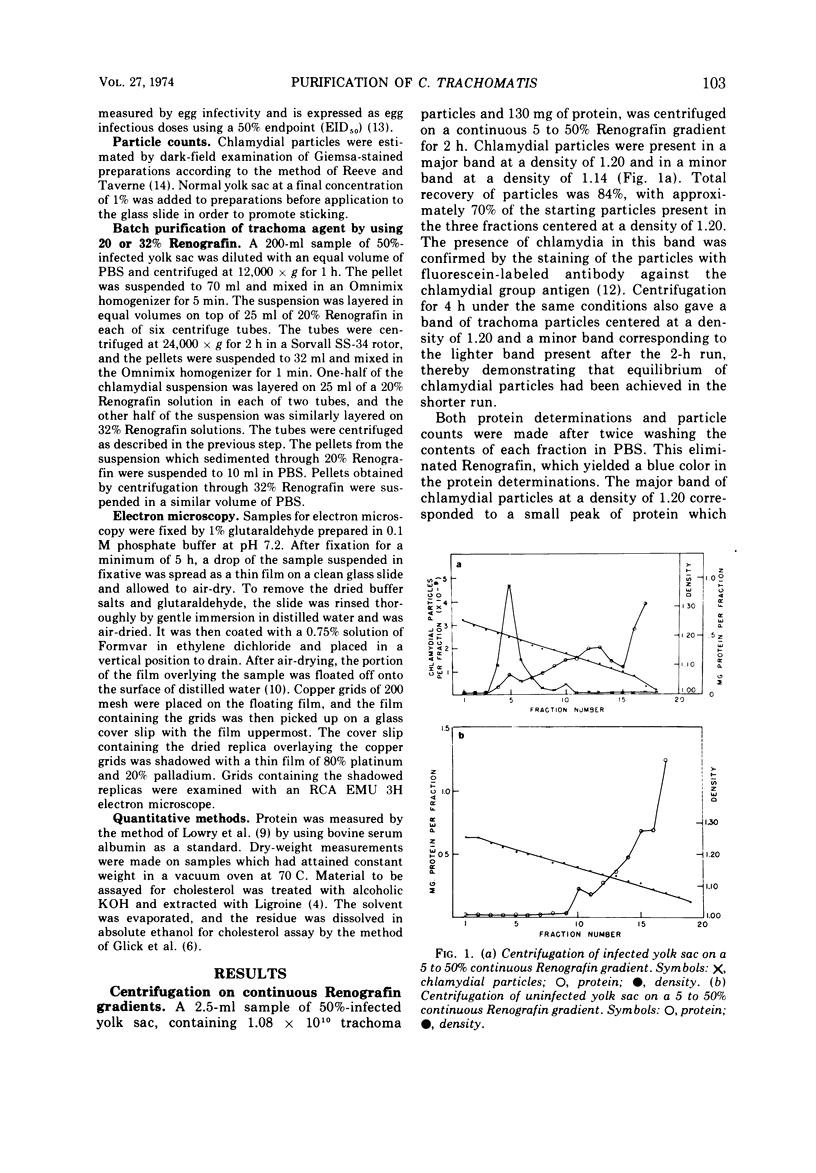
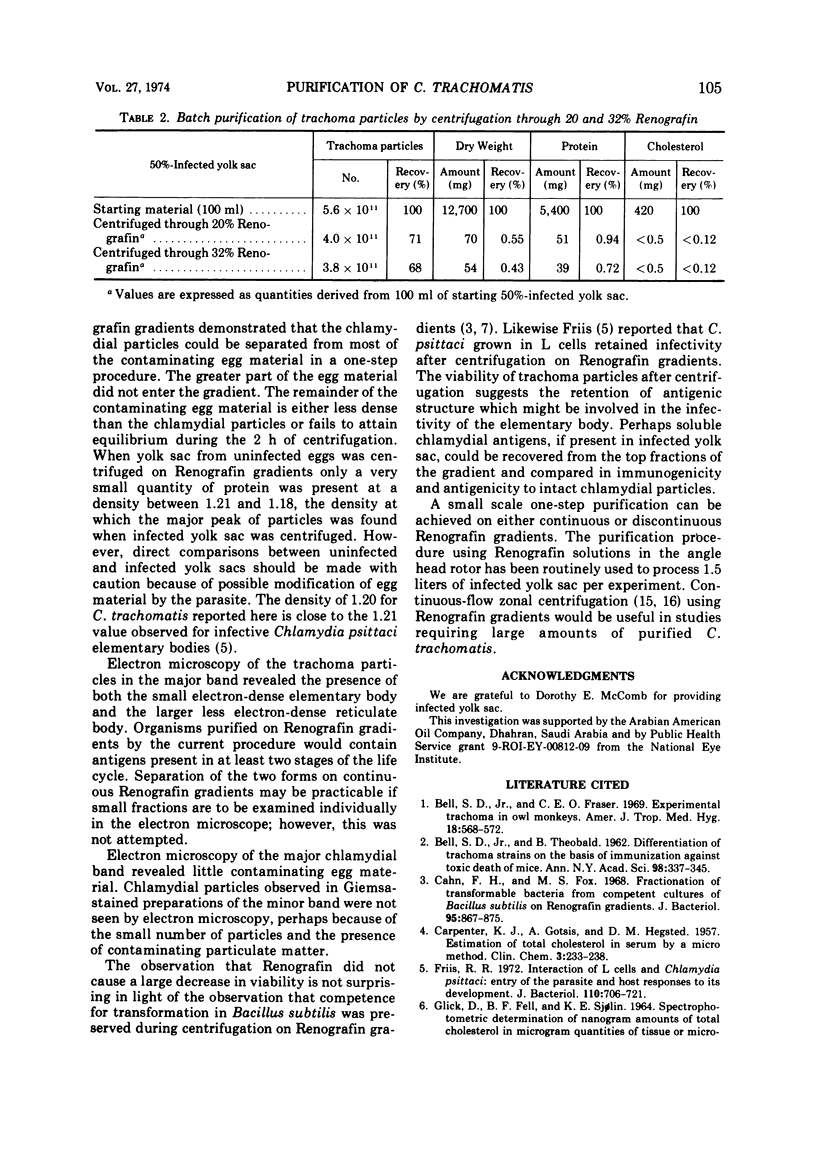
Chlamydia trachomatis grown in the yolk sac of embryonated eggs was purified by centrifugation on continuous isopycnic Renografin density gradients. A band of chlamydial particles with a buoyant density of 1.20 contained 70% of the starting particles, and electron microscopy revealed the virtual absence of contaminating egg material. Centrifugation on Renografin gradients caused only a moderate decrease in infectivity. For large-scale purification, infected yolk sac was centrifuged through Renografin solutions, resulting in greater than 60% recovery of starting chlamydial particles, but less than 1% recovery of the dry weight and protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL S. D., Jr, THEOBALD B. Differentiation of trachoma strains on the basis of immunization against toxic death of mice. Ann N Y Acad Sci. 1962 Mar 5;98:337–346. doi: 10.1111/j.1749-6632.1962.tb30556.x. [DOI] [PubMed] [Google Scholar]
- Bell S. D., Fraser C. E. Experimental trachoma in owl monkeys. Am J Trop Med Hyg. 1969 Jul;18(4):568–572. doi: 10.4269/ajtmh.1969.18.568. [DOI] [PubMed] [Google Scholar]
- CARPENTER K. J., GOTSIS A., HEGSTED D. M. Estimation of total cholesterol in serum by a micro method. Clin Chem. 1957 Aug;3(4 Pt 1):233–238. [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZENELSON E., BERNKOPF H. SEROLOGIC DIFFERENTIATION OF RACHOMA STRAINS AND OTHER AGENTS OF THE PSITTACOSIS - LYMPHOGRANULOMA VENERERUMTRACHOMA GROUP WITH THE AID OF THE DIRECT FLUORESCENT ANTIBODY METHOD. J Immunol. 1965 Mar;94:467–474. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manire G. P. Structure of purified cell walls of dense forms of meningopneumonitis organisms. J Bacteriol. 1966 Jan;91(1):409–413. doi: 10.1128/jb.91.1.409-413.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS R. L., McCOMB D. E. Immunofluorescent studies with trachoma and related antigens. J Immunol. 1962 Oct;89:545–554. [PubMed] [Google Scholar]
- REEVE P., TAVERNE J. A simple method for total particle counts of trachoma and inclusion blennorrhoea viruses. Nature. 1962 Sep 1;195:923–924. doi: 10.1038/195923a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Clark J. W., Jr, Cline G. B., Anderson N. G., Russell H. Separation of Treponema pallidum from tissue substances by continuous-flow zonal centrifugation. Appl Microbiol. 1972 Apr;23(4):714–720. doi: 10.1128/am.23.4.714-720.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplin I., Sottong P. Large-volume purification of tumor viruses by use of zonal centrifuges. Appl Microbiol. 1972 May;23(5):1010–1014. doi: 10.1128/am.23.5.1010-1014.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. A potency test for trachoma vaccine utilizing the mouse toxicity prevention test. Am J Ophthalmol. 1967 May;63(5 Suppl):1443–1454. doi: 10.1016/0002-9394(67)94130-x. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kenny G. E., Grayston J. T. Characterization of trachoma antigens protective against mouse toxicity. Am J Ophthalmol. 1967 May;63(5 Suppl):1454–1461. doi: 10.1016/0002-9394(67)94131-1. [DOI] [PubMed] [Google Scholar]



