Abstract
Cancer cells and immune cells modulate their metabolism according to specific needs during cancer progression and immune responses. The ability to measure cellular metabolic function in vivo would enable the evaluation of tumors and their response to therapy and also the effectiveness of cellular immune responses to cancer. Positron emission tomography (PET) is a highly sensitive clinical imaging modality that enables whole-body, quantitative measurements of tissue biochemical function. Here, we review work using PET probes for specific metabolic pathways to measure cell function in cancer and immunity. We focus on the use of probes for glycolysis and nucleoside salvage and then discuss the development of new metabolic probes that visualize distinct parameters of cell function during disease.
Cancer and the immune system: the need for functional imaging tools
Cancer results from aberrant cellular genetic activity that occurs in specific tissue micro-environments. Interactions between transformed cells and innate and adaptive immune cells play a critical role in determining tumor progression. Malignant transformation can trigger cellular immune responses that are capable of killing cancer cells early, before the emergence of detectable lesions [1•]. Cancer, as it appears clinically however, occurs in a state of immune dysfunction. Helper T cells (CD4+), cytotoxic T cells (CD8+), macrophages, and natural killer cells (NK cells) are often found in tumors [1•]. While the presence of cytotoxic T cells or NK cell infiltrates often indicates a good prognosis [2], tumors can grow in the presence of immune cells. It is now known that tumors deploy an arsenal of weapons against immune cells to suppress their function and evade killing [3,4]. Moreover, specific types of immune activity, particularly chronic inflammation arising from innate immune cell infiltration, aid tumor growth and invasion [5].
Identification of key mechanisms underlying immune dysfunction in cancer has led to several therapeutic strategies that arm the immune system against tumor-induced immunosuppression. Vaccination with dendritic cells pulsed with proteins from a patient’s tumor can mobilize endogenous T cells to attack that tumor [6]. Vaccination approaches have been coupled with the blockade of T cell inhibitory signals (e.g. by treating with monoclonal antibodies against CTLA-4) in order to augment T cell number and function in tumors [7]. Another promising immune modulatory strategy is adoptive cell transfer (ACT) therapy using tumor-reactive T cells primed in vitro to kill tumor cells [8,9]. Combining ACT therapy with dendritic cell vaccination and blockade of T cell inhibitory signals offers the potential for treating a variety of cancers that are typically difficult to manage with conventional therapies.
The central role of the immune system in cancer progression and anticancer therapies encourages the development of technologies that can measure parameters pertaining to both tumor cells and immune cells for diagnosing disease and monitoring treatment. Whole-body imaging modalities such as magnetic resonance imaging (MRI), computerized tomography (CT), single photon emission computerized tomography (SPECT), and positron emission tomography (PET) can detect pathological lesions throughout the body [10]. PET is particularly applicable to the task of measuring cell function because it provides clinically relevant measurements of tissue biochemistry [11]. This brief review will highlight the work that has led to the development of PET into a technology to measure distinct functional parameters of cancer and immune cells in vivo and evaluate the efficacy of cancer immunotherapy.
PET imaging of cell function
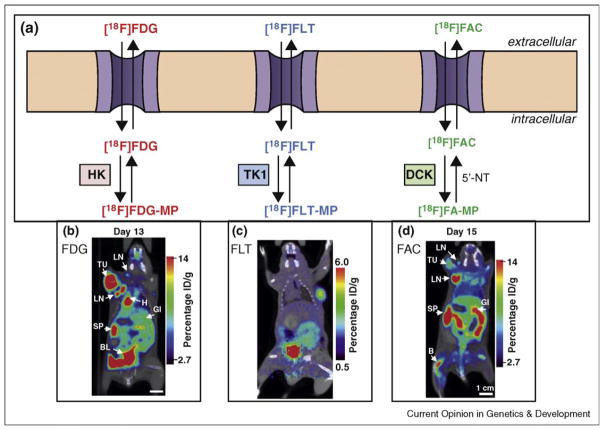
PET is a noninvasive imaging modality used in preclinical and clinical settings to measure site-specific accumulation of probes labeled with positron emitting radioisotopes at subnanomolar concentrations in the body. The biodistribution of a particular probe is a function of unique biochemical interactions between the probe and tissues. Numerous PET probes have been developed that measure reporter gene activity, cell surface receptor expression, and metabolism (reviewed in [12,13]). The following sections will focus on PET probes that measure the activity of metabolic pathways that are critical to cancer and immune cell function (Figure 1).
Figure 1.
(a) Schematic showing the metabolic pathways measured by FDG, FLT, and FAC. HK = hexokinase; TK1 = thymidine kinase-1; DCK = deoxycytidine kinase. (b) Preclinical FDG-PET scan of a mouse after injection with the MoMSV oncoretrovirus. At the period of peak immune response, high FDG uptake is seen in the tumor, lymph nodes, and spleen. Reproduced from Radu et al. [56••]. (c) FLT-PET scan of a LN229 xenograft implanted in a scid/ scid mouse. (d) Preclinical FAC-PET scan of a mouse after injection with the MoMSV oncoretrovirus. At day 15, the peak of the immune response, increased FAC uptake is seen in the lymph nodes (LN) and spleen (SP) compared to tumor (TU). Reproduced from Radu et al. [56••].
[18F]FDG-PET in cancer imaging
In the 1930s, the German biochemist Otto Warburg first observed that cancer cells metabolize glucose nonoxidatively through glycolysis, even in the presence of oxygen [14]. Over 70 years after its discovery, the significance of the Warburg effect for cancer development and progression is still under intense investigation. Since the ATP yield of glycolysis is many folds lower than that of oxidative phosphorylation, tumor cells take up massive amounts of glucose to generate the same amount of energy that normal tissues can produce by oxidative phosphorylation of far fewer glucose molecules [15]. High intracellular concentrations of metabolic byproducts of glucose breakdown provide the building blocks for biosynthetic pathways necessary for cell growth and proliferation [16,17]. Also, upregulation of glycolysis may enable cancer cells to survive hypoxic tumor environments while their metabolic byproducts such as lactate and protons are thought to inhibit the growth of normal cells and the function of immune cells (reviewed in [15,18]).
Gain of function of oncogenes and/or loss of function of tumor suppressor genes play key roles in regulating the cellular biochemical machinery required to sustain high rates of glycolysis [19,20••]. Elevated glycolysis in cancer cells, relative to neighboring normal tissues, provides the rationale for metabolic PET imaging using [18F]-2-fluorodeoxyglucose ([18F]FDG). [18F]FDG is a radiolabeled glucose analog that is taken into cells through glucose transporters (GLUTs) and phosphorylated by hexokinase (HK), thus trapping it within the cell [21]. GLUTs and HK are downstream targets of several transcriptional pathways activated during cellular transformation. As a result, [18F]FDG accumulates at high levels in greater than 90% of tumors. [18F]FDG accumulation in cancer cells provides a quantitative, visual readout of oncogenic activity on a whole-body level.
[18F]FDG-PET has been used for the last three decades to diagnose and stage an array of cancers, including gastrointestinal stromal tumors (GIST), lymphoma, melanoma, sarcoma, lung, and breast cancer [22–32]. Some tumor types, such as prostate carcinoma, are often indolent with low levels of glycolytic activity, making them difficult to detect with [18F]FDG-PET. Alternative probes for the detection of prostate cancer are needed.
Recently [18F]FDG-PET has become a powerful technique to monitor the efficacy of anticancer therapies. One of the most notable examples of this application is visualizing the effect of targeted small molecule therapy on GIST. Imatinib inhibits c-kit, a receptor tyrosine kinase that contains activating mutations in more than 85% of cases of GIST [22]. GISTs are typically highly glycolytic tumors, and treatment with Imatinib leads to a dramatic reduction in tumor [18F]FDG accumulation in responding patients. It is important to note that the functional perturbation determined by diminished [18F]FDG accumulation often occurs before tumor shrinkage is observed with anatomical imaging modalities such as CT and MRI [22]. In nonresponding patients (e.g. individuals harboring Imatinib resistance mutations), [18F]FDG accumulation in the tumor does not diminish with drug treatment. [18F]FDG has been used to monitor and predict the effects of several other anticancer therapies, providing early readouts of treatment efficacy and helping guide decisions that eliminate unnecessary or ineffective treatment [33].
[18F]FDG-PET to visualize immune function
Phenotypically diverse cell types of the innate and adaptive immune systems rely heavily on glycolytic metabolism. Innate immune cells such as macrophages and granulocytes are critically dependent on HIF-1α-mediated induction of glycolytic genes to infiltrate inflamed tissue [34]. Adaptive immune cells upregulate the expression of GLUTs and HK in response to mitogenic signals [35]. This has enabled the use of [18F]FDG-PET in several immune-mediated disorders. In preclinical settings, [18F]FDG-PET was shown to detect specific sites of immune activity in animal models of rheumatoid arthritis [36], experimental autoimmune encephalitis [37], colitis [38], and graft-versus-host disease [39•]. PET imaging in these instances can also measure the effects of systemic immunosuppressive therapy. In the clinic, [18F]FDG-PET has been used to evaluate inflammation in rheumatoid arthritis [40], GHVD [39•], atherosclerosis [41], and infectious diseases [42].
The central role of glycolysis in cancer and immune cell function can lead to false positives when using [18F]FDG-PET for cancer diagnostics [43,44]. Two areas that are particularly problematic are firstly, differentiating viable tumor cells from colocalized immune cells and secondly, distinguishing lymph node metastasis from immune activation in reactive lymph nodes [45]. These limitations have led to the design of metabolic PET probes that provide readouts of cellular metabolic function distinct from glycolysis, to potentially allow discrimination of tumor from immune activity.
Imaging cell proliferation with [18F]FLT
Fluorothymidine (FLT) is an analog of thymidine that accumulates in cells via the thymidine salvage pathway. Similar to endogenous thymidine, FLT is taken up by nucleoside transporters expressed on the cell surface and is phosphorylated by the S-phase specific enzyme thymidine kinase 1 (TK1), trapping the probe within the cell [46,47]. [18F]FLT accumulates in highly proliferative tissues and largely correlates with positive staining for the proliferation antigen Ki67 [48]. [18F]FLT-PET has been evaluated as a probe for cell proliferation in diagnostic studies of several types of cancer and compared with [18F]FDG for the ability to specifically label tumors [49,50]. In some preclinical studies, measuring cell proliferation with [18F]FLT offers better tumor-specificity than measuring glycolytic activity with [18F]FDG. This is believed to be due to lower accumulation of FLT compared to FDG in tumor-infiltrating immune cells [51]. Nonetheless, [18F]FLT has limitations in cancer imaging. High levels of probe accumulation in actively proliferating marrow cavities limit the detection of lesions to areas away from central skeletal structures. Given the importance of thymidine and bromodeoxyuridine (BrDU) incorporation techniques in basic immunological studies, and the accumulation of [18F]FLT in bone marrow, it is surprising that [18F]FLT-PET has not been used more extensively to visualize immune responses in vivo. [18F]FLT-PET has shown promise in diagnosing extramedullary sites of leukemia, especially in areas such as the brain that have high levels of physiologic FDG accumulation [52]. [18F]FLT-PET may allow the detection of therapies designed to enhance immune cell proliferation during cancer immunotherapy.
Design of new metabolic PET probes to visualize the immune system
To further metabolic PET imaging technology, we have designed probes for metabolic pathways distinct from glycolysis and thymidine salvage. We focused on metabolic pathways that could enable the distinction of the immune cells from tumors. Previous studies have shown that hematolymphoid tissues are particularly enriched for deoxycytidine salvage and that the activation of the immune system augments the activity of this pathway [53,54]. Analogous to thymidine salvage, deoxycytidine salvage mediates the uptake of deoxycytidine, and related nucleosides, through specific transporters expressed on the cell surface. Intracellularly, deoxycytidine is phosphorylated and trapped by deoxycytidine kinase (DCK), a rate-limiting enzyme in deoxycytidine salvage [55].
Using a differential screening assay, we identified 1-(2-deoxy-2-fluoro-arabinofuranosyl)-cytosine (FAC), a deoxycytidine analog that accumulates in cells based on DCK activity [56••]. 18F-labeled FAC can be used as a PET probe to visualize DCK activity in vivo. Preclinical imaging in mice showed that [18F]FAC accumulates in primary and secondary lymphoid organs, including the thymus, bone marrow, spleen, and lymph nodes as well as in the gastrointestinal tract. [18F]FAC-PET signal in peripheral lymph nodes and spleens of mice increased with immune activation due to viral infection and lymphoproliferative disorders. Importantly, in an antitumor immune response model, [18F]FAC showed intense signal in lymphoid organs but little accumulation in the tumor compared to [18F]FDG [56••]. A comparison of FAC and FDG showed that these probes had different specificities for immune cell subtypes during an antitumor immune response. While deoxycytidine salvage was most prevalent in adaptive immune cells in active cell cycle, glycolysis was highest in innate immune cells (Nair-Gill et al., unpublished data). This finding demonstrates that imaging probes for distinct metabolic pathways can provide complementary functional information about the immune system in vivo, encouraging the use of multiple imaging probes in clinical situations to gather comprehensive information about immune function.
[18F]FAC also has a potential role in stratifying tumors based on the expression of DCK. Nucleoside analog prodrugs such as gemcitabine, cytosine arabinoside (cytarabine), and 5-aza-2′-deoxycytidine (decitabine) are DCK substrates and this enzyme is critically required for their activation [55,57]. Loss of DCK activity in tumor cells is a common cause of resistance to these prodrugs. Thus, a noninvasive imaging method to detect functional DCK in tumors could guide the treatment of patients with nucleoside analog therapy. We have demonstrated that [18F]FAC preferentially accumulates in DCK-positive cells. Cells lacking DCK expression are unable to phosphorylate and trap [18F]FAC and show no probe accumulation. Using a preclinical cancer treatment model, we found that [18F]FAC-PET was able to predict responses to gemcitabine [58••]. DCK-positive tumors accumulated [18F]FAC during a pretreatment scan and responded to gemcitabine treatment, while tumors that were deficient for DCK were not detected with [18F]FAC-PET and continued to grow despite gemcitabine treatment. Importantly, [18F]FDG imaging could not distinguish the gemcitabine sensitive and resistant tumors, demonstrating that functional imaging of distinct metabolic pathways can predict the effect of specific anticancer therapies [58••].
Concluding remarks
Measuring cellular metabolism can provide insight into the function of cancer and the immune system during disease. Whole-body imaging with metabolic PET can isolate sites of disease and monitor how therapeutic intervention affects cell function. We have summarized work on the development and application of PET probes with defined specificities for different metabolic pathways. Further understanding of the biological role of these pathways during cancer progression and immune responses should guide the use of metabolic imaging for diagnostics and the evaluation of specific therapies. Identification and characterization of metabolic pathways other than glycolysis and nucleoside salvage will provide opportunities to design new PET imaging probes. The development of new PET probes is essential: the ability to make several distinct measurements in the same subject will further the understanding of cancer and immunity on a personalized basis.
Acknowledgments
We acknowledge all colleagues who contributed to the work presented here. We acknowledge Andrew Tran for help with figure preparation and Barbara Anderson for help with manuscript preparation. REL is supported by the Tumor Immunology Training Grant (5-T32-CA009120-34). ENG is supported by US National Institutes of Health T32 GM08042 UCLA Medical Scientist Training Program and Interdisciplinary Training in Virology and Gene Therapy T32 A1065067. CGR is supported by National Cancer Institute Grant No. 5U54 CA119347, In Vivo Cellular and Molecular Imaging Centers (ICMIC) Developmental Project Award NIH P50 CA86306, the CIRM Tools and Technology Grant and the Dana Foundation. ONW is an Investigator of the Howard Hughes Medical Institute.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. This is a comprehensive review of the complex interactions between tumors and diverse cell types of the immune system. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 3.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 4.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 6.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 7.Peggs KS, Segal NH, Allison JP. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell. 2007;12:192–199. doi: 10.1016/j.ccr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Paulos CM, Suhoski MM, Plesa G, Jiang T, Basu S, Golovina TN, Jiang S, Aqui NA, Powell DJ, Jr, Levine BL, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42:182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis DJ, Hoffman JM, Herfkens RJ, Jeffrey RB, Quon A, Gambhir SS. Molecular imaging techniques in body imaging. Radiology. 2007;245:333–356. doi: 10.1148/radiol.2452061117. [DOI] [PubMed] [Google Scholar]
- 11.Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–681. [PubMed] [Google Scholar]
- 12.Nair-Gill ED, Shu CJ, Radu CG, Witte ON. Non-invasive imaging of adaptive immunity using positron emission tomography. Immunol Rev. 2008;221:214–228. doi: 10.1111/j.1600-065X.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang JH, Chung JK. Molecular-genetic imaging based on reporter gene expression. J Nucl Med. 2008;49(Suppl 2):164S–179S. doi: 10.2967/jnumed.107.045955. [DOI] [PubMed] [Google Scholar]
- 14.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 15.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 17.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 18.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20••.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. This review covers the important concepts that link oncogenic transformation to programmed changes in cellular metabolic pathways necessary for tumor progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czernin J, Phelps ME. Positron emission tomography scanning: current and future applications. Annu Rev Med. 2002;53:89–112. doi: 10.1146/annurev.med.53.082901.104028. [DOI] [PubMed] [Google Scholar]
- 22.Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17–21. [PubMed] [Google Scholar]
- 23.Goerres GW, Stupp R, Barghouth G, Hany TF, Pestalozzi B, Dizendorf E, Schnyder P, Luthi F, von Schulthess GK, Leyvraz S. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32:153–162. doi: 10.1007/s00259-004-1633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stumpe KD, Urbinelli M, Steinert HC, Glanzmann C, Buck A, von Schulthess GK. Whole-body positron emission tomography using fluorodeoxyglucose for staging of lymphoma: effectiveness and comparison with computed tomography. Eur J Nucl Med. 1998;25:721–728. doi: 10.1007/s002590050275. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi E, Pregno P, Penna D, Deandreis D, Chiappella A, Limerutti G, Vitolo U, Mancini M, Bisi G, Gallo E. Role of whole-body [18F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) and conventional techniques in the staging of patients with Hodgkin and aggressive non Hodgkin lymphoma. Radiol Med. 2008;113:578–590. doi: 10.1007/s11547-008-0264-7. [DOI] [PubMed] [Google Scholar]
- 26.Damian DL, Fulham MJ, Thompson E, Thompson JF. Positron emission tomography in the detection and management of metastatic melanoma. Melanoma Res. 1996;6:325–329. doi: 10.1097/00008390-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JD, Schauwecker D, Hutchins G, Coleman JJ., 3rd Initial assessment of positron emission tomography for detection of nonpalpable regional lymphatic metastases in melanoma. J Surg Oncol. 1997;64:181–189. doi: 10.1002/(sici)1096-9098(199703)64:3<181::aid-jso2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Benz MR, Evilevitch V, Allen-Auerbach MS, Eilber FC, Phelps ME, Czernin J, Weber WA. Treatment monitoring by 18F-FDG PET/ CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J Nucl Med. 2008;49:1038–1046. doi: 10.2967/jnumed.107.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evilevitch V, Weber WA, Tap WD, Allen-Auerbach M, Chow K, Nelson SD, Eilber FR, Eckardt JJ, Elashoff RM, Phelps ME, et al. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14:715–720. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- 30.Bury T, Dowlati A, Paulus P, Hustinx R, Radermecker M, Rigo P. Staging of non-small-cell lung cancer by whole-body fluorine-18 deoxyglucose positron emission tomography. Eur J Nucl Med. 1996;23:204–206. doi: 10.1007/BF01731846. [DOI] [PubMed] [Google Scholar]
- 31.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, Mattfeldt T, Neumaier B, Reske SN, Hetzel M. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 32.Dose Schwarz J, Bader M, Jenicke L, Hemminger G, Janicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nucl Med. 2005;46:1144–1150. [PubMed] [Google Scholar]
- 33.Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Elashoff D, Chow K, Evilevitch V, Eckardt JJ, Phelps ME, et al. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–2863. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Matsui T, Nakata N, Nagai S, Nakatani A, Takahashi M, Momose T, Ohtomo K, Koyasu S. Inflammatory cytokines and hypoxia contribute to 18F-FDG uptake by cells involved in pannus formation in rheumatoid arthritis. J Nucl Med. 2009;50:920–926. doi: 10.2967/jnumed.108.060103. [DOI] [PubMed] [Google Scholar]
- 37.Radu CG, Shu CJ, Shelly SM, Phelps ME, Witte ON. Positron emission tomography with computed tomography imaging of neuroinflammation in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2007;104:1937–1942. doi: 10.1073/pnas.0610544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brewer S, McPherson M, Fujiwara D, Turovskaya O, Ziring D, Chen L, Takedatsu H, Targan SR, Wei B, Braun J. Molecular imaging of murine intestinal inflammation with 2-deoxy-2-[18F]fluoro-D-glucose and positron emission tomography. Gastroenterology. 2008;135:744–755. doi: 10.1053/j.gastro.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Stelljes M, Hermann S, Albring J, Kohler G, Loffler M, Franzius C, Poremba C, Schlosser V, Volkmann S, Opitz C, et al. Clinical molecular imaging in intestinal graft-versus-host disease: mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood. 2008;111:2909–2918. doi: 10.1182/blood-2007-10-119164. This study translates the evaluation of tissue-specific immune function during GVHD with [18F]FDG-PET from an animal model to clinical practice. It highlights the advantages of functional whole-body imaging in diagnosis and prediction of therapeutic efficacy compared to analysis of biopsy samples. [DOI] [PubMed] [Google Scholar]
- 40.Elzinga EH, van der Laken CJ, Comans EF, Lammertsma AA, Dijkmans BA, Voskuyl AE. 2-Deoxy-2-[F-18]fluoro-D-glucose joint uptake on positron emission tomography images: rheumatoid arthritis versus osteoarthritis. Mol Imaging Biol. 2007;9:357–360. doi: 10.1007/s11307-007-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1009–1016. doi: 10.1161/ATVBAHA.108.165563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S, Chryssikos T, Moghadam-Kia S, Zhuang H, Torigian DA, Alavi A. Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. Semin Nucl Med. 2009;39:36–51. doi: 10.1053/j.semnuclmed.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 44.Tumeh PC, Radu CG, Ribas A. PET imaging of cancer immunotherapy. J Nucl Med. 2008;49:865–868. doi: 10.2967/jnumed.108.051342. [DOI] [PubMed] [Google Scholar]
- 45.Mamede M, Saga T, Ishimori T, Nakamoto Y, Sato N, Higashi T, Mukai T, Kobayashi H, Konishi J. Differential uptake of (18)F-fluorodeoxyglucose by experimental tumors xenografted into immunocompetent and immunodeficient mice and the effect of immunomodification. Neoplasia. 2003;5:179–183. doi: 10.1016/s1476-5586(03)80010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 47.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49(Suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 48.Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, Peterson LM, Vallieres E, Wood DE. In vivo validation of 3′ deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–3323. [PubMed] [Google Scholar]
- 49.Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging. 2004;31:1659–1672. doi: 10.1007/s00259-004-1687-6. [DOI] [PubMed] [Google Scholar]
- 50.Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am. 2005;43:153–167. doi: 10.1016/j.rcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 51.van Waarde A, Cobben DC, Suurmeijer AJ, Maas B, Vaalburg W, de Vries EF, Jager PL, Hoekstra HJ, Elsinga PH. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45:695–700. [PubMed] [Google Scholar]
- 52.Buck AK, Bommer M, Juweid ME, Glatting G, Stilgenbauer S, Mottaghy FM, Schulz M, Kull T, Bunjes D, Moller P, et al. First demonstration of leukemia imaging with the proliferation marker 18F-fluorodeoxythymidine. J Nucl Med. 2008;49:1756–1762. doi: 10.2967/jnumed.108.055335. [DOI] [PubMed] [Google Scholar]
- 53.Arner ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 54.Eriksson S, Munch-Petersen B, Kierdaszuk B, Arner E. Expression and substrate specificities of human thymidine kinase 1, thymidine kinase 2 and deoxycytidine kinase. Adv Exp Med Biol. 1991;309B:239–243. doi: 10.1007/978-1-4615-7703-4_53. [DOI] [PubMed] [Google Scholar]
- 55.Jordheim LP, Dumontet C. Review of recent studies on resistance to cytotoxic deoxynucleoside analogues. Biochim Biophys Acta. 2007;1776:138–159. doi: 10.1016/j.bbcan.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 56••.Radu CG, Shu CJ, Nair-Gill E, Shelly SM, Barrio JR, Satyamurthy N, Phelps ME, Witte ON. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nat Med. 2008;14:783–788. doi: 10.1038/nm1724. The authors present the design and evaluation [18F]FAC, a probe for the deoxycytidine salvage pathway that shows tissue accumulation patterns distinct from [18F]FDG or [18F]-FLT. This is a unique study showing the de novo development of a metabolic PET probe with enhanced immune imaging characteristics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faderl S, Gandhi V, Keating MJ, Jeha S, Plunkett W, Kantarjian HM. The role of clofarabine in hematologic and solid malignancies — development of a next-generation nucleoside analog. Cancer. 2005;103:1985–1995. doi: 10.1002/cncr.21005. [DOI] [PubMed] [Google Scholar]
- 58••.Laing RE, Walter MA, Campbell DO, Herschman HR, Satyamurthy N, Phelps ME, Czernin J, Witte ON, Radu CG. Noninvasive prediction of tumor responses to gemcitabine using positron emission tomography. Proc Natl Acad Sci U S A. 2009;106:2847–2852. doi: 10.1073/pnas.0812890106. Using [18F]FAC, the authors demonstrate the ability to stratify tumors based on deoxycytidine salvage activity into groups that will or will not respond to therapy with nucleoside-based chemotherapy. This study demonstrates that analysis of metabolic pathways distinct from glycolysis can provide important information about the function of cancer cells and predict their response to specific treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]