Although DNA methylation is a relatively stable epigenetic modification, global DNA demethylation has been observed in two stages during embryogenesis. One takes place in zygotes, when the paternal genome is preferentially demethylated (1). Despite great efforts in identifying the responsible enzymes, the identity of the putative DNA demethylase has remained elusive (2). Recent demonstration that the Tet (ten eleven translocation) family proteins are capable of converting 5-methylcytosine (5mC) of DNA to 5-hydroxymethylcytosine (5hmC) raises the possibility that Tet proteins might be involved in this process (3, 4).
To address this possibility, we performed immunostaining that demonstrated, similar to recently published results (5, 6), that loss of 5mC staining in the male pronucleus correlates with the appearance of 5hmC (fig. S1), indicating the conversion of 5mC to 5hmC at the male pronucleus. Several possibilities, including base excision repair (BER), further oxidation, and replication-dependent dilution, have been proposed for the fate of 5hmC (2, 4, 7). Indeed, two recent studies have demonstrated that Tet proteins can oxidize 5mC and 5hmC further to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) (8, 9) and that 5caC can be excised by thymine-DNA glycosylase (TDG)–mediated BER (8).
To distinguish between these possibilities, we prepared mitotic chromosome spreads at various stages of preimplantation embryos. Co-staining of the chromosome spreads with antibodies against 5mC or 5hmC revealed that, at the one-cell stage, the two sets of chromosomes are compartmentalized, with 5hmC specifically enriched in the sperm-derived chromosomes and 5mC specifically enriched in the egg-derived chromosomes (Fig. 1A). Similar staining with parthenogenetic one-cell embryos revealed uniform 5mC staining in the two sets of chromosomes, with little staining of 5hmC antibodies (fig. S2A). This result is consistent with previous studies demonstrating relative enrichment of 5hmC in the sperm-derived chromosomes (5, 6). Similar staining of mitotic chromosomes at the two-cell stage revealed that only one of the two sister chromatids of sperm-derived chromosomes is enriched for 5hmC (Fig. 1B), indicating that the 5hmC mark is not maintained during DNA replication. Further analysis of the four-cell– and eight-cell–stage embryo blastomeres revealed that the chromosomes containing 5hmC are gradually reduced (Fig. 1, C and D).
Fig. 1.
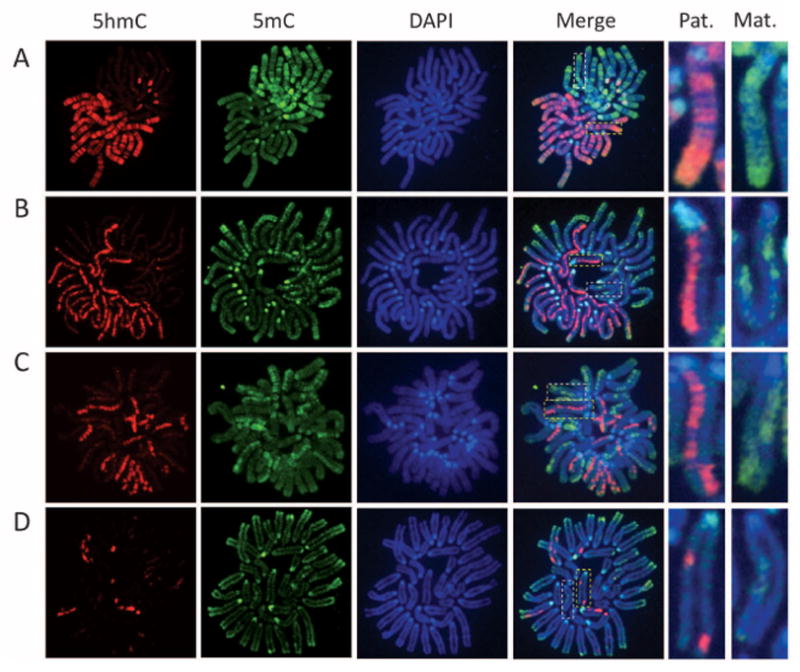
Replication-dependent loss of 5-hmC during mouse preimplantation development. Representative confocal microscopy images of mitotic chromosome spreads derived from in vitro fertilization mouse embryos co-stained with 5hmC (red) or 5mC (green) antibodies or DAPI (4′,6′-diamidino-2-phenylindole, blue) at one-cell (A), two-cell (B), four-cell (C), and eight-cell (D) stages. Shown are the images of chromosomes from one blastomere at each developmental stage. As a result of sister chromatid exchange, only part of the sister chromatids stained positively for 5hmC at the four-cell and eight-cell stages (C and D). The dotted squares represent enlarged paternal (Pat.) or maternal (Mat.) chromosomes.
We note that, at the four- and eight-cell stages, 5hmC appears to be present only in part of the chromatids, likely because of sister chromatid exchange (Fig. 1, C and D; note the enlarged images on right of the panels). Although the exact numbers of 5hmC-positive chromatids vary among individual blastomeres, the total length of the 5hmC-enriched part in chromatids in each blastomere is very close to the theoretical number of 5hmC-positive chromatids expected from the original 5hmC-containing chromatids present in the zygote (table S1).
The above results, together with previous studies (5, 6), support a model by which Tet3 converts 5mC to 5hmC in the male pronucleus in zygotes followed by replication-dependent dilution during preimplantation development (fig. S3).
Supplementary Material
Acknowledgments
We thank S. Wu for critical reading of the manuscript. This work was supported by HHMI and NIH (GM68804). Y.Z. is an Investigator of HHMI.
Footnotes
www.sciencemag.org/cgi/content/full/science.1212483/DC1
Materials and Methods
SOM Text
Figs. S1 to S3
Table S1
References
References
- 1.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Nature. 2000;403:501. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 2.Wu SC, Zhang Y. Nat Rev Mol Cell Biol. 2010;11:607. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito S, et al. Nature. 2010;466:1129. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahiliani M, et al. Science. 2009;324:930. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal K, Jin SG, Pfeifer GP, Szabó PE. Proc Natl Acad Sci USA. 2011;108:3642. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wossidlo M, et al. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 7.Guo JU, Su Y, Zhong C, Ming GL, Song H. Cell. 2011;145:423. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He YF, et al. Science. 2011;333:1303. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, et al. Science. 2011;333:1300. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


