Abstract
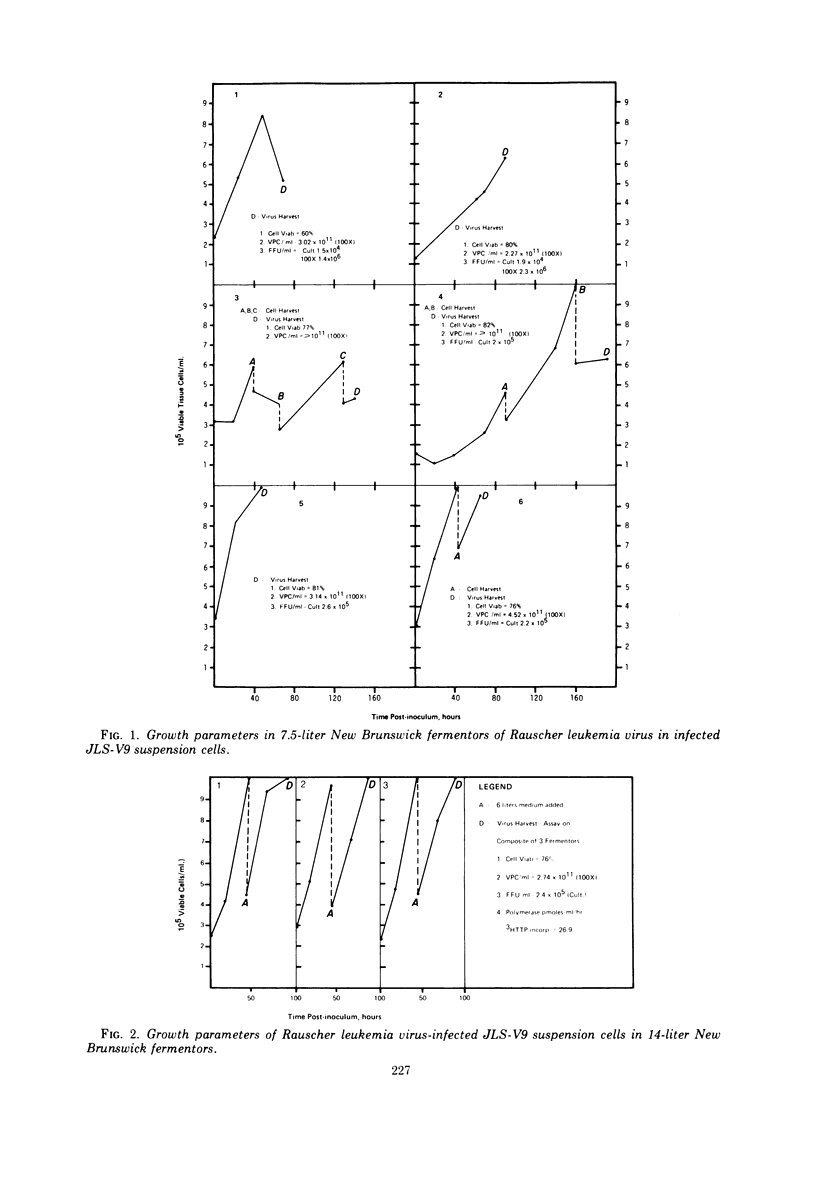
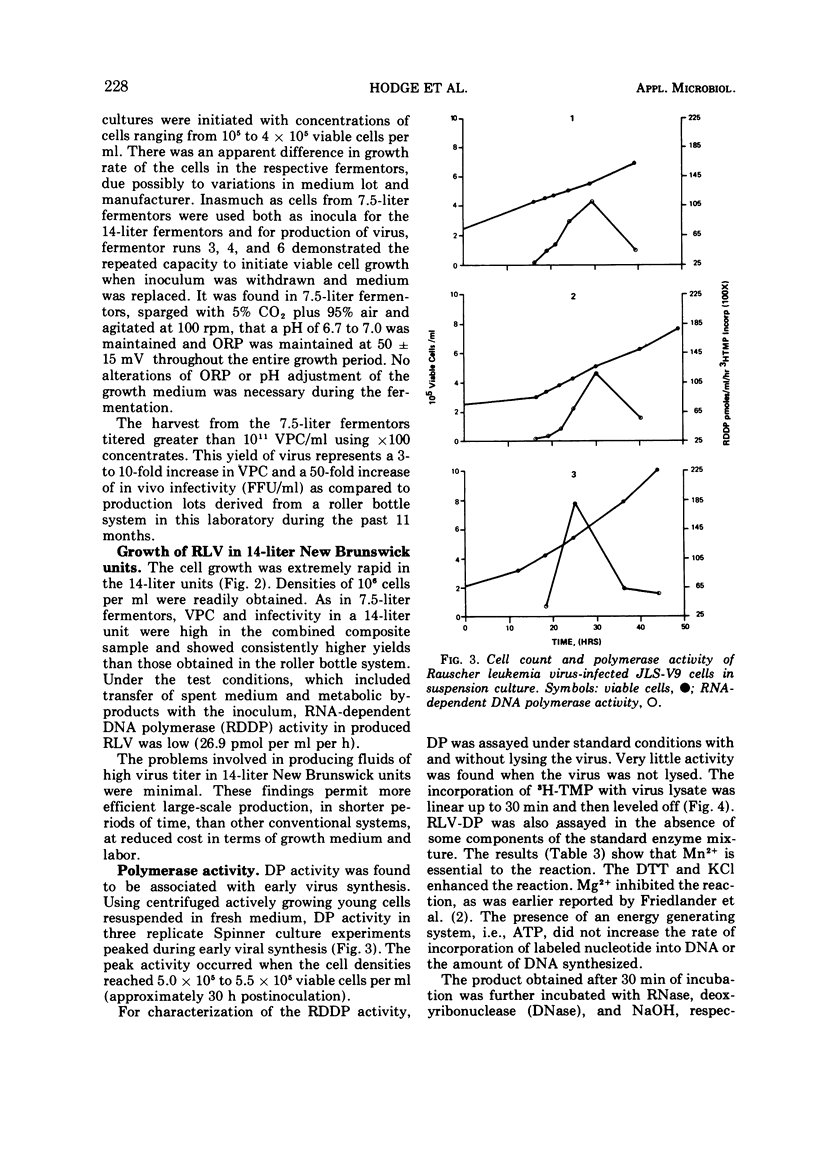
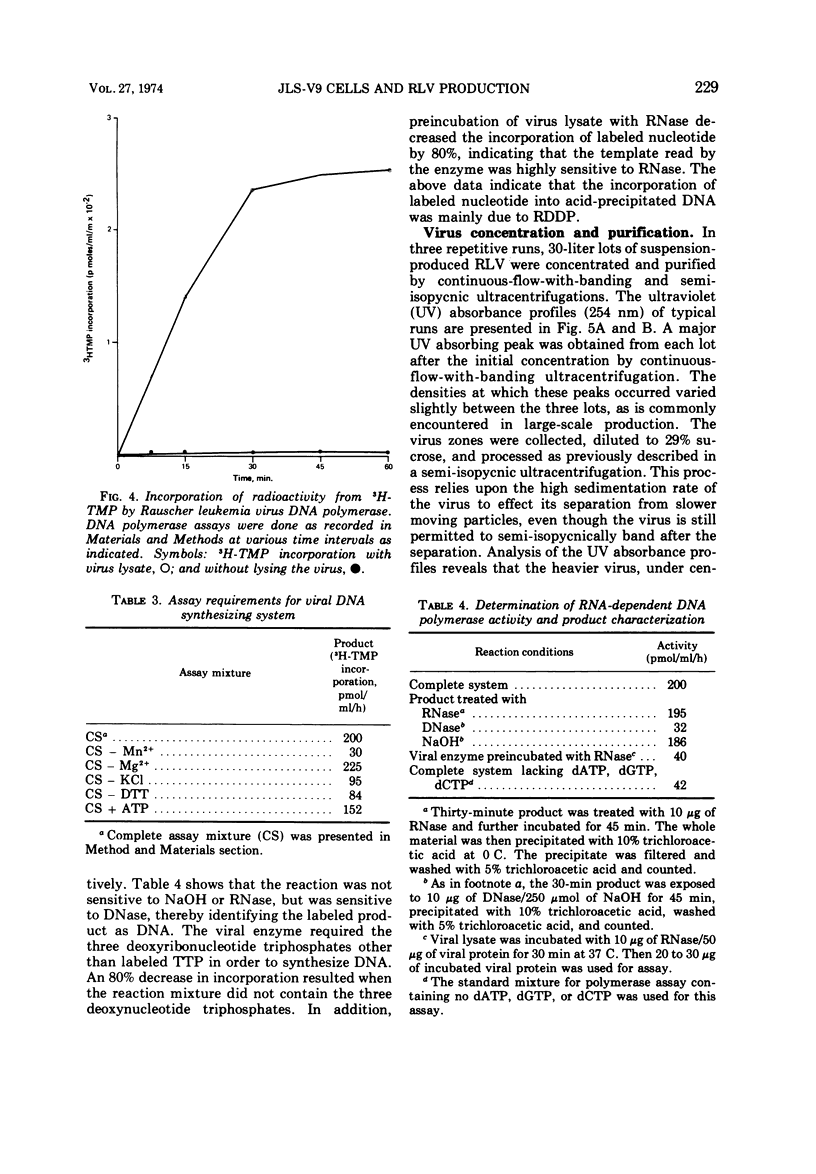
JLS-V9 mouse bone marrow cells were readily adapted to suspension culture, chronically infected with Rauscher leukemia virus (RLV), and subsequently grown in 7.5- and 14-liter New Brunswick fermentors. The suspension-type cell system can be modified to produce virus with clearly defined properties, such as high ribonucleic acid-dependent deoxyribonucleic acid polymerase (RDDP) activity, high particle count, and high infectious particle count. Biological and biophysical properties of suspension-produced RLV were not affected by concentration and purification employing continuous-flow and rate-zonal centrifugation procedures. The RDDP assay was standardized and showed a linear incorporation of 3H-thymidine 5′-monophosphate (3H-TMP) up to 30 min. Further characterization indicated that a high percentage of 3H-TMP incorporation was due to RDDP.
Full text
PDF


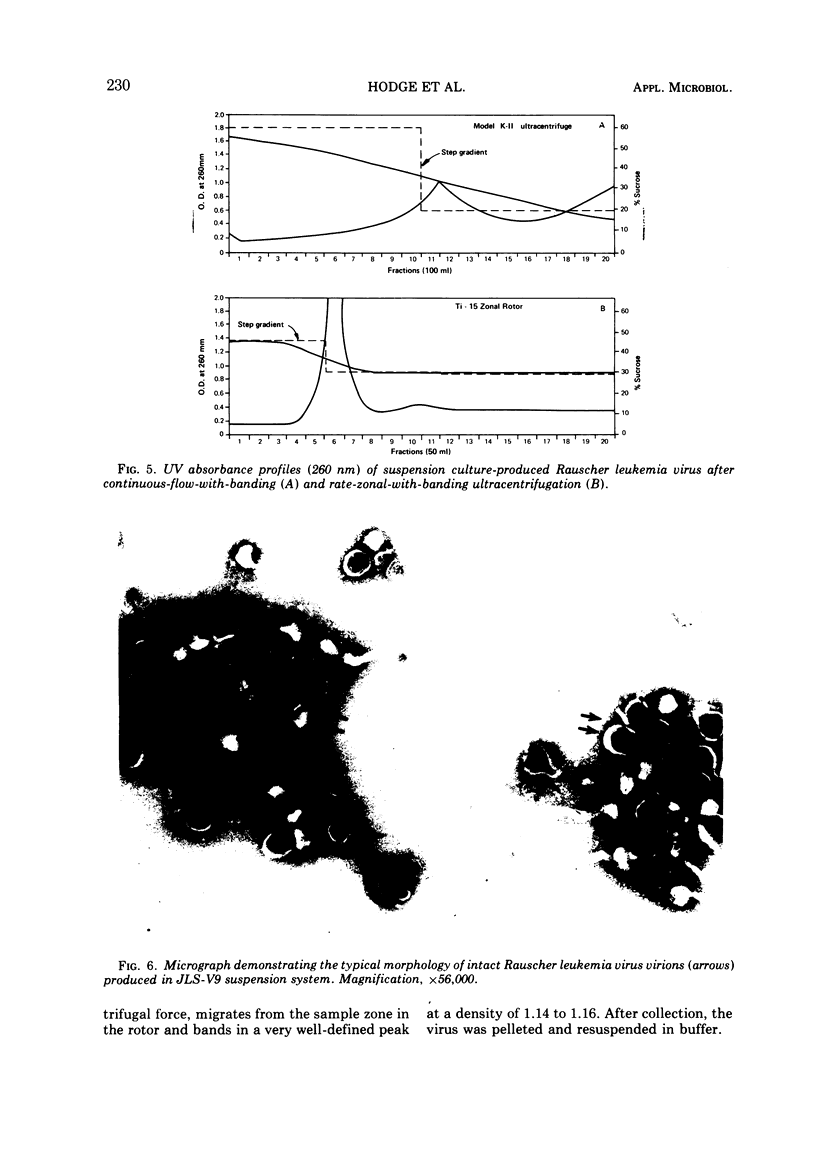
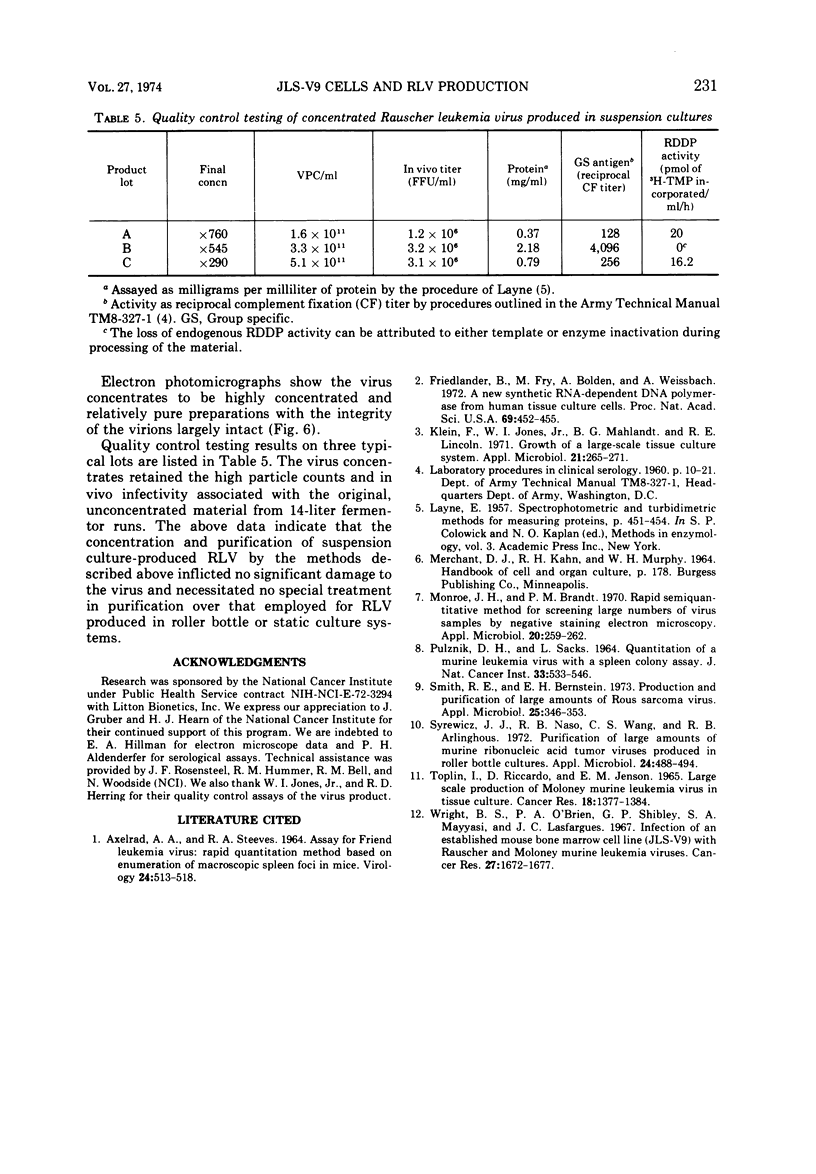
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Fridlender B., Fry M., Bolden A., Weissbach A. A new synthetic RNA-dependent DNA polymerase from human tissue culture cells (HeLa-fibroblast-synthetic oligonucleotides-template-purified enzymes). Proc Natl Acad Sci U S A. 1972 Feb;69(2):452–455. doi: 10.1073/pnas.69.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F., Jones W. I., Jr, Mahlandt B. G., Lincoln R. E. Growth of pathogenic virus in a large-scale tissue culture system. Appl Microbiol. 1971 Feb;21(2):265–271. doi: 10.1128/am.21.2.265-271.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrewicz J. J., Naso R. B., Wang C. S., Arlinghaus R. B. Purification of large amounts of murine ribonucleic acid tumor viruses produced in roller bottle cultures. Appl Microbiol. 1972 Sep;24(3):488–494. doi: 10.1128/am.24.3.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplin I., Riccardo D., Jensen E. M. Large-scale production of Moloney murine leukemia virus in tissue culture. Cancer. 1965 Oct;18(10):1377–1384. doi: 10.1002/1097-0142(196510)18:10<1377::aid-cncr2820181023>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]