Abstract
Hypomagnesemia continues to cause difficult clinical problems, such as significant cardiac arrhythmias where intravenous magnesium therapy can be lifesaving. Nutritional deficiency of magnesium may present with some subtle symptoms such as leg cramps and occasional palpitation. We have investigated dietary-induced magnesium deficiency in rodent models to assess the pathobiology associated with prolonged hypomagnesemia. We found that neuronal sources of the neuropeptide, substance P (SP), contributed to very early prooxidant/proinflammatory changes during Mg deficiency. This neurogenic inflammation is systemic in nature, affecting blood cells, cardiovascular, intestinal, and other tissues, leading to impaired cardiac contractility similar to that seen in patients with heart failure. We have used drugs that block the release of SP from neurons and SP-receptor blockers to prevent some of these pathobiological changes; whereas, blocking SP catabolism enhances inflammation. Our findings emphasize the essential role of this cation in preventing cardiomyopathic changes and intestinal inflammation in a well-studied animal model, and also implicate the need for more appreciation of the potential clinical relevance of optimal magnesium nutrition and therapy.
Keywords: Mg-deficient rodent model, substance P and substance P receptor, N-methyl-D-aspartate (NMDA) receptor/channel, neutrophil superoxide, neutral endopeptidase, endotoxin
Neuropeptide Responses in Experimental Magnesium Deficiency
Severe dietary Mg deficiency leads to diverse pathology in animal models: myocardial necrosis, neuromuscular hyperexcitability, arrhythmia, hyperirritability, erythema, hemorrhagic skin lesions, increased oxidative stress, and myocardial susceptibility to ischemia/reperfusion stress1,2 and significant systolic dysfunction.3 Our early investigations with Mg deficient (MgD) rodents, led to the discovery that circulating levels of the pro-inflammatory neuropeptide, substance P (SP),4,5 were significantly elevated 6–8 within 3 days after initiating a diet (Harlan Teklad, Madison, WI) containing only 9% of Recommended Daily Allowance for Mg (100% RDA ≈ 500 mg/kg feed).2,9 Calcitonin gene-related peptide was also elevated at this time, and probably emanated from sensory neuron fibers which are rich in both neuropeptides.10 Our later studies indicated that SP may be inducing many of the inflammatory/oxidative events which eventually promote cardiomyopathy. These early and late pathological events are described in Figure 1. The acute stage release of SP during Mg deficiency may initiate the events that determine the early and late stage responses. Interestingly, rats placed on moderate MgD (40% RDA) and severe MgD (9 % RDA) diets for 3 weeks exhibited proportionately elevated circulating SP levels that were 2.43- and 5.14- fold higher than Mg sufficient controls (MgS ≥ 100% RDA), respectively. Indeed our findings and those of others suggested that even moderate dietary Mg deficiency induced significant systemic inflammation: increases in PGE2,11 circulating histamine,12 and erythema.13 In addition to the elevated levels of SP, the neurokinin-1 (NK-1) receptor for SP is increasingly expressed during Mg deficiency. Multi-label flow cytometry was used for estimation of the SP receptor in peripheral neutrophils of rats using a multi-label FACS assay with different fluorophores. The circulating neutrophils from the early stage of severe MgD indicated a 2-fold elevation of the SP receptor (Figure 2). These results are consistent with our observations that superoxide production in neutrophils from Mg deficient rats was significantly activated during the early stage of the diet. Thus, both the elevated SP levels, coupled with increased expression of the SP receptor, amplified the oxidative stress during the early stage of Mg deficiency.
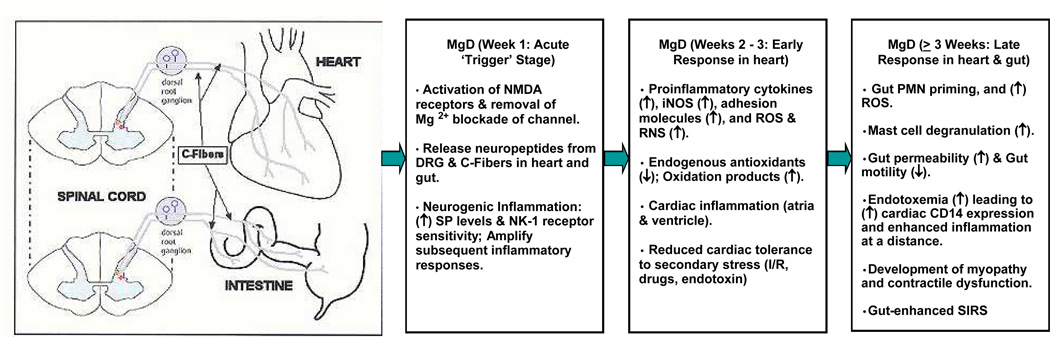
Figure 1.
Temporal sequence and interplay of pathologic events involving the neuronal, hematopoietic, gastrointestinal, and cardiovascular systems during acute and chronic stages of Mg deficiency (MgD), and which may lead to enhanced ‘cardiac inflammation at a distance’ and eventual myopathy and cardiac dysfunction.
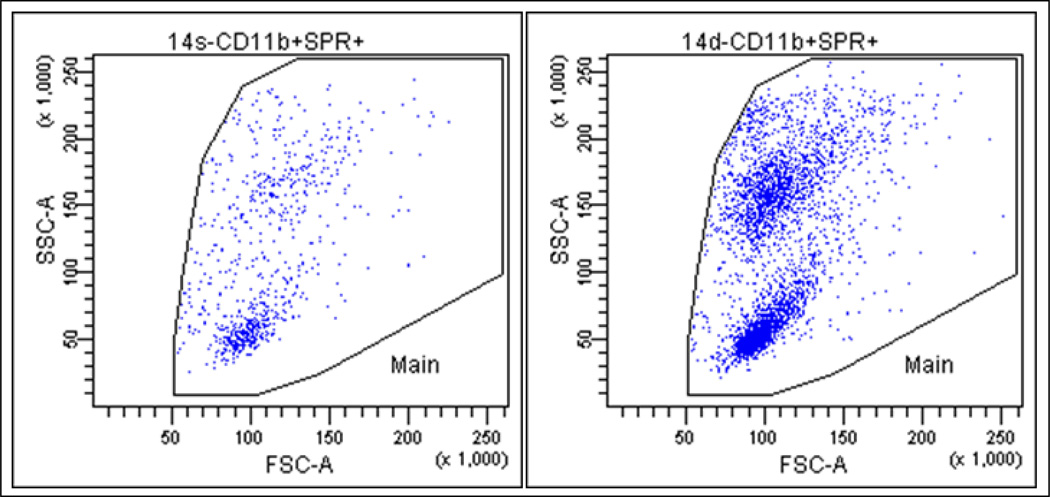
Figure 2.
Representative data showing substance P receptor (SPR) up-regulation in neutrophils of Mg deficient (MgD) rats. Whole blood samples from 2 wk Mg sufficient (MgS, left panel) and MgD (right) rats were analyzed by flow cytometry. A multi-label FACS assay using simultaneously 4 different fluorophores with a mixture of fluorophore-tagged anti CD3 (for T lymphocytes), CD45RA (B Lymphocytes), CD11b (neutrophils), and indirectly tagged SPR (3 µg/ml) were used during the analysis.
N-methyl-D-aspartate (NMDA) Receptor and SP–mediated Pathobiology during Mg deficiency
The NMDA receptor / channel complex is an important mediator of neuropeptide release. The NMDA channel is blocked by Mg2+ in a voltage-dependent manner14 and specific NMDA receptor/channel blockers are protective. For example, Dizocilpine (MK-801), a non-competitive NMDA receptor/channel blocker, prevented noise-mediated seizures15–16 and depressed the acoustic startle reflex17 in Mg deficient rats. The influence of NMDA receptor inhibition on neutrophil superoxide production in severe MgD rats was examined. The enriched blood neutrophil fractions displayed a 5.3 fold (p<0.02) elevated basal superoxide production and pretreatment with MK-801 attenuated this superoxide response by 69%, p<0.01 (Figure 3). Neuronal localization revealed significant concentrations around blood vessels, and staining with PGP-9.5, a pan neuronal stain, demonstrated a rich perivascular innervation throughout cardiac tissue. Using serial sections to correlate neuronal regions with areas of SP distribution revealed substantial co-localization of SP and inflammation around vascular elements during Mg deficiency. The acute stage elevation in circulating SP 18 may arise, in part, from C-fibers of the dorsal root ganglia.19 These ganglia were isolated from early stage and late stage severe MgD rats where the SP staining intensity was substantially reduced; pre-treatment of these MgD rats with MK-801 prevented much of the loss in SP staining intensity during the early and late response stages. We concluded that SP depletion from the DRG was due to unblocking of the NMDA receptor channel during Mg deficiency leading to enhanced neuronal SP release.20 Chronic Mg deficiency also produced significant cardiac apoptosis with significantly elevated caspase 3 enzyme activity.21 The effect of MK-801 treatment during severe Mg deficiency on DNA fragmentation in rat cardiac tissue was also examined; 33% apoptotic nuclei were seen in severe MgD hearts, and MK-801treatment significantly decreased the number of apoptotic nuclei by 40%. Thus, cardiac apoptosis was inhibited by NMDA receptor blockade, and is likely modulated by neuronal SP release.
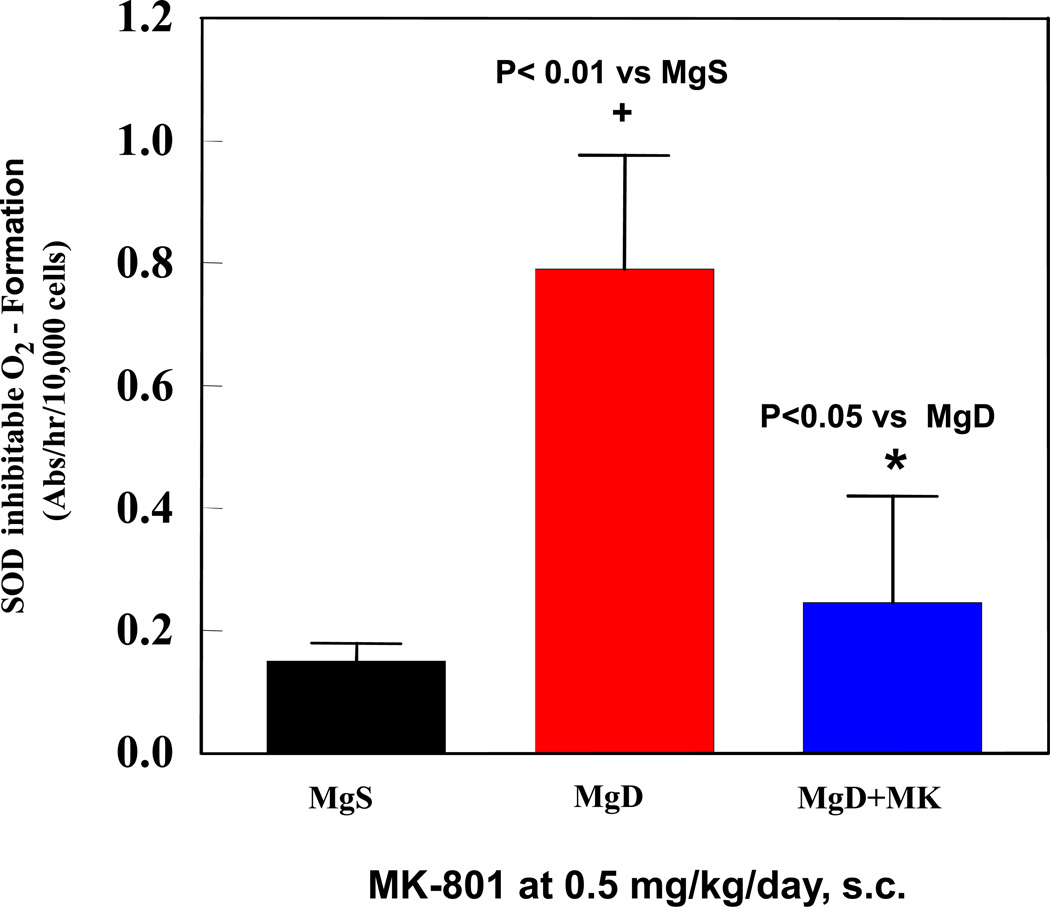
Figure 3.
Effect of NMDA receptor blockade (MK-801, 0.5 mg/kg/day, s.c.) on PMN (superoxide production) activity during 3 wks of severe MgD treatment. Whole blood PMNs were obtained using a modified ficoll hypaque reagent and a step-gradient centrifugation.18 Basal and stimulated superoxide anion production was assayed in the absence or presence of 100 ng/ml phorbol myristate acetate plus 75 µM cytochrome c with or without 50 µg SOD. Superoxide production was estimated as SOD-inhibitable reduction of cytochrome c using the extinction coefficient: E550 = 2.1×104M−1cm−1. Values are means ± SD from 3–5 rats/group.
Neutral Endopeptidase during Mg deficiency
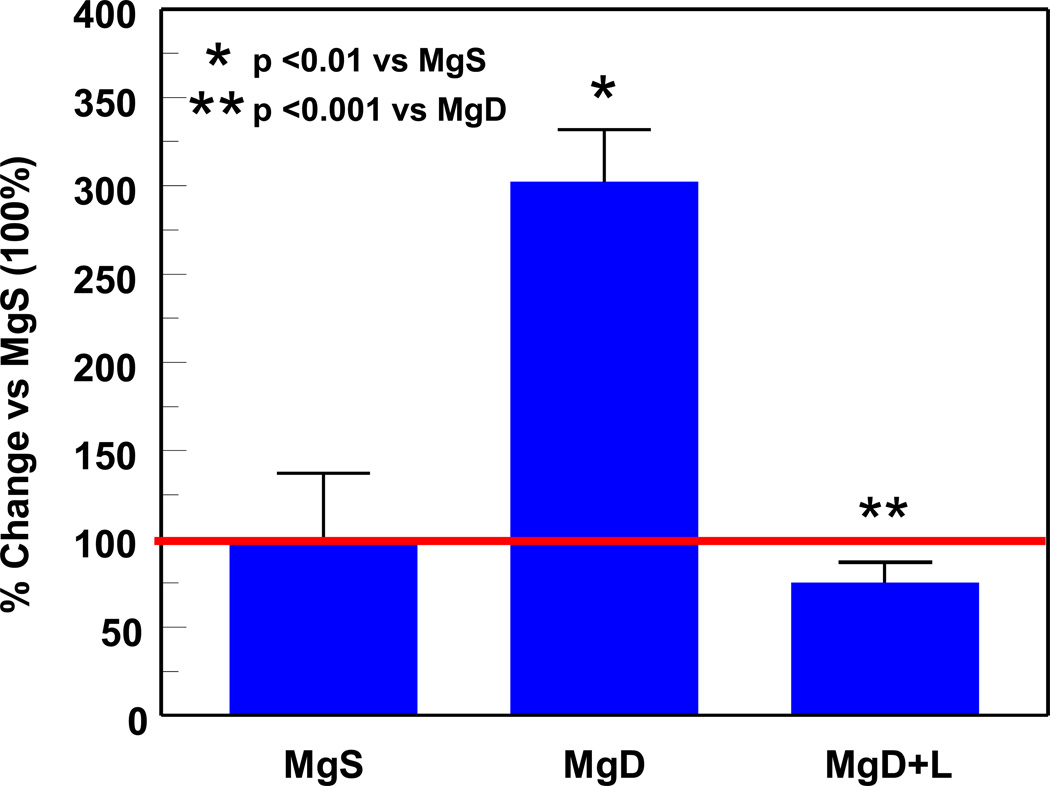
In addition to neuronal release of SP during the acute stage, heightened elevations of SP during the early and late response stages of Mg deficiency may also involve inactivation of neutral endopeptidase (NEP), the principal proteolytic SP-degrading enzyme.22 Other proteases (angiotensin converting enzyme, dipeptidyl aminopeptidase IV, aminopeptidase M) may degrade SP, but only to a lesser extent.23 NEP is a metalloprotease expressed by various tissues and cells, including heart, small intestine, kidney, brain, airway epithelium, vascular endothelium,24 neutrophils25 and macrophages.26 We found that NEP inactivation can lead to enhanced SP-mediated systemic inflammation during Mg deficiency.27 Moreover, NEP can be a target of oxidative damage by circulating lipid peroxidation products (conjugated dienes, MDA-like materials),28,29 and during the late response stage of MgD, we showed that NEP activity was significantly depressed in circulating neutrophils (Figure 4).
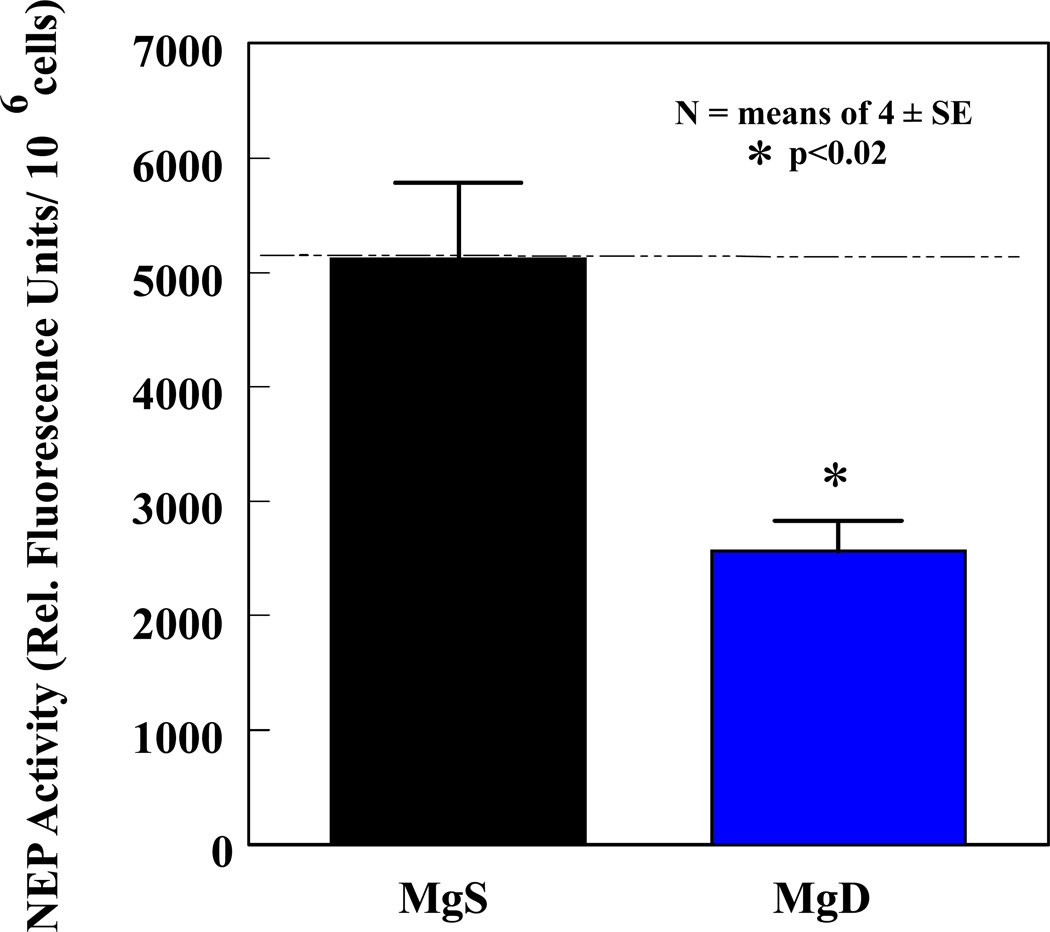
Figure 4.
PMN neutral endopeptidase (NEP) activity was assessed in 3 wk MgS and severe MgD rats. The fluorogenic peptide substrate (Mca-Arg-Pro-Gly-Phe-Ser-Ala-Phe-Lys-(Dnp)-OH dissolved in HEPES buffer was used to measure NEP activity in PMN samples on microtiter plates (incubated 30 min, 37°C), and control samples preincubated with a NEP-specific inhibitor (100 nM thiorphan) was included.29,30 Fluorescence was read at 320 nm (excitation) and 405 nm (emission) on a Victor 3 Perkin Elmer plate reader. Values are means ± SE of 4–6 rats and are reported as % of MgS activity. *p <0.02 vs MgS.
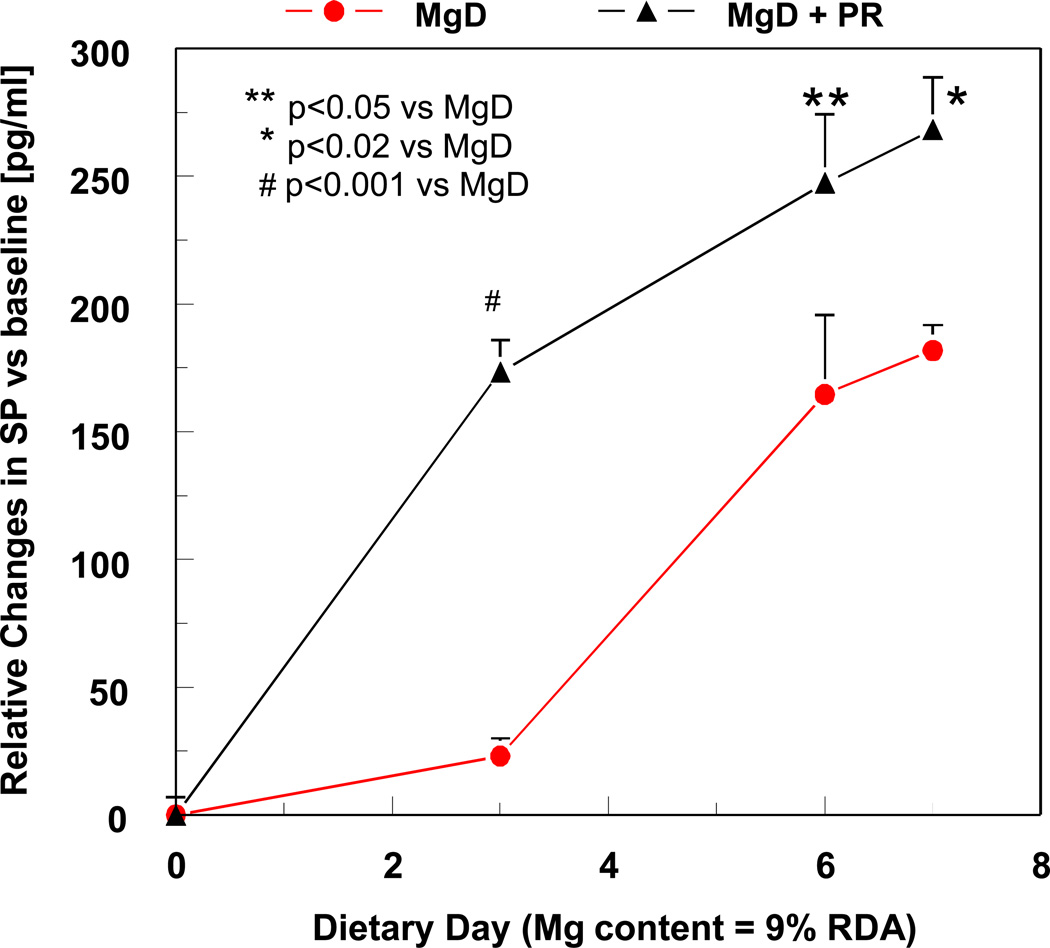
In additional studies we used the NEP inhibitor, phosphoramidon, to investigate its effect on circulating SP levels in conjunction with changes in oxidative stress (circulating prostaglandin E2 [PGE2], RBC glutathione [GSH] loss, and neutrophil activation). During the acute stage of Mg deficiency we found a 7.5-fold elevation in SP versus MgS control, but after phosphoramidon treatment a further 2.45-fold increase in total SP was seen versus untreated MgD (Figure 5). These findings supported the notion that NEP strongly influences SP bio-availability during Mg deficiency. It remains to be determined if similar reductions in NEP activity occur in other tissues. Phosphoramidon treatment of control rats produced no significant superoxide production, indicating that, in the absence of elevated SP, the basal activity of neutrophils remained unchanged.
Figure 5.
Effect of the NEP inhibitor, phosphoramidon (PR: 5 mg/kg/day, s.c.) on plasma SP levels during week 1 of severe MgD. SP levels were determined using an ELISA kit from R&D Systems (Minneapolis, MN).31 The assay is a competitive binding technique in which sample SP competes with a fixed amount of horseradish-labeled SP for sites on a murine monoclonal antibody. Color development is inversely proportional to SP concentration and absorbance is read at 450 nm. Values were normalized to baseline and are means ± SE of 3–6 rats. To obtain total levels for week 1, time-courses were area integrated.
Intestinal Inflammation in Mg deficiency
The gut is also richly innervated32 and during Mg deficiency, the C-fibers may contribute to the early rise in plasma SP. Even during the acute stage of Mg deficiency, mucosal inflammation by neutrophils was evident, and pronounced infiltration occurred by the late response stage.33 Our prior studies had shown elevations of TNFα in Mg deficient animals. Since endotoxin (lipopolysaccharide, LPS) is known to induce a systemic elevation of TNFα34 by stimulating cytokine secretion, we wondered whether elevated levels of LPS promoted the secretion of TNFα in Mg deficiency. Our studies using Evan’s blue dye showed a dramatic increase (19-fold > control) in capillary leak of gut tissue harvested from severe Mg deficient rats during the early response stage.33 Indeed, we found significantly elevated plasma LPS in the late response stage of Mg deficient rats and these elevated levels of LPS were significantly inhibited by treatment with SP receptor blockade (Figure 6). We reported previously that in vivo SP receptor blockade (CP-96,345) in Mg deficient rats significantly lowered plasma TNFα levels during the early response stage.6 Taken together, these findings indicate a role for SP in the elevations of both LPS and TNFα during Mg-deficiency.
Figure 6.
Effect of extending exposure to 5 wks on plasma endotoxin (LPS) levels during severe MgD with or without neurokinin-1 (NK-1) receptor blockade (L-703,606; 0.5 mg/kg/day, s.c. pellet). Systemic endotoxemia was measured in blood samples obtained with endotoxin-free needles, syringes, and glassware, then diluted 1:10 in endotoxin-free water, and heated for 5 min to remove interfering plasma components. Endotoxin was determined using limulus amebocyte lysate.34 A quantitative colorimetric “Pyrochrome” assay (Associates of Cape Cod, Woods Hole, MA) was performed in 96-well microplates and read at 540 nm using a plate reader (Versa max; Molecular Devices). The detection limit for “Pyrochrome” is 0.3 pg/ml. Values are means ± SE of 3–5 rats.
Since endotoxin receptor (CD14) is known to be up-regulated in cells exposed to LPS, we used immunohistochemical staining to monitor the expression of this LPS receptor.35 We found prominent CD14 positive macrophage-type cells in the intestine of severe Mg deficient rats during the early response stage;36 we also observed enhanced CD14 receptor expression in the cardiac tissue. The importance of CD14 in LPS-induced responses was shown previously using transgenic mice over-expressing CD14 receptor,37 where the known cardio-depressant effects of LPS were dependent on this CD14 signaling.38 These observed significant elevations in plasma LPS in Mg deficient rats indicated that prolonged Mg deficiency results in increased intestinal permeability to bacterial products leading to increased circulating LPS, along with elevated endotoxin receptor expression in the heart; this CD14 elevation may further amplify the cardiomyopathy leading to eventual contractile dysfunction. Recently, we used trans-thoracic echocardiography to study cardiac contractility during severe Mg deficiency in rats. We found that significantly impaired diastolic function occurred during the early response stage, and systolic function (% ejection fraction) was significantly decreased during the late response stage.3 We anticipate that prolonged exposure (> 7 weeks) to a moderate MgD diet will also lead to impaired cardiac function, based on our observations of depressed baseline contractility in perfused hearts from these animals.3
In conclusion, the animal studies of dietary Mg deficiency indicate that an inflammatory process is triggered by neuronal release of SP which may affect cardiovascular and intestinal tissues. In the event that gut permeability is enhanced, endotoxemia may result in further systemic inflammatory responses. The clinical literature has documented that patients with congestive heart failure may have elevated endotoxin levels.39–40 and that hypomagnesemia41 may also be present due to diuretic therapy and other concurrent disease processes. We suggest that some of the findings from animal studies of Mg deficiency may have some clinically relevant parallels and we submit that the careful assessment of Mg status in patients may indicate the need for more frequent oral and intravenous therapy with this important electrolyte.
Acknowledgments
Supported by NIH Grants: RO1 HL062282 and HL 065178
REFERENCES
- 1.Kramer JH, Phillips TM, Weglicki WB. Magnesium-deficiency enhanced postischemic myocardial injury is reduced by substance P receptor blockade. J. Mol. Cell. Cardiol. 1997;29:97–110. doi: 10.1006/jmcc.1996.0255. [DOI] [PubMed] [Google Scholar]
- 2.Kramer JH, Mak IT, Phillips TM, Weglicki WB. Dietary Mg-intake influence circulating pro-inflammatory neuropeptide levels and loss of myocardial tolerance to postischemic stress. Exp. Biol. Med. 2003;228:665–673. doi: 10.1177/153537020322800604. [DOI] [PubMed] [Google Scholar]
- 3.Kramer JH, Spurney C, Iantorno M, Tziros C, Mak IT, Tejero-Taldo MI, Chmielinska JJ, Komarov AM, Weglicki WB. Neurogenic inflammation and cardiac dysfunction due to hypomagnesemia. Am. J. Med. Sci. 2009;338(1):22–27. doi: 10.1097/MAJ.0b013e3181aaee4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantyh PW. Substance P and the inflammatory and immune response. Ann. NY Acad. Sci. 1991;632:263–271. doi: 10.1111/j.1749-6632.1991.tb33114.x. [DOI] [PubMed] [Google Scholar]
- 5.Bost KL, Pascual DW. Substance P: A late-acting B lymphocyte differentiation cofactor. Am. J. Physiol. Cell Physiol. 1992;262:C537–C545. doi: 10.1152/ajpcell.1992.262.3.C537. [DOI] [PubMed] [Google Scholar]
- 6.Weglicki WB, Mak IT, Stafford RE, Dickens BF, Cassidy MM, Phillips TM. Neurogenic peptides and the cardiomyopathy of Mg-deficiency: Effects of substance P-receptor inhibition. Mol. Cell. Biochem. 1994;130:103–109. doi: 10.1007/BF01457391. [DOI] [PubMed] [Google Scholar]
- 7.Weglicki WB, Phillips TM. Pathobiology of magnesium deficiency: a cytokine/neurogenic inflammation hypothesis. Am. J. Physiol. 1992;263:R734–R737. doi: 10.1152/ajpregu.1992.263.3.R734. [DOI] [PubMed] [Google Scholar]
- 8.Weglicki WB, Mak IT, Phillips TM. Blockade of cardiac inflammation in Mg-deficiency by substance P receptor inhibition. Circ. Res. 1994;24:1009–1013. doi: 10.1161/01.res.74.5.1009. [DOI] [PubMed] [Google Scholar]
- 9.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 10.Furness JB, Costa M, Papka RE, Della NG, Murphy R. Neuropeptides contained in peripheral cardiovascular nerves. Clin. Exp. Hypertens. [A] 1984;6(1–2):91–106. doi: 10.3109/10641968409062553. [DOI] [PubMed] [Google Scholar]
- 11.Soma M, Cunnane SC, Horrobin DF, Manku MS, Honda M, Hatano M. Effects of low magnesium diet on the vascular prostaglandin and fatty acid metabolism in rats. Prostaglandins. 1988;36:431–441. doi: 10.1016/0090-6980(88)90041-x. [DOI] [PubMed] [Google Scholar]
- 12.Kraeuter SL, Schwartz R. Blood and mast cell histamine levels in magnesium-deficient rats. J. Nutr. 1980;110:851–858. doi: 10.1093/jn/110.5.851. [DOI] [PubMed] [Google Scholar]
- 13.Classen CU, Abele C, Schimatschek HF, Friedberg KD, Classen HG, Haubold W. Erythema formation in magnesium-deficient albino rats. A non-invasive model for screening anti-inflammatory agents and oral mineral supplements. Arzneimittelforschung. 1993;43:672–675. [PubMed] [Google Scholar]
- 14.McIntosh TK. Novel pharmacologic therapies in the treatment of experimental traumatic brain injury: a review. J. Neurotrauma. 1993;10:215–261. doi: 10.1089/neu.1993.10.215. [DOI] [PubMed] [Google Scholar]
- 15.Pierson M, Swann J. Sensitization to noise-mediated induction of seizure susceptibility by MK-801 and phencyclidine. Brain Res. 1991;560:229–236. doi: 10.1016/0006-8993(91)91237-u. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M. Cardiac arrhythmias in magnesium deficiency. In: Theophanides T, Anastassopoulou J, editors. Magnesium: Current Status and New Developments. Theoretical, Biological and Medical Aspects. Dordrecht, Netherlands: Kluwer Academic Publishers; 1997. pp. 215–221. [Google Scholar]
- 17.Miserendino MJ, Davis M. NMDA and non-NMDA antagonists infused into the nucleus reticularis pontis caudalis depress the acoustic startle reflex. Brain Res. 1993;623:215–222. doi: 10.1016/0006-8993(93)91430-z. [DOI] [PubMed] [Google Scholar]
- 18.Mak IT, Kramer JH, Chmielinska JJ, Khalid H, Landgraf K, Weglicki WB. Inhibition of Neutral endopeptidase potentiates neutrophil activation during Mg-deficiency in the rat. Inflamm. Res. 2008;57(7):300–305. doi: 10.1007/s00011-007-7186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeman SE. Substance P and neurotensin: discovery, isolation, chemical characterization and physiological studies. J. Exp. Biol. 1980;89:193–200. doi: 10.1242/jeb.89.1.193. [DOI] [PubMed] [Google Scholar]
- 20.Tejero–Taldo MI, Chmielinska JJ, Gonzalez G, Mak IT, Weglicki WB. N-Methyl D- Aspartate Receptor Blockade Inhibits Cardiac Inflammation in the Mg2+ - Deficient Rat. JPET. 2004;311:8–13. doi: 10.1124/jpet.104.070003. [DOI] [PubMed] [Google Scholar]
- 21.Tejero-Taldo MI, Chmielinska JJ, Weglicki WB. Chronic dietary Mg2+ deficiency induces cardiac apoptosis in the rat heart. Magnes. Res. 2007;20(3):208–212. [PubMed] [Google Scholar]
- 22.Scholzen TE, Steinhoff M, Bonaccorsi P, Klein R, Amadesi S, Geppetti P, Lu B, Gerard NP, Olerud JE, Luger TA, Bunnett NW, Grady EF, Armstrong CA, Ansel JC. Neutral endopeptidase terminates substance-P induced inflammation in allergic contact dermatitis. J Immunol. 2001;166:1285–1291. doi: 10.4049/jimmunol.166.2.1285. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Sadoun E, Stephens RE, Ward PE. Metabolism of substance P and neurokinin A by human vascular endothelium and smooth muscle. Peptides. 1994;15:497–503. doi: 10.1016/0196-9781(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 24.Nadel JA. Peptidase modulation of neurogenic inflammation. In: Geppetti P, Holzer P, editors. Neurogenic inflammation. Boca Raton: CRC Press; 1996. pp. 115–127. [Google Scholar]
- 25.Iwamoto I, Kimura A, Ochiai K, Tomioka H, Yoshida S. Distribution of NEP activity in human blood leukocytes. J Leuk. Biol. 1991;49:116–125. doi: 10.1002/jlb.49.2.116. [DOI] [PubMed] [Google Scholar]
- 26.Lesser M, Fung K, Choi HS, Yoo OH, Cardozo C. Identification of two zinc metalloendopeptidases in alveolar macrophages of rats, guinea pigs, and human beings. J. Lab. Clin. Med. 1992;120:597–603. [PubMed] [Google Scholar]
- 27.Weglicki WB, Chmielinska JJ, Tejero-Taldo MI, Kramer JH, Spurney C, Viswalingham K, Lu B, Mak IT. Neutral endopeptidase inhibition enhances substance P mediated inflammation due to hypomagnesemia. Magnes. Res. 2009;22(3):167–173. [PMC free article] [PubMed] [Google Scholar]
- 28.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Butterfield DA. The glial glutamate transporter, GLT-1 is oxidatively modified by 4-HNA in the Alzheimer disease brain: the role of Abeta 1. J. Neutrochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang DS, Iwata N, Hama E, Saido TC, Dickson DW. Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2003;310:236. doi: 10.1016/j.bbrc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Miners JS, Van Helmond Z, Chalmers K, Wilcock G, Love S, Kehoe PG. Decreased Expression and Activity of Neprilysin in Alzheimer Disease are Associated with Cerebral Amyloid Angiopathy. J. Neuropathol. Exp. Neurol. 2006;65(10):1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- 31.R&D Systems Catalog # KGE007 User Manual. Minneapolis, MN: 2007. Substance P Assay. [Google Scholar]
- 32.Zhou M, Arthur AJ, Ba ZF, Chaudry IH, Wang P. The small intestine plays an important role in upregulating CGRP during sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R382–R388. doi: 10.1152/ajpregu.2001.280.2.R382. [DOI] [PubMed] [Google Scholar]
- 33.Scanlan BJ, Kim SY, Tuft B, Elfrey JE, Smith A, Zhao A, Morimoto M, Chmielinska JJ, Tejero-Taldo MI, Mak IT, Weglicki WB, Shea-Donohue T. Intestinal inflammation caused by magnesium deficiency alters basal and oxidative stress-induced intestinal function. Mol. Cell. Biochem. 2007;306(1–2):59–66. doi: 10.1007/s11010-007-9554-y. [DOI] [PubMed] [Google Scholar]
- 34.Malpuech-Brugère C, Nowacki W, Rock E, Gueux E, Mazur A, Rayssiguier Y. Enhanced tumor necrosis factor-alpha production following endotoxin challenge in rats is an early event during magnesium deficiency. Biochim. Biophys. Acta. 1999;1453(1):35–40. doi: 10.1016/s0925-4439(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 35.Opal SM, Scannon PJ, Vincent J-L, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Diseases. 1999;180(5):1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 36.Chmielinska JJ, Tejero-Taldo MI, Mak IT, Weglicki WB. Intestinal and cardiac inflammatory response shows enhanced endotoxin receptor (CD14) expression in magnesium deficiency. Mol. Cell. Biochem. 2005;278:53–57. doi: 10.1007/s11010-005-2733-9. [DOI] [PubMed] [Google Scholar]
- 37.Ferrero E, Jiao D, Tsuberi BZ, Tesio L, Rong GW, Haziot A, Goyert SM. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc. Natl. Acad. Sci. USA. 1993;90:2380–2384. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comstock KL, Krown KA, Page MT, Martin D, Ho P, Pedraza M, Castro EN, Nakajima N, Glembotski CC, Quintana PJ, Sabbadini RA. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J. Mol. Cell. Cardiol. 1998;30:2761–2775. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- 39.Peschel T, Schönauer M, Thiele H, Anker S, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur. J. Heart Failure. 2003;5:609–614. doi: 10.1016/s1388-9842(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 40.Bolger AP, Anker SD. Tumour necrosis factor in chronic heart failure: a peripheral view on pathogenesis, clinical manifestations and therapeutic implications. Drugs. 2000;60:1245–1257. doi: 10.2165/00003495-200060060-00002. [DOI] [PubMed] [Google Scholar]
- 41.Liebscher DH, Liebscher DE. About the misdiagnosis of magnesium deficiency. J. Am. Coll. Nutr. 2004;23(6):730S–731S. doi: 10.1080/07315724.2004.10719416. [DOI] [PubMed] [Google Scholar]