Abstract
Reports of low quality pharmaceuticals have been on the rise in the last decade with the greatest prevalence of substandard medicines in developing countries, where lapses in manufacturing quality control or breaches in the supply chain allow substandard medicines to reach the marketplace. Here, we describe inexpensive test cards for fast field screening of pharmaceutical dosage forms containing beta lactam antibiotics or combinations of the four first-line antituberculosis (TB) drugs. The devices detect the active pharmaceutical ingredients (APIs) ampicillin, amoxicillin, rifampicin, isoniazid, ethambutol, and pyrazinamide, and also screen for substitute pharmaceuticals such as acetaminophen and chloroquine that may be found in counterfeit pharmaceuticals. The tests can detect binders and fillers like chalk, talc, and starch not revealed by traditional chromatographic methods. These paper devices contain twelve lanes, separated by hydrophobic barriers, with different reagents deposited in the lanes. The user rubs some of the solid pharmaceutical across the lanes and dips the edge of the paper into water. As water climbs up the lanes by capillary action, it triggers a library of different chemical tests and a timer to indicate when the tests are completed. The reactions in each lane generate colors to form a “color bar code” which can be analyzed visually by comparison to standard outcomes. While quantification of the APIs is poor compared to conventional analytical methods, the sensitivity and selectivity for the analytes is high enough to pick out suspicious formulations containing no API or a substitute API, as well as formulations containing APIs that have been “cut” with inactive ingredients.
Keywords: pharmaceutical, antiinfective, antibiotic, tuberculosis, substandard, counterfeit, falsified, fake, screening test, field test, colorimetric, paper, paper-based, microfluidic, millifluidic
The problem of substandard and counterfeit medicines is worldwide,1,2 but most acute in developing countries.3–8 State of the art pharmaceutical testing, such as high performance liquid chromatography or mass spectrometry, provides powerful tools to identify active pharmaceutical ingredients (APIs), but is expensive and requires skilled technicians, reliable laboratory infrastructure, and resources to buy, operate, repair, and maintain instruments. To address the problem of substandard medicines around the world, detecting poor quality medication in low resource settings is an urgent public health issue in need of inexpensive, user-friendly tools to increase screening capacity within the pharmaceutical supply chain.
One approach, taken by many manufacturers, incorporates security features into product packaging and labeling, such as fluorescent inks, microprinting, holographic stickers and scratch off labels9,10. However, without chemical testing, these security features cannot prevent fake medicines slipping into legitimate bottles or production and distribution of poor quality pharmaceuticals by unscrupulous manufacturers. Among methods well suited for chemical analysis in low resource environments are portable X-ray powder diffraction11, hand-held X-ray fluorescence12, Raman13 and infrared14 spectroscopy devices. A recent study of one hand-held Raman spectrometer concluded that it is limited to qualitative analysis of counterfeits, appropriate only for indicating medicines containing substitute or no API 15. Portable spectrometers also come with a high initial investment cost and must be preloaded with a library of sample spectra sufficient to allow variations of genuine pharmaceuticals to be distinguished from fakes and substandard formulations. This technology has proven helpful to regulatory agencies in on-site testing, but is too costly to be the answer to monitoring medicine quality in the many clinics and pharmacies scattered in rural areas of developing countries. Inspection of pharmaceuticals that is more economically feasible in a low resource setting involves packaging analysis followed by rapid tests involving dissolution testing, colorimetric testing16–20 and/or thin layer chromatography, as is found in the Minilab®21,22 This technology, however, requires reagents, solvents, and standards, and must be run and interpreted by a trained technician. To further simplify pharmaceutical testing we have incorporated libraries of colorimetric reactions onto a paper-based device that qualitatively tests pharmaceutical chemical content.
Paper test strips such as litmus paper have a long history. These tests are cheap, easy to use, and don’t require a lab setting or instrumentation. However, they are generally restricted to use with liquids and lack chemical specificity. Current research has creatively expanded the capabilities of paper based tests to carry out analyses of complex samples by incorporating biochemical selectivity (enzymatic reactions, antibody/antigen binding) into paper millifluidic devices.23–30 We show that paper analytical devices can provide a qualitative chemical profile of pharmaceutical formulations (figure 1).31 The paper device is patterned using wax printing32 with many channels to allow multiplexing, 33,34 while millifluidic flow assembles reagents in situ. These paper analytical devices (PADs) are a novel technology for rapid field screening of medicines in the developing world.
Figure 1.
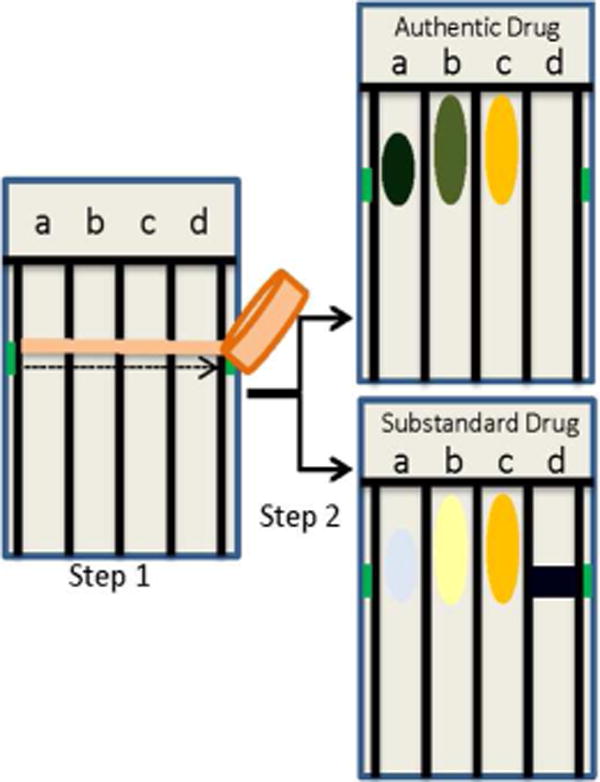
Schematic of PAD testing procedure in which sample is “swiped” to deposit material for analysis in several lanes (step 1). The bottom edge of the device is dipped in water to bring together reagent and analyte by capillary flow (step 2). Colors produced by an authentic versus a substandard drug will vary in one or more lanes, reflecting differences in chemical make-up of samples.
The goal of this first generation of PADs is rapid field screening: qualitative determination of the presence of APIs, detection of substitute APIs, and detection of unapproved excipients. Such a tool could be used at various points in the supply chain to detect very low quality pharmaceuticals that have the most potential to harm patients. A pharmaceutical screening test that does not require a lab would be useful to hospitals, clinics, pharmacies and non-governmental organizations as added quality assurance during the purchasing process. Health care workers could use the devices when patients do not respond to a medication to determine if the cause is a substandard medicine as opposed to drug resistance. Additionally, such a tool could be used by regulatory agencies to implement low-cost, higher frequency testing of pharmaceuticals along the supply chain.
EXPERIMENTAL
Materials and Methods
The PADs were wax printed using the methodology of Carrilho et al. and Lu et al32,34 The three layers of printing (front color laser, front wax, and back wax) aligned within 0.5 mm tolerance 93% of the time. Batches of pages were baked at 100°C for 9 minutes to form hydrophobic barriers, and then 20–30 reagents per PAD were spotted at a rate of 1 PAD per min and an error rate under 6% by a liquid delivery robot. A batch of 60 PADs can be made start to finish in under 2 hours and at a materials cost of under $0.12 per PAD. With labor at $1o/hour, the cost per PAD is about $0.45. The Supporting Information contains complete description of the materials used for the fabrication of the PADs and the lab validation study as well as details of lane development and stability testing.
PAD analysis
Solids are “swiped” across the PAD about 1–1.5 cm below the top of the lanes, depositing at least 0.5 mg of the solid in each lane. Once the sample is applied, the PAD is set in water up to the blue line and held upright for about four minutes. Capillary action carries water up the hydrophilic test lanes and brings together test reagents and analyte. The outcome is a “color bar code” at the top third of the PAD, providing the basis of sample characterization (Figure 2).
Figure 2.

Examples of beta-lactam PADs (top panel) and TB PADs (lower panel), (a) Beta-lactam PAD with 100% amoxicillin gives dark green in lane A, green in lane B, and orange in lane G. (b) A 2:1 w/w mixture of ampicillin and calcium carbonate gives blue-green in lane A, orange in lane B, and no color in lane G (indicating ampicillin rather than amoxicillin) and dark orange at the swipe line in lanes I and J (indicating carbonate), (c) Maize flour gives no API colors and dark purple at the swipe line in lane H (indicating starch). (d) TB PAD with isoniazid/ethambutol combo gives royal blue at the top of lane A (ethambutol), green near swipe line in lane B as well as bright orange-red in lane E (isoniazid). (e) A 4-drug combo formulation produces orange across the entire swipe area and black in lanes C and I (rifampicin), orange and orange-red coloration in lanes D and E indicating pyrazinamide and isoniazid respectively. Rifampicin interferes with detection of ethambutol in lane A. (f) Diphenhydramine (an antihistamine) produces a bright aqua color in lane K, indicating an API not expected in TB medications. This PAD also shows a failed talc test in lane F. In all PADs lane L exhibits the pink timer spot, indicating that the PAD testing was carried out to completion.
The variety of chemical tests and transient nature of many colorimetric tests provide a challenge to PAD analysis. To obtain the best test results the timing of test run duration and test imaging are both important factors. One lane houses a control reaction that generates a strong pink color; this lane serves as a timer. A spot of nioxime or dimethyl glyoxime is placed below the swipe line, and a spot of Ni(II) is deposited at the very top of the lane. The nickel has a very pale color and is not visible in the lane. When the chromogenic ligand reaches the metal, an intense pink spot is formed, which indicates to the user that the PAD should be removed from the water. This lane also serves to detect user errors such as failing to run the PAD completely (no spot appears) placing the PAD in the water upside-down (spot shows up in the wrong location) or dunking the entire PAD into water (reagents wash off and spot does not appear).
To indicate the best time for analysis of the “color bar code” we measured color intensity of the two tests whose color outputs 1) decayed most rapidly (the starch/iodine test) and 2) appeared most slowly (the talc/eosin red test). The optimum time for capturing the image of the PAD for color analysis was determined by the intersection of the color development of these two tests, and was found to be between 5–7 minutes (Figure S4, Supporting Information). The blue cobalt thiocyanate reagent turns pink when it is eluted up the lane by the water, but dries to a blue color that begins at the top of the lane. A distinct blue rim is visible at 15–20 minutes after removal from water, and indicates that a user has waited too long to take the image (Figure S5, Supporting Information). In PAD analysis, the appearance of the timer blaze and pink at the top of the cobalt thiocyanate lane both indicate that the user is compliant with PAD testing requirements.
Lab validation-Generation of fringe diagrams
For each lane, positive and negative outcomes were first defined based on images of standard test results obtained with known chemicals in a lab setting. PADs were run in deionized water until the timer spot appeared and were allowed to dry flat for 5 minutes for optimal color development. Each PAD was imaged with a cell phone and individual lane images were extracted for different samples. Once these standards had been generated, unknown samples were run and two readers compared each lane to standards to determine the presence or absence of specific functional groups in the unknowns (Figure 3a). Lane results that did not resemble a positive or negative outcome were classified as negative for the analyte in question. Some lane tests, such as the copper (II) tests in lanes A and B on the TB PAD, gave different colors (sometimes in different locations) depending on which pharmaceutical was applied. These tests could no longer be treated as binary tests, and a more complex set of standards known as the “fringe” diagram (due to the image’s resemblance to carpet fringe) was used to categorize results (Figure 3b and Figure S2, Supporting Information). The “fringe” analysis was generated by running multiple samples containing pure pharmaceutical ingredients, combinations of pharmaceutical ingredients and pure excipients. Replicate tests were carried out using light, medium and heavy swiping of the analyte, generating six test results per sample. Light swipes consisted of a barely visible dusting of the powdered pharmaceutical onto the PAD; gravimetric analysis showed deposition of an average of about 0.5 mg of material in each lane. Heavy swipes consisted of a layer of sample thick enough to obscure the grey color of the wax lines beneath the “swipe”, which provided 2–6 mg of material in each lane (Table S3). Medium swipes were intermediate in coverage. Images from each lane were laid side by side to produce a colorful “fringe” of outcomes based on the identity and quantity of the sample. The readers could then match an observed lane outcome with colorimetric outcomes associated with the known samples.
Figure 3.
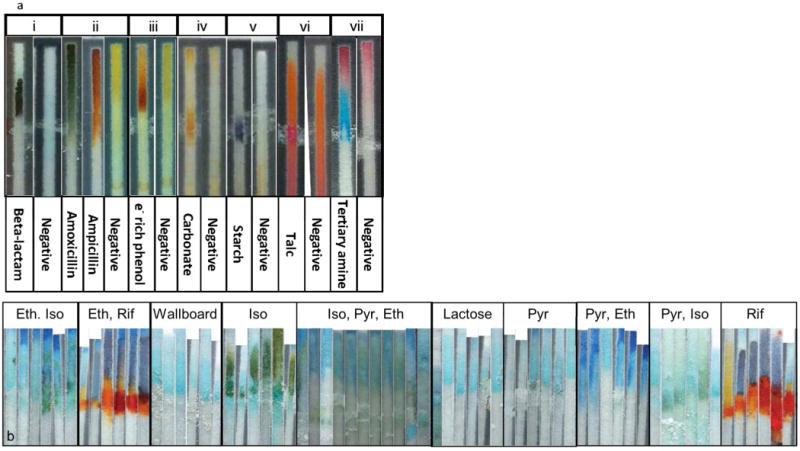
Validation standards used by readers for the analysis of PAD colorimetric results. Tests with binary or ternary outcomes are compiled into a small color key. Lane test reagents are copper sulfate (i), ninhydrin (ii), nitroaniline (iii), iron (III) chloride (iv), tri-iodide (v), eosine red (vi) and cobalt thiocyanate (a). Tests of the TB PAD present a more complex array of results and are evaluated using the “fringe” diagram. Represented here is a portion of the fringe diagram for the copper sulfate test for ethambutol, lane B of the TB PAD (b). The “fringe” diagram is a collection of outcomes from testing different quantities of all 15 combinations of the four main TB drugs, plus a library of excipients and substitute APIs. The reader matches each lane of the PAD to the most similar group of images to identify analytes present. See See Figure S2, Supporting Information, for the complete fringe diagram.
Analysis of unknowns
Each sample was coded so the person evaluating the PAD did not know the sample identity, and the serial numbers of the PADs were randomized so readers would not encounter clusters of identical PADs. A medium swipe of each sample (0.5–2 mg per lane) was applied. The PADs were run in de-ionized water until the timer spot was fully formed, and each PAD was laid flat for 3–5 minutes for color development, and then imaged with a cell phone camera. The photos were acquired in a home-built light box to provide uniform illumination and focal length. Two readers evaluated the PAD images lane by lane, comparing the appearance of each lane to standard positive/negative images or to a fringe diagram. If the readers did not agree on test outcomes, a third reader was used as a tie-breaker. The tiebreaker was only required for about 1% of the lanes.
RESULTS
Paper analytical devices show high sensitivity and specificity for active pharmaceutical ingredients and excipients
Lane tests from both TB PADs and beta lactam PADs were evaluated in a blinded validation study. The samples used in the validation study are listed in Figure 4 and Tables S4a and S4b (Supporting Information). Lanes were scored as positive or negative for each of the analytes they were designed to detect (eg, the copper (II) test was scored for detection of any beta lactam antibiotic, but not for detection of carbonate). Ambiguous or weak test results were scored as negatives (see scoring examples in Figure S3, Supporting Information). After unblinding the samples, the sensitivity of each lane was determined based on the number of true positives divided by the number of samples that should have been detected by that lane, and the selectivity by the number of true negatives divided by the number of samples that should have produced negative results.
Figure 4.
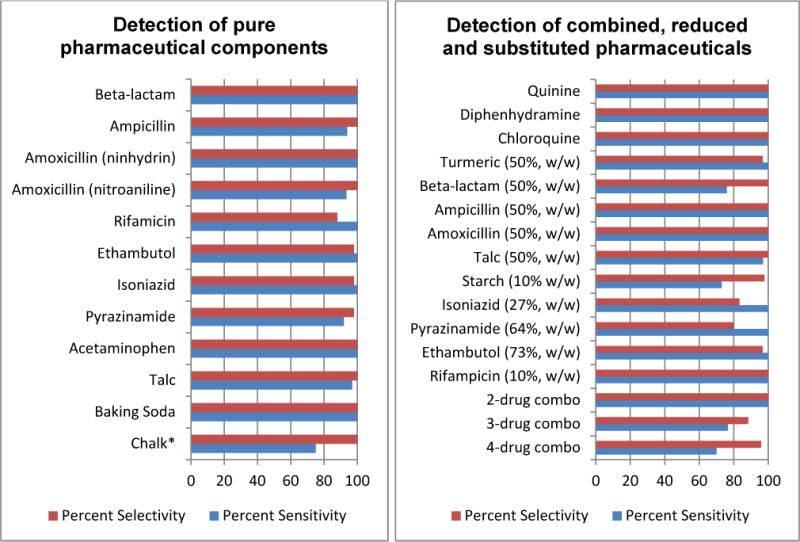
PAD performance in the detection of pure and combined pharmaceutical ingredients. Measurements were based on sample sizes of 15–135; complete details of these studies can be found in the Supporting Information, Tables S4a and S4b. *Deposition of ≤0.5mg chalk produced a false negative with this test, all other tests worked when 0.5mg was deposited.
Every pure API and every excipient was detected with sensitivity of 92%–100% and selectivity of 88%–100%. For the beta lactam antibiotics ampicillin and amoxicillin, the basic Cu(II) lane gave 100% sensitivity and specificity for detection of the beta lactam functionality. Ampicillin and amoxicillin have very similar chemical structures, but were reliably distinguished both by the different colors produced in the ninhydrin lane and by the positive nitroaniline test obtained for amoxicillin. The four major TB drugs rifampicin (Rif, R), isoniazid (Iso, I), pyrazinamide (Pyr, P) and ethambutol (Eth, E) were detected with sensitivity and selectivity ranging from 85%–100%. Isoniazid, in addition to giving a 100% sensitive and 98% selective response on the napthaquinone sulfonate lane, also showed a characteristic green color in the Cu(II) lane used in beta-lactam analysis. Acetaminophen forms a grey or brown colored complex with iron which gave false positive results for rifampicin on the Fe(III) lane. However, acetaminophen was reliably distinguished from rifampic-in by its positive nitroaniline test and by the lack of the orange color of rifampicin.
Anti-tuberculosis medications for uncomplicated TB are almost always given as combination tablets containing two, three, or all four of the first-line drugs (Table S2, Supporting Information). The two-drug combination of ethambutol and isoniazid was properly detected 30 out of 30 times with no false positives for this combo. Isoniazid and pyrazinamide were both reliably detected in the presence of each other and in the presence of rifampicin. Ethambutol was missed 30% of the time and falsely reported as present 17% of the time in the presence of rifampicin. The ethambutol test is therefore not sufficiently reliable to detect presence/absence of ethambutol in formulations that contain rifampicin. Neglecting the ethambutol, formulations containing RIP were properly identified in 58/60 cases (Table S4b, Supporting Information).
The presence of unapproved fillers or substitute APIs in a pharmaceutical formulation is a critical warning sign.* Several of the samples were designed to simulate falsified drugs, which are often adulterated with fillers such as calcite, talc, or starch, or may contain inexpensive drugs like acetaminophen in place of more expensive APIs.35,36 Samples of ampicillin and amoxicillin were cut with 50% w/w talc to simulate an adulterated antibiotic; the PADs detected the talc in 81% of the samples. To mimic poor quality anti-TB medications, rifampicin was mixed with 90% w/w potato starch; even though the dark orange color of rifampicin makes it difficult to see the starch/iodine color, 73% of the adulterated samples were detected. Pyrazinamide was cut with 50% w/w turmeric to mimic the orange color of rifampicin expected in an RP combination medicine. Although we had not tested turmeric on the PADs and it was not present on the fringe diagrams, all of the readers agreed that the suspect formulation did not match any of the possible anti-TB drug combinations and flagged 100% of them as suspicious. Finally, a set of substitute pharmaceuticals was tested, including the analgesic acetaminophen, antimalarial drugs quinine sulfate and chloroquine phosphate, and the antihistamine diphenhydramine. All of these inexpensive pharmaceuticals have been used as substitute APIs in counterfeit drug formulations, and each of these substitute APIs was detected with 100% sensitivity and specificity.
Development of lane tests for the beta lactam and TB PADs
The paper test devices must operate reliably in low resource settings, and this imposes a very stringent set of requirements on prospective color reactions:
The reaction must work at room temperature. Controlled heating is not common in many field settings, and a chemical heater would increase the cost of the PAD.
All reagents and the overall reaction must tolerate water, as non-aqueous solvents (even ethanol) are not reliably available in the developing world. Including solvents in a kit form would increase costs and introduce hazard and waste disposal issues.
All reagents must be stable during storage on paper, which eliminates use of concentrated acids (which eat away the cellulose), volatile materials such as iodine (but see below), or highly reactive species such as nitrous acid (see below).
The quantity of reagents on each PAD must be small enough that the PAD would not be treated as hazardous waste according to US EPA standards, limiting use of most heavy metals and highly toxic reagents.
And finally, the array of reagents used in the available lane tests has to provide good sensitivity and specificity for identification of not only the targeted APIs, but for excipients and substitute APIs that might be present in low quality pharmaceuticals.
Many color-generating reactions have been used in low-resource settings for the detection of APIs and excipients.17,19,20,37 The World Health organization (WHO) has published a series of monographs on simple color reactions and precipitation test procedures for pharmaceutical substances and dosage forms, intended for use in developing countries.16,18 Many of the classic color tests used in these procedures originated before the 1960’s, and survive today as TLC development procedures.38 The adaptation of inorganic and organic color tests to microliter scale using spot plates and filter or reagent papers, is comprehensively described in Fritz Feigl’s monograph.39 However, many of these laboratory-based spot tests involve digestion in strong acids, heating, and toxic, volatile or unstable reagents, and are not suitable for field use. Details of the test development and formulation for each lane on the beta lactam and TB drug PADs are given in the supporting information.
Overview of reactions used on the beta lactam PAD
The beta lactam PAD used in validation testing contained five lanes for functional groups expected to appear in the targeted antibiotics, six lanes for excipients and substitute drugs, and one timer lane. Lane A used copper(II) to test for the beta lactam group; as shown in figure 2a, this test gives a dark forest green color for either ampicillin or amoxicillin. In lane B, ninhydrin was used to discriminate between ampicillin and amoxicillin, as shown in Figure 2b. At room temperature in water, ampicillin forms an orange colored species, while amoxicillin gives a forest-green color, presumably due to formation of Schiff base intermediates. The lanes retain the ability to differentiate the two antibiotics for at least 2 months at room temperature (Figure S6, Supporting Information), at least 104 days at 37°C and at least 30 days at 60°C (Figure S7, Supporting Information). Lanes C and D contained ninhydrin plus either ampicillin or amoxicillin, and were intended as standards to show the proper colors of the ninhydrin reaction. However, even light swipe samples show the same color outcomes in lanes B, C, and D (all green or all orange) because the swipe deposits so much ampicillin or amoxicillin in all three ninhydrin lanes that the control reactions are masked. Lane E contained an ion-pairing reagent that was not tested in this study. In lane F, a modification of the Scott test for alkaloids was used to indicate tertiary amines such as quinine sulfate, chloroquine, amodiaquine, and diphenhydramine. Lane G was developed to indicate the presence of the phenol functional group found in both amoxicillin and acetaminophen (a substitute API that has been found in several counterfeit anti-infective drugs19,36,40). This lane contains four reagents: at the bottom, tosic acid (a strong acid that is stable on paper), then NaNO2, nitroaniline, and at the swipe line, sodium hydroxide. As the water moves up the lane by capillary action it successively mixes these reagents, forming the unstable acid HONO, diazotizing the nitroaniline to form a diazonium salt, and finally reaching the basic region where the excess acid is neutralized and any phenol groups in the sample are deprotonated. This lane produces a strong orange color with amoxicillin or acetaminophen (Figure 2c); the two drugs can be differentiated by cross-checking with the ninhydrin lane and the copper lane (both are negative for acetaminophen). The lane shows good stability for at least 104 days at 37°C (Figure S7, Supporting Information). Lane H contains tri-iodide ion stored in a poly(vinylpyrolidone) matrix as a test for soluble and insoluble starches (see figure 2e). Even trace quantities of starch, such as the binder in commercial wallboard, are strongly detected. This lane is stable for at least 2 months at 37°C (Figure S7, Supporting Information). Lanes I and J contained FeCl3, either mixed with a trace of detergent (in lane I) or alone (in lane J). Ferric ion forms an insoluble carbonate salt which is orange in color (Figure 2d); this test can detect adulterants such as baking soda, chalk, or calcite. The detergent was included to help the iron solution wet the analytes, but the two lanes did not give significantly different results after the 4 min color development period. False negatives reported with this test occurred in cases of light sample swiping and suggest that all testing of unknown samples should be carried out using medium to heavy swiping of the pharmaceutical to produce the expected colorimetric output. Lane K contained a test for sulfate ions which was too unstable for validation testing and hence is not discussed here. Finally, in the timer lane L, separate spots of nioxime and nickel were combined by capillary flow to provide a visual indicator to the operator that water had run up the lane all the way to the top.
Overview of reactions used on the TB PAD
The TB PAD included both lane A from the beta lactam PAD (intended to detect beta lactam antibiotics that might be used as substitute APIs) and a modified copper(II) test in Lane B as an indicator for the substituted ethylenediamine group present in ethambutol. The optimal formulation for detection of ethambutol was to place copper below the swipe line and carbonate or hydroxide above the swipe line; in the presence of ethambutol, a royal blue compound that moved at the solvent front was formed. Both of these copper lanes also give a green color with low chromatographic mobility when isoniazid is present at the swipe line, and a light green mobile complex forms with salicylic acid (degraded aspirin). In lane C, iron(III) was used to indicate the macrolide antibiotic rifampicin through formation of a purple-black complex. Lane D included pentacyanoaquoferroate Na3Fe(CN)5H2O, a moderately labile complex that undergoes replacement of the coordinated water by strong nucleophiles. Isoniazid, which contains an acyl hydrazine, gives a strong yellow-orange color which develops within five minutes, while pyrazinamide, like pyridine, gives a reddish-orange color (Figure S2, Supporting Information) which is stronger than the yellow isoniazid color. Tests remain viable for at least 104 days at 37°C (Figure S7, Supporting Information). In lane E, sodium naphthoquinone sulfonate (NQS) was used The presence of isoniazid can be confirmed by checking lanes A and B for the characteristic light green precipitate at the swipe line, and in Lane E for a strong orange-red color formed by reaction with 1,2-napthoquinone-4-sulfonate (NQS). NQS undergoes coordinated Michael addition/elimination reactions and/or Schiff base substitution reactions with amines and hydrazines in basic solution. At room temperature, the reaction with strong nucleophiles like the acyl hydrazine group in isoniazid is rapid, while primary amines like ampicillin or amoxicillin react slowly. Lane F shows the presence of talc, a magnesium silicate mineral with formula Mg3Si4O10(OH)2. It is insoluble in water and has only sparing solubility in strong mineral acids, so detection is a challenge. Eosin Red gives a pink color on the paper, but if it passes over a “swipe” of talc, a vibrant red color develops at the swipe mark after about 5 minutes (Figure 2F). Freshly spotted tests performed well in lab validation, however, in PADs that were tested one or more days following fabrication eosin red did not migrate well. In tests carried out 2–7 days following fabrication, 43% (65/150) of eosin red tests did not run to completion. Lane G contains the nitroaniline test for phenols, and lanes H and I duplicate the starch test and carbonate test used on the beta lactam PAD; lane I also responded strongly to rifampicin, just like lane C. Lane J contained a test which was not used in this validation study. Lanes K and L contained the cobalt thiocyanate test for tertiary amines and the timer lane from the beta lactam PAD.
CONCLUSIONS
Overall, these tests show high sensitivity and specificity for analytes and provide a useful chemical profile of APIs and excipients within a few minutes. The analytical metrics are good enough to detect very low quality drugs in wide-scale field screening. Although the tests do not directly address the issues of patent violation and unauthorized manufacturing—a counterfeit product that contained the correct APIs and excipients in the correct amounts would not be detected as suspicious—and do not detect slight under- or over-dosing, they are able to detect medications with substitute APIs, unapproved fillers, and adulterated APIs.
The scale of testing necessary to detect a given prevalence of “bad” drugs can be estimated statistically.41 If the fraction of pharmaceuticals in a region that are very low quality is θ and one wishes to measure the prevalence to within ±u, the number of samples m that must be examined in order to estimate the prevalence with 95% confidence is given by:
| (1) |
If the true prevalence of very low quality drugs is 3%, prevalence can be measured with 95% confidence in the interval 3±2% by testing 291 samples. The low rates of false positives for the excipient tests is important because the prevalence of very low quality or fake pharmaceuticals is, in most studies35, in the 0–7% range, so a high rate of false positives would cause needless alarm and expensive follow-up testing. For small prevalence rates, the chance that a bad drug will be missed due to a false negative can be compensated for by increasing both the sample size and the observed prevalence by a factor of 1/sensitivity. Assuming a PAD sensitivity of 75% (the worst value in our testing) a randomly selected sample42 of under 400 would enable reliable detection of very low quality pharmaceuticals across a region. The fabrication and labor costs of making a single PAD are under $0.50, so this scale of testing is feasible. In practice, a “suspicious” PAD result would be followed up by retesting the sample with a fresh PAD; two suspicious PAD tests should trigger confirmatory testing by an analytical lab.
Ideally, analytical devices for use in field settings should be viable for several years with proper storage. This is currently a limitation of PADs that may be solved through the use of reagent stabilizing polymers and improved packaging. Though the current generation of PAD tests are qualitative rather than quantitative, they are capable of detecting very low quality pharmaceuticals of the types that have been previously found in many locations in the developing world. We believe that it will be possible to add quantitative detection capabilities to detect substandard pharmaceuticals and to use mobile phone technology and image analysis software to automate the evaluation of the “color bar codes” in the next generation of devices. The minimal training for users and ease of manufacturing scale-up of these paper test cards could provide additional quality assurance within the supply chain in developing countries and exert economic pressures on manufacturers and distributors involved in the widespread trade in falsified pharmaceuticals.
Supplementary Material
Acknowledgments
We would like to thank Prof. Fang Liu for discussions about sampling statistics and Shannon Huey, Revathi Kollipara and Sarah Halweg for their assistance in this project.
Funding Sources
Support from the Notre Dame FSRP and the Eck Institute for Global Health is gratefully acknowledged. AW is a fellow of the GLOBES program, the Eck Institute for Global Health, and the Chemistry-Biochemistry-Biology Interface (CBBI) Program at the University of Notre Dame, supported by training grant T32GM075762 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. BJ received summer support from ACS Project SEED; MB and MH were supported by the ND Nanocenter NURF program, and TB acknowledges the support of the Mathile Foundation.
Footnotes
ASSOCIATED CONTENT
The Supporting Information as noted in the text is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript
“Some circumstantial evidence is very strong, as when you find a trout in the milk” (H. D. Thoreau, Journal, 11/11/1854)
References
- 1.World Health Organization WHO. Medicines spurious falsely-labelled falsified counterfeit (SFFC) medicines. www.who.int/mediacentre/factsheets/fs275/en (accessed Mar 21, 2013).
- 2.Gostin LO, Buckley GJ, Products CM. Countering the Problem of Falsified and Substandard Drugs Committee on Understanding the Global Public Health Implications of. The National Academies Press; 2013. [PubMed] [Google Scholar]
- 3.Newton PN, Green MD, Fernández FM. Trends in pharmacological sciences. 2010;31:99–101. doi: 10.1016/j.tips.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldhous P. Nature. 2005;434:132–136. doi: 10.1038/434132a. [DOI] [PubMed] [Google Scholar]
- 5.Newton PN, McGready R, Fernandez F, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJM, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NPJ, Greenwood BM, Nosten F, White NJ. PLoS medicine. 2006;3:e197. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton PN, Green MD, Fernández FM, Day NPJ, White NJ. The Lancet infectious diseases. 2006;6:602–13. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- 7.Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. PLoS medicine. 2005;2:e100. doi: 10.1371/journal.pmed.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayyar GML, Breman JG, Newton PN, Herrington J. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. The Lancet infectious diseases. 2012;12:488–96. doi: 10.1016/S1473-3099(12)70064-6. [DOI] [PubMed] [Google Scholar]
- 9.Deisingh AK. The Analyst. 2005;130:271–9. doi: 10.1039/b407759h. [DOI] [PubMed] [Google Scholar]
- 10.Business Call to Action Sproxil?: Combating Counterfeit Drugs with Mobile Phones. http://www.businesscalltoaction.org/wpcontent/files_mf/sproxilcasestudy2.23.2012forweb17.pdf (accessed Mar 22, 2013).
- 11.Blake D, Sarrazin P, Boyer B, Jennison T. PPXRD-9. Hilton Head Island; South Carolina: 2010. [Google Scholar]
- 12.Ida H, Kawai J. Forensic science international. 2005;151:267–272. doi: 10.1016/j.forsciint.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Ricci C, Nyadong L, Yang F. Analytica Chimica Acta. 2008;623:178–186. doi: 10.1016/j.aca.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Moffatt A, Assi S, Watt R. Journal of near infrared spectroscopy. 2010;18:1–15. [Google Scholar]
- 15.Hajjou M, Qin Y, Bradby S. Journal of Pharmaceutical and Biomedical Analysis. 2012;74:47–55. doi: 10.1016/j.jpba.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Basic Tests for Pharmaceutical Dosage Forms. Geneva: World Health Organization; 1991. pp. 18–19. [Google Scholar]
- 17.Green MD, Nettey H, Wirtz R. a Emerging infectious diseases. 2008;14:552–6. doi: 10.3201/eid1404.061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Basic Tests for Drugs: Pharmaceutical Substances, Medicinal Plant Materials, and Dosage Forms. 1998. [Google Scholar]
- 19.Ioset J-R, Kaur H. PloS one. 2009;4:e7270. doi: 10.1371/journal.pone.0007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green M, Mount D, Wirtz R, White N. Journal of Pharmaceutical and Biomedical Analysis. 2000;24:65–70. doi: 10.1016/s0731-7085(00)00360-5. [DOI] [PubMed] [Google Scholar]
- 21.Jähnke R, Küsters G, Fleischer K. Drug information journal. 2001;35:941–945. [Google Scholar]
- 22.Risha PG, Msuya Z, Clark M, Johnson K, Ndomondo-Sigonda M, Layloff T. Health policy (Amsterdam, Netherlands) 2008;87:217–22. doi: 10.1016/j.healthpol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Analytical Chemistry. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 24.Martinez AW, Phillips ST, Whitesides GM. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19606–11. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez AW, Phillips ST, Nie Z, Cheng C-M, Carrilho E, Wiley BJ, Whitesides GM. Lab on a Chip. 2010;10:2499–504. doi: 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- 26.Osborn JL, Lutz B, Fu E, Kauffman P, Stevens DY, Yager P. Lab on a Chip. 2010;10:2659–65. doi: 10.1039/c004821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutz BR, Trinh P, Ball C, Fu E, Yager P. Lab on a Chip. 2011 doi: 10.1039/c1lc20758j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe K, Suzuki K, Citterio D. Analytical chemistry. 2008;80:6928–34. doi: 10.1021/ac800604v. [DOI] [PubMed] [Google Scholar]
- 29.Apilux A, Ukita Y, Chikae M, Chailapakul O, Takamura Y. Lab on a chip. 2013;13:126–35. doi: 10.1039/c2lc40690j. [DOI] [PubMed] [Google Scholar]
- 30.Fu E, Liang T, Spicar-Mihalic P, Houghtaling J, Ramachandran S, Yager P. Analytical chemistry. 2012;84:4574–9. doi: 10.1021/ac300689s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization Department of Essential Medicines and Pharmaceutical Policies. The International Pharmacopoeia. 4. World Health Organization; 2011. [Google Scholar]
- 32.Carrilho E, Martinez AW, Whitesides GM. Analytical Chemistry. 2009;81:7091–5. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Shi W, Qin J, Lin B. Analytical Chemistry. 2010;82:329–35. doi: 10.1021/ac9020193. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Shi W, Jiang L, Qin J, Lin B. Electrophoresis. 2009;30:1497–500. doi: 10.1002/elps.200800563. [DOI] [PubMed] [Google Scholar]
- 35.Kelesidis T, Kelesidis I, Rafailidis PI, Falagas ME. The Journal of antimicrobial chemotherapy. 2007;60:214–36. doi: 10.1093/jac/dkm109. [DOI] [PubMed] [Google Scholar]
- 36.Newton PN, Fernández FM, Plançon A, Mildenhall DC, Green MD, Ziyong L, Christophel EM, Phanouvong S, Howells S, McIntosh E, Laurin P, Blum N, Hampton CY, Faure K, Nyadong L, Soong CWR, Santoso B, Zhiguang W, Newton J, Palmer K. PLoS medicine. 2008;5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green MD, Nettey H, Villalva Rojas O, Pamanivong C, Khounsaknalath L, Grande Ortiz M, Newton PN, Fernández FM, Vongsack L, Manolin O. Use of refractometry and colorimetry as field methods to rapidly assess antimalarial drug quality. Journal of pharmaceutical and biomedical analysis. 2007;43:105–10. doi: 10.1016/j.jpba.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 38.Kirschner J. In: Technique in Organic Chemistry. Perry ES, Weissberger A, editors. XII. Interscience Publishers; New York: 1967. [Google Scholar]
- 39.Feigl F. In: Qualitative Analysis by Spot Tests. 3. Oesper RE, editor. Elsevier Publishing; New York: 1947. [Google Scholar]
- 40.Newton PN, Green MD, Mildenhall DC, Plançon A, Nettey H, Nyadong L, Hostetler DM, Swamidoss I, Harris Ga, Powell K, Timmermans AE, Amin Aa, Opuni SK, Barbereau S, Faurant C, Soong RCW, Faure K, Thevanayagam J, Fernandes P, Kaur H, Angus B, Stepniewska K, Guerin PJ, Fernández FM. Malaria journal. 2011;10:352. doi: 10.1186/1475-2875-10-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aitken CG. Journal of forensic sciences. 1999;44:750–60. [PubMed] [Google Scholar]
- 42.Newton PN, Lee SJ, Goodman C, Fernández FM, Yeung S, Phanouvong S, Kaur H, Amin AA, Whitty CJM, Kokwaro GO, Lindegårdh N, Lukulay P, White LJ, Day NPJ, Green MD, White NJ. PLoS Medicine. 2009:6. doi: 10.1371/journal.pmed.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


