Abstract
Rationale and Objectives
Modafinil (MOD) and its R-enantiomer (R-MOD) are approved medications for narcolepsy and other sleep disorders. They have also been used, off label, as cognitive enhancers in populations of patients with mental disorders, including substance abusers that demonstrate impaired cognitive function. A debated non-medical use of MOD in healthy individuals to improve intellectual performance is raising questions about its potential abuse liability in this population.
Results and Conclusions
MOD has low micromolar affinity for the dopamine transporter (DAT). Inhibition of dopamine (DA) reuptake via the DAT explains the enhancement of DA levels in several brain areas, an effect shared with psychostimulants like cocaine, methylphenidate and the amphetamines. However, its neurochemical effects and anatomical pattern of brain area activation differ from typical psychostimulants and are consistent with its beneficial effects on cognitive performance processes such as attention, learning, and memory. At variance with typical psychostimulants, MOD shows very low, if any, abuse liability, in spite of its use as a cognitive enhancer by otherwise healthy individuals. Finally, recent clinical studies have focused on the potential use of MOD as a medication for treatment of drug abuse, but have not shown consistent outcomes. However, positive trends in several result measures suggest that medications that improve cognitive function, like MOD or R-MOD, may be beneficial for treatment of substance use disorders in certain patient populations.
Keywords: ADHD, Addiction, Cocaine, Cognition, Dopamine, Drug abuse, Methamphetamine, Modafinil, Psychostimulant
1) Introduction
Translational research in neuroscience has recently provided valuable information about drugs that improve cognitive function in subjects affected by specific pathological conditions as well as in healthy individuals (Partridge et al. 2011). These findings, along with increased non-medical-use of “smart drugs” (Cakic 2009; Randall et al. 2003), which are being used to improve cognitive performance, learning, memory and attention, are raising concerns of abuse potential in otherwise healthy individuals (Morein-Zamir et al. 2007; Sahakian and Morein-Zamir 2011). Among these drugs are several well-known psychostimulants, including methylphenidate and the amphetamines, as well as drugs like modafinil (MOD, Provigil™) and its R-enantiomer (R-MOD, Armodafinil, Nuvigil™). MOD and R-MOD (Fig. 1) are FDA approved for the treatment of narcolepsy and sleep-related disorders, but MOD has also been tested off-label as a cognitive enhancer (Turner et al. 2003).
Figure 1.
Chemical Structures of modafinil (MOD) and armodafinil (R-MOD).
The cognitive enhancing effects of MOD and their neural correlates are being extensively investigated preclinically and in humans (Minzenberg and Carter 2008). For example, MOD-induced improvement of cognitive performance has been explored in several brain disorders in humans that impair cognitive function. Promising results have been obtained using MOD as an adjunct therapy to antipsychotic treatments (Farrow et al. 2006; Rosenthal and Bryant 2004) in order to ameliorate cognitive impairments in schizophrenic patients. Other clinical studies have also documented beneficial effects of MOD on cognitive performance in human subjects who have been experimentally sleep deprived (Wesensten 2006). In addition, MOD appears to also enhance cognitive performance in healthy adults who are not sleep-deprived (Makris et al. 2007; Wesensten 2006). For instance, improvement on digit span, visual recognition memory, spatial planning, and the Stop-Signal Reaction Time (SSRT) task suggest MOD-related improvement of working memory and inhibition of pre-potent responding (Turner et al. 2003). Evidence for enhanced working memory, impulse control, vigilance and sustained attention in healthy volunteers has also been reported (Baranski et al. 2004; Muller et al. 2013; Randall et al. 2003; Turner et al. 2003). Thus, the effects of MOD on improving attention and cognitive function in healthy subjects have provided insight into its neuropsychological actions, free from confounds of any underlying pathology (Morein-Zamir et al. 2007). Moreover, treatment (or adjunctive treatment) of neuropsychological disorders such as drug abuse and addiction with cognitive enhancers such as MOD or R-MOD may be more effective than current strategies (Brady et al. 2011). This idea is particularly appealing for the treatment of cocaine and/or methamphetamine abuse, as there are no effective medications currently available (Dean et al. 2011; Ghahremani et al. 2011).
2) Effects of MOD on neurotransmitters related to cognitive function
Though MOD has no measurable affinity at monoamine receptors (Duteil et al. 1990; Korotkova et al. 2007; Zolkowska et al. 2009), direct inhibition of several neurotransmitter transporter systems that lead to increased monoamine transmission have been described (Minzenberg and Carter 2008). The following subchapters review how MOD-induced changes in neurotransmission are related to activation of receptors and brain pathways that play critical roles in modulating cognitive function. It is clear that this simple molecule either directly or indirectly affects many neurotransmitter systems that are likely involved in the expression of cognitive enhancing effects of MOD. In Fig. 2, the following potential targets of MOD in mediating cognitive enhancement are depicted.
Figure 2.
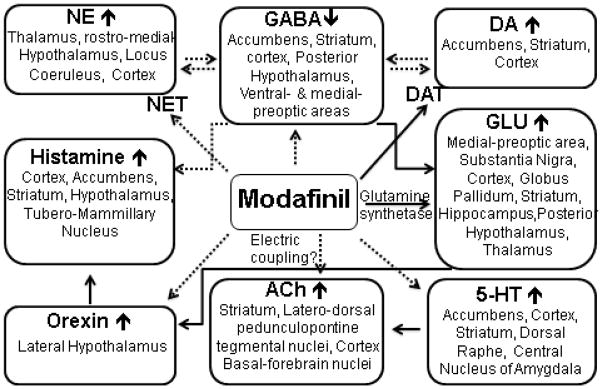
Targets of MOD’s actions as a cognitive enhancer
Brain areas and related neurotransmitter systems that are potentially involved in mediating the therapeutic actions of MOD as a cognitive enhancer. NE= norepinephrine; DA= Dopamine; GABA= gamma-amino-butyric-acid; GLU= glutamate; NET= norepinephrine transporter; DAT= dopamine transporter, 5-HT=serotonin; Ach= Acetylcholine.
Solid lines indicate direct interactions, while dashed lines indicate observed effects that appear to be via indirect interactions or for which a mechanism has not yet been elucidated.
2.1) MOD effects on dopaminergic neurotransmission
The dopaminergic system includes several well-characterized brain pathways, with DA neurons that are concentrated in specific midbrain areas, the substantia nigra and the ventral tegmental area, and from which axons project to selected terminal areas, for example striatum, nucleus accumbens (NAC), and medial prefrontal cortex to name a few (See for review Cooper et al. 1996a). DA neurotransmission is involved in several important brain functions including somatomotor activity, learning, memory, reward, motivation and emotions (Smythies 2005a). Thus it is not surprising that DA is also involved in the etiology of several neurological disorders such as Parkinson’s disease, schizophrenia, ADHD, and addiction (Smythies 2005a).
Though the precise mechanism(s) underlying MOD’s clinical effects are not fully understood, several studies indicate that MOD inhibits the reuptake of DA via the DAT (Loland et al. 2012; Madras et al. 2006; Mignot et al. 1994; Volkow et al. 2009; Zolkowska et al. 2009). It should be noted that in vitro studies show that MOD binds to the DAT with lower affinity than methylphenidate and other psychostimulants drugs (Minzenberg and Carter 2008). Despite its relatively low affinity for the DAT, in human PET studies MOD shows DAT occupancy comparable to that of methylphenidate at clinically relevant doses (Volkow et al. 2009). It has also been suggested that DA uptake inhibition improves learning of inhibitory avoidance and increases hippocampal acetylcholine release (Nail-Boucherie et al. 1998), thus suggesting that binding to the DAT might be related to the cognitive enhancing activity of MOD.
In vitro, MOD does not appear to stimulate the release of DA from preloaded synaptosomes (Simon et al. 1995), but in vivo MOD and R-MOD have been repeatedly shown to significantly increase DA levels in the NAC shell in mouse brain and in other brain areas in rat where increased DA neurotransmission is observed (de Saint Hilaire et al. 2001; Dopheide et al. 2007; Ferraro et al. 1997; Loland et al. 2012; Murillo-Rodriguez et al. 2007; Zolkowska et al. 2009). MOD has also been reported to increase DA levels in the frontal cortex suggesting this brain region as a viable target for improving cognitive performance, since DA is a potential substrate for synaptic plasticity and mechanisms underlying memory processes (Jay 2003). Moreover, mice lacking the DAT do not exhibit MOD-induced wakefulness, suggesting that the DAT is also involved in this specific behavioral effect of MOD (Wisor et al. 2001). Although compensatory neuroadaptations, in this particular case involving changes in the DA D1 and D2 receptor levels, might be a confounding factor in the experimental outcome (Fauchey et al. 2000; Jones et al. 1999; Wisor et al. 2001). It should also be noted that increased wakefulness elicited by MOD might influence cognitive performance through alternative mechanisms, including activation of the orexin system (Ferraro et al. 1997; Ishizuka et al. 2012; Lin et al. 1992; Taylor and Russo 2000). Future investigation into mechanisms related to the dopaminergic system in specific brain regions and downstream effects that contribute to MOD’s cognitive enhancing effects in healthy subjects and/or in populations with specific cognitive disorders are underway (Farrow et al. 2006).
2.2) MOD effects on noradrenergic neurotransmission
The noradrenergic system uses norepinephrine (NE) as its primary chemical messenger and serves multiple brain functions, including arousal, attention, mood, learning, memory and stress-related responses (see for example Smythies 2005b; Sofuoglu and Sewell 2009). Noradrenergic neurons are localized in brainstem nuclei such as the locus coeruleus (LC) and noradrenergic axons project diffusely throughout the brain (See for review: Cooper et al. 1996b; Smythies 2005b).
As noted above, binding studies showed MOD had no detectable binding affinity (>20 μM) for the NE transporter (NET) (Loland 2012). Nevertheless, Madras and colleagues (2006) measured inhibition of NE uptake in vivo by displacement of the NET selective positron emission tomography ligand [11C](S,S)-2-(α-(2-methoxyphenoxy)benzyl)-morpholine (MeNER), in the monkey thalamus, suggesting activation of the NE system by MOD. An alternative mechanism underlying this effect could be secondary to MOD-induced blockade of the DAT, which in turn produces an accumulation of extracellular synaptic DA resulting in reduced [11C]MeNER binding. In fact, DA has been shown to be taken up in vitro and in vivo by the NET with a potency similar to NE (Rothman et al. 2001; Tanda et al. 1997), suggesting that the in vivo binding of [11C]MeNER might be sensitive to endogenous NET substrates (Seneca et al. 2006) including DA. Interestingly, MOD does not affect the activity of NE neuronal single unit recordings in the LC of anaesthetized rats (Akaoka et al. 1991). However, R-MOD appears to activate LC activity in experimental animals and in humans, as shown by induction of Fos-like immunochemistry in rat brain (Fiocchi et al. 2009), and stimulation of extracellular NE levels in prefrontal cortex (along with DA) and rostromedial hypothalamus (de Saint Hilaire et al. 2001). Moreover, in a study on arousal and autonomic functions in humans (Hou et al. 2005) MOD was shown to activate noradrenergic neurons in the LC, without affecting extra-coerulear noradrenergic neurons, compared to clonidine. Hence, presently it is unclear how MOD affects the noradrenergic system, as it does not appear to directly inhibit the reuptake of NE via the transporter, but stimulates downstream neurotransmission that may be related to its cognitive enhancing effects. Alvarez and colleagues (Alvarez et al. 2002) have suggested the possibility that synchronization of the spontaneous firing of LC neurons is obtained with the strengthening of electronic coupling, which might result in improved neurotransmission. MOD has been suggested to enhance the activity of different neurotransmitter pathways by acting on gap-junctions increasing the firing synchrony in selected brain areas (Garcia-Rill et al. 2007; Urbano et al. 2007). Thus, even though the direct effect of MOD on NE receptors or uptake is limited, it is possible that a selective action of MOD on electronic coupling in the gap-junctions of the NE neurons in the LC might positively influence NE neurotransmission.
Another indirect suggestion regarding the NE modulation of MOD effects on memory processes related to cognition in human subjects has been shown by Muller and colleagues (Muller et al. 2004). In that report, the improvement in working memory, in healthy individuals, was characterized by tasks involving levels of high difficulty and demanding high levels of effort. Thus, under these conditions the authors related the improvement obtained with MOD to a possible NE mechanism of action (Smith and Nutt 1996). Moreover, the authors propose the involvement of the orexin system in these actions (Muller et al. 2004), since this system shows the most dense arborization of orexin axons in the LC (Horvath et al. 1999; Sutcliffe and de Lecea 2002).
2.3) MOD and serotonin neurotransmission
Serotonin (5-hydroxytryptamine, 5-HT) neurotransmission and 5-HT receptors have been reported to be involved in modulation of cognitive function, especially memory and learning (see for example: Meneses 1999; Sirvio et al. 1994). Moreover, interactions of 5-HT-receptor ligands and 5-HT neurotransmission with other neurotransmitter systems have been systematically studied and shown to modulate different cognitive functions (Cassel and Jeltsch 1995; Sirvio et al. 1994; Zarrindast 2006).
Several studies have reported that MOD dose-dependently increases extracellular 5-HT in brain regions that are functionally related to cognition. For example the effects of MOD on 5-HT have been described in the frontal cortex, central nucleus of the amygdala and dorsal raphe nucleus, and to a lesser extent, in the hypothalamus (de Saint Hilaire et al. 2001; Ferraro et al. 2000; Ferraro et al. 2002). However, the authors of these papers only discuss their data in terms of MODs involvement in wakefulness and regarding potential treatment of depressive states (de Saint Hilaire et al. 2001; Ferraro et al. 2013). Hence, although MOD may influence 5-HT neurotransmission, no direct evidence supporting 5-HT mediation of MOD’s effects on cognition is described. It is noteworthy that MOD has been reported to inhibit [3H]DA uptake, with an IC50 value of 6.4 μM, while inhibition for [3H]NE and [3H]5-HT uptake was shown only at very high micromolar concentrations (>30 and >500 μM, respectively) in human embryonic kidney cells expressing cloned human DAT, NET, and SERT (Madras et al. 2006). Similar data were obtained for MOD inhibition of DA, NE and 5-HT reuptake in rat brain or in COS7 cells transfected with human DAT, NET or SERT (Loland et al. 2012; Zolkowska et al. 2009). Also an early study by Mignot and colleagues (1994) demonstrated that MOD, up to a concentration of 100 μM, did not display any affinity for the SERT in rat forebrain. These very low in vitro potencies of MOD at NET and SERT would predict insignificant binding to these sites in vivo, which contrasts with results from those reports described above where MOD was found to increase 5-HT levels in selected brain areas. In summary, based on data currently available, it is unlikely that the serotoninergic system directly mediates MOD’s cognitive enhancing actions. Nevertheless, proposed indirect serotonergic actions of MOD are described in the appropriate section below for each neurotransmitter system in which modulation of cognitive functions by 5-HT receptors have been reported (see, for example, section 2.5 and 2.6 below).
2.4) MOD effects on glutamatergic neurotransmission
As discussed by Homayoun and Moghaddam (2010), the excitatory transmission through glutamate receptors represents the main mode of synaptic signaling in brain regions involved in cognitive functions. Glutamate receptors have been implicated in several forms of mental disease, including dissociative thought disorder, schizophrenia and various forms of dementia (Homayoun and Moghaddam 2010). For instance, NMDA receptors are believed to play a key role in several disorders characterized by the occurrence of cognitive deficits such as in schizophrenia (Coyle 1996; Tamminga 2006). NMDA and AMPA receptors are also involved in long-term neuronal adaptations or synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD) (Luscher and Malenka 2011; Malenka 1994) that could be related to learning, memory, cognitive processes, and behavioral flexibility (Bowers et al. 2010; Brasil-Neto 2012). To this end, it is interesting that MOD-induced wakefulness, both acute and prolonged, results in a LTP of glutamatergic synapses on orexin LH neurons (Rao et al. 2007). These neurons are indeed involved in several physiological functions, as an arousal and wake-promoting center, but also in metabolic health and achievement of natural and drug reinforced behaviors (Boutrel et al. 2013). Thus, it would be interesting to better understand the effects of the glutamatergic LTP on LH orexin neurons in models of reinforcement, since orexin antagonist ligands have been found to be effective in reducing the addictive actions of several drugs of abuse. Gerrard and Malcom (2007) also pointed out the impairment of sleep observed in drug dependents, and the improvement in cognition that MOD might have in this population.
As observed by Gerrard & Malcom (2007), an interesting feature about MOD’s effects on glutamate transmission is found in the inconsistent pattern of activation of this brain neurotransmitter. Different directions for glutamate level modulation are described depending on the brain region. For instance, likely as a result of a reduction in GABAergic tone, MOD elevates extracellular glutamate in the medial preoptic area and in the posterior hypothalamus(Ferraro et al. 1996b). MOD-induced increases in glutamate levels in the thalamus and hippocampus, instead, appear to be independent from modulation of GABAergic tone (Ferraro et al. 1997). Furthermore, administration of MOD leads to an increase in glutamate synthesis (Pierard et al. 1995; Touret et al. 1994) and striatal glutamate brain levels (Ferraro et al. 1998; Ferraro et al. 1996a) through an unknown mechanism. On the other hand, it was observed that only high doses (300 mg/kg) of MOD increased glutamate release in the substantia nigra, or in the pallidum (Ferraro et al. 1998). This effect on glutamate levels has been suggested to mediate some of the effects of MOD in neurobiological processes related to cognitive dysfunction in drug addicted subjects (Mahler et al. 2012a; Tahsili-Fahadan et al. 2010)(see also section 4.2, below). Increases in the cerebral glutamate-glutamine pool have been reported and may result from MOD-induced increases in glutamine synthetase activity (Touret et al. 1994). Thus, by enhancing the glutamate-glutamine cycle in astrocytes, MOD could also modulate glutamatergic tone in the synapse, an effect that might be involved in modulation of cognition (Homayoun and Moghaddam 2010). This modulation improves especially executive functions (as reviewed by Gerrard and Malcolm 2007) and involves mainly individuals with average or lower intelligence quotient (IQ) but not people with higher IQ (Randall et al. 2005).
2.5) MOD effects on GABAergic neurotransmission
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the brain (Wong et al. 2003). Administration of GABAergic agents affects memory retention and learning (Havekes et al. 2011). For instance, GABA receptor agonists impair, while antagonists facilitate memory consolidation (Brioni and McGaugh 1988). Furthermore, tiagabine, a GABA transport inhibitor impairs spatial learning in rats in the Morris water maze (Schmitt and Hiemke 2002), and baclofen, a selective GABA-B receptor agonist, has been shown to impair spatial learning in rats (McNamara and Skelton 1996). In contrast, selective GABA-B receptor antagonists can enhance cognitive performance in a variety of learning paradigms (Getova and Bowery 1998).
MOD has consistent lowering effects on central GABAergic neurotransmission and one possible mechanism/s of action is related to the alteration of GABAergic neurons in specific brain regions. Indeed, previous studies have indicated that MOD dose-dependently inhibits the activity of GABA neurons in the cerebral cortex, the basal ganglia (striatum, globus pallidus, NAC, substantia nigra), medial preoptic area and posterior hypothalamus in rats (Ferraro et al. 1997; 1998; Ferraro et al. 1999; Ferraro et al. 1996a; Ferraro et al. 1996b; Tanganelli et al. 1992). However, other in vitro studies suggested that MOD was unable to modify GABA-uptake as well as basal and K-evoked [³H]GABA release (Antonelli et al. 1998; Tanganelli et al. 1995), though a reduction in the cortical GABA outflow was observed in freely moving guinea-pigs (Tanganelli et al. 1992). This suggests that MOD’s effects on cortical GABA neurons might result from an indirect interaction (Tanganelli et al. 1995). Indeed, to exert its effects on cortical GABA MOD requires intact catecholamine neurons, i.e. NE and likely DA neurons, as demonstrated by intra-cerebroventricular (icv) pre-treatments with 6-hydroxydopamine that abolished MOD-induced reductions in GABA (Tanganelli et al. 1994). This has also been shown with pretreatments of the α1 noradrenergic receptor antagonist prazosin (Tanganelli et al. 1995). MOD’s effects on GABA are also influenced by the 5-HT system and can be abolished in the cortex by pre-treatment with the 5-HT2 receptor antagonist ketanserin (Tanganelli et al. 1992) and in the hypothalamus by the 5-HT3 antagonist MDL72222, which alone has no effect on GABA levels (Ferraro et al. 1996b).
Studies showing that MOD prevented MPTP-induced increases in GABA-A receptor binding in the internal globus pallidus (Zeng et al. 2004) have provided evidence for an interaction at the striatal area level GABAergic system in MPTP-treated marmosets. In this study, the authors suggest that the neuroprotection and pro-cognitive effects of MOD are mediated by glutamatergic and GABAergic pathways within the basal ganglia, thus explaining its potential beneficial effects in Parkinson’s disease.
2.6) MOD interactions with the cholinergic systems
The cholinergic system has been linked to brain processes important for cognitive functions, such as attention, learning and mnemonic processes (see, for review: Bentley et al. 2011; Bubser et al. 2012; Furey 2011; Graef et al. 2011; Klinkenberg et al. 2011; Sarter and Paolone 2011; Terry 2006). However, only a few studies have been performed to test if the effects of MOD in behavioral procedures involving cognitive function are mediated or modulated by the brain cholinergic system.
In a report by Waters and colleagues (2005), it was shown that MOD treatment did not affect attentional processes in normal awake rats. The experiments were conducted with the 5-choice serial reaction time task, a preclinical model extensively used for studying several behavioral/brain processes related to cognition and attention (Robbins 2002). In the same report the authors also found no evidence for improvement in response control by MOD, which instead seemed to facilitate impulsive responding under conditions of increased attentional load. Moreover, in this model MOD failed to improve performance deficits induced by scopolamine, an antagonist of muscarinic acetylcholine receptors, suggesting that MOD does not directly interact with the cholinergic system (Waters et al. 2005). Indeed, at doses that significantly reduce cortical GABAergic outflow MOD does not interact with cortical acetylcholine release (Tanganelli et al. 1992). It is also of interest to note that brain neurotransmitter systems that are affected by MOD administration, such as the 5-HT system (de Saint Hilaire et al. 2001; Ferraro et al. 2000; Ferraro et al. 2002), might interact with cholinergic neurotransmission. As suggested above, though 5-HT may have direct effects on cognitive function (Meneses 1999), it may also indirectly produce specific behavioral actions by interacting with the cholinergic system (Cassel and Jeltsch 1995; Sirvio et al. 1994). Indeed, 5-HT neurotransmission has been involved in several physiological and neurological processes in different brain regions including, for example, basal forebrain nuclei, hippocampus, striatum and selected cortical areas. Thus, 5-HT transmission, indirectly stimulated by MOD, might interact with and influence cholinergic function related to cognition (Cassel and Jeltsch 1995; Ricaurte et al. 1993).
As described above, the wake-promoting actions of MOD might be related to improvement in cognition as a consequence of increased attention. In this regard, it is interesting that among the many neurochemical targets identified that may explain its spectrum of pharmacological actions, it has been recently suggested that MOD increases electrical coupling between cortical interneurons, thalamic reticular neurons, subcoerulear nucleus neurons, and inferior olivary neurons (Beck et al. 2008; Garcia-Rill et al. 2007; Urbano et al. 2007). This action of MOD on GAP-junctions is suggested to be opposite to the actions of anesthetics, which reduce the synchronous activity of neurons (Evans and Boitano 2001; He and Burt 2000). Thus, MOD would increase electrical coupling in the pedunculopontine nucleus, which contains medium and large cholinergic neurons. This finding on one-hand supports a role for these neurons, belonging to the so-called “reticular activating system”, in functions related to the sleep-wake cycle. On the other hand, the increased vigilance/attention due to MOD modulation of neuronal ensemble activities and the possibility of a rhythmic firing under a cholinergic input control (Beck et al. 2008; Garcia-Rill et al. 2007; Urbano et al. 2007) suggest an important new modality of MOD interaction with a neurotransmitter and in a brain area that play a direct role in cognitive function and dysfunction.
2.7) MOD interactions with histamine neurotransmission
Histamine has long been known to trigger peripheral actions like allergic reactions and gastric acid secretion. The discovery of histamine as a neurotransmitter released by brain histaminergic neurons onto its receptors has completely changed the landscape around the physiological and therapeutic importance of the histaminergic system (Tiligada et al. 2011). Histamine neurotransmission is mediated by three main receptor subtypes, which differ in pharmacology, localization (selected mesolimbic areas, but also pre- and post-synaptic), and intracellular responses (Leurs et al. 1995; Raddatz et al. 2010). Histamine regulates basic homeostatic and higher brain functions, including cognition, arousal, circadian and feeding rhythms (See for review: Blandina and Passani 2006; Passani et al. 2007). Histaminergic brain neurons are exclusively located in the hypothalamic tuberomammillary nucleus (TMN) (Tiligada et al. 2011).
MOD’s effects on brain histamine levels and neurotransmission have been the focus of several reports. For instance, evidence that MOD could induce c-Fos expression in the TMN (Scammell et al. 2000), where histaminergic cell bodies are located, suggests that MOD increases histamine transmission in this area. Moreover, several studies reported that systemic MOD administration would increase histamine release in hypothalamic areas in anesthetized (Ishizuka et al. 2003), but also in freely-moving rats (Ishizuka et al. 2008; Ishizuka et al. 2010). It is however noteworthy that Ishizuka and colleagues (2003) were not able to demonstrate a significant stimulation of histamine levels when MOD was directly infused locally into the TMN at a concentration of 1 nM. This lack of local effect on TMN histamine neurons might indicate that when injected systemically MOD is likely stimulating the increase in histamine levels through activation of an indirect pathway. In agreement with these observation, and in favor of a possible downstream effect, in the same report it was shown that MOD does not bind to histamine receptors (Ishizuka et al. 2003). The authors suggest the orexin system as a possible mechanism of MOD actions on the histamine system, and indeed, the same research group (Ishizuka et al. 2010) later found that MOD increased hypothalamic histamine release and c-Fos expression of the TMN in wild-type mice, but not in genetically-modified orexin neuron-deficient mice. These findings indicate that MOD-induced increases in histamine release may require intact orexinergic neurons (see for review: Ishizuka et al. 2012). However, how MOD interacts with the orexin system responsible for the activation of histamine neurons and in turn for the increase in hypothalamic histamine release is not known, since MOD does not directly interact with orexin receptors. An indirect action through the glutamatergic system has been suggested (Rao et al. 2007), but more studies are necessary to better understand the pharmacological relevance of this interaction.
Ishizuka and colleagues (2012), have suggested an alternative mechanism to explain MOD effects on histamine neurotransmission. This complex mechanism would involve MOD-induced decreases in GABA levels in the ventral preoptic area, where Scammell et al (2000) observed a MOD-induced decrease in C-Fos-like immunohistochemistry due to inhibition of GABA neurons. These GABA neurons, in turn, promote inhibition of activity of TMN histaminergic neurons (Sherin et al. 1998), and a reduction in their inhibitory activity would result in increased activity of TMN neurons and increased levels of histamine in TMN-histamine terminal areas. Regardless of mechanism, histamine levels are only slightly increased in the presence of MOD and may not be a major contributor to its cognitive enhancing effects. For example, other cognitive enhancing agents, such as methylphenidate that share mechanisms of action e.g. DAT inhibition, do not induce histamine release (Ishizuka et al. 2012).
3) Effects of MOD on brain areas involved in cognitive processes
Several brain areas, identified through modern imaging technologies, are activated by MOD even though MOD does not have sufficient affinity to bind and activate amine receptors in those regions (Loland et al. 2012). However, it is apparent that MOD affects the activity of several neurotransmitters without directly interacting with their specific receptors (see chapter above), and as suggested by several authors, electronic coupling and neuronal synchrony effects might explain some of these activities (Garcia-Rill et al. 2007; Urbano et al. 2007). To this end, it is interesting that MOD administration, even at very high doses, specifically activates selected brain areas, as shown by Engber and colleagues (1998b). In that report the effects of MOD (300 mg/kg) were related to a selective increase in c-Fos-like immune-reactivity on a limited set of brain targets: central nucleus of the amygdala (ACe), paraventricular nucleus of the hypothalamus, and the anterior hypothalamus. This activation of neurotransmission suggests a distinct action profile of MOD as compared to the typical psychostimulant amphetamine administered at a dose of 5 mg/kg showing similar effects on wakefulness (Engber et al. 1998b). Amphetamine, in addition, could activate frontal cortex, striatum, lateral habenula and basolateral amygdala. Compared to MOD, these amphetamine effects suggest a broader, less selective activation, which includes brain regions belonging to the basal ganglia, a large brain structure that includes all of the striatal areas (caudate, putamen, NAC), substantia nigra, and subthalamic nucleus. The basal ganglia play important roles in motor function, cognition, learning and reward-related behaviors (Afifi 2003; Brooks 1995; Grahn et al. 2009).
In a different study, using 2-deoxyglucose (2-DG) utilization as a measure of synaptic activity, Engber and colleagues (1998a) confirmed the broader activation with amphetamine, monitoring altered metabolic rates in regions of the basal ganglia, frontal cortex, NAC, ventral tegmental area, pontine reticular fields, and other nuclei of the thalamus. In the same report MOD but not amphetamine administration increased 2-DG utilization in the ACe. Thus, while not directly affecting areas related to regulation of sleep and wakefulness MOD affected brain areas interconnected to those, and at variance with amphetamine, MOD had no effect on regions involved in controlling motor function. In agreement with this, a systemic administration of a low dose of MOD, 75 mg/kg, increased the induction of c-Fos-like immunochemistry in the lateral division of the ACe, as well as in the latero-dorsal division of the bed nucleus of the stria terminalis (BNST) (Scammell et al. 2000), an integral region of the so-called “extended Amygdala” (Alheid and Heimer 1988). This latter brain structure has been defined from neuroanatomical data and functional observations, and is represented by several interconnected brain regions, the BNST, the ACe, the NAC shell, and the sublenticular substantia innominata. This brain structure plays a fundamental role in rewarded, motivated behaviors (See for example: Koob 1999), and activation of this area by MOD might play a role in its suggested therapeutic efficacy as a psychostimulant medication (see chapter 4).
A more recent observation by Gozzi and colleagues (2012) showed that a strong activation of fronto-cortical areas involved in higher cognitive function could be found upon MOD administration in rats at doses producing plasma level concentrations resembling those found to be efficacious in clinical studies. Thus, even though this study was performed in anesthetized animals, it shows that MOD would be able to activate brain areas related to arousal that are neurobiological targets underlying the wake-promoting and pro-cognitive effects of this drug. In fact, the frontal cortex is proposed to mediate executive functions that are essential in enhancing performance of complex tasks that might comprise of psychological and neurological processes (See for review: Chudasama and Robbins 2006; Dalley et al. 2004). Control of cognitive processes and attention could also be modulated by subcortical catecholamine systems (Aston-Jones and Cohen 2005; Robbins 2005). For instance, the locus coeruleus (LC) and the ventral tegmental area exert a gating function on cortical ensembles arising from projecting systems involved in cognitive processing (Aston-Jones and Cohen 2005). Cortical function can be regulated by LC neurons, and disturbances in these systems are central to major psychiatric disorders, such as schizophrenia, depression and bipolar disorders (Aston-Jones and Cohen 2005). Recently Minzenberg and Carter (2008) demonstrated that MOD shifts the function of the LC-NE system toward a low-tonic/high-phasic pattern of activity to optimize task performance. In a follow up to that study, Minzeberg and colleagues (2011) tested the hypothesis that MOD could modulate the default mode network (DMN). The DMN is a distributed network of functionally connected cortical regions identified in functional neuroimaging studies (Minzenberg et al. 2011). It has been found that by increasing the task-induced deactivation in the DMN, MOD would also increase the speed of sensorimotor processing (Minzenberg et al. 2011). These effects are believed to be dependent from changes in vmPFC activity. The importance of changes in this brain region comes from its particular structure, in which there are projections from several neurotransmitter systems, including catecholamine systems, and output connections able to modify and control the function of many subcortical areas, including the basal ganglia (Gu 2002).
In another related and recent clinical study, Rasetti and colleagues (2010) examined the effect of a chronic low-dose of MOD (100 mg/day for 1 week) on emotion information-processing as well as cognitive information processing circuits in healthy volunteers. The authors suggest that MOD improved the efficiency of cognitive information processing mediated by the frontal cortex, while reducing external influences from anxiety-like stimuli by dampening reactivity of the amygdala, without causing any changes in blood pressure or heart rate.
4) MOD as treatment for substance abuse and addiction
Several studies now indicate that substance abusers can suffer from significant cognitive deficits that either preceded or are caused by chronic substance abuse (Brady et al. 2011). It is well documented that the reinforcing effects of drugs can lead to addiction (Di Chiara et al. 1993; Le Moal and Koob 2007) and that a role for the brain’s DA system is unequivocal. In addition, dopaminergic modulation of emotional responses and behavioral effects (Di Chiara et al. 1998; Wise 2006), can lead to the progression from recreational use to drug dependence, including withdrawal states, craving and relapse (Gould 2010). As noted above, the direct effects of drugs of abuse on the dopaminergic system can have multiple downstream effects on other neurotransmitter systems that can be long term and are integrally involved in both addiction and resulting cognitive deficits. Impairment of attention, memory, abstract reasoning and information processing can all conceivably interfere with treatment success. Hence, understanding neural correlates to these deficits and implementing cognitive enhancement through therapeutic intervention may therefore be beneficial in the treatment strategy for at least some individuals (Anderson et al. 2009; Brady et al. 2011; Heinzerling et al. 2010).
We have discussed the interactions of MOD with the dopaminergic system, and with other brain neurotransmitters involved in different phases of substance use disorders. As shown in Fig. 3, the targets of MOD potentially involved in the treatment of substance abuse are depicted. Here we focus on recent preclinical and clinical reports and on clinical trials related to MOD use as a potential medication for drug-dependence, and as a specific enhancer of cognitive functions in addicted individuals. Moreover, given the limited, if any, abuse liability of MOD, we have reviewed recent reports in which it has been successfully substituted for psychostimulant drugs, like methylphenidate or amphetamine, both of which are more likely to be abused in therapies where a psychostimulant is required.
Figure 3.
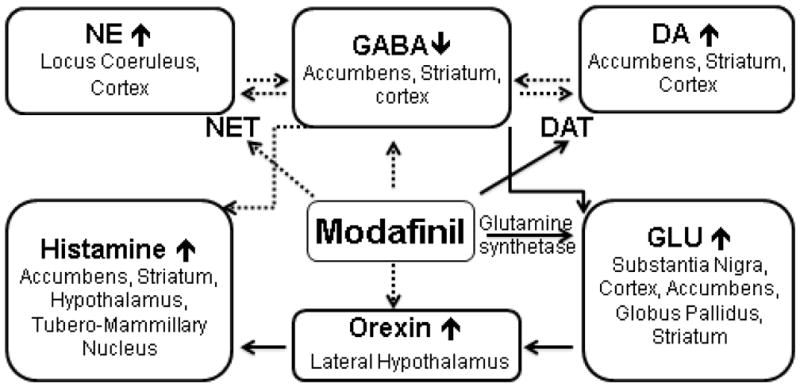
Targets of MOD’s actions as a potential medication for substance use disorders.
Brain areas and related neurotransmitter systems that are potentially involved in mediating the actions of MOD related to its potential therapeutic effects on psychostimulant and opiate abuse. NE= norepinephrine; DA= Dopamine; GABA= gamma-amino-butyric-acid; GLU= glutamate; NET= norepinephrine transporter; DAT= dopamine transporter.
Solid lines indicate direct interactions, while dashed lines indicate observed effects that appear to be via indirect interactions or for which a mechanism has not yet been elucidated.
4.1) MOD as a potential medication for psychostimulant abuse
Several interesting clinical reports of testing MOD’s effects in addicted individuals have been published in the last decade, with equivocal findings about its ability to influence drug-taking, drug-craving etc. (Brady et al. 2011; Shearer et al. 2009). For example, the subjective effects of MOD have been studied in human cocaine-using volunteers, trained to recognize the cocaine discriminative stimulus (150 mg, orally) from placebo (Rush et al. 2002b). The results showed that MOD did not share subjective effects with cocaine in the therapeutic dose-range of (200–600 mg, orally), while in the same study methylphenidate (60 mg) fully generalized to the cocaine cue. Similar results were obtained by other researchers when comparing subjective and physiological effects of MOD with amphetamine (Warot et al. 1993) or methylphenidate (Jasinski 2000). These results point toward a unique mechanism of action for MOD as compared to other psychostimulants, even though all of these drugs interact in humans with the DAT (Spencer et al. 2010; Volkow et al. 2009).
It is important to note, however, that even though the subjective effects of MOD did not overlap with those of other known psychostimulants, it has been reported to elicit dose-dependent subjective-rated measures of “Like the drug”, and “High” (Jasinski 2000). Further, in a more recent report MOD’s reinforcing effects have been studied in a double blind fashion in healthy volunteers not reporting any use of illicit substances at the time the research took place (Stoops et al. 2005). A progressive-ratio procedure for earning MOD capsules was studied under two different conditions (a relaxation and a performance condition). In this study, MOD elicited dose-related reinforcing effects, measured as increases in break point, under performance, but not relaxation conditions. In both subject groups, however, MOD elicited similar stimulant-like, subject-rated effects. Thus, based on the results of this report, the authors conclude that MOD might have abuse potential under specific circumstances and especially in healthy subjects (Stoops et al. 2005). A prosperous black market for MOD and other stimulants used as cognitive enhancers (Cakic 2009; Chatterjee 2004; 2007), facilitated by their availability online (Randall et al. 2005), might also raise concerns about the abuse-liability of these substances (Partridge et al. 2011).
To date there are no reports describing MOD-induced intoxication, dependence or withdrawal (Myrick et al. 2004). This could be a result of its physico-chemical properties, i.e. poor water solubility - it is only administered orally - and its instability at high temperatures, thus preventing diversion to routes of administration (e.g. smoking or injecting) that might increase its delivery time and concentration to the brain (Jasinski and Kovacevic-Ristanovic 2000). Indeed, the chemical structure of MOD (Fig. 1) has been identified as a factor in its unique pharmacological profile as well as providing a challenge to in vivo investigation (Cao et al. 2010; Loland et al. 2012).
Taken together these reports support the notion that MOD maybe useful as an agonist-like medication for the treatment of stimulant abuse/dependence. For instance, its “pleasant” subjective effects (Jasinski 2000) and its longer duration of action, which is especially true for R-MOD (Robertson and Hellriegel 2003), might improve compliance, a significant challenge in treatment of substance abusing individuals (Loland et al. 2012; Sofuoglu et al. 2013). Several clinical trials using MOD for both methamphetamine and cocaine users have been reported (Brady et al. 2011). For example, a clinical report on non-treatment seeking, cocaine-using volunteers showed how MOD, administered for 5 consecutive days (200, 400, or 800 mg orally) significantly reduced the effects of randomized blinded infusion of cocaine (20 or 40 mg IV) (Malcolm et al. 2006). Similarly, Hart and colleagues (2008), showed that under controlled laboratory conditions, during 48 days of study, MOD (200 and 400 mg/day) could markedly attenuate the subjective-effect ratings of smoked cocaine self-administration. MOD (400 mg/day), associated to a biweekly cognitive psychosocial behavioral therapy in a double-blind trial in cocaine-dependent subjects (n=62), was more efficacious than placebo in significantly increasing the number of negative benzoyl-ecgonine (cocaine metabolite) urine samples, and in maintaining protracted periods of cocaine abstinence (Dackis et al. 2005). A more recent trial was performed by the same research group in a larger number of cocaine-dependent subjects (n=210). While this study confirmed a very good safety and tolerability use of MOD during the 8 weeks of its administration, providing adverse events in <5% of subjects, it failed to confirm the previously reported significant differences between MOD and placebo over the same measures/effects of cocaine abstinence (Dackis et al. 2012). In the latter study MOD treatment was combined with only once a week (instead of biweekly) cognitive/behavioral therapy, a factor that might have influenced the outcome. Also, though without statistical significance, the authors suggested a positive trend in some of the measures taken, including higher odds of attaining abstinence from cocaine. Moreover, when data were elaborated by gender, the male subjects (n=155) showed an almost (p=0.06) significant difference between MOD 400 mg and placebo treatments, in respect to odd ratios for abstinence, thus suggesting that MOD might be efficacious in attaining cocaine abstinence under specific conditions.
Several clinical studies have also explored the potential effects of MOD on different stages of methamphetamine dependence. While all of these studies found MOD to be safe and well tolerated in methamphetamine dependent subjects, none of them found statistically significant improvements in urine detection of metabolites, abstinence, depressive symptoms, or craving (Anderson et al. 2012; De La Garza et al. 2010; Heinzerling et al. 2010; Hester et al. 2010; Lee et al. 2013; McGaugh et al. 2009; Shearer et al. 2009). Some of these reports are placebo controlled trials, like for example in the study by Heinzerling and colleagues (2010) who did not find significant differences between MOD (400 mg/day) and placebo effects in methamphetamine dependent, treatment seeking subjects (n=71) over several measures of methamphetamine use, retention, depressive symptoms, and craving. Other reports were performed without a placebo control, or with a limited number of subjects. Most if not all of these reports show interesting trends of MOD toward a significant effect on reduction in methamphetamine use or methamphetamine effects. In addition, Anderson and colleagues (2012) recently reported that ad hoc analysis of medication compliance wherein a significant improvement in maximum duration of abstinence in those subjects who were in the top quartile for compliance was demonstrated. This study not only provides positive results but underlines clinical trial challenges that must be addressed in the future with better biomarkers for medication compliance.
A recent preclinical report shows how MOD treatment associated with behavioral training to extinction, blocked the reinstatement of extinguished opiate-seeking behavior in rats (Tahsili-Fahadan et al. 2010), but only at a 300 mg/kg dose. Interestingly, this anti-reinstatement effect of MOD could be fully suppressed by the selective mGlu2/3R antagonist LY 341495 (Tahsili-Fahadan et al. 2010). These effects led the authors to hypothesize that MOD predominantly increased extrasynaptic glutamate levels especially in the NAC, which would result in preferential stimulation of extrasynaptic mGlu2/3Rs, due to a concomitant downregulation of mGlu1/5Rs that occurred during the extinction training (Knackstedt et al. 2010). This effect of MOD is specific for reinstatement of opiate-seeking behavior in animals trained to extinction, since it does not affect the expression of morphine preference and the reinstatement of opiate-seeking behavior in animals not trained to extinction (Tahsili-Fahadan et al. 2010).
4.2) MOD effects on cognitive function in substance abusers
Based on reports describing the concurrence of cognitive dysfunction in the addicted population, several clinical procedures and preclinical animal models have been developed to study cognition and especially neurocognitive deficits produced by acute and chronic administration of abused substances (Beveridge et al. 2008; Garavan and Hester 2007; Nic Dhonnchadha and Kantak 2011; Recinto et al. 2012; Verdejo-Garcia et al. 2012).
Cognitive function may be of particular relevance in understanding neuro-behavioral mechanism/s underlying the transition from recreational to dependent use, withdrawal and relapse (Garavan and Hester 2007). Impulsivity and sensation-seeking traits, and a kind of pathological learning are also very well targeted as important factors during this transition to addiction (Di Chiara et al. 1999; Ersche et al. 2010; Jupp and Dalley 2013; Le Moal and Koob 2007). Thus, it is not surprising that drug addicted individuals display neurobiological impairments in those brain circuits required for normal decision-making and cognitive function (Garavan and Hester 2007). As indicated by Gould (2010) the brain regions and the neural processes related to cognitive functions (including learning, memory, and reasoning) overlap extensively with those that are involved in mediating drug abuse and addiction processes (Gould 2010) see Figs. 2 and 3. For example, it has been shown that continued drug use, i.e. methamphetamine or cocaine, may cause cognitive impairment or deficits, especially in the young, wherein the developing brain is particularly susceptible to the effects of drugs of abuse. Thus, alteration of cognitive functions can be found as a result of long-lasting changes produced by exposure to drugs of abuse in early stages of life (Gould 2010; Porter et al. 2011). For example, repeated drug use may elicit disruption of frontal cortex processes known to regulate and control cognitive activities such as decision-making, response inhibition, planning and memory, as indicated by brain-imaging studies in humans and neuro-behavioral studies in experimental animals (Gould 2010; Jupp and Dalley 2013). Therefore, the emerging emphasis on cognitive impairment in neuropsychiatric disorders, including addiction (Brady et al. 2011) has stimulated investigation into the potential pro-cognitive effects of MOD in this population (Ghahremani et al. 2011; Rasetti et al. 2010; Turner et al. 2003). It has been repeatedly shown in both clinical and preclinical studies that MOD may serve as a useful adjunct to behavioral therapies in the treatment of drug dependencies.
Deficits in cognition and prefrontal cortical functions have also been described in methamphetamine- (MA) dependent individuals (Monterosso et al. 2005; Salo et al. 2002). In a recent report on a limited number of MA-dependent, non-treatment seeking subjects, a three-day 400 mg MOD treatment significantly improved working memory in those individuals showing poor performance at baseline, but not in those with relatively higher performance at baseline (Kalechstein et al. 2010). The same research group more recently (Kalechstein et al. 2013) showed that a lower dose of MOD, 200 mg, administered daily for five days in a larger group of cocaine-dependent individuals could improve performance in two specific sets of working memory measures tested immediately after washout. MOD improved learning by enhancing neural functions in brain regions related to learning and cognitive control, suggesting that MOD may be a suitable pharmacological adjunct for enhancing the efficacy of cognitive-based therapies for psychostimulant-dependence (Ghahremani et al. 2011). Therefore, medications that improve cognition in these subjects may also improve the success of therapy for their addiction. It should be noted that in the cigarette smoking population, where cognitive deficits have been consistently found in young and older smokers (Chamberlain et al. 2012; Durazzo et al. 2012), MOD does not seem to ameliorate these deficits (Schnoll et al. 2008). In a randomized placebo-controlled trial for smoking cessation in 157 treatment-seeking tobacco smokers, MOD administered at a dose of 200 mg daily for 8 weeks did not increase abstinence from smoking more than placebo (Schnoll et al. 2008). Moreover, MOD worsened withdrawal conditions and affect, suggesting it might not be a drug of choice in smoker population with comorbid psychiatric disorders. It is interesting that MOD lacks any positive effects in this population, where the cognitive deficits might appear as a result of long term exposure to nicotine, an agonist at Ach-nicotinic receptors (Chamberlain et al. 2012; Durazzo et al. 2012). It is then plausible that neural adaptations of Ach-nicotinic receptors render this patient population less likely to respond to treatment with MOD, even though no direct link between Ach receptors and MOD has been reported (see section 2.6 above) Thus, the neurobiology underlying dependence from different classes of drugs may be a factor in MOD actions, and for this reason, more studies on this topic may be warranted.
In this regard, the suggestions of therapeutic applications for MOD coming from the well-known link between patients with mental illnesses (schizophrenia, bipolar disorder, depression) impairing cognition functions and their high risk for substance abuse (see for example: Fenton et al. 2012; O’Brien et al. 2004; Pulay et al. 2009) deserve more research at both clinical and preclinical leves. Certainly an additional adverse impact on cognition, due to chronic substance abuse would be particularly deleterious in combination with pre-existing cognitive problems characteristic of such mental disorders (Gould 2010).
4.3) MOD as a potential and safer alternative to other psychostimulant medications
One of the most interesting aspects of MOD pharmacology stands in its ability to occupy the DAT (Volkow et al. 2009), and to increase DA levels in dopaminergic brain terminal areas (Loland et al. 2012; Volkow et al. 2009), without apparent consequence for abuse (Deroche-Gamonet et al. 2002; Myrick et al. 2004).
We have discussed the importance of the physico-chemical characteristics of MOD that might reduce its propensity for abuse (Jasinski and Kovacevic-Ristanovic 2000). There might be, however, other important pharmacological targets that play a significant role in this apparent lack of abuse liability. Among these targets, histamine and orexin/hypocretin neurotransmission have been repeatedly shown to be involved in modulation of the reinforcing effects of drugs (Boutrel et al. 2013; Brabant et al. 2010). We have also discussed their involvement in the cognitive effects of MOD. A precise role for increased histamine neurotransmission in drug abuse and in the reinforcing effects of MOD is not clear. There are known effects of specific histamine receptors and their antagonist ligands, especially for the H3 receptor, in modulating the reinforcing and behavioral actions of psychostimulants (Campbell et al. 2005; Ellenbroek 2013; Munzar et al. 2004; Tanda et al. 2008). Also, like MOD there have been suggestions that amphetamines too might increase brain histamine transmission (Munzar et al. 2004). However, it is of interest to note that histamine H3 inverse agonists/antagonists like MOD show specific effects on arousal and attention, are devoid of abuse liability, and have been clinically tested and found efficacious as narcoleptic or ADHD medications (Uguen et al. 2013; Weisler et al. 2012). Similarly, the orexin/hypocretin system has been recently demonstrated to modulate and participate in brain mechanisms and reinforcing actions of drugs abused by humans (Hollander et al. 2012; see for review: Mahler et al. 2012b). For example, orexin antagonists have been shown to negatively modulate the reinforcing efficacy and behavioral effects of several drugs of abuse, suggesting that orexin transmission plays a facilitating role in reward-related behaviors, including reinstatement of drug intake in abstinent animals (Mahler et al. 2012b). MOD has been suggested to increase orexin release through an unknown mechanism (see the already discussed papers by Ishizuka et al. 2010; 2012). An indirect action on LH orexin neurons through MOD-induced LTP in glutamategic synapses has been reported (Rao et al. 2007). Interestingly, this effect is suppressed by pharmacologic blockade of the melanin concentrating hormone (MCH) or is not present in genetically modified mice lacking the MCH-1 receptors (Rao et al. 2008). Even more interesting is the evidence that suggests the MCH system is involved in cocaine abuse and in natural and drug reinforcement (Chung et al. 2009; Karlsson et al. 2012). Thus, while it is not clear how an increase in orexin neurotransmission might be responsible, at least partially, for the reduced abuse liability of MOD, it is possible that complex interactions among several pharmacological targets have a role in this effect. For instance, when taken together, the histaminergic and orexinergic properties of MOD might play a primary role in its adjunct therapeutic effects in specific populations of drug abusers, where cognitive dysfunction is evident. Also, this might be true for treating those brain disorders that benefit from associating psychostimulant and cognitive therapies, for example ADHD or specific substance use disorders (Brady et al. 2011).
Alteration of normal DAT function is related to a number of neurological disorders that are commonly treated with psychostimulant drugs. For example, the most commonly used medications in the treatment of ADHD in children and adults are methylphenidate and amphetamine, both of which function primarily through the DAT to express their therapeutic actions. However, misuse of prescription ADHD medications in the U.S. is very common, especially among young adults, 18 to 25 years of age (See for example Sweeney et al. 2013). The question is, can the psychostimulant actions of MOD efficaciously substitute for the therapeutic effects of amphetamine or methylphenidate in ADHD patients? The answer to this question comes from several trials and reports showing positive effects of MOD treatments in the therapy of ADHD. For example, MOD has been tested as an ADHD medication in comparison to amphetamine, in adult-subjects who met DSM-IV criteria for ADHD (Taylor and Russo 2000). Results from this report show improvement for both MOD and amphetamine vs. placebo in several symptoms related to ADHD. The authors conclude that these results support MOD as a reasonable alternative to the more addictive psychostimulants for treatment of ADHD. Several other reports and meta-analyses show mostly positive effects and efficacy of MOD as a treatment for ADHD (Faraone and Glatt 2010; Lindsay et al. 2006; Mann and Bitsios 2009; Turner 2006; but see: Wilens et al. 2011), thus making it a potentially safer candidate for this therapeutic application.
An extension of the concept that MOD might also be useful in the treatment of other disorders typically treated with methylphenidate or amphetamine comes from its military use. It is well known that veterans from war operations are at high risk for post-traumatic stress-disorders and for developing other mental illnesses (see for example: Seal et al. 2011). Moreover, compared to active duty forces, veterans might also be at higher risk for alcohol and substance use disorders. We are not aware of the potential role that psychostimulants used during combat operations might have on this specific population. However, since MOD has not been shown to be abused or to elicit withdrawal signs after its repeated use (Martinez-Raga et al. 2008), the possibility to substitute psychostimulants showing higher abuse liability with a safer compound makes MOD a very promising candidate for use in military settings where sleep deprivation is common. This is especially true since one of the important features of MOD is to promote wakefulness without preventing sleep, if this opportunity is available (Batejat and Lagarde 1999). Thus, a random combination of periods of sleep-deprivation, for example, during combat operations, and an opportunity for periodic naps would make MOD the drug of choice. As discussed above, the recent discovery that histamine H3 receptor inverse-agonist/antagonist are safe and clinically efficacious anti-narcoleptic therapies (Uguen et al. 2013), suggest that safe histaminergic enhancing effects of MOD may contribute to its therapeutic wake-promoting effects.
During the last 10–15 years MOD has been tested as an alternative to d-amphetamine by aircrews of the United States military services during helicopter flight operations (Estrada et al. 2012), and its use for sustained military operations has been tested in several studies (Batejat and Lagarde 1999; Buguet et al. 2003; Caldwell et al. 2000). In one of these studies MOD was administered at high doses, 3 × 200 mg of MOD/day, and it was shown that helicopter pilots maintained their alertness and accuracy at pre-deprivation sleep-levels, after 40 hours without sleep (Caldwell et al. 2000). In the same study MOD elicited some side effects, dizziness, nausea, and vertigo that, as also suggested by Buguet and colleagues (2003) were likely the result of a too high dose of MOD. Indeed, when compared to the effects of d-amphetamine in sustained military flight operations (Estrada et al. 2012), administration of MOD was well tolerated at 3 × 100 mg doses given every 4 hours. In the same report MOD performed very well as a d-amphetamine alternative counteracting the sleep deprivation effects (40 hours without sleep) on mood and cognition (Estrada et al. 2012). In a different report by Whitmore and colleagues (2006) the effects of MOD (400 mg/day) were studied on military personnel during a prolonged, 88-hour sleep deprivation to simulate military ground operations. In this report subjects receiving MOD treatment did not maintain a better state of alertness and performance compared to subjects treated with placebo. While this dosage has been suggested by Buguet and colleagues (2003) as a safe and efficacious, Whitmore and colleagues (2006) concluded that the complete sleep loss could not be counteracted by the low dose of MOD employed (Whitmore et al. 2006).
Based on these reports, MOD appears to be a superior medication for improving performance upon sleep deprivation as compared to methylphenidate or amphetamine that are more likely to be abused by the general population. This is even more important in certain populations such as young adults that may be more vulnerable to develop substance use disorders, or military personnel that might show a higher likelihood to develop post-traumatic stress disorders, and comorbid alcohol or drug abuse (Seal et al. 2011).
5) Conclusions
MOD appears to have multiple effects on different brain areas and neurotransmitter systems in the brain. MOD activates different circuits and brain areas as compared to amphetamine and methylphenidate, while sharing inhibition of dopamine reuptake as a common mechanism underlying its pharmacological effects. Indeed, intriguing preliminary findings suggest that MOD may activate cortical over subcortical areas in the brain and might produce cognitive improvement in a number of neurological and psychiatric disorders with relatively low, if any, abuse liability. Thus, MOD and its longer acting R-enantiomer, R-MOD, offer significant potential as cognitive enhancers suggesting alternative therapies for ADHD and other neuropsychiatric disorders (Loland et al. 2012). Recent research identified several non-dopaminergic effects of MOD, such as the increase of electrical neuronal coupling, or the enhancement of histamine and orexin neurotransmission that might be of primary importance to explain its efficacy as a wake-promoting and cognitive enhancing medication, even in non-sleep deprived individuals. Moreover, its ability to selectively activate specific brain areas, like the extended amygdala and the lateral hypothalamus would likely contribute to its unique pharmacological profile and to its efficacy as a psychostimulant medication. Indeed, the reviewed clinical literature has shown that MOD may hold promise as a medication to treat psychostimulant-induced cognitive dysfunctions and also as an adjunct to behavioral therapies for psychostimulant abuse. Additional clinical studies need to be designed to better understand how and to what extent MOD improves cognitive performance. Understanding the mechanism/s underlying the therapeutic efficacy of MOD will help to better address the needs of multiple patient populations affected by cognitive disorders, including substance abuse.
Table N. 1.
Clinical studies testing the reinforcing/subjective effects of MOD or its effects as a treatment for substance use disorders
| Number and information on subjects | Experimental information, measures taken | MOD Treatment, Dosage, route, length of study | Main effect of the treatment | Secondary effect of the treatment | reference |
|---|---|---|---|---|---|
| 6 cocaine-using volunteers | Discrimination of subjective effects of cocaine 150 mg PO | 200–600 mg PO | No significant cocaine-like subjective effect (but MOD fully substituted for cocaine in 3 subjects) | (Rush et al. 2002b) | |
| 9 cocaine-using volunteers | Placebo controlled Self reported drug (cocaine 100–300 mg PO, MOD 200–600 mg PO) effects | 200–600 mg PO | MOD did not show significant induction of psychoactive effects | (Rush et al. 2002a) | |
| 16 healthy volunteers, no history of drug abuse | Subjective effects of amphetamine (15 mg PO) caffeine (300 mg PO) and placebo | 300 mg PO | No significant induction of amphetamine-like psychoactive effects | (Warot et al. 1993) | |
| 24 male volunteers with polysubstance abuse history | Double blind placebo controlled subjective effects of methylphenidate, 45–90 mg PO | 200, 400 or 800 mg PO | Lack of amphetamine-like subjective effects reported in responses to the Addiction Research Center Inventory questionnaire | (Jasinski 2000) | |
| 6 Healthy, no drug user volunteers | Reinforcing effects studied in progressive-ratio tests | Significant dose-related reinforcing effects under performance conditions | No effects under relaxation condition | (Stoops et al. 2005) | |
| 12 Non-treatment seeking cocaine-using volunteers | Interaction with cocaine 20–40 mg i.v. | 5 days 200, 400, 800 mg PO | Significant reduction in cocaine randomized blinded infusion | (Malcolm et al. 2006) | |
| 8 Non-treatment seeking cocaine-dependent volunteers | Subject-rated effects of smoked cocaine | 48 days, 200 and 400 mg/day PO | Attenuation of subjective ratings of smoked cocaine | (Hart et al. 2008) | |
| 62 cocaine-dependent subjects | Double blind placebo controlled tests on cocaine abstinence, craving, withdrawal | 400 mg daily over 8 weeks + bi-weekly cognitive behavioral therapy | Significant higher number of patients with cocaine-negative urine-samples, and more likely to achieve longer periods of abstinence compared to placebo | No serious adverse events, full patients compliance | (Dackis et al. 2005) |
| 210 cocaine-dependent subjects | Outpatient double blind placebo controlled tests on cocaine abstinence, craving, withdrawal | 200–400 mg daily over 8 weeks, + weekly cognitive behavioral therapy | None of the measures taken were significantly different from placebo. There were gender differences; male subjects receiving 400 mg were more abstinent than women | (Dackis et al. 2012) | |
| 210 treatment seeking cocaine dependents | Double blind placebo outpatient facilitation of cocaine abstinence | 200 or 400 mg/day PO for 12 weeks, 4 weeks follow-up | No significant difference in cocaine free days between MOD and placebo | Significant increase in the MOD 200 mg group for increasing cocaine free days and reducing cocaine craving in individuals without a history of comorbid alcohol abuse | (Anderson et al. 2009) |
| 210 treatment seeker MA dependents | Double blind placebo controlled efficacy in decreasing MA use | 200 or 400 mg/day PO for 12 weeks, 4 weeks of follow up | No significant difference in drug-free days between MOD and placebo | Study was found inconclusive due to inadequate compliance in the subjects taking MOD | (Anderson et al. 2012) |
| 13 non treatment seeking MA dependent volunteers | Placebo controlled subjective effects of MA | 200 mg PO for 3 days | Non significant trend toward reduction of MA-induced “high” and “want MA” | (De La Garza et al. 2010) De La Garza 2010 | |
| 62 cocaine dependent subjects | Double blind outpatient placebo controlled cocaine abstinence | 400 mg daily combined with psychosocial behavioral therapy for 8 weeks | Significant increase in cocaine consecutive abstinent days | (Heinzerling et al. 2010) | |
| 13 MA-dependent subjects | Neuropsychological performance tests during Double blind inpatient placebo controlled MA abstinence | 200 mg daily | Improvement shown only in one measure, immediate verbal memory recall. | Confirmed previous evidence of cognitive deficits in active and abstinent MA users | (Hester et al. 2010) |
| 19 MA-dependent subjects | Treatment retention, severity of withdrawal and physiological functions during double blind inpatient placebo controlled MA abstinence | 200 mg daily, days 1–5, 100 mg days 6 and 7 | No significant differences in any measure taken between MOD and placebo. | (Lee et al. 2013) | |
| 8 MA-dependent subjects | Assessment of Safety and tolerability of MOD during self-reported MA use in an open-label pilot clinical trial | 400 mg daily during 6 weeks + adjunctive contingency management | This dose of MOD was confirmed safe and tolerable. Self-reported use of MA was improved, even though there was no change in amphetamine positive urine analysis. | (McGaugh et al. 2009) | |
| 80 MA-dependent subjects | Assessment of Safety and efficacy in reducing MA use in a double blind placebo controlled trial | 200 mg daily during 10 weeks, + follow up 12 weeks after treatment | Treatment retention and adherence to medication were equal for MOD and placebo groups. MOD showed better outcomes in subjects with only MA dependency than polysubstance users | (Shearer et al. 2009) | |
| 11 MA-dependent individuals | Working memory test performance after washout, placebo-study controlled inpatient | 3 days 400 mg | Significant memory improvement in subjects with poor-performance at baseline | No improvement in subjects with higher-performance at baseline | (Kalechstein et al. 2010) |
| 61 High-dose, long-term cocaine-dependents | Working memory test performance after washout, placebo-controlled inpatient study | 5 days 200 mg and/or escitalopram 20 mg | Significant improvement in several measures of working memory performance; reduced impulsivity. No significant changes were elicited by escitalopram alone or in combination | (Kalechstein et al. 2013) | |
| 157 treatment seeking smokers | Abstinence from smoking in a placebo controlled clinical trial | 200 mg daily during 8 weeks | No difference in abstinence rates between MOD and placebo. Smoking rate and withdrawal symptoms were higher at the end of the study in the MOD compared to placebo group. | Schnoll 2008 |
PO= Oral administration; i.v = intravenous administration; MOD= modafinil; MA= methamphetamine
Acknowledgments
Funding. NIDA Intramural Research Program, NIH/DHHS.
Footnotes
Disclosure/conflicts of interest: All the authors declare no conflict of interest.
References
- Afifi AK. The basal ganglia: a neural network with more than motor function. Semin Pediatr Neurol. 2003;10:3–10. doi: 10.1016/s1071-9091(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Roussel B, Lin JS, Chouvet G, Jouvet M. Effect of modafinil and amphetamine on the rat catecholaminergic neuron activity. Neurosci Lett. 1991;123:20–2. doi: 10.1016/0304-3940(91)90148-m. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Chow CC, Van Bockstaele EJ, Williams JT. Frequency-dependent synchrony in locus ceruleus: role of electrotonic coupling. Proc Natl Acad Sci U S A. 2002;99:4032–6. doi: 10.1073/pnas.062716299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E, Kahn R, Chiang N, Beresford T, Campbell J, Haning W, Mawhinney J, McCann M, Rawson R, Stock C, Weis D, Yu E, Elkashef AM. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–41. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–9. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli T, Ferraro L, Hillion J, Tomasini MC, Rambert FA, Fuxe K. Modafinil prevents glutamate cytotoxicity in cultured cortical neurons. Neuroreport. 1998;9:4209–13. doi: 10.1097/00001756-199812210-00038. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baranski JV, Pigeau R, Dinich P, Jacobs I. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol. 2004;19:323–32. doi: 10.1002/hup.596. [DOI] [PubMed] [Google Scholar]
- Batejat DM, Lagarde DP. Naps and modafinil as countermeasures for the effects of sleep deprivation on cognitive performance. Aviat Space Environ Med. 1999;70:493–8. [PubMed] [Google Scholar]
- Beck P, Odle A, Wallace-Huitt T, Skinner RD, Garcia-Rill E. Modafinil increases arousal determined by P13 potential amplitude: an effect blocked by gap junction antagonists. Sleep. 2008;31:1647–54. doi: 10.1093/sleep/31.12.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94:360–88. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–66. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandina P, Passani MB. Central histaminergic system interactions and cognition. Exs. 2006;98:149–63. doi: 10.1007/978-3-7643-7772-4_8. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Steiner N, Halfon O. The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci. 2013;7:59. doi: 10.3389/fnbeh.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Alleva L, Quertemont E, Tirelli E. Involvement of the brain histaminergic system in addiction and addiction-related behaviors: a comprehensive review with emphasis on the potential therapeutic use of histaminergic compounds in drug dependence. Prog Neurobiol. 2010;92:421–41. doi: 10.1016/j.pneurobio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacol Biochem Behav. 2011;99:285–94. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP. Learning, memory, and transcranial direct current stimulation. Front Psychiatry. 2012;3:80. doi: 10.3389/fpsyt.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioni JD, McGaugh JL. Post-training administration of GABAergic antagonists enhances retention of aversively motivated tasks. Psychopharmacology (Berl) 1988;96:505–10. doi: 10.1007/BF02180032. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. The role of the basal ganglia in motor control: contributions from PET. J Neurol Sci. 1995;128:1–13. doi: 10.1016/0022-510x(94)00206-4. [DOI] [PubMed] [Google Scholar]
- Bubser M, Byun N, Wood MR, Jones CK. Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb Exp Pharmacol. 2012:121–66. doi: 10.1007/978-3-642-23274-9_7. [DOI] [PubMed] [Google Scholar]
- Buguet A, Moroz DE, Radomski MW. Modafinil--medical considerations for use in sustained operations. Aviat Space Environ Med. 2003;74:659–63. [PubMed] [Google Scholar]
- Cakic V. Smart drugs for cognitive enhancement: ethical and pragmatic considerations in the era of cosmetic neurology. J Med Ethics. 2009;35:611–5. doi: 10.1136/jme.2009.030882. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Jr, Caldwell JL, Smythe NK, 3rd, Hall KK. A double-blind, placebo-controlled investigation of the efficacy of modafinil for sustaining the alertness and performance of aviators: a helicopter simulator study. Psychopharmacology (Berl) 2000;150:272–82. doi: 10.1007/s002130000450. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Kopajtic TA, Newman AH, Katz JL. Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs. J Pharmacol Exp Ther. 2005;315:631–40. doi: 10.1124/jpet.105.090829. [DOI] [PubMed] [Google Scholar]
- Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, Newman AH. Structure-Activity Relationships at the Monoamine Transporters for a Novel Series of Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) Analogues. ACS Med Chem Lett. 2010;2:48–52. doi: 10.1021/ml1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel JC, Jeltsch H. Serotonergic modulation of cholinergic function in the central nervous system: cognitive implications. Neuroscience. 1995;69:1–41. doi: 10.1016/0306-4522(95)00241-a. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between Tobacco Smoking and Cognitive Functioning in Young Adults. Am J Addict. 2012;21(Suppl 1):S14–9. doi: 10.1111/j.1521-0391.2012.00290.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Cosmetic neurology: the controversy over enhancing movement, mentation, and mood. Neurology. 2004;63:968–74. doi: 10.1212/01.wnl.0000138438.88589.7c. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Cosmetic neurology and cosmetic surgery: parallels, predictions, and challenges. Camb Q Healthc Ethics. 2007;16:129–37. doi: 10.1017/s0963180107070156. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009;106:6772–7. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. Dopamine. The Biochemical Basis of Neuropharmacology. Oxford University Press; Oxford: 1996a. pp. 293–351. [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford University Press; Oxford: 1996b. Norepinephrine and Epinephrine; pp. 226–292. [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–53. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–11. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43:303–12. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2010;106:173–80. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12:3533–7. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- Dean AC, Sevak RJ, Monterosso JR, Hellemann G, Sugar CA, London ED. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72:943–53. doi: 10.15288/jsad.2011.72.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology (Berl) 2002;161:387–95. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G, Cadoni C. Drugs of abuse: biochemical surrogates of specific aspects of natural reward? Biochem Soc Symp. 1993;59:65–81. [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–85. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Cadoni C, Acquas E, Bassareo V, Carboni E. Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependence. Adv Pharmacol. 1998;42:983–7. doi: 10.1016/s1054-3589(08)60911-4. [DOI] [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–23. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–11. doi: 10.1016/j.drugalcdep.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duteil J, Rambert FA, Pessonnier J, Hermant JF, Gombert R, Assous E. Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 1990;180:49–58. doi: 10.1016/0014-2999(90)90591-s. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA. Histamine H3 receptors. The complex interaction with dopamine and its implications for addiction. Br J Pharmacol. 2013 doi: 10.1111/bph.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engber TM, Dennis SA, Jones BE, Miller MS, Contreras PC. Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience. 1998a;87:905–11. doi: 10.1016/s0306-4522(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV. Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett. 1998b;241:95–8. doi: 10.1016/s0304-3940(97)00962-2. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–3. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada A, Kelley AM, Webb CM, Athy JR, Crowley JS. Modafinil as a replacement for dextroamphetamine for sustaining alertness in military helicopter pilots. Aviat Space Environ Med. 2012;83:556–64. doi: 10.3357/asem.3129.2012. [DOI] [PubMed] [Google Scholar]
- Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans. 2001;29:606–12. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71:754–63. doi: 10.4088/JCP.08m04902pur. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Hunter MD, Haque R, Spence SA. Modafinil and unconstrained motor activity in schizophrenia: double-blind crossover placebo-controlled trial. Br J Psychiatry. 2006;189:461–2. doi: 10.1192/bjp.bp.105.017335. [DOI] [PubMed] [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Fenton MC, Keyes K, Geier T, Greenstein E, Skodol A, Krueger B, Grant BF, Hasin DS. Psychiatric comorbidity and the persistence of drug use disorders in the United States. Addiction. 2012;107:599–609. doi: 10.1111/j.1360-0443.2011.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Beggiato S, Cristina Tomasini M, Fuxe K, Tanganelli S. The vigilance promoting drug modafinil modulates serotonin transmission in the rat prefrontal cortex and dorsal raphe nucleus. Possible relevance for its postulated antidepressant activity. Mini Rev Med Chem. 2013;13:478–92. doi: 10.2174/1389557511313040002. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O’Connor WT, Tanganelli S, Rambert FA, Fuxe K. Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biol Psychiatry. 1997;42:1181–3. doi: 10.1016/s0006-3223(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O’Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135–8. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O’Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–56. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, Tanganelli S, Fernandez M, Rambert FA, Antonelli T. Amplification of cortical serotonin release: a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology. 2000;39:1974–83. doi: 10.1016/s0028-3908(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Fuxe K, Tanganelli S, Tomasini MC, Rambert FA, Antonelli T. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res. 2002;68:107–12. doi: 10.1002/jnr.10196. [DOI] [PubMed] [Google Scholar]
- Ferraro L, O’Connor WT, Li XM, Rimondini R, Beani L, Ungerstedt U, Fuxe K, Tanganelli S. Evidence for a differential cholecystokinin-B and -A receptor regulation of GABA release in the rat nucleus accumbens mediated via dopaminergic and cholinergic mechanisms. Neuroscience. 1996a;73:941–50. doi: 10.1016/0306-4522(96)00098-x. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O’Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996b;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- Fiocchi EM, Lin YG, Aimone L, Gruner JA, Flood DG. Armodafinil promotes wakefulness and activates Fos in rat brain. Pharmacol Biochem Behav. 2009;92:549–57. doi: 10.1016/j.pbb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Furey ML. The prominent role of stimulus processing: cholinergic function and dysfunction in cognition. Curr Opin Neurol. 2011;24:364–70. doi: 10.1097/WCO.0b013e328348bda5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–45. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–14. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007;3:349–64. [PMC free article] [PubMed] [Google Scholar]
- Getova D, Bowery NG. The modulatory effects of high affinity GABA(B) receptor antagonists in an active avoidance learning paradigm in rats. Psychopharmacology (Berl) 1998;137:369–73. doi: 10.1007/s002130050632. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36:950–9. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Colavito V, Seke Etet PF, Montanari D, Fiorini S, Tambalo S, Bifone A, Zucconi GG, Bentivoglio M. Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology. 2012;37:822–37. doi: 10.1038/npp.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef S, Schonknecht P, Sabri O, Hegerl U. Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: an overview of preclinical and clinical findings. Psychopharmacology (Berl) 2011;215:205–29. doi: 10.1007/s00213-010-2153-8. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res. 2009;199:53–60. doi: 10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–35. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–8. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Havekes R, Abel T, Van der Zee EA. The cholinergic system and neostriatal memory functions. Behav Brain Res. 2011;221:412–23. doi: 10.1016/j.bbr.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ Res. 2000;86:E104–9. doi: 10.1161/01.res.86.11.e104. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–9. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18:489–97. doi: 10.1037/a0021791. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol. 2010;639:33–9. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–59. [PubMed] [Google Scholar]
- Hou RH, Freeman C, Langley RW, Szabadi E, Bradshaw CM. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology (Berl) 2005;181:537–49. doi: 10.1007/s00213-005-0013-8. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Murakami M, Yamatodani A. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol. 2008;578:209–15. doi: 10.1016/j.ejphar.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Murotani T, Yamatodani A. Modanifil activates the histaminergic system through the orexinergic neurons. Neurosci Lett. 2010;483:193–6. doi: 10.1016/j.neulet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Murotani T, Yamatodani A. Action of modafinil through histaminergic and orexinergic neurons. Vitam Horm. 2012;89:259–78. doi: 10.1016/B978-0-12-394623-2.00014-7. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339:143–6. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Kovacevic-Ristanovic R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23:149–56. doi: 10.1097/00002826-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–90. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–55. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Jupp B, Dalley JW. Behavioral endophenotypes of drug addiction: Etiological insights from neuroimaging studies. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Newton TF. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am J Addict. 2010;19:340–4. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd, Yoon JH, Bennett R, De la Garza R., 2nd Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology. 2013;64:472–8. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Zook M, Ciccocioppo R, Gehlert DR, Thorsell A, Heilig M, Cippitelli A. Melanin-concentrating hormone receptor 1 (MCH1-R) antagonism: reduced appetite for calories and suppression of addictive-like behaviors. Pharmacol Biochem Behav. 2012;102:400–6. doi: 10.1016/j.pbb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res. 2011;221:430–42. doi: 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–92. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–60. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Klyuch BP, Ponomarenko AA, Lin JS, Haas HL, Sergeeva OA. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology. 2007;52:626–33. doi: 10.1016/j.neuropharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–93. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Lee N, Pennay A, Hester R, McKetin R, Nielsen S, Ferris J. A pilot randomised controlled trial of modafinil during acute methamphetamine withdrawal: feasibility, tolerability and clinical outcomes. Drug Alcohol Rev. 2013;32:88–95. doi: 10.1111/j.1465-3362.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Leurs R, Smit MJ, Timmerman H. Molecular pharmacological aspects of histamine receptors. Pharmacol Ther. 1995;66:413–63. doi: 10.1016/0163-7258(95)00006-3. [DOI] [PubMed] [Google Scholar]
- Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–26. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- Lindsay SE, Gudelsky GA, Heaton PC. Use of modafinil for the treatment of attention deficit/hyperactivity disorder. Ann Pharmacother. 2006;40:1829–33. doi: 10.1345/aph.1H024. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G, Newman AH. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72:405–13. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–9. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, Lalumiere RT, Thomas C, Fallon RV, Kalivas PW, Aston-Jones G. Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addict Biol. 2012a doi: 10.1111/j.1369-1600.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012b;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris AP, Rush CR, Frederich RC, Taylor AC, Kelly TH. Behavioral and subjective effects of d-amphetamine and modafinil in healthy adults. Exp Clin Psychopharmacol. 2007;15:123–33. doi: 10.1037/1064-1297.15.2.123. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, Khan R, Mojsiak J, Myrick DL, Hedden S, Cochran K, Woolson RF. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32:577–87. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–8. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Mann N, Bitsios P. Modafinil treatment of amphetamine abuse in adult ADHD. J Psychopharmacol. 2009;23:468–71. doi: 10.1177/0269881108091258. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Knecht C, Cepeda S. Modafinil: a useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Curr Drug Abuse Rev. 2008;1:213–21. doi: 10.2174/1874473710801020213. [DOI] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, Oliveto A. Open-label pilot study of modafinil for methamphetamine dependence. J Clin Psychopharmacol. 2009;29:488–91. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53:303–8. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–25. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–7. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215:23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Turner DC, Sahakian BJ. A review of the effects of modafinil on cognition in schizophrenia. Schizophr Bull. 2007;33:1298–306. doi: 10.1093/schbul/sbm090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology. 2013;64:490–5. doi: 10.1016/j.neuropharm.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology (Berl) 2004;177:161–9. doi: 10.1007/s00213-004-1926-3. [DOI] [PubMed] [Google Scholar]
- Munzar P, Tanda G, Justinova Z, Goldberg SR. Histamine h3 receptor antagonists potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsychopharmacology. 2004;29:705–17. doi: 10.1038/sj.npp.1300380. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Haro R, Palomero-Rivero M, Millan-Aldaco D, Drucker-Colin R. Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increases wakefulness in rats. Behav Brain Res. 2007;176:353–7. doi: 10.1016/j.bbr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance--a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101–9. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K, Dourmap N, Jaffard R, Costentin J. The specific dopamine uptake inhibitor GBR 12783 improves learning of inhibitory avoidance and increases hippocampal acetylcholine release. Brain Res Cogn Brain Res. 1998;7:203–5. doi: 10.1016/s0926-6410(98)00023-8. [DOI] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav. 2011;99:229–44. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Charney DS, Lewis L, Cornish JW, Post RM, Woody GE, Zubieta JK, Anthony JC, Blaine JD, Bowden CL, Calabrese JR, Carroll K, Kosten T, Rounsaville B, Childress AR, Oslin DW, Pettinati HM, Davis MA, Demartino R, Drake RE, Fleming MF, Fricks L, Glassman AH, Levin FR, Nunes EV, Johnson RL, Jordan C, Kessler RC, Laden SK, Regier DA, Renner JA, Jr, Ries RK, Sklar-Blake T, Weisner C. Priority actions to improve the care of persons with co-occurring substance abuse and other mental disorders: a call to action. Biol Psychiatry. 2004;56:703–13. doi: 10.1016/j.biopsych.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Partridge BJ, Bell SK, Lucke JC, Yeates S, Hall WD. Smart drugs “as common as coffee”: media hype about neuroenhancement. PLoS One. 2011;6:e28416. doi: 10.1371/journal.pone.0028416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P. Histamine in the brain: beyond sleep and memory. Biochem Pharmacol. 2007;73:1113–22. doi: 10.1016/j.bcp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pierard C, Satabin P, Lagarde D, Barrere B, Guezennec CY, Menu JP, Peres M. Effects of a vigilance-enhancing drug, modafinil, on rat brain metabolism: a 2D COSY 1H-NMR study. Brain Res. 1995;693:251–6. doi: 10.1016/0006-8993(95)00711-x. [DOI] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–34. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulay AJ, Stinson FS, Dawson DA, Goldstein RB, Chou SP, Huang B, Saha TD, Smith SM, Pickering RP, Ruan WJ, Hasin DS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV schizotypal personality disorder: results from the wave 2 national epidemiologic survey on alcohol and related conditions. Prim Care Companion J Clin Psychiatry. 2009;11:53–67. doi: 10.4088/pcc.08m00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddatz R, Tao M, Hudkins RL. Histamine H3 antagonists for treatment of cognitive deficits in CNS diseases. Curr Top Med Chem. 2010;10:153–69. doi: 10.2174/156802610790411027. [DOI] [PubMed] [Google Scholar]
- Randall DC, Shneerson JM, Plaha KK, File SE. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol. 2003;18:163–73. doi: 10.1002/hup.456. [DOI] [PubMed] [Google Scholar]
- Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, File SE. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J Clin Psychopharmacol. 2005;25:175–9. doi: 10.1097/01.jcp.0000155816.21467.25. [DOI] [PubMed] [Google Scholar]
- Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–33. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, Picciotto MR, Gao XB. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–10. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, Arrillaga-Romany IC, Goldberg TE, Callicott JH, Apud JA, Weinberger DR. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology. 2010;35:2101–9. doi: 10.1038/npp.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–87. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Markowska AL, Wenk GL, Hatzidimitriou G, Wlos J, Olton DS. 3,4-Methylenedioxymethamphetamine, serotonin and memory. J Pharmacol Exp Ther. 1993;266:1097–105. [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–6. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–37. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- Rosenthal MH, Bryant SL. Benefits of adjunct modafinil in an open-label, pilot study in patients with schizophrenia. Clin Neuropharmacol. 2004;27:38–43. doi: 10.1097/00002826-200401000-00011. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002a;13:105–15. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002b;67:311–22. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morein-Zamir S. Neuroethical issues in cognitive enhancement. J Psychopharmacol. 2011;25:197–204. doi: 10.1177/0269881109106926. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Sarter M, Paolone G. Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci. 2011;125:825–35. doi: 10.1037/a0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Hiemke C. Tiagabine, a gamma-amino-butyric acid transporter inhibitor impairs spatial learning of rats in the Morris water-maze. Behav Brain Res. 2002;133:391–4. doi: 10.1016/s0166-4328(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Wileyto EP, Pinto A, Leone F, Gariti P, Siegel S, Perkins KA, Dackis C, Heitjan DF, Berrettini W, Lerman C. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98:86–93. doi: 10.1016/j.drugalcdep.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93–101. doi: 10.1016/j.drugalcdep.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Seneca N, Gulyas B, Varrone A, Schou M, Airaksinen A, Tauscher J, Vandenhende F, Kielbasa W, Farde L, Innis RB, Halldin C. Atomoxetine occupies the norepinephrine transporter in a dose-dependent fashion: a PET study in nonhuman primate brain using (S,S)-[18F]FMeNER-D2. Psychopharmacology (Berl) 2006;188:119–27. doi: 10.1007/s00213-006-0483-3. [DOI] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–33. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Hemet C, Ramassamy C, Costentin J. Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 1995;5:509–14. doi: 10.1016/0924-977x(95)00041-m. [DOI] [PubMed] [Google Scholar]
- Sirvio J, Riekkinen P, Jr, Jakala P, Riekkinen PJ. Experimental studies on the role of serotonin in cognition. Prog Neurobiol. 1994;43:363–79. doi: 10.1016/0301-0082(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Smith A, Nutt D. Noradrenaline and attention lapses. Nature. 1996;380:291. doi: 10.1038/380291a0. [DOI] [PubMed] [Google Scholar]
- Smythies J. Section II. The dopamine system. Int Rev Neurobiol. 2005a;64:123–72. doi: 10.1016/S0074-7742(05)64002-0. [DOI] [PubMed] [Google Scholar]
- Smythies J. Section III. The norepinephrine system. Int Rev Neurobiol. 2005b;64:173–211. doi: 10.1016/S0074-7742(05)64003-2. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–63. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–29. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Madras BK, Bonab AA, Dougherty DD, Clarke A, Mirto T, Martin J, Fischman AJ. A positron emission tomography study examining the dopaminergic activity of armodafinil in adults using [(1)(1)C]altropane and [(1)(1)C]raclopride. Biol Psychiatry. 2010;68:964–70. doi: 10.1016/j.biopsych.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;182:186–93. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–49. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Sembower MA, Ertischek MD, Shiffman S, Schnoll SH. Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. J Addict Dis. 2013;32:1–10. doi: 10.1080/10550887.2012.759858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G. Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology. 2010;35:2203–10. doi: 10.1038/npp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67:e11. [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, Katz JL. Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem. 2008;106:147–57. doi: 10.1111/j.1471-4159.2008.05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–85. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Ferraro L, Bianchi C, Fuxe K. 6-hydroxy-dopamine treatment counteracts the reduction of cortical GABA release produced by the vigilance promoting drug modafinil in the awake freely moving guinea-pig. Neurosci Lett. 1994;171:201–4. doi: 10.1016/0304-3940(94)90639-4. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Fuxe K, Ferraro L, Janson AM, Bianchi C. Inhibitory effects of the psychoactive drug modafinil on gamma-aminobutyric acid outflow from the cerebral cortex of the awake freely moving guinea-pig. Possible involvement of 5-hydroxytryptamine mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:461–5. doi: 10.1007/BF00176625. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Perez de la Mora M, Ferraro L, Mendez-Franco J, Beani L, Rambert FA, Fuxe K. Modafinil and cortical gamma-aminobutyric acid outflow. Modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol. 1995;273:63–71. doi: 10.1016/0014-2999(94)00675-w. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol. 2000;10:311–20. doi: 10.1089/cap.2000.10.311. [DOI] [PubMed] [Google Scholar]
- Terry AV., Jr . Muscarinic Receptor Antagonists in Rats Animal Models of Cognitive Impairment. Taylor & Francis Group, LLC; Boca Raton FL: 2006. [Google Scholar]
- Tiligada E, Kyriakidis K, Chazot PL, Passani MB. Histamine pharmacology and new CNS drug targets. CNS Neurosci Ther. 2011;17:620–8. doi: 10.1111/j.1755-5949.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret M, Sallanon-Moulin M, Fages C, Roudier V, Didier-Bazes M, Roussel B, Tardy M, Jouvet M. Effects of modafinil-induced wakefulness on glutamine synthetase regulation in the rat brain. Brain Res Mol Brain Res. 1994;26:123–8. doi: 10.1016/0169-328x(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Turner D. A review of the use of modafinil for attention-deficit hyperactivity disorder. Expert Rev Neurother. 2006;6:455–68. doi: 10.1586/14737175.6.4.455. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–9. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Uguen M, Perrin D, Belliard S, Ligneau X, Beardsley PM, Lecomte JM, Schwartz JC. Preclinical evaluation of the abuse potential of Pitolisant, a histamine H(3) receptor inverse agonist/antagonist compared with Modafinil. Br J Pharmacol. 2013;169:632–44. doi: 10.1111/bph.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinas RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104:12554–9. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Contreras-Rodriguez O, Fonseca F, Cuenca A, Soriano-Mas C, Rodriguez J, Pardo-Lozano R, Blanco-Hinojo L, de Sola Llopis S, Farre M, Torrens M, Pujol J, de la Torre R. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00472.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warot D, Corruble E, Payan C, Weil JS, Puech AJ. Subjective effects of modafinil, a new central adrenergic stimulant in healthy volunteers: a comparison with amphetamine, caffeine and placebo. European psychiatry. 1993;8:201–208. [Google Scholar]
- Waters KA, Burnham KE, O’Connor D, Dawson GR, Dias R. Assessment of modafinil on attentional processes in a five-choice serial reaction time test in the rat. J Psychopharmacol. 2005;19:149–58. doi: 10.1177/0269881105048995. [DOI] [PubMed] [Google Scholar]
- Weisler RH, Pandina GJ, Daly EJ, Cooper K, Gassmann-Mayer C. Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs. 2012;26:421–34. doi: 10.2165/11631990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wesensten NJ. Effects of modafinil on cognitive performance and alertness during sleep deprivation. Curr Pharm Des. 2006;12:2457–71. doi: 10.2174/138161206777698819. [DOI] [PubMed] [Google Scholar]
- Whitmore J, Hickey P, Doan B, Harrison R, Kisner J, Beltran T, McQuade J, Fischer J, Marks F Air Force Research Laboratory HED, Biosciences and Protection Division, Fatigue Countermeasures Branch, editor. A DOUBLE-BLIND PLACEBO-CONTROLLED INVESTIGATION OF THE EFFICACY OF MODAFINIL FOR MAINTAINING ALERTNESS AND PERFORMANCE IN SUSTAINED MILITARY GROUND OPERATIONS. 2006. [Google Scholar]
- Wilens TE, Morrison NR, Prince J. An update on the pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2011;11:1443–65. doi: 10.1586/ern.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CG, Bottiglieri T, Snead OC., 3rd GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54(Suppl 6):S3–12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR. Neurotransmitters and cognition. Exs. 2006;98:5–39. doi: 10.1007/978-3-7643-7772-4_2. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Smith LA, Pearce RK, Tel B, Chancharme L, Moachon G, Jenner P. Modafinil prevents the MPTP-induced increase in GABAA receptor binding in the internal globus pallidus of MPTP-treated common marmosets. Neurosci Lett. 2004;354:6–9. doi: 10.1016/j.neulet.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–46. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]


