Abstract
Classic IL-6 signaling is conditioned by the transmembrane receptor (IL-6R) and homodimerization of gp130. During trans-signaling, IL-6 binds to soluble IL-6R (sIL-6R) enabling activation of cells expressing solely gp130. Soluble gp130 (sgp130) selectively inhibits IL-6 trans-signaling. To characterize amniotic fluid IL-6 trans-signaling molecules (IL-6, sIL-6R, sgp130) in normal gestations and pregnancies complicated by intra-amniotic inflammation (IAI) we studied 301 women during second trimester (n=39), third trimester (n=40) and preterm labor with intact (n=131, 85 IAI negative & 46 IAI positive) or preterm premature rupture of membranes (PPROM: n=91, 61 IAI negative & 30 IAI positive). ELISA, Western blotting and RT-PCR were used to investigate amniotic fluid, placenta and amniochorion for protein and mRNA expression of sIL-6R, sgp130, IL-6R and gp130. Tissues were immunostained for IL-6R, gp130, CD15+ (polymorphonuclear) and CD3+ (T-cell) inflammatory cells. The ability of sIL-6R and sgp130 to modulate basal and LPS-stimulated release of amniochorion matrix-metalloprotease-9 (MMP-9) was tested ex-vivo. We showed that in physiologic gestations amniotic fluid sgp130 decreases toward term. Amniotic fluid IL-6 and sIL-6R were elevated in IAI whereas sgp130 was decreased in PPROM. Our results suggested that fetal membranes are the probable source of amniotic fluid sIL-6R and sgp130. Immunohistochemistry and RT-PCR revealed increased IL-6R and decreased gp130 expression in amniochorion of women with IAI. Ex-vivo, sIL-6R and LPS augmented amniochorion MMP-9 release whereas sgp130 opposed this effect. We conclude that IL-6 trans-signaling molecules are physiologic constituents of the amniotic fluid regulated by gestational age and inflammation. PPROM likely involves functional loss of sgp130.
INTRODUCTION
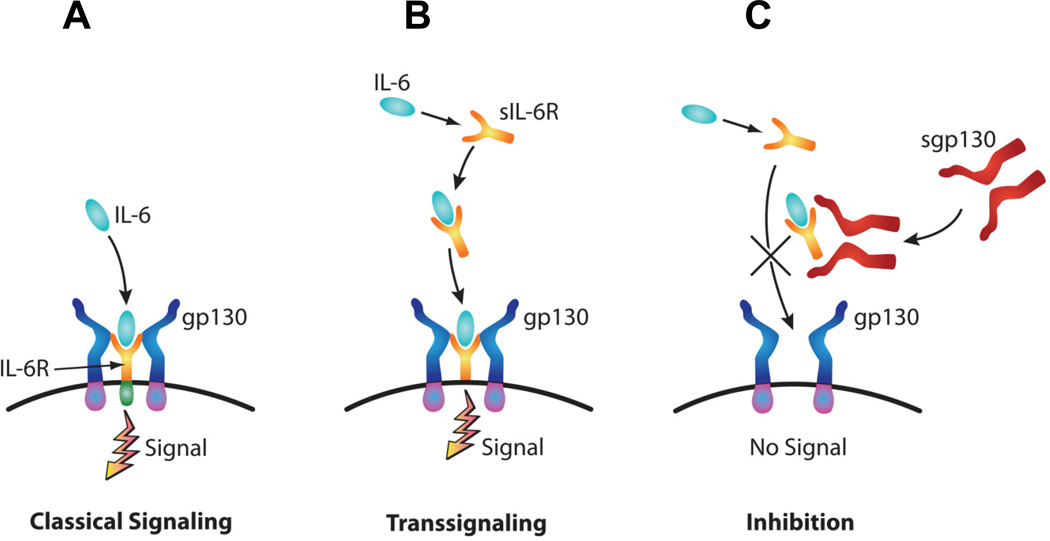
IL-6 is a multifunctional cytokine involved in regulation of acute phase responses, hematopoiesis, cell regeneration and transition from innate to acquired immunity (1,2,3,4). To achieve these functions, IL-6 engages in two types of downstream signaling pathways: classic and trans-signaling (Fig. 1) (5). In the classic pathway, IL-6 binds to a transmembrane cognate receptor, IL-6R, resulting in homodimerization of a signal-transducing glycoprotein, gp130 (6). This triggers a complex intracellular cascade involving STAT factors, JAK and MAPK (6). The end result is a concerted transcriptional increase of genes with critical roles in inflammation (6). In the trans-signaling pathway, IL-6 complexes to a soluble form of IL-6R (sIL-6R) produced by alternative splicing or proteolytic cleavage of IL6-R (5,7). This IL-6/sIL-6R complex then triggers dimerization of gp130 and downstream signaling thereby circumventing the need for IL-6R (5,7).
Figure 1. Diagram illustrating the downstream pathways of IL-6 signaling.
In the classic pathway, binding of the IL-6 to its transmembrane receptor IL-6R induces dimerization of the signal transducing receptor subunit gp130 (A). In the trans-signaling pathway, IL-6 binds a soluble form of IL-6R (sIL-6R). The complex IL-6/sIL-6R complex enables activation of cells expressing solely gp130 (B). A soluble form of gp130 (sgp130) selectively blocks trans-signaling (C).
Unlike gp130 which is ubiquitously expressed, IL-6R is restricted to a few cell types such as hepatocytes, megakaryocytes, neutrophils, macrophages, leukocytes and trophoblast (5,8). Thus, the potential of IL-6 acting through its classical cognate receptor is limited. The molecular interaction of IL-6/sIL-6R complex with gp130 confers IL-6 sensitivity to many cell types that do not express IL-6R. Importantly, the induction of acute phase response genes via trans-signaling pathway is controlled by an inhibitory mechanism (6). Generated by enzymatic shedding and alternative splicing, soluble gp130 (sgp130) is a natural inhibitor of IL-6 trans-signaling by preventing the agonistic IL-6/sIL-6R complex from interacting with membrane bound gp130 (6,9). This selective antagonistic effect of sgp130 distinguishes between downstream effects of IL-6 trans-signaling and those controlled via the classical pathway. In particular, IL-6 trans-signaling defines the nature of inflammatory infiltrates by modulating expression of chemokines and adhesion molecules, polymorphonuclear neutrophil function and apoptosis and T-cell recruitment (1,10,11,12).
The height of the inflammatory response elicited by microbial invasion of the amniotic fluid (AF) cavity and amniochorion is critical for pregnancy outcome (1). For instance, cytokine activation triggers release of matrix-metalloproteases (MMPs) which in turn leads to degradation of extracellular matrix (ECM) and preterm premature rupture of the membranes (PPROM) (13). That the levels of AF IL-6 in pregnancies complicated by IAI are significantly elevated remains undisputed (1,14). Still, our understanding of IL-6’s biological function in AF remains incomplete unless placed in the context of its two signaling pathways. We hypothesized that aside from IL-6, human AF contains IL-6 trans-signaling molecules with independent ability to modulate downstream endpoints with pathogenic significance for preterm birth (PTB).
In this study, we investigated the presence and activation of IL-6 trans-signaling in AF and reproductive tissues of pregnancies complicated by IAI. Furthermore, we provide functional evidence that sgp130 is a modulator of fetal membrane MMP-9 release, thereby highlighting the involvement of IL-6 trans-signaling in PPROM and PTB.
PATIENTS AND METHODS
Patient population and AF samples
A flowchart of the women enrolled in the study outlining the subgroups of analyzed samples is presented in the Supplemental Fig. 1. Amniotic fluid was retrieved from 301 consecutive women who had an indicated amniocentesis at Yale-New-Haven- Hospital from March 2004 to December 2008. All women provided informed consent. The study was approved by the Human Investigational Committee of Yale University.
We studied the following clinical groups: i) second trimester women undergoing genetic amniocentesis who delivered a healthy baby at term [gestational age (GA) median [interquartile range (IQR)]: 19 [17–20] weeks, n=39); ii) third trimester women undergoing fetal lung maturity testing prior to delivery (GA: 36 [36–37] weeks, n=40) and iii) women presenting with preterm labor or PPROM who had an amniocentesis to rule-out infection (GA: 28 [25–31] weeks, n=222). This last group was further divided into: women with negative IAI and intact membrane (GA: 28 [25–32] weeks, n=85), women with negative IAI and PPROM (GA: 30 [28–32] weeks, n=61), women with positive IAI and intact membrane (GA: 26 [24–29] weeks, n=46) and women with positive IAI and PPROM (GA: 28 [26–31] weeks, n=30). Of the 85 cases with negative IAI and intact membranes, 31 women delivered a healthy term baby. These cases were analyzed along with the genetic and lung maturity samples for GA-regulation of AF IL-6, sIL-6R and sgp130.
Second and third trimester control groups were designed to identify possible GA mediated changes in AF levels of IL-6, sIL-6 and sgp130. A cut-off of 27 completed weeks GA was chosen to delineate the second (<28 wks GA) from third trimester (≥28 wks GA). The indications for the second trimester amniocentesis included: advanced maternal age, personal preference for testing, abnormal serum screening, or presence of minor ultrasound markers suggestive of aneuploidy (choroid plexus cyst, intra-cardiac echogenic foci). Amniocentesis results for all women included in this group showed normal fetal karyotypes. The third trimester group consisted of healthy women where confirmation of fetal lung maturity was clinically indicated prior to induction of labor or surgical delivery (Cesarean Section). Preterm labor was defined as the presence of regular uterine contractions and documented cervical effacement and/or dilatation in patients <37 wks GA. Preterm birth was defined as delivery of the fetus <37 wks GA. Preterm premature rupture of membranes was confirmed by visualization of vaginal “pooling” at sterile speculum examination, and positive “nitrazine” or “ferning” tests. When necessary an intra-amniotic “dye test” (infusion of indigo carmine) was used (n=25). Exclusion criteria for the study population included: chromosomal aneuploidy, fetal structural abnormalities, multiple gestation, uterine contractions for the 2nd and 3rd trimester controls, known maternal medical conditions, anhydramnios, viral infection (human immunodeficiency or hepatitis), and fetal heart rate abnormalities at enrollment (i.e. bradycardia, prolonged/repeated variable decelerations) requiring emergent delivery.
Protocol implemented for the clinical care of the preterm labor patients
Management of the patients was left to the clinical team. Based on the American Congress of Obstetrics and Gynecology recommendations, in the absence of clinical laboratory results suggestive of infection, or signs/symptoms of clinical chorioamnionitis (fever >100.4°F, abdominal tenderness, fetal tachycardia), and/or abnormalities of fetal heart rate (variable or late decelerations), and/or abruption, PPROM was managed expectantly (15). If PPROM, digital exams were not allowed. Patients received corticosteroids for lung maturity if ≤32 weeks GA and antibiotic therapy as clinically indicated. The vast majority of preterm labor/PPROM women (80%) had their amniocentesis procedure performed prior to antibiotic or steroid therapy. Inpatient women were monitored by cardiotocography at least twice daily for the presence of fetal heart abnormalities and/or uterine contractions.
The decision for amniocentesis, expectant management, tocolysis or indicated delivery was made by the primary physician independent of our research protocol. The clinical management team was not aware of our research test results. Following amniocentesis, each woman was followed prospectively to delivery. Indicated delivery through induction of labor or Cesarean section was performed for such clinical indications as AF laboratory results traditionally considered to indicate IAI/infection, fetal lung maturity, prolapsed umbilical cord and/or GA ≥34 weeks.
Biochemical and microbiological studies of AF
A diagnosis of intra-amniotic infection and/or IAI was established based biochemical and microbiological test panels. Amniotic fluid was analyzed for glucose concentration, lactate dehydrogenase activity, white blood cell count and Gram stain. Standard culturing methods for aerobic and anaerobic bacteria, including Ureaplasma and Mycoplasma species were employed. Positive Gram stain and/or culture results were considered suggestive of intra-amniotic infection. Once the clinical requirements were satisfied, the remaining AF was centrifuged at 3, 000g, 4°C for 10 min., aliquoted and stored at − 80°C for research purposes. The results for these studies were used for clinical case management of the cases.
Diagnosis of IAI by mass spectrometry. Mass restricted (MR) score
In previous studies, our group demonstrated that proteomic profiling of AF using surface-enhanced laser-desorbtion ionization time of flight mass spectrometry was the most accurate research method for diagnosing IAI (14,16). Briefly, the AF MR score is comprised of 4 proteomic biomarkers: defensin-2, defensin-1, S100A12 (calgranulin C) and S100A8 (calgranulin A) and ranges from 0 to 4, depending upon the presence or absence of each of the 4 protein biomarkers. A value of 1 was assigned if a biomarker peak was present and 0 if absent. An MR score of 3 or 4 indicates the presence of IAI. All mass spectrometry tracings were scored by one investigator (IAB) who was unaware of the results of the biochemical or microbiological tests used to diagnose IAI/infection.
Immunoassays for IL-6, sIL-6R, sgp130 and MMP-9
ELISA assays for IL-6 (eBioscience, San Diego, CA), sIL-6R (eBioscience) and sgp130 (R&D systems, Minneapolis, MN) were performed to measure their levels in AF and explant media. The molar ratio between AF sIL-6R and sgp130 was calculated for each patient as previously described (17). Explant media was also immunoassayed for MMP-9. The assays were run in duplicate according to the manufacturer’s protocols. For all assays, samples were diluted from 1:10 to 1:100 to fall within the range of the standard curves. The inter- and intra-assay coefficients of variation was <10% for all the analytes.
Evaluation of histological chorioamnionitis
Paraffin-embedded tissues were available from 119/146 (82%) of the negative IAI and 76/76 (100%) of the positive IAI patients who provided AF samples. Placental and fetal membranes tissues biopsies were collected immediately after delivery. Tissues were formalin fixed and embedded in paraffin. For clinical purposes, a diagnosis of histological inflammation of the placenta and fetal membranes was based on well-established criteria (18).
Placental and fetal membranes IL-6R, gp130, CD3 and CD15 immunohistochemistry
Immunohistochemistry for IL-6R, gp130, CD15 [polymorphonuclear marker) (19) and CD3 (mature T-cell marker) (1,20) was performed in tissues of women with idiopathic PTB (negative IAI and absent histological chorioamnionitis, n=5), PTB in the setting of positive IAI and histological choirioamniontis (n=15) and healthy women with Cesarean delivery and absence of labor (n=9). The third trimester group (GA: 38–40 weeks) consisted of healthy, term, non-laboring women, undergoing a scheduled elective Cesarean delivery for indications such as fetal malpresentation (i.e breech) or prior Cesarean delivery. All their infants were appropriately grown for GA and had reassuring fetal heart rate patterns prior to surgery. Clinical characteristics of these cases are provided in Supplemental Table 1.
Five µm paraffin tissue sections were deparaffinized in xylene and rehydrated with graded ethanol to potassium-phosphate-buffered saline solution, pH 7.2. Following antigen retrieval with citrate buffer (pH=6), the sections were pretreated with 1% hydrogen peroxide for 15 min. followed by 1 hour blocking with 5% goat serum. The following primary antibodies were used: rabbit anti-human IL-6R (sc-661, Santa Cruz Biotechnology Inc., Santa Cruz, CA, 1:200, overnight incubation at 4°C), mouse anti-human gp130 (sc-9994, Santa Cruz, 1:50 dilution, overnight incubation at room temperature), rabbit anti-human CD3 (T-cell marker; A0452, Dako, Denmark, 1:200, overnight incubation at 4°C), mouse monoclonal CD15 (polymorphonuclear neutrophil marker; ab53997, 1:50, overnight incubation at 4°C). Following 1 hour incubation with appropriate secondary antibodies (biotinylated goat anti-rabbit or antimouse IgG 1:600 dilution, Jackson Immunochemicals, West Grove, PA), the sections were developed using the avidin-biotin-peroxidase system (Vectastain Elite ABC, Vector Laboratories, Burlingame, CA) with Vector NovaRed (Vector Laboratories) as chromogen and hematoxylin as counterstain. The tissue sections were dehydrated in graded ethanol, cleared, and mounted. Specificity of staining was confirmed by replacing the primary antibodies with equivalent concentrations of mouse or rabbit non-immune IgG (Novus Biologicals Littleton, CO). Immunohistochemical staining of the intensity of the chromogen deposited in the amnion epithelium, chorio-decidua and placental villous trophoblast, stromal and endothelial cells was evaluated semiquantitatively in a blind fashion by examining 3 fields/slide and subjectively scoring on a scale from 0 (no staining) to 3 (intense red-brown staining) the intensity of the chromogen deposited in the amnion epithelium, choriodecidua and placental villous trophoblast, as previously described (21). Vimentin immunostaining was performed to identify decidual cells while cytokeratin staining identified trophoblast cells (data not shown).
Quantitative RT-PCR and Western blotting for IL-6R and gp130
For the RT-PCR experiments we used tissues (placenta and amniochorion membranes) retrieved from the same cases where the IL-6R level of expression was evaluated by immunohistochemistry. Clinical characteristics of these cases are provided in Supplemental Table 1. Immediately after delivery, tissues were frozen in liquid nitrogen and kept at −80°C. RNA was extracted and reverse transcribed into cDNA with random hexamer primers using standard procedures. Quantitative RT-PCR was performed using TaqMan® (Applied Biosystems) chemistry in 20 µL reactions composed of 10 µL mastermix (TaqMan® Fast Universal PCR 2x Master Mix), 8 µL water, 1µL cDNA template normalized and 1 µL PCR probe set (TaqMan® Gene Expression Assays on Demand). For the detection of IL-6R and gp130 mRNAs, TaqMan® probes from Applied Biosystems were used: hs00169842_m1 (IL-6R) (22) and hs00174360_m1 (gp130) (23). For controls, we used Taqman® probes hs99999907_m1 (β-2 microglobulin, B2M) and hs00265497_m1 (and ribosomal protein L30, RPL30). The combination of the two endogenous control mRNAs (B2M and RPL30) was validated in preliminary experiments using pools of cDNA amplified in the TaqMan® Human Endogenous Control Plate (Applied Biosystems). Selection of the two reference genes was based on low cycle threshold (Ct) values that were not different among the six cDNA pools.
For each target, amplification was performed in duplicate reactions in a 2-step cycle (denaturation, 95°C for 15 seconds, annealing/extension at 62°C for 60 seconds) for 40 cycles. Post-processing calculations were performed using the StepOne Software (v2.1). The values obtained were normalized to the geometric mean of the endogenous control RNAs using calculations of ΔCt (Ct of the target – Ct mean of endogenous controls). A ΔCt of 0 indicates a ratio of 1 between the target and housekeeping genes. This ratio may be used as an indication of relative abundance between different targets among different tissues. Calculation of ΔΔCt (ΔCt of individual sample –ΔCt of same target in reference sample) adds an additional normalization within targets. Therefore, ΔΔCt improves estimation of relative mRNA abundance among different biological groups (24,25). For ΔΔCt calculations we used our reference samples RNA pools of the same tissue type.
Western blotting for IL-6R and gp130 isoforms
We used fetal membrane, placental tissue lysates and corresponding AF samples of a select group of preterm women with (n=6) and without (n=6) IAI and/or histological chorioamnionitis. Western blotting was performed on AF (5 µl/lane), fetal membrane and placenta tissue lysates (20 µg total protein/lane) prepared by homogenization in a buffer containing 50 mM Tris-buffer (pH 7.4), 0.1 mM EGTA, 0.14 µL/mL β-mercaptoethanol and Complete® protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN). Samples were mixed 1:2 with reducing sample buffer (Bio-Rad), boiled and applied to 4–20% SGS-PAGE gels. After electrophoretic transfer, nitrocellulose membranes were blocked with 5% milk and then incubated overnight at 4°C with either polyclonal rabbit anti-IL-6R antibody (sc-661, 1:200, Santa Cruz) or mouse monoclonal anti-gp130 antibody (clone B-P4, ab34325, 1:300, Abcam, Cambridge MA). Detection was performed using appropriate horseradish peroxidase-linked secondary antibodies and chemiluminescence (ECL-Plus, Amersham Biosystems). Optical density of the bands of interest (sIL-6R: approx. 44–55 kDa (26); sgp130: approx. 100 kDa (27) was analyzed with Image J software (NIH, http:\\rsb.info.nih.gov).
Fetal membranes explant culture system
Fetal membranes were obtained from healthy women (n=7) without any significant past medical history undergoing scheduled, elective Cesarean delivery in the absence of labor (GA: 39 [38–40] weeks). Indications for abdominal delivery included elective repeat or primary Cesarean delivery for fetal malpresentation (i.e. breech) or prior uterine scar. No patient had abnormal placentation (i.e placenta previa, abruption). All their infants were appropriately grown for GA and had reassuring fetal heart rate patterns prior to surgery.
The setup for the fetal membrane culture system was established based on previously published procedures (28,29). Membranes, distal from the point of iatrogenic rupture, were harvested under sterile conditions and dissected free from the placenta within 10 min of delivery. Ten-mm-diameter pieces were washed thoroughly to eliminate blood clots using cold Hank's Buffered Salt Solution (HBSS) with antibiotic-antimycotic solution (100U/ml penicillin, 100ug/ml streptomycin, and 0.25ug/ml amphotericin B). Two pieces of fetal membrane tissue were placed into each well of a 24-well tissue culture dish in 1ml of culture medium containing a 1:1 mixture of Dulbecco's Modified Eagle Medium (DMEM) with low glucose, 10% fetal bovine serum and antibiotic-antimycotic solution. Prior studies demonstrated that relative cellular quiescence is achieved in untreated membrane cultures after a minimum period of 48h (30). Therefore, in the present study, fetal membranes were initially maintained in the medium for 48h (37°C, 5% CO2–95% air) prior to treatment. Subsequently, the tissues were placed in fresh culture medium and stimulated for 24h with LPS (50ng/ml Sigma, St. Louis, MO), sIL-6R alone (10 ng/mL, R&D systems) or sgp130 alone (500 ng/mL, R&D systems). In addition, the effect of sgp130 in the presence of LPS or sIL-6R was also investigated. The LPS dose was chosen based on a dose response experiment (5, 50, 500 ng/mL LPS) where we determined that the peak of MMP-9 and IL-6 release in the explants medium was seen following stimulation with 50 ng/mL LPS (Supplemental Fig. 2). Optimal concentrations of sIL-6R and sgp130 were chosen in our attempt to mimic as close as possible our in vivo data.
After 24h of incubation, the supernatants were collected, centrifuged to remove cellular debris and stored at –80°C. The incubated tissue was immediately homogenized in 1-ml cell extraction buffer (20mmol/l Tris-HCl, 150mmol/l NaCl, 1% Triton X-100, 1mmol/l phenylmethylsulfonyl fluoride, and complete protease inhibitor cocktail (Roche, Indianapolis, IN). Specimens were spun at 1, 000g at 4°C for 15 min. and protein quantification of incubated tissue performed using Bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions. To correct for variations in tissue incubated per each well, the explant medium concentration of the sought analytes (MMP-9, IL-6, sIL-6R, sgp130) was normalized to total protein in tissue extract. For each experimental condition, values were derived by averaging normalized values from duplicate wells either without (untreated) or with the various treatments. Values were further interpreted as percent change from basal release.
To assess tissue viability during in vitro incubations, the release of the intracellular enzyme LDH into the incubation medium was determined as previously described using the LDH Liqui-UV® Assay (Stanbio, Boerne, TX) (31). The inter-assay and intra-assay coefficients of variation were <5%. We found no significant change in LDH release during the incubation period and after treatments in support of sustained tissue viability.
Statistical analysis
Normality testing was performed using the Kolmogorov-Smirnov test. Data were compared with one-way or two-way ANOVA followed by Student Newman Keuls tests (parametric) or Kruskal-Wallis on ranks followed by Dunn’s tests (non-parametric), to adjust for multiple comparisons as appropriate. Statistical analyses of data derived from immunoassays were performed after logarithmic transformation. Spearman or Pearson correlations were used to measure co-linearity between the selected independent variables as well as other relevant relationships between dependent and independent variables. Comparisons between proportions were done with Chi-square tests. A two-step clustering method analysis was undertaken to identify an unbiased GA separation point that partitioned the levels of AF sgp130 into three clusters (“low”, “mid”, “high”) (32). Stepwise multivariable regression analysis was used to determine concurrent relationships between variables and to correct for possible influences of GA. Our results were reported as either mean with SEM or as median with IQR, as appropriate. A P value of <0.05 was considered significant throughout the analysis. Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc., Chicago, IL) and MedCalc (Broekstraat, Belgium) statistical softwares.
RESULTS
Gestational regulation of AF trans-signaling molecules IL-6, sIL-6R and sgp130
Demographic clinical and outcome characteristics of the women who provided AF samples are presented in Table I. Women in second trimester genetic amniocentesis group were significantly older compared to those who had an amniocentesis to rule-out infection. Third trimester control women were of higher parity compared to the second trimester genetic amniocentesis group, and as expected, were evaluated at a more advanced GA compared to the other two groups. Given the clinical circumstances at the time of recruitment, the preterm labor group had a higher frequency of uterine contractions, advanced degree of cervical dilatation, PPROM, clinical symptoms of chorioamnionitis and a higher frequency of spontaneous or induced PTB. Seventy-five percent of the third trimester patients had reassuring AF fetal lung maturity test results (lecytin/sphynomyelin ratio >2.5). Thus, the third trimester group experienced a higher frequency of medically-indicated PTB and the shortest amniocentesis-to-delivery interval.
Table I.
Demographic clinical and outcome characteristics of the women who provided AF samples for sgp130, sIL-6R and IL-6 levels (n=301).
| Variable | 2nd trimester genetic amniocenteses n = 39 |
3rd trimester lung maturity amniocenteses n = 40 |
Rule-out infection amniocenteses n = 222 |
P value |
|---|---|---|---|---|
| Characteristics at amniocentesis | ||||
| Maternal age, years, † | 33 [28 – 38] | 30 [26 – 36] | 28 [22 – 33] | 0.002 |
| Parity † | 0 [0 – 1] | 1 [0 – 2] | 1 [0 – 1] | 0.017 |
| Gravidity † | 2 [1 – 3] | 3 [2 – 4] | 2 [1 – 3] | 0.162 |
| GA, weeks † | 19 [17 – 20] | 36 [36 – 37] | 29 [25 – 31] | <0.001 |
| Uterine contractions ‡ | 0 (0) | 0 (0) | 109 (49) | <0.001 |
| Cervical dilatation > 3 cm ‡ | 0 (0) | 0 (0) | 72 (32) | <0.001 |
| PPROM ‡ | 0 (0) | 0 (0) | 91 (41) | <0.001 |
| Clinical chorioamnionitis ‡ | 0 (0) | 0 (0) | 18 (8) | <0.001 |
| Outcome characteristics | ||||
| Term delivery (≥ 37 weeks) ‡ | 39 (100) | 23 (58) | 31 (14) | <0.001 |
| Preterm birth at < 34 weeks ‡ | 0 (0) | 0 (0) | 175 (79) | <0.001 |
| GA at delivery, weeks † | 39 [39 – 40] | 37 [36 – 37] | 31 [27 – 33] | <0.001 |
| Amniocentesis-delivery time, days † | 143 [134 – 154] | 1 [1 – 4] | 3 [0 – 17] | <0.001 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA on Ranks.
Data presented as n (%) and analyzed by Chi square tests.
Abbreviations: PPROM, preterm premature rupture of membrane; GA, gestational age
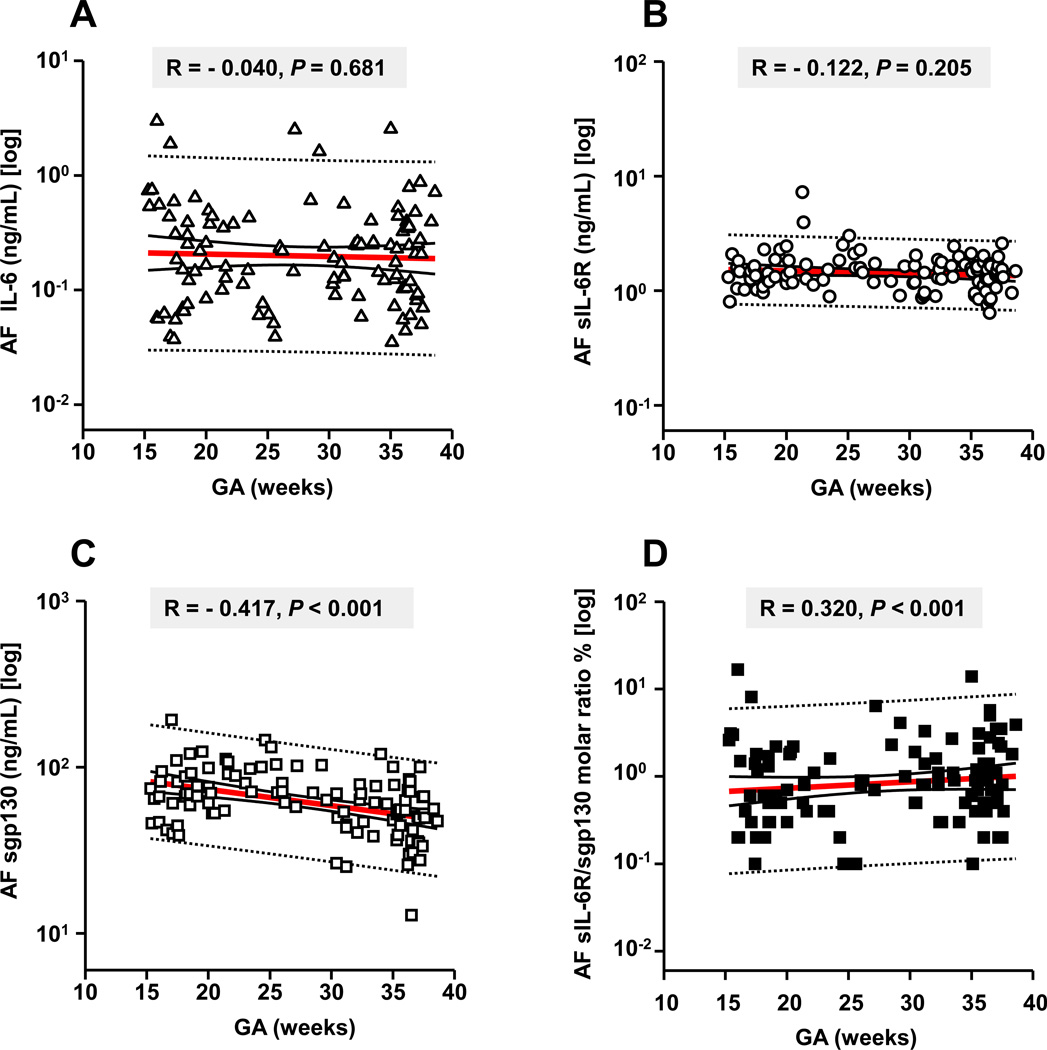
Our analysis of GA regulation of IL-6 trans-signaling molecules was limited to genetic (n=39), lung maturity (n=40) and rule-out infection amniocenteses from women who ultimately had a term delivery (n=31). All three trans-signaling analytes were constitutively present in human AF across gestation The AF IL-6 and sIL-6R concentrations did not vary significantly among groups or with GA (Fig. 2A&B; absolute levels are provided in Supplemental Table II). Conversely, AF sgp130 was inversely correlated with GA with lower levels approaching term (Fig. 2C, P<0.001). Because the balance between the stimulatory sIL-6R and the inhibitory sgp130 may have considerable functional relevance, we were interested in how their relative ratio varied with GA (17). There was a significant GA regulation of sIL-6R/sgp130 molar ratio which increased toward term (Fig. 2D, P<0.001).
Figure 2. Relationships between amniotic fluid (AF) levels of IL-6, sIL-6R, sgp130, sIL-R/ sgp130 molar ratio and gestational age (GA) in pregnancies with normal outcomes (n=110).
We restricted this analysis to amniocenteses samples from women with normal outcomes: second trimester genetic (n=39), third trimester lung maturity (n=40) and preterm women with amniocentesis to rule-out infection who ultimately delivered at term (n=31). Data for IL-6 (x-axis of A) and sIL-6R (x-axis of B) demonstrated no significant correlations between the levels of these two analytes and GA (y-axes of all). The AF levels of sgp130 were inversely correlated with GA with significantly lower levels at term (x-axis of C). Using the two-step clustering method, we identified unbiased GA separation points that partitioned AF sgp130 concentration into 3 clusters: “high”: 104 [100–120] ng/mL, GA: 22 [18–26] weeks “mid”: 67 [60–74] ng/mL, GA: 27 [19–35] weeks and “low”: 39 [34–45] ng/mL, GA: 35 [31–37] weeks. There was a significant GA regulation of the sIL-6R/sgp130 molar ratio (x-axis of D) with higher values at term. This trend was primarily due to the decrease in sgp130 as gestation progressed. For each graph the Spearman correlation coefficient R and the level of statistical significance is shown. Data presented in logarithmic format. Panels A-D: Thick lines: 1st order regression line; Thin continuous lines: 95% confidence intervals; dotted lines: 95% prediction intervals.
Changes in AF IL-6 trans-signaling molecules in human pregnancies complicated by IAI and/or PPROM
The demographic, clinical and laboratory characteristic of the preterm women are presented in Table II. PPROM patients were significantly older irrespective of IAI status (two-way ANOVA PPROM: P<0.001, IAI: P=0.171). We also determined that both IAI and PPROM impacted on latency interval (time from PPROM-to-delivery) with significant interaction between the two variables (IAI: P<0.001, ROM: P=0.010, interaction P=0.002). Moreover, women in the IAI groups delivered at earlier GA and their babies had lower birthweights. Both GA and birthweight were synergistically impacted by IAI and membrane status (IAI: P<0.001, PPROM: P<0.001, interaction P=0.001).
Table II.
Demographic, clinical, laboratory and outcome characteristics of with symptoms of preterm labor or PPROM who provided AF samples for sgp130, sIL-6R and IL-6 levels.
| Variable | Rule out infection amniocenteses (n = 222) | ||||
|---|---|---|---|---|---|
| no IAI & intact n = 85 |
no IAI & PPROM n = 61 |
yes IAI & intact n = 46 |
yes IAI & PPROM n = 30 |
P value | |
| Clinical and outcome characteristics at amniocentesis and at delivery | |||||
| Maternal age, years, † | 25 [20 – 32] | 30 [26 – 33] | 24 [21 –- 32] | 33 [25 – 38] | <0.001 |
| Parity † | 1 [0 – 1] | 1 [0 – 2] | 0 [0 – 1] | 1 [0 – 3] | 0.030 |
| Gravidity † | 2 [1 – 3] | 3 [1 – 4] | 2 [1 – 3] | 2 [2 – 5] | 0.365 |
| History of preterm birth ‡ | 21 (25%) | 16 (26%) | 11 (24%) | 7 (23%) | 0.989 |
| GA at amniocentesis, weeks † | 28 [25 – 32] | 30 [28 – 32] | 26 [24 – 29] | 28 [26 – 31] | <0.001 |
| Expectant management ‡ | 72 (87%) | 42 (69%) | 16 (36%) | 10 (33%) | <0.001 |
| GA at delivery, weeks † | 33 [28 – 38] | 32 [30 – 33] | 26 [24 – 29] | 29 [27 – 32] | <0.001 |
| Amniocentesis-delivery interval, hours† | 428 [52 – 1,379] | 107 [31 – 223] | 6 [4 – 11] | 18 [7 – 69] | <0.001 |
| Amniocentesis-delivery < 7 days | 33 (39%) | 37 (61%) | 43 (93%) | 26 (87%) | <0.001 |
| Birthweight, grams † | 2,210 [1,285 –3,060] | 1,820 [1,430 –2,126] | 955 [731 – 1,246] | 1,350 [1,000 –1,710] | <0.001 |
| AF laboratory test results | |||||
| Glucose, mg/dL † | 30 [22 – 39] | 28 [21 – 40] | 3 [2 – 13] | 8 [2 – 18] | <0.001 |
| LDH activity, U/L † | 168 [126 – 236] | 150 [105 – 257] | 924 [541 – 2,083] | 639 [424 – 1,118] | <0.001 |
| WBC count, cells/mm3 † | 3 [1 – 7] | 8 [2 – 22] | 715 [194 – 1,445] | 687 [52 – 2,380] | <0.001 |
| Positive Gram stain ‡ | 0 (0%) | 4 (7%) | 8 (57%) | 11 (37%) | <0.001 |
| Positive cultures ‡ | 1 (1%) | 14 (23%) | 30 (65%) | 22 (73%) | <0.001 |
| Placental histology results | n=59 | n=60 | n=46 | n=30 | |
| Chorionic plate inflammation, stage † | 1 [0 – 2] | 1 [0 – 3] | 3 [3 – 3] | 3 [2 – 3] | <0.001 |
| Amnionitis, grade † | 0 [0 – 0] | 0 [0 – 2] | 3 [2 – 4] | 3 [2 – 3] | <0.001 |
| Chorio-deciduitis, grade † | 1 [0 – 3] | 2 [0 – 3] | 3 [3 – 4] | 3 [2 – 4] | <0.001 |
| Funisitis, grade † | 0 [0 – 0] | 0 [0 – 2] | 3 [0 – 4] | 2 [0 – 4] | <0.001 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA on Ranks.
Data presented as n (%) and analyzed by Chi square test.
Abbreviations: IAI, intra-amniotic inflammation; PPROM, preterm premature rupture of membrane, GA, gestational age; LDH, lactate dehydrogenase
The results of the biochemical and microbiological studies of the AF showed lower glucose and higher LDH activity in the IAI groups which were significantly influenced by membrane status (IAI: P<0.001, PPROM: P<0.001, interaction P=0.001). Higher AF WBC counts were seen in the IAI groups with significant interaction with PPROM (IAI: P<0.001, PPROM: P<0.001, interaction P=0.005). Lastly, women in IAI groups experienced a higher frequency of positive Gram stain and positive microbial culture results, again significantly influenced by the membrane status (IAI: P<0.001, PPROM: P<0.001, interaction P=0.004). The pathological examination of the placenta revealed higher stages and grades of histological chorioamnionitis and funisitis in the IAI groups, independent of membrane status.
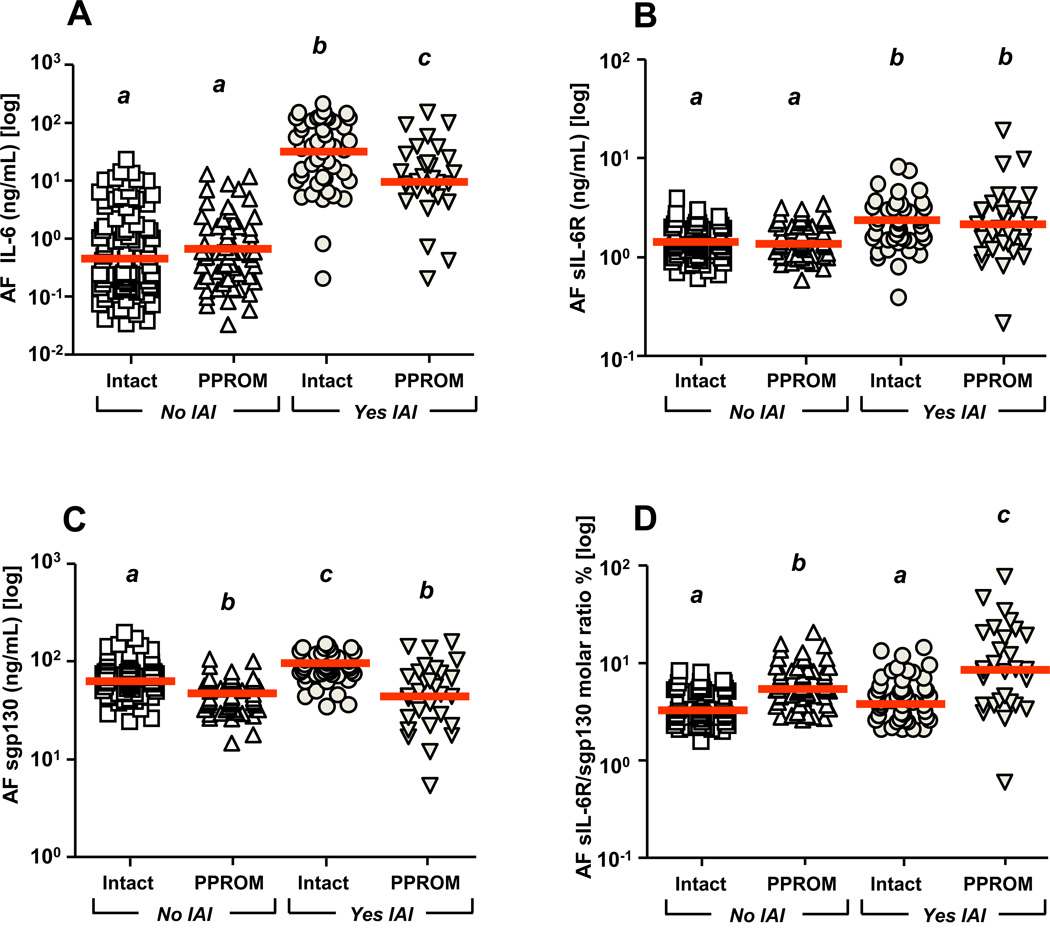
The absolute values for IL-6, sIL-6R and sgp130 in AF of preterm women evaluated for IAI are presented in Supplemental Table III. IL-6 was significantly elevated in women with IAI, although its levels were lower in IAI with PPROM compared to IAI with intact membranes (two-way ANOVA, Fig. 3A, IAI: P<0.001, membrane status P<0.001, interaction P<0.001). The levels of AF sIL-6R were elevated in women with IAI independent of PPROM (Fig. 3B, IAI: P<0.001, membrane status P=0.866, interaction P=0.973). Conversely, PPROM women had decreased sgp130 levels whether or not they had IAI (Fig. 3C, IAI: P=0.247, membrane status P<0.001, interaction P=0.129). The sIL-6R/sgp130 molar ratio was significantly elevated by PPROM alone and further elevated in the setting of PPROM with IAI (Fig 3D, IAI: P<0.001, membrane status P<0.001, interaction P<0.001). When we restricted our analysis to PPROM cases with negative Gram stain and microbial culture results (n=40) managed expectantly post-amniocentesis, we determined that women with amniocentesis-to-delivery intervals longer than 7 days (n=16) had higher AF sgp130 concentrations compared to cases with shorter latencies (n=24) (latency≥7 days: 50.1[38.4–61.4] vs. latency<7 days: 39.2[31.1–51.2] ng/mL, P=0.034). All results maintained following GA correction.
Figure 3. Levels of amniotic fluid (AF) IL-6, sIL-6R, sgp130 and sIL-6R/sgp130 molar ratio in pregnancies complicated by intra-amniotic inflammation (IAI) and preterm premature rupture of the membranes (PPROM).
The following groups were studied: no IAI & intact membrane (n=85); no IAI & PPROM (n=61); IAI & intact membrane (n=46); IAI & PPROM (n=30). The AF IL-6 levels were significantly elevated in women with IAI. IL-6 concentrations were lower in women with IAI & PPROM compared to those with IAI & intact membranes (A). The levels of AF sIL-6R were elevated in women with IAI independent of PPROM (B). Pregnancies complicated by PPROM were characterized by lower AF levels of sgp130 and this phenomenon was observed in both pregnancies complicated or not by IAI (C). The sIL-6R/sgp130 molar ratio was significantly elevated by PPROM and further elevated in the setting of PPROM & IAI (D). All results maintained following correction for GA. Different letters reflects statistically significant differences among groups. Thick line represents the median value for the group. Data presented in logarithmic format. Data were analyzed by two-way ANOVA.
Transcriptional changes in IL-6R and gp130 in human fetal membranes and placenta
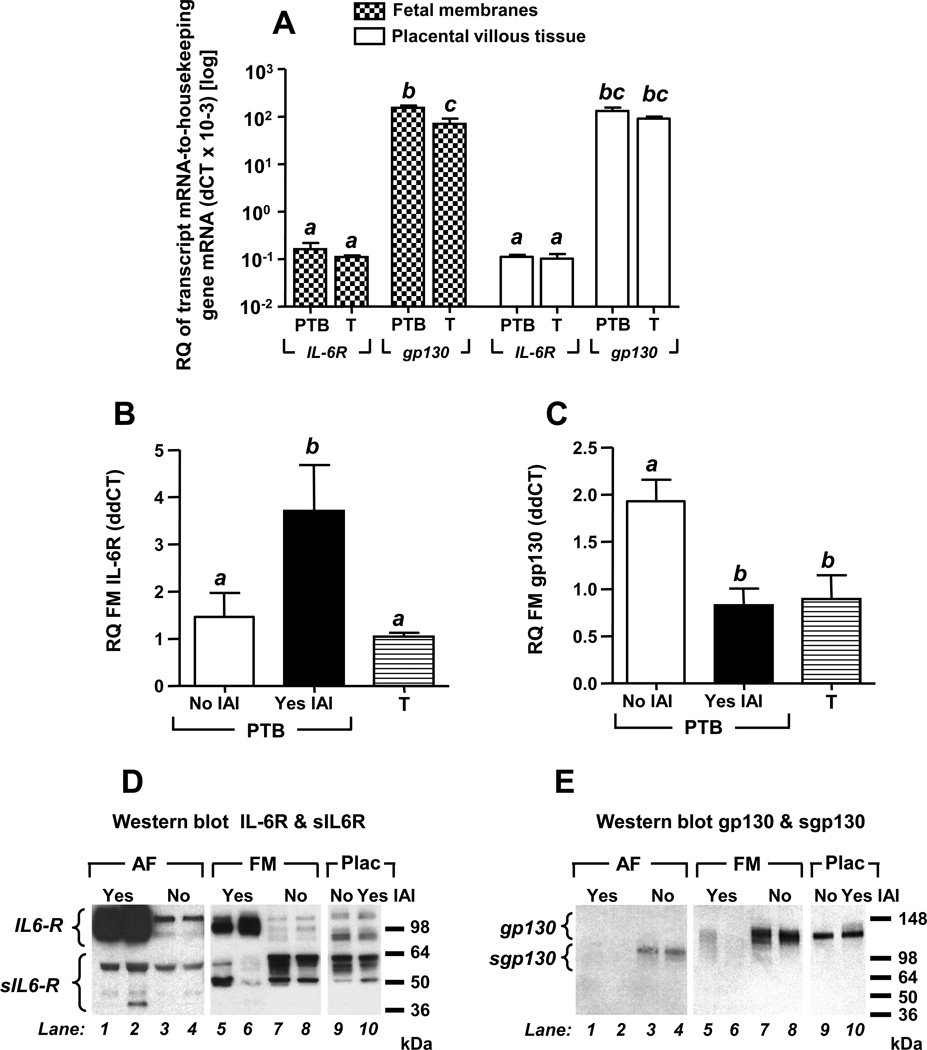
Figure 4 displays mRNA levels of the full length IL-6R and gp130 in reproductive tissues of term and preterm women with and without IAI. In the absence of IAI, transcripts of IL-6R and gp130 were present in both placenta and fetal membranes (Fig. 4A). Compared to IL-6R, gp130 mRNA was more abundant (~3 orders of magnitude) in both tissue types (P<0.001). IL-6R mRNA levels were similar in fetal membranes and placental villous tissues with absent GA regulation (Fig. 4A). The gp130 mRNA was significantly decreased in term amniochorion compared to preterm tissues, (P=0.002). This change was not observed in placental villous tissue. We noted a significant increase in IL-6R mRNA (P=0.029, Fig. 4B) concurrent with significantly decreased gp130 expression in amniochorion of IAI cases (P=0.018, Fig. 4C). The differences remained significant after correcting for GA. Placental IL-6R and gp130 mRNA levels remained unaffected by inflammation (Supplemental Fig. 3).
Figure 4. mRNA (quantitative RT-PCR) and protein (Western blot) expression of IL-6R and gp130.
Real time quantitative RT-PCR was used to study tissues retrieved from 9 term (T) third trimester healthy women and a subset of 20 preterm birth (PTB) cases [no intra-amniotic inflammation (IAI) & no histological chorioamnionitis n=5; yes IAI, & yes histological chorioamnionitis n=15]. IL-6R and gp130 mRNA was identified in both fetal membranes and placental villous trophoblast (A). gp130 mRNA was more abundant than the IL-6R mRNA in both fetal membranes and placental villous tissues. In the fetal membranes the gp130 mRNA levels were significantly decreased at term (T). There was a significant increase in IL-6R mRNA (B) and a significant decrease in the expression of gp130 (C) in the fetal membranes of women with IAI. Relative quantitation (RQ) ΔCT values are reported relative to expression of the housekeeping genes for each tissue (A). ΔΔCT RQ values were reported relative to a reference RNA pool of the same tissue (B-C). Data presented as mean + SEM and analyzed by one-way ANOVA followed by post-hoc Student-Newman-Keuls tests. Means marked with different superscripts are statistically significant (P<0.05). Panels D and E show representative Western blots of AF (AF), fetal membrane (FM) and placental (Plac) proteins. Membranes were probed with compatible polyclonal anti-IL-6R (D) or monoclonal anti-gp130 antibody (E). Specificity was confirmed in identical blots with omitted primary antibodies.
Immunoreactive forms of sIL-6R and sgp130 in human AF, placenta and fetal membranes
The Western blots in Fig. 4D depict several specific bands corresponding to IL-6R (80–110kDa) and sIL-6R (38–55 kDa). The multiple bands likely resulted from post-translational modifications of both targets as previously described (33). In the setting of IAI, we observed marked differences in banding patterns in both AF (Fig. 4D, lanes 1–4) and fetal membranes (Fig. 4D, lanes 5–8) but not in placental lysates (Fig. 4D, lanes 9–10). A significant increase in 80–110 kDa bands was identified in both AF and fetal membranes of patients with IAI compared to those without IAI (P<0.001). In the absence of IAI, immunoreactive bands corresponding to sgp130 (~100 kDa) were present in the AF (Fig. 4E, lanes 1–4) but absent in the fetal membrane and placenta which appear to express only the full length gp130 isoform (~130 kDa, Fig. 4E, lanes 5– 10). Amniotic fluid bands characteristic to sgp130 were significantly decreased in the context of IAI (Fig. 4E, lanes 1–4, P=0.026). This phenomenon appeared to be mirrored by a decrease in the expression of gp130 protein in fetal membranes but not placenta (Fig. 4E, lanes 5–10).
Immunostaining of IL-6R and gp130 in human placenta and fetal membranes
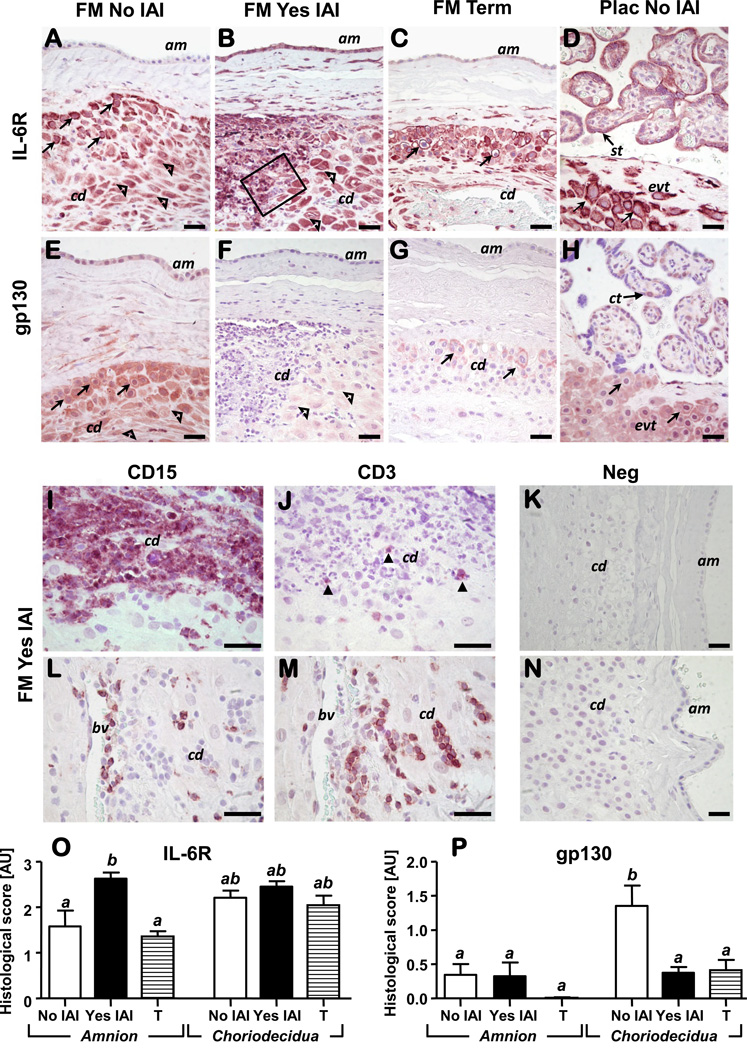
Figure 5A-D shows IL-6R immunostaining in fetal membranes and placental tissue. In preterm fetal membranes without IAI, IL-6R was localized predominantly in extravillous trophoblasts (EVTs) and decidual cells while the amnion epithelium exhibited little positive staining (Fig. 5A). In IAI, a marked increase in IL-6R immunoreactivity was observed in preterm amnion epithelium and in infiltrating inflammatory cells (Fig. 5B). In term fetal membranes, IL-6R remained localized only at the periphery of vacuolated EVTs while decidual cells maintained their staining intensity (Fig. 5C). In the placenta, IL-6R was identified in EVTs and villous synctiotrophoblast (FIG. 5D) without notable changes with either IAI or GA (data not shown). Figure 5E-H illustrates gp130 immunostaining in fetal membranes and placental tissues in the same tissues as shown for IL-6R. In preterm fetal membranes absent IAI, amnion and choriodecidual cells displayed conspicuous gp130 staining (Fig. 5E), which decreased significantly with IAI (Fig. 5F) and at term (Fig. 5G). In the placenta, the strongest gp130 signal was noted in villous cytotrophoblasts and in EVTs of the basal and chorionic plate without remarkable impact from histological chorioamnionitis or GA. Given the role of the IL-6 trans-signaling in directing transition from innate to acquired immunity, we characterized the nature of the inflammatory infiltrates in tissues with confirmed histological chorioamnionitis. Figures 5I&J demonstrate concurrent choriodecidual homing for both CD15+ (Fig. 5I) and CD3+ inflammatory cells (Fig. 5J). Both CD15+ and CD3+ cells populated perivascular areas in the decidua as shown in Figs. 5L&M. Results of histological scoring of the amnion and choriodecidua for IL-6R and gp130 are shown in Fig. 5O&P.
Figure 5. Representative photomicrographs of IL-6R and gp130 immunoreactivity in fetal membranes and placental sections of preterm and term pregnancies.
Preterm amnion (am) stained higher for IL-6R in the presence of IAI compared to no IAI (A-B) and term fetal membranes (C). Intense IL6-R immunostaining was identified in extravillous trophoblasts (evt, arrows) and decidual cells (open arrowheads) of the choriodecidua (cd) as identified by cytokeratin or vimentin positive staining (data not shown). In the placenta (D), IL-6R was identified in evt and villous synctiotrophoblasts (st) without notable changes with either IAI or GA (data not shown). Localization of IL-6R staining in extravillous trophoblasts (evts) was both peri-membranar and intra-cytoplasmic consistent with the ability of the antibody to recognize both membrane bound IL6-R and sIL-6R. The amnion (am) stained less positive for gp130 than choriodecidua (cd) with no discernable differences with histological inflammation or GA (E-G). Both extravillous trophoblasts (arrows) and decidual cells (open arrowheads) stained intensely for gp130 in the absence of histological inflammation (E). In the placenta (H), the strongest gp130 signal was noted in villous cytotrophoblasts (ct) and extravillous trophoblasts (evt, arrows) of chorionic plate. The outlined area in B is shown in panels I&J at higher magnification aimed to illustrate concurrent homing of neutrophils (CD15+) (I) and T lymphocytes (CD3+, closed arrowheads) (J) cells in the inflammatory infiltrate of the choriodecidua (cd) of women effected by IAI. CD15+ and CD3+ cells were also identified in the vicinity of choriodecidual (cd) blood vessels (bv) suggestive of trans-endothelial migration of inflammatory cells (L-M). Specificity of staining was confirmed by incubation of slides with rabbit IgG as control for IL- 6R & CD3 staining (K). Mouse non-immune IgG served as negative control for gp130 and CD15 mono-clonal antibodies (N). IL-6R and gp130 histological scores in the amnion and choriodecidua of preterm (NO & YES IAI) and term patients (T) is shown in panel O and P, respectively. Data presented as mean + SEM and analyzed by one-way ANOVA followed by post-hoc Student-Newman-Keuls tests. Means with at least one common superscript are not statistically significant (P > 0.05). Scale bars: 30 µm for all panels (A-N).
Effect of sIL-6R and sgp130 on MMP-9 release by fetal membrane explants
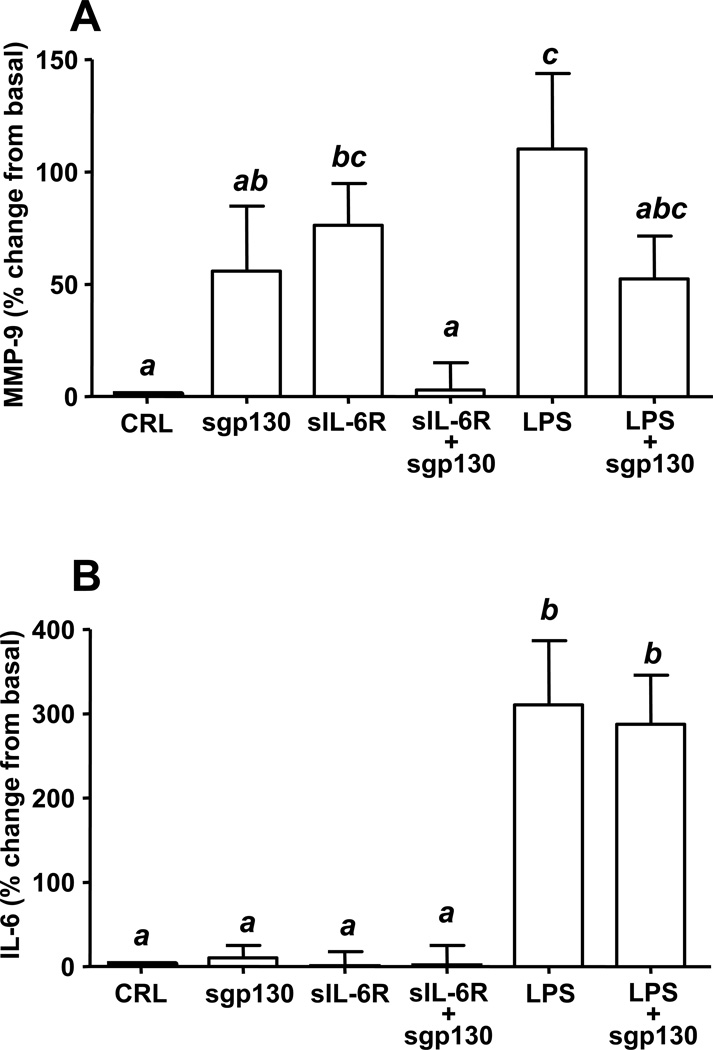
We explored the functional role of sIL-6R and sgp130 in modulating the release of MMP-9 and IL-6 by using an amniochorion explant system. Recombinant sIL-6R alone but not sgp130 significantly upregulated MMP-9 (IL-6R vs. basal P=0.035, Fig. 6A), an effect similar to that of LPS alone (LPS vs basal: P=0.020; LPS vs. sIL6-R: P=0.330). Addition of recombinant sgp130 over sIL-6R or LPS had an antagonistic effect by reversing the MMP-9 release (P<0.001 for both) to the basal level (sIL-6R +sgp130 vs. basal P=0.910; LPS+sgp130 vs. basal P=0.141, Fig. 6A). sgp130, sIL-6R and their combination had no effect on IL-6 (Fig 6B). In contrast, LPS alone significantly upregulated IL-6 levels (LPS vs. basal P<0.001), an effect unaltered by addition of sgp130 (LPS+sgp130 vs. LPS P=0.706).
Figure 6. Ex-vivo production of MMP-9 and IL-6 following incubation of the fetal membranes with sgp130, sIL-6R and LPS.
Recombinant sIL-6R and LPS stimulated the release of MMP-9 (A). Addition of recombinant sgp130 over sIL-6R or LPS reversed the release of MMP-9. Incubation of the tissues with sgp130, sIL-6R and their combination had no effect of IL-6 release in the conditional media of the amniochorion explant (B). The IL-6 levels were significantly upregulated following incubation with LPS. sgp130 did not impact the stimulatory effect of LPS. These results indicate that sIL-6R and sgp130 modulate MMP-9 activity through mechanisms independent of IL-6 levels in the system. Data presented as mean + SEM of 7 independent experiments and analyzed by one-way ANOVA followed by post-hoc Student-Newman-Keuls tests. Means with at least one common superscript are not statistically significant (P > 0.05).
DISCUSSION
Considerable progress has been made in understanding the downstream molecular events of a large array of decidual, placental, fetal membranes and AF cytokines including TNF-α, IL-1, IL-8 and in particular IL-6 (1,3,8,34,35). The latter is a pleiotropic factor that belongs to the family of gp130 cytokines (36). Investigation of IL-6’s bioactivity has been focused primarily on its pro-inflammatory properties given that augmented AF IL-6 levels are associated with PTB (37). However, as our understanding continues to evolve we have gained a better appreciation of the physiologic as well as the pathologic role of IL-6 in human gestation. Consistent with previous reports, our study demonstrated that IL-6 was present in AF of pregnancies with normal outcomes and absent infection (38,39). That the human amnion, choriodecidua and fetus are potential sites of IL-6 synthesis and probable sources of AF IL-6 has been previously proposed (34,35). Therefore, a relevant question relates to the physiologic function of AF IL-6 across normal human gestation. The current study provides first hand evidence that, if any, the biological role of AF IL-6 can be exercised through both classic and trans-signaling pathways. In support of the classic pathway we demonstrated that both IL-6R and gp130 are expressed in resident cells of the fetal membranes and placental villous tissues. It is critical to recognize that IL-6 exercises not only a pro-, but also an anti-inflammatory role (1). The anti-inflammatory function of IL-6 is facilitated through down-regulation in expression of pro-inflammatory cytokines (i.e. TNF-α, interferon-γ) and induction of soluble IL-1 and TNF-α receptor antagonists (40). These mediators act then in an autocrine/paracrine fashion and target normal biological processes occurring within villous trophoblast, decidua and amnion (41). As a result, regardless of its source, the AF and decidual IL-6 could be of significant relevance for fetal development.
Our novel finding that sIL-6R and sgp130 are constituents of the normal human AF supports the notion that any biological role assigned to AF IL-6 could be initiated or repressed via the trans-signaling pathway. Similar to IL-6, the source of sIL-6R and sgp130 in AF is debatable. Our RT-PCR, Western blot and immunohistochemistry results point to fetal membranes as primary and placenta as secondary source. Cleavage of the amniochorion and villous trophoblast IL-6R or gp130 could be responsible for the presence of these two soluble factors in AF (9,42,43). That this mechanism occurs in fetal membranes and placental tissues needs to be confirmed. However, in the case of sIL-6R, the inducers of the shedding process [i.e. TNF-α converting-enzyme (TACE)] are expressed in amnion and choriodecidua of healthy women (42). There is a high regulatory complexity of the splicing mechanisms responsible for sIL-6R and sgp130 synthesis (9). Inducers of the IL-6R mRNA splicing process include oncostatin-M (cytokine synthesized by activated T lymphocytes and monocytes), and IL-1 (44). The splicing process for the sgp130 mRNA continues to be poorly understood, but oncostain-M, IL-1 and IL-6 may play a role (44). The well-documented expression of oncostatin-M, IL-1 and IL-6 in the human decidua (34,45) suggest that physiologic immune processes occurring at the maternal-fetal interface may be responsible for the synthesis of sIL-6R and sgp130, and their subsequent release into the AF. The contribution of enzymatic cleavage and mRNA splicing mechanisms to the total pool of AF sIL-6R and sgp130, in addition to the rates of metabolism and clearance for the two aforementioned factors, needs to be clarified in the future.
We showed that AF levels of sgp130 progressively decrease toward term. This suggests that the inflammatory modulator effect of this molecule is GA-dependent. This observation is remarkable because sgp130 specifically inhibits sIL-6R-reliant responses and does not interfere with the cell-surface expressed IL-6R (6). Our data raise important questions regarding the identity of the inhibitory mechanisms leading to the decreased cellular release or synthesis of sgp130 at term. Understanding the subtleties of this regulatory process is important because an increased level of sgp130 can render cells unresponsive to IL-6 and the family of gp130 cytokines. Data derived from the human endometrium demonstrated that sgp130 was downregulated in the proliferative but upregulated during the secretory phase of the menstrual cycle (46). This suggests that hormonal factors such as estrogen and progesterone might impact the synthesis and release of sgp130 (46,47). Whether the functional progesterone withdrawal characteristic to human pregnancy is responsible or linked to the decreased AF levels of sgp130, at term, remains unknown.
Mitchell et al. indicated that IL-6 signaling through the classic pathway was responsible for the increased production of prostaglandins by amnion and decidual cells both term and preterm (48,49). The observed increased sIL-6R/sgp130 molar ratio at term implies that the IL-6 trans-signaling pathway becomes more active towards the end of gestation. Withdrawal of the trans-signaling system inhibition could enhance the process of IL-6-induced prostaglandin production and subsequently facilitate the onset of term human parturition. The MMP-9 release was inhibited when amniochorion was incubated with sgp130 plus sIL-6R in the absence of LPS stimulation. Therefore, we propose that lower levels of AF sgp130 toward term may predispose fetal membranes to rupture.
There is evidence to sustain the view that the sharp increase in the AF IL-6 levels occurs secondary to microbial invasion of the amnion and choriodecidua (34,35). This is further supported by our in-vitro experiments that demonstrate the amplified release of IL-6 following incubation of fetal membranes with LPS. What mediates the trans-membrane passage of IL-6 into AF remains largely unknown (35), but angiopoietins may play a significant role (50). We determined that the fetal membrane IL-6R mRNA levels were upregulated while the expression of gp130 was reduced in IAI. First, these results imply that in IAI the amniochorion is a functional target for IL-6 classic signaling. Second, incubation of the amniochorion with sIL-6R, sgp130 and their combination did not enhance the release of IL-6. From this perspective, components of the IL-6 trans-signaling pathway play a limited role in the process of amniochorion IL-6 protein synthesis and/or release.
In our study it was evident that AF sIL-6R levels were upregulated in relationship with IAI and the steady-state amniochorion, but not placental IL-6R mRNA and sIL-6R protein levels were significantly enhanced by IAI. The mechanisms responsible for the proteolytic release of the ecto-domain of IL-6R and IL-6R alternative splicing rearrangements are likely to play an integral role in promoting an elevation of the AF sIL-6R levels during microbial (i.e. Streptococcus, Escherichia coli) invasion of fetal membranes. The proof of concept that exacerbation of the TACE enzymatic activity and shedding of the IL-6R could be evoked by bacterial MMPs and toxins (i.e. streptolysin-O, Escherichia coli-hemolysin) was previously provided (51). In addition, bioavailability of the AF sIL-6R could occur through functional amplification of the alternative splicing apparatus in the amnion and choriodecidua. Experiments to substantiate this hypothesis are needed.
We found that women with PPROM and IAI have lower IL-6 levels than women with IAI but intact membranes. No prospective study has targeted a comparison of the two clinical categories. Yet, studies in PPROM women report lower optimal diagnostic cut-offs for IL-6 than studies limited to intact subjects, which would be in agreement with our data (14). Our finding that PPROM and especially PPROM & IAI women have lower sgp130 in AF is novel. One possible explanation may be that similar to other soluble antagonists (i.e. soluble RAGE) (21), sgp130 becomes consumed while binding the IL-6/IL-6R complex in the process of trans-signaling inhibition (52). Alternatively, gp130 itself may be down-regulated as processes terminating IL-6 signaling become activated (53). Lastly, transcriptional and/or shedding processes responsible for synthesis and release of sgp130 may be altered in a subgroup of women and these events may facilitate rupture of the membranes.
RT-PCR and Western blotting results revealed down-regulation in expression of fetal membrane gp130 in the setting of IAI. In addition, by Western blotting we demonstrated a significant down-regulation in the synthesis and release of sgp130 protein in relationship to IAI. This could be an alternative explanation for the decreased sgp130 in women with PPROM & IAI. Binding of IL-6 to sIL6-R is known to prolong IL6’s half-life (54). Although relevant for complex systems harboring all IL-6 trans-signaling components (i.e. amniotic cavity), the direct role played by sgp130 in modulating the clearance of IL-6 or IL-6/sIL6-R complexes has not been explored.
A relevant question is whether IL-6 plays a direct role in events leading to PTB. In an infection-induced mouse model of PTB, acute administration of recombinant IL-6 did not result in significant shortening of gestation (55). This observation would argue that IL-6 has limited biological relevance for parturition. However, recent evidence suggests the contrary (56). By using IL-6 deficient animals, Robertson et al. demonstrated that IL-6 deletion results in delayed normal timing of delivery (~24 hours) (56). Chronic infusion of IL-6 in IL-6-/-mice, restored the normal timing of delivery. Maternal progesterone levels remained unaltered by IL-6 manipulation, despite remarkable changes of a wide array of genes with critical roles in parturition. Together, this data support the argument that IL-6 is instrumental in regulating the timing of delivery in normal gestation and in infection-induced PTB in a manner independent of luteolysis.
We found that women with PPROM and longer latency intervals had higher levels of sgp130. This argues that in these women, the inflammatory process involved in activation of uterine contractility could be suppressed for a longer period. The observation that PPROM women had decreased AF sgp130 levels and an increased sIL-6R/sgp130 molar ratio was provocative. Our in-vitro experiments provide evidence for a mechanism through which AF sgp130 may prevent weakening of the fetal membranes in pregnancies complicated by infection. As shown, incubation of the fetal membranes with LPS induced a significant increase in the release of MMP-9. The recombinant sgp130 reversed the process.
The acute inflammatory response involves immediate access of the neutrophils at the site of a bacterial attack (1,3,18,34). Our immunohistochemistry results established concurrent homing of CD15+ neutrophils and CD3+ T-cells in the choriodecidua of women with IAI. Given that resident cells of these tissues express IL-6, IL-6R, sIL-6R, gp130 and sgp130, we argue that in chorioamnionitis the classic and trans-signaling mechanisms are both engaged in the process of leukocyte trafficking and activation (34). Generation of sIL-6R may therefore represent a ratelimiting event in the regulation of these processes distinguishing between physiological and pathological events orchestrated by IL-6.
Decidual T cells are sparse in early pregnancy (57). At term, however, 45–50% of the leukocytes in the decidua basalis are CD3+ representing one of the most abundant leukocyte populations. The choriodecidua T cells population is thought to participate in defense mechanisms against pathogens, particularly during parturition and in regulation of chronic inflammatory processes localized at the fetal-maternal interface (57). Studies have documented key roles for IL-6 trans-signaling in leukocyte trafficking and activation. In particular, IL-6 trans-signaling driven STAT3 activity is important for T cell recruitment during successful resolution of any inflammatory response and transition from innate to acquired immunity (58). Therefore, we posit that downregulation of gp130 approaching term may facilitate IL-6 trans-signaling and physiological T-cell recruitment in the choriodecidua, in anticipation of parturition.
In summary, we found that IL-6 trans-signaling molecules are physiologic constituents of the AF with both GA and inflammatory regulation. The decreased AF levels of sgp130 in women with ruptured membranes and the demonstrated inhibitory effect of sgp130 on MMP-9 release suggest that IL-6 trans-signaling may play a critical role in PPROM. Similar to inflammatory arthritis, where intra-articular injection of recombinant sgp130 effectively prevented joint destruction (59), we suggest that targeting of IL-6 trans-signaling pathway may have potential for preventing PTB and PPROM (56).
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the nurses, fellows, residents and faculty at Yale-New Haven Hospital, the Yale Department of Obstetrics and Gynecology and Reproductive Sciences and to all women who participated in the study.
This work was supported from National Institutes of Health Grant RO1 HD 047321 (IAB), R01 HD062007-01(CSB & IAB) and Departmental funds.
ABBREVIATIONS USED IN THIS PAPER
- PTB
preterm birth
- GA
gestational age
- ECM
extracellular matrix
- PPROM
preterm premature rupture of membranes
- AF
amniotic fluid
- IAI
intra-amniotic inflammation
- sIL-6R
soluble IL-6R
- sgp130
soluble gp130
Footnotes
CONTRIBUTIONS TO AUTHORSHIP
SYL, IAB and CSB formulated the hypothesis, designed the study, analyzed and interpreted the data and drafted the manuscript. SYL, CSB, ATD, UAA, SAA-R, MOB, ST and EF collected biological specimens, recruited and followed the patients prospectively to the point of delivery. SYL, GZ, UA, ATD, CSB and IAB conducted the ELISA assays, the immunohistochemistry, Western blot and PCR experiments. All the co-authors participated with aspects of study design, critical interpretation of the data, contributed to writing of the paper and have reviewed and approved the final version.
DISCLOSURES: The authors have nothing to disclose.
REFERENCES
- 1.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod. Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 3.Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S, Buhimschi IA. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J. Immunol. 2009;182:7244–7253. doi: 10.4049/jimmunol.0803517. [DOI] [PubMed] [Google Scholar]
- 4.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 5.Rose-John S, Waetzig GH, Scheller J, Grötzinger J, Seegert D. The IL-6/sIL- 6R complex as a novel target for therapeutic approaches. Expert. Opin. Ther. Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lust JA, Donovan KA, Kline MP, Greipp PR, Kyle RA, Maihle NJ. Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4:96–100. doi: 10.1016/1043-4666(92)90043-q. [DOI] [PubMed] [Google Scholar]
- 8.Nishino E, Matsuzaki N, Masuhiro K, Kameda T, Taniguchi T, Takagi T, Saji F, Tanizawa O. Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. J. Clin. Endocrinol. Metab. 1990;71:436–441. doi: 10.1210/jcem-71-2-436. [DOI] [PubMed] [Google Scholar]
- 9.Diamant M, Rieneck K, Mechti N, Zhang XG, Svenson M, Bendtzen K, Klein KB. Cloning and expression of an alternatively spliced mRNA encoding a soluble form of the human interleukin-6 signal transducer gp130. FEBS Lett. 1997;412:379–384. doi: 10.1016/s0014-5793(97)00750-3. [DOI] [PubMed] [Google Scholar]
- 10.Hurst SM, Wilkinson TS, McLoughlin SM, Jones RM, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 11.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008;181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, Rose-John S, von Andrian UH, Baumann H, Evans SS. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat. Immunol. 2006;7:1299–1308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 13.Myatt L, Sun K. Role of fetal membranes in signaling of fetal maturation and parturition. Int. J. Dev. Biol. 2010;54:545–553. doi: 10.1387/ijdb.082771lm. [DOI] [PubMed] [Google Scholar]
- 14.Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, Pettker CM, Magloire L, Funai E, Norwitz ER, Paidas M, Copel JA, Weiner CP, Lockwood CJ, Buhimschi IA. Proteomic Profiling of the Amniotic Fluid to Detect Inflammation, Infection, and Neonatal Sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACOG Practice Bulletin No. 80: premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. ACOG Committee on Practice Bulletins- Obstetrics. Obstet. Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 16.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of AF for identification of intra-amniotic inflammation. BJOG. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 17.von Bismarck P, Claass A, Schickor C, Krause MF, Rose-John S. Altered pulmonary interleukin-6 signaling in preterm infants developing bronchopulmonary dysplasia. Exp. Lung. Res. 2008;34:694–706. doi: 10.1080/01902140802389693. [DOI] [PubMed] [Google Scholar]
- 18.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet. Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 19.Lockwood CJ, Toti P, Arcuri F, Paidas M, Buchwalder L, Krikun G, Schatz F. Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombinenhanced interleukin-8 expression in term decidua. Am. J. Pathol. 2005;167:1443–1449. doi: 10.1016/S0002-9440(10)61230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum. Pathol. 1998;29:1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 21.Buhimschi IA, Zhao G, Pettker CM, Bahtiyar MO, Magloire LK, Thung S, Fairchild T, Buhimschi CS. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am. J. Obstet. Gynecol. 2007;196:181, e1–e13. doi: 10.1016/j.ajog.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Lemmers A, Gustot T, Durnez A, Evrard S, Moreno C, Quertinmont E, Vercruysse V, Demetter P, Franchimont D, Le Moine O, Geerts A, Devière J. An inhibitor of interleukin-6 trans-signalling, sgp130, contributes to impaired acute phase response in human chronic liver disease. Clin. Exp. Immunol. 2009;156:518–527. doi: 10.1111/j.1365-2249.2009.03916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wånggren K, Lalitkumar PG, Hambiliki F, Ståbi B, Gemzell-Danielsson K, Stavreus- Evers A. Leukaemia inhibitory factor receptor and gp130 in the human Fallopian tube and endometrium before and after mifepristone treatment and in the human preimplantation embryo. Mol. Hum. Reprod. 2007;13:391–397. doi: 10.1093/molehr/gam013. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Ponchel F, Toomes C, Bransfield K, Leong FT, Douglas SH, Field SL, Bell SM, Combaret V, Puisieux A, Mighell AJ, Robinson PA, Inglehearn CF, Isaacs JD, Markham AF. Real-time PCR based on SYBR-Green I fluorescence: an alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003;3:18. doi: 10.1186/1472-6750-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horiuchi S, Koyanagi Y, Zhou Y, Miyamoto H, Tanaka Y, Waki M, Matsumoto A, Yamamoto M, Yamamoto N. Soluble interleukin-6 receptors released from T cell or granulocyte/macrophage cell lines and human peripheral blood mononuclear cells are generated through an alternative splicing mechanism. Eur. J. Immunol. 1994;24:1945–1948. doi: 10.1002/eji.1830240837. [DOI] [PubMed] [Google Scholar]
- 27.Narazaki M, Yasukawa K, Saito T, Ohsugi Y, Fukui H, Koishihara Y, Yancopoulos GD, Taga T, Kishimoto T. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82:1120–1126. [PubMed] [Google Scholar]
- 28.Stephenson CD, Lockwood CJ, Ma Y, Guller S. Thrombin-dependent regulation of matrix metalloproteinase (MMP)-9 levels in human fetal membranes. J Matern. Fetal. Neonatal. Med. 2005;18:17–22. doi: 10.1080/14767050500123632. [DOI] [PubMed] [Google Scholar]
- 29.Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol. Reprod. 2002;67:1952–1958. doi: 10.1095/biolreprod.102.004721. [DOI] [PubMed] [Google Scholar]
- 30.Fortunato SJ, Menon R, Swan KF, Lyden TW. Organ culture of amniochorionic membrane in vitro. Am. J. Reprod. Immunol. 1994;32:184–187. doi: 10.1111/j.1600-0897.1994.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 31.Magloire LK, Buhimschi CS, Pettker CM, Sfakianaki AK, Hamar BD, Bhandari V, Buhimschi IA. Lactate dehydrogenase isoform activity mapping in patients with intra-amniotic infection. Am. J. Obstet. Gynecol. 2006;195:1045–1052. doi: 10.1016/j.ajog.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 32.El Mansouri L, Bahiri R, Abourazzak FE, Abouqal R, Hajjaj-Hassouni N. Two distinct patterns of ankylosing spondylitis in Moroccan patients. Rheumatol. Int. 2009;29:1423–1429. doi: 10.1007/s00296-009-0873-z. [DOI] [PubMed] [Google Scholar]
- 33.Müllberg J, Oberthür W, Lottspeich F, Mehl E, Dittrich E, Graeve L, Heinrich PC, Rose-John S. The soluble human IL-6 receptor. Mutational characterization of the proteolytic cleavage site. J. Immunol. 1994;152:4958–1468. [PubMed] [Google Scholar]
- 34.Lockwood CJ, Murk WK, Kayisli UA, Buchwalder LF, Huang SJ, Arcuri F, Li M, Gopinath A, Schatz F. Regulation of Interleukin-6 Expression in Human Decidual Cells and Its Potential Role in Chorioamnionitis. Am. J. Pathol. 2010;177:1755–1764. doi: 10.2353/ajpath.2010.090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol. Reprod. 1997;57:1438–1444. doi: 10.1095/biolreprod57.6.1438. [DOI] [PubMed] [Google Scholar]
- 36.Tenhumberg S, Waetzig GH, Chalaris A, Rabe B, Seegert D, Scheller J, Rose-John S, Grötzinger J. Structure-guided optimization of the interleukin-6 trans-signaling antagonist sgp130. J. Biol. Chem. 2008;283:27200–27207. doi: 10.1074/jbc.M803694200. [DOI] [PubMed] [Google Scholar]
- 37.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 38.Chow SS, Craig ME, Jones CA, Hall B, Catteau J, Lloyd AR, Rawlinson WD. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. 2008;44:78–84. doi: 10.1016/j.cyto.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Opsjłn SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am. J. Obstet. Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 40.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simón C, Frances A, Piquette G, Hendrickson M, Milki A, Polan ML. Interleukin-1 system in the materno-trophoblast unit in human implantation: immunohistochemical evidence for autocrine/paracrine function. J. Clin. Endocrinol. Metab. 1994;78:847–854. doi: 10.1210/jcem.78.4.8157710. [DOI] [PubMed] [Google Scholar]
- 42.Hung TH, Chen SF, Hsu JJ, Hsieh CC, Hsueh S, Hsieh TT. Tumour necrosis factor-alpha converting enzyme in human gestational tissues from pregnancies complicated by chorioamnionitis. Placenta. 2006;27:996–1006. doi: 10.1016/j.placenta.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Müllberg J, Dittrich E, Graeve L, Gerhartz C, Yasukawa K, Taga T, Kishimoto T, Heinrich PC, Rose-John S. Differential shedding of the two subunits of the interleukin-6 receptor. FEBS Lett. 1993;332:174–178. doi: 10.1016/0014-5793(93)80507-q. [DOI] [PubMed] [Google Scholar]
- 44.Geisterfer M, Richards CD, Gauldie J. Cytokines oncostatin M and interleukin 1 regulate the expression of the IL-6 receptor (gp80, gp130) Cytokine. 1995;7:503–509. doi: 10.1006/cyto.1995.0068. [DOI] [PubMed] [Google Scholar]
- 45.Ogata I, Shimoya K, Moriyama A, Shiki Y, Matsumura Y, Yamanaka K, Nobunaga T, Tokugawa Y, Kimura T, Koyama M, Azuma C, Murata Y. Oncostatin M is produced during pregnancy by decidual cells and stimulates the release of HCG. Mol. Hum. Reprod. 2000;6:750–757. doi: 10.1093/molehr/6.8.750. [DOI] [PubMed] [Google Scholar]
- 46.Sherwin JR, Smith SK, Wilson A, Sharkey AM. Soluble gp130 is upregulated in the implantation window and shows altered secretion in patients with primary unexplained infertility. J. Clin. Endocrinol. Metab. 2002;87:3953–3960. doi: 10.1210/jcem.87.8.8766. [DOI] [PubMed] [Google Scholar]
- 47.Canellada AI Alvarez, Berod L, Gentile T. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J. Steroid Biochem. Mol. Biol. 2008;2111:255–261. doi: 10.1016/j.jsbmb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 48.McEwan M, Lins RJ, Munro SK, Vincent ZL, Ponnampalam AP, Mitchell MD. Cytokine regulation during the formation of the fetal-maternal interface: focus on cell-cell adhesion and remodeling of the extra-cellular matrix. Cytokine Growth Factor Rev. 2009;20:241–249. doi: 10.1016/j.cytogfr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur. J. Pharmacol. 1991;192:189–191. doi: 10.1016/0014-2999(91)90090-d. [DOI] [PubMed] [Google Scholar]
- 50.Buhimschi CS, Bhandari V, Dulay AT, Thung S, Abdel-Razeq SS, Rosenberg V, Han CS, Ali UA, Zambrano E, Zhao G, Funai EF, Buhimschi IA. Amniotic fluid angiopoietin-1, angiopoietin-2, and soluble receptor tunica interna endothelial cell kinase-2 levels and regulation in normal pregnancy and intraamniotic inflammation-induced preterm birth. J. Clin. Endocrinol. Metab. 2010;95:3428–3436. doi: 10.1210/jc.2009-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walev I, Vollmer P, Palmer M, Bhakdi S, Rose-John S. Pore-forming toxins trigger shedding of receptors for interleukin 6 and lipopolysaccharide. Proc. Natl. Acad. Sci. U S A. 1996;93:7882–7887. doi: 10.1073/pnas.93.15.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, Bhandari V, Ehrenkranz RA, Weiner CP, Madri JA, Buhimschi IA. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am. J. Pathol. 2009;175:958–975. doi: 10.2353/ajpath.2009.090156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrich PC, Bode J, Decker M, Graeve L, Martens A, Müller-Newen G, Pflanz S, Schaper F, Schmitz J. Termination and modulation of IL-6-type cytokine signaling. Exp. Med. Biol. 2001;495:153–160. doi: 10.1007/978-1-4615-0685-0_20. [DOI] [PubMed] [Google Scholar]
- 54.Peters M, Jacobs S, Ehlers M, Vollmer P, Müllberg J, Wolf E, Brem G, Meyer zum Büschenfelde KH, Rose-John S. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J. Exp. Med. 1996;183:1399–1406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura K, Hirsch E. Interleukin-6 is neither necessary nor sufficient for preterm labor in a murine infection model. J. Soc. Gynecol. Investig. 2003;10:423–427. doi: 10.1016/s1071-5576(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 56.Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology. 2010;151:3996–4006. doi: 10.1210/en.2010-0063. [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J. Leukoc. Biol. 2010;88:625–633. doi: 10.1189/jlb.1209796. [DOI] [PubMed] [Google Scholar]
- 58.McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nowell MA, Richards PJ, Horiuchi S, Yamamoto N, Rose-John S, Topley N, Williams AS, Jones SA. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J. Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.