Abstract
Background
Using three independent methods, prior studies in Swedish sibling pairs indicate that environmental factors contribute substantially to familial aggregation for drug abuse (DA). Could we replicate these results in cousin pairs?
Method
Using multiple Swedish public databases (1964–2011), we defined DA using medical, legal or pharmacy registry records and examined concordance in full cousin pairs as a function of age differences, younger–older relationships and geographical proximity while growing up.
Results
Replicating prior results in siblings, cousin pairs were significantly more similar in their history of DA if they were (i) closer versus more distant in age and (ii) grew up in high versus low geographical proximity to one another. Furthermore, controlling for background factors, having an older cousin with DA conveys a greater risk for DA than having a younger drug-abusing cousin. The greater transmission of DA from older to younger versus younger to older cousin was more prominent in pairs who grew up close to one another. In age difference and geographical proximity analyses, effects were consistently strongest in male–male cousin pairs. In analyses of older → younger versus younger → older transmission, effects were stronger in male–male and male–female than in female–female or female–male relative pairs.
Conclusions
In accord with prior results in siblings, environmental factors contribute substantially to the familial aggregation of DA in cousins and these effects are, in general, stronger in males than in females.
Keywords: Drug abuse, environmental transmission, epidemiology, genetics, Sweden
Introduction
Drug abuse (DA) is strongly familial (Merikangas et al. 1998; van den Bree et al. 1998). Although twin and adoption studies consistently show that genetic factors contribute substantially to this familial aggregation (Cadoret et al. 1986, 1995, 1996; Tsuang et al. 1996; Kendler & Prescott, 1998; Kendler et al. 2000; Lynskey et al. 2002), most, but not all, studies also show significant familial–environmental effects (Cadoret et al. 1986, 1996; Tsuang et al. 1996; Kendler et al. 2000; Lynskey et al. 2002). In a national Swedish adoption study of DA, we recently found that, in addition to strong genetic effects, familial environmental risk, indexed by disruptions in the adoptive home and DA in adoptive siblings, contributed significantly to liability to DA in the adoptees (Kendler et al. 2012).
We have further examined the role of familial–environmental factors in the etiology of DA in Swedish sibling pairs using three distinct and novel designs. First, within sibling pairs, familial resemblance for DA was inversely related to age difference (Kendler et al. 2013). That is, risk for DA was more highly correlated in siblings born within a few years of one another than in pairs born many years apart. Second, we observed stronger transmission of risk for DA from older to younger than from younger to older siblings (Kendler et al. 2013). Third, within full- and half-sibling pairs, risk for DA was positively correlated with the length of time they resided together in the same household or small geographical region when growing up (Kendler et al., unpublished observations).
The aim of the current study was to replicate and extend these findings by applying these three same designs to familial resemblance for DA in first-cousin pairs in a Swedish national sample. We sought to address the following four questions:
Will resemblance for DA within first-cousin pairs be greater in those closer versus more distant in age?
Will risk for DA be greater when a drug-abusing cousin is older versus younger?
In first-cousin pairs, will resemblance for DA be correlated with their geographical proximity when growing up?
Would the impact of age differences on DA resemblance or the older to younger versus younger to older transmission of DA would be more potent in cousin pairs who grew up closer versus more distant geographically from one another?
Method
As in previous studies, we used linked data from multiple Swedish nationwide registries and healthcare data (Kendler et al. 2012, 2013, unpublished observations). Linkage was achieved by using the unique individual Swedish 10-digit personal ID number assigned at birth or immigration to all Swedish residents. This ID number was replaced by a serial number to preserve confidentiality.
The following sources were used to create our database: the Total Population Register, containing annual data on family and geographical status from 1990 to 2010; the Multi-Generation Register, providing information on family relationships; the Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish inhabitants from 1964 to 2010; the Swedish Prescribed Drug Register, containing all prescriptions in Sweden picked up by patients from 2005 to 2009; the Out-patient Care Register, containing information from all out-patient clinics from 2001 to 2009; the Primary Health Care Register, which includes out-patient primary care data on diagnoses and time for diagnoses 2001–2007 for 1 million patients from Stockholm and middle Sweden; the Swedish Crime Register, which includes national complete data on all convictions from 1973 to 2011, and the Swedish Suspicion Register, which includes national complete data on all individuals strongly suspected of crime from 1998 to 2011 (for convenience, these two registers are referred to as the ‘criminal register’); the Swedish Mortality Register, containing causes of death; and the Population and Housing Censuses, providing information on household and geographical status in 1960, 1965, 1970, 1975, 1980 and 1985. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409).
DA was identified in the Swedish medical and mortality registries by ICD codes [ICD-8: drug dependence (304); ICD-9: drug psychoses (292) and drug dependence (304); ICD-10: mental and behavioral disorders due to psychoactive substance use (F10–F19), except those due to alcohol (F10) or tobacco (F17)]; in the Suspicion register by codes 3070, 5010, 5011 and 5012, which reflect crimes related to DA; and in the Crime register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). DA was identified in individuals (excluding those suffering from cancer) in the Prescribed Drug Register who had retrieved (on average) more than four defined daily doses a day for 12 months from either Hypnotics and Sedatives [Anatomical Therapeutic Chemical (ATC) Classification System N05C and N05BA] or Opioids (ATC: N02A). We restricted the diagnosis of DA to individuals above the age of 10 years except from the prescribed drug register, where the age limit was set at 18 years.
Sample
We created our cousin database by entering all first-cousin pairs born between 1950 and 1993 in the Swedish population and we required that (1) both cousins in the pair were alive after 1972; (2) both cousins could, sometime during the period 1960–2010, be linked to a household and a geographical area; and (3) the age difference between the cousins did not exceed 18 years. A full-cousin pair was identified through their sharing of the same two grandparents.
From the Population and Housing Census and the Total Population Register, we included information on small area market statistics (SAMS) for each cousin within each pair. In Sweden, SAMS are geographical units that have boundaries defined by homogeneous types of buildings and have an average of 1000 to 2000 inhabitants. However, between 1960 and 1985 we had no information on SAMS, and therefore used parishes as a proxy for SAMS. The parishes serve as districts for the Swedish census and elections, and have approximately the same number of inhabitants as SAMS. This information allowed us to calculate the number of years each cousin pair resided within the same SAMS. As the median age of leaving home was approximately 21 in Sweden during this time period (Yi et al. 1994), we set the upper limit for the number of years living in the same SAMS to 21. However, as the age differences between cousins set a limit on the number of possible years of living together, we subtracted the number of years between cousins from 21. That is, for example, cousins born 2 years apart had only 19 possible years of living in the same SAMS.
The procedure for retrieving and filtering data on SAMS was repeated for municipalities to calculate the number of years each cousin pair resided within the same municipality. The municipalities of Sweden are its lower-level local government entities and are responsible for a large proportion of local services. Today there are 290 municipalities in Sweden with populations ranging from approximately 3000 to 870 000. The data structure was hierarchical with SAMS nested within municipalities.
We divided all pairs into three geographical proximity groups: high, medium and low. High proximity was defined as pairs who both spent ➮75% of their possible years during childhood and adolescence residing in the same SAMS. Intermediate proximity was defined as pairs not meeting criteria for high proximity but spending ➮75% of their possible years residing in the same municipality. The low proximity group includes all pairs who met criteria for neither high nor intermediate proximity.
Statistical methods
We conducted two main analyses. First, we looked at aggregation of DA among cousins and examined resemblance as a function of their age differences. Probands were defined as individuals registered for DA from 1973 to 2011, who had at least one cousin living in Sweden 1973–2011. For each DA proband, we specified all possible case-cousin pairs consisting of the proband and each of their cousins (who were or were not registered as DA cases). For each pair, we randomly selected five control-cousin pairs matched to case pairs by gender, year and country of birth, geographical proximity and education. Individuals were eligible as controls if they lived in Sweden at the time of the case’s DA registration, and were not registered as DA prior to the time of the case’s registration. They could, however, be registered later as DA.
Analyses were conducted using conditional logistic regression. In the first model, DA in the cousin (yes/no) was used as the independent variable and we also included an interaction term between age difference (between cousins in the pair) and DA in the cousin. We examined linear and quadratic effects for this age difference. In the second step we stratified our models into four different gender groups: male–male, male–female (i.e. the case is male, the cousin female), female–male and female–female.
Our second set of analyses examined the difference in risk to a younger versus older cousin of a proband with DA. From our cousin database, we selected all pairs where at least one individual, the proband, had DA. For cousin pairs where both had DA, the first individual found in the DA register was selected as the proband and the second as the cousin. Because of the possibility of differential right censoring (the older cousin will always have more years of exposure), we used a Cox proportional hazards regression model with DA in the cousin as the outcome. The key predictor was a dummy variable that defined whether the cousin was younger or older than the proband. Control variables included linear effects of age difference between the cousin and the proband, the gender of the proband and of the cousin, birth cohort and relevant interactions. The grandparents were used as the cluster variable when adjusting for the non-independence of the cousin pairs. We tested the proportional hazards assumption for the different variables in the models. In situations where it was not fulfilled, we included an interaction term between follow-up time and the variable of interest.
All analyses were repeated for the three previously described geographical proximity groups. As a proband could be included several times in the analyses, we adjusted for the non-independence with a robust sandwich estimator in all models. The statistical analyses were performed using SAS version 9.2 (SAS Institute, 2008). Although we had clear directional hypotheses for all of our tests, to be conservative we report two-tailed p values.
Results
Descriptive statistics
Our total sample included 18842692 first-cousin pairs, of whom 39366 were concordant for DA. The tetrachoric correlation (±s.e.) for DA in these pairs was +0.141±0.001 and the odds ratio (OR) was 2.00 [95% confidence interval (CI) 1.97–2.02]. The mean age difference between these pairs was 7.73 years (s.d.=6.06). Resemblance for DA was highest in male–male pairs (0.179±0.002, OR 2.14, 95% CI 2.11–2.18), intermediate in female–female pairs (0.117±0.004, HR 1.95, 95% CI 1.88–2.02) and lowest in opposite-sex pairs (0.084±0.002, HR 1.53, 95% CI 1.50–1.55).
The prevalence of DA in this cousin sample was 3.4% with the following prevalences in the different registry sources: discharge 1.06%, out-patient 0.76%, prescription 0.14%, primary care 0.01%, mortality 0.04%, and criminal 2.68%. The ORs for cross-registration from DA from these six sources are shown in Table 1. With the exception of the prescription and mortality registries, the ORs were all in excess of 5.0 and all but one greater than 10.0.
Table 1.
Odds ratios (and 95% confidence intervals) for the registration of drug abuse between the five registers used in this study
| Out-patient | Prescribed | Primary care | Mortality | Criminal | |
|---|---|---|---|---|---|
| Discharge | 221.9 (215.3–228.7) | 44.2 (41.3–47.3) | 130.6 (106.5–160.0) | 158.1 (140.3–178.1) | 50.3 (49.2–51.5) |
| Out-patient | 52.2 (48.6–56.0) | 123.2 (106.6–159.2) | 22.9 (19.5–27.0) | 73.5 (70.5–74.5) | |
| Prescribed | 58.7 (39.9–86.4) | 1.3 (0.3–5.1) | 11.6 (10.7–12.4) | ||
| Primary care | 6.6 (0.9–47.1) | 78.8 (63.5–97.8) | |||
| Mortality | 30.9 (27.6–34.7) |
Age differences in cousin pairs
We identified 219607 proband-cousin pairs with DA (mean year of birth 1977 and s.d.=10.3; 51% male) who could be matched with 1098035 control proband-cousin pairs. We found a modest and significant interaction between DA in the cousin and age difference in the pair (OR 0.99, 95% CI 0.98–0.997, p=0.004), such that the risk for DA was higher in cousins born closer together. For cousins born in the same year, the predicted OR for DA was 1.88, in comparison to 1.79, 1.70 and 1.62 for those born 5, 10 and 15 years apart respectively. Quadratic effects of age were tested and were not significant.
We then examined this association by the sex composition of the cousin. We found a significant linear interaction between DA in the cousin and age differences in both same-sex pairs (male–male: OR 0.99, 95% CI 0.99–0.998, p=0.01) and female–female pairs (OR 0.99, 95% CI 0.98–0.998, p=0.02) but in neither of the opposite-sex pairs [male–female (i.e. male proband, female cousin) p=0.76; female–male pairs p=0.64].
Transmission of DA from older to younger versus younger to older cousins
We examined 565346 pairs containing at least one proband cousin with DA. Our Cox model predicted the hazard ratio (HR) for DA in the cousin of an affected proband. Controlling for the linear effect of the age difference between the cousins, sex combination of the cousin pair, birth cohort and interaction terms, the HR for DA in the cousin was significantly greater when the proband was older than when the proband was younger (HR 1.42, 95% CI 1.37–1.47) (Table 2). However, the proportional hazards assumption was not fulfilled for this variable and the interaction term between follow-up time and DA in the older cousin (HR 1.18, 95% CI 1.15–1.20) produced a final HR that became slowly stronger the longer the follow-up period (Fig. 1). The effect of having an older versus a younger cousin with DA was similar in male–male and male–female pairs (male older, female younger) but significantly weaker in female–female pairs (HR 1.21) and in female–male pairs (HR 1.31).
Table 2.
Results from our full Cox proportional hazards model for risk for drug abuse (DA) as a function of whether the DA-affected cousin was older versus younger
| Variable | HR (95% CI) | χ2, df=1 | p value |
|---|---|---|---|
| Older versus younger cousin | 1.42 (1.37–1.47) | 341.1 | <0.0001 |
| Older versus younger cousin × log(follow-up) | 1.18 (1.15–1.20) | 268.4 | <0.0001 |
| Age differences | 0.94 (0.93–0.94) | 923.0 | <0.0001 |
| Age differences × log(follow-up) | 1.03 (1.03–1.03) | 502.3 | <0.0001 |
| Gender | |||
| Male–male | Reference | ||
| Male–female | 0.35 (0.34–0.37) | 3017.4 | <0.0001 |
| Female–female | 0.41 (0.37–0.44) | 429.7 | <0.0001 |
| Female–male | 0.90 (0.85–0.96) | 11.5 | <0.0001 |
| Interaction terms | |||
| Older cousin × female–female | 0.85 (0.76–0.94) | 8.9 | 0.0029 |
| Older cousin × female–male | 0.92 (0.85–0.99) | 4.6 | 0.0322 |
HR, Hazard ratio; CI, confidence interval; df, degrees of freedom.
Fig. 1.
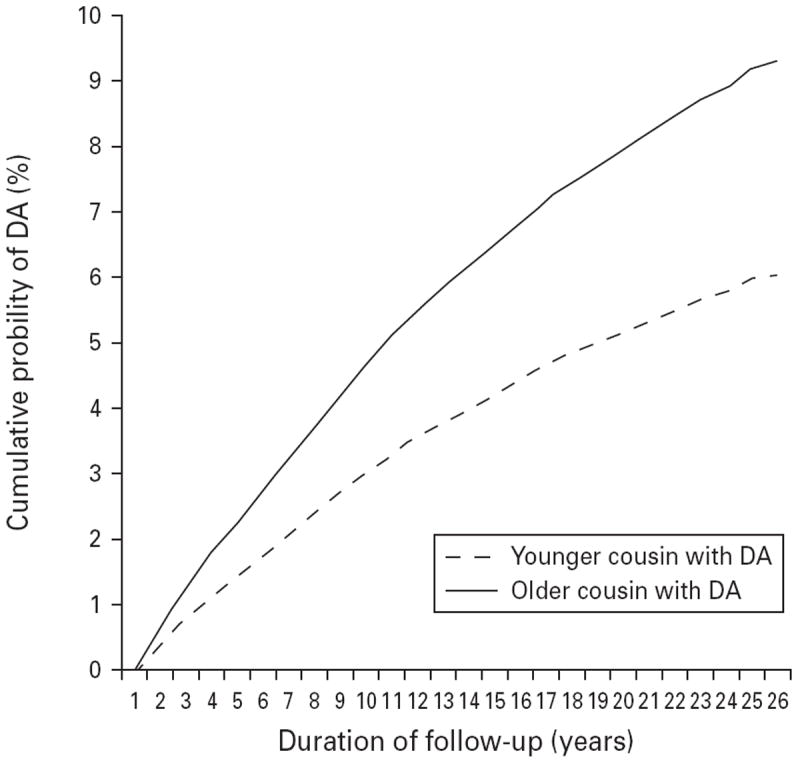
The cumulative probability of receiving a drug abuse (DA) diagnosis over a 26-year follow-up period for siblings, where the older versus younger sibling had DA as predicted from the full Cox model, the results of which are given in Table 2.
Resemblance and geographical proximity while growing up
We divided the cousin pairs into three groups as a function of their geographical proximity up to the year when the older member turned 21. Those with high, intermediate and low proximity constituted 13, 33 and 54% of all cousin pairs respectively. The ORs for DA in the cousin pairs with high, intermediate and low geographical proximity while growing up were 2.68 (95% CI 2.27–3.15), 1.90 (95% CI 1.81–1.99) and 1.79 (95% CI 1.76–1.83) respectively. The ORs for the latter two groups differed significantly from the OR found for the first group (both p<0.0001). The resemblance for DA differed by geographical proximity in male–male pairs (ORs of 3.37, 2.00 and 1.88 respectively; p<0.001) and in male–female pairs (ORs of 3.25, 1.73 and 1.75 respectively; p<0.03) but not for female–female or female–male pairs (all n.s.).
Age differences and older–younger transmission of DA by geographical proximity
We explored the association between age difference and resemblance for DA in first-cousin pairs in our three geographical proximity groups. The associations were not significant and did not differ across the three groups. We also repeated the analyses for older–younger transmission in our three cousin-pair groups divided by proximity of rearing. The increased risk of having an older versus a younger cousin with DA was substantially greater for those cousin pairs who grew up in high geographical proximity (OR 1.58, 95% CI 1.31–1.89) than in those with intermediate or low proximity (OR 1.36, 95% CI 1.27–1.45 and OR 1.37, 95% CI 1.32–1.43, respectively).
Discussion
Prior evidence largely from twin studies suggests that shared environmental factors contribute to the familial aggregation of drug use and DA. In these studies, the environment is not measured directly but is inferred from patterns of resemblance in monozygotic and dizygotic twins. We have developed new lines of evidence for familial environmental effects on DA using Swedish national data, first in an adoption sample (Kendler et al. 2012) and second by the application of three new methods to assess shared environmental exposures in sibling pairs unconfounded by genetic effects (Kendler et al. 2013, unpublished observations). In the current study we sought to replicate and extend these findings to pairs of first cousins. More specifically, we attempted to address four questions, which we review in turn.
First, we found in first-cousin pairs, consistent with our prior results in full siblings (Kendler et al. 2013), a significant inverse association between age differences and resemblance for DA. These results would make sense if age differences in relative pairs are a rough proxy for the degree of co-socialization. Given the strong evidence for the impact of peer groups on risk for DA and other externalizing behaviors (Kandel 1985; Hawkins et al. 1998; Petraitis et al. 1998; Harris 2002; Allen et al. 2003), it is plausible that resemblance for DA would relate to the degree of sharing of peer groups, which in turn would be roughly reflected by their age differences. In full siblings, the main linear effect on age differences on risk for DA was substantial, with an OR of 0.62/year when expressed in the model used in this study (but reduced in impact by an inverse quadratic effect). In first-cousin pairs, the association, although significant, was much weaker (and only linear), with an OR of 0.99. Given the greater average contact and peer sharing that would be expected between siblings than between cousins, it seems likely that the impact of age differences on resemblance for DA would be much stronger in the former than in the latter. However, we have no direct evidence that our findings arise from sharing of peers and they could be the result of other environmental factors that impact more strongly on individuals of similar age.
Second, we replicated in our cousin pairs prior results from full siblings where, controlling for background factors including age differences, having an older sibling with DA conveys a substantially greater risk for DA than having a younger drug-abusing sibling (Kendler et al., unpublished observations). These findings are consistent with several prior reports showing that siblings in general, or older siblings in particular, are frequently important role models and suppliers of drugs (Clayton & Lacy, 1982; Needle et al. 1986; Gfroerer, 1987; Duncan et al. 1996; Brook et al. 1999; Windle, 2000). However, unexpectedly, the magnitude of this effect (a 42% increased risk with an older versus a younger relative with DA) was exactly the same in the sibling and cousin pairs. While reflecting a ratio of risks, we nonetheless would have expected the impact of having a drug-abusing older versus younger cousin to be somewhat less robust than that seen in siblings.
Third, consistent with our prior analyses in full- and half-sibling pairs (Kendler et al., unpublished observations), resemblance for DA in first cousins was correlated with their geographical proximity when growing up. In this case, direct comparison of findings between these two groups of relatives is difficult. Our analytic approaches to the two groups of relatives differed substantially in part because siblings typically and cousins very rarely reside together in the same household through much of their childhood. For full and half-siblings, for each year of living in the same household or SAMS unit, the probability of sibling concordance for DA increased from 2% to 5%. Extrapolated over the expected years of cohabitation, we estimated much higher total ORs (~1.6–2.0). We used a cruder approach here, dividing our cousin pairs into three groups with high, intermediate and low geographical proximity for which we observed ORs for DA of 2.68, 1.90 and 1.79 respectively. Of note, the ratio of ORs between our high and low geographical proximity is 2.68/1.79=1.50, suggesting that the magnitude of the effect of geographical proximity on resemblance for DA might be only slightly less in cousins than is seen in full and half-siblings.
One methodological point in common to these three analyses is worth emphasizing. They are all able to isolate environmental from genetic sources of pair resemblance. In each case, genetic factors were controlled for by examining only first-cousin pairs, all of whom had the same degree of genetic relationship.
Our fourth and final question was: if the impact of age differences and older → younger versus younger → older transmission of resemblance for DA results from environmental contact and/or peer sharing between relatives, would these effects then be more potent in pairs growing up in closer versus more distant geographic areas? We found results consistent with our hypothesis in only one of our two relevant analyses. When we examined the relationship between age differences and sibling resemblance for DA in pairs divided into our three proximity groups, none of the comparisons alone were statistically significant (presumably due to loss of power), nor did they differ from one another. However, the risk for DA when an older versus a younger cousin also had DA was stronger in cousin pairs living in high versus intermediate or low proximity. We have no ready explanation for these somewhat contradictory findings except to note that the older → younger versus younger → older transmission for DA was much more robust in our cousin pairs than the association with age differences. Furthermore, we cannot be overly confident about this overall hypothesis because cousins residing far from one another might have a close relationship, meeting at family gatherings or vacations, and substantially influencing one another’s risk for DA. Alternatively, cousins living nearby might, for a range of reasons, have little to no contact.
We can also usefully compare results we obtained here in cousins to those found previously in same-and opposite-sex sibling pairs. We found much stronger associations between age differences and resemblance for DA in same-sex than in opposite-sex sibling pairs (Kendler et al. 2013). We obtained very similar findings here, where age differences significantly related to pair resemblance only in same-sex and not in opposite-sex cousin pairs. In our prior analysis of older → younger versus younger → older transmission for DA in sibling pairs, we found the largest effect in male–male and male–female pairs, which was significantly stronger than that found in female–female and female–male pairs. That is, the differences in having an older versus a younger drug-abusing brother were consistently greater than having an older versus a younger drug-abusing sister. We saw the same pattern in cousins, where the effect on risk for DA in an older versus younger cousins was greater when the affected member of the pair was male than when it was female. In our analysis of cohabitation histories in sibling pairs, we found the strongest effects in male–male pairs, intermediate effects in female–female pairs and the weakest effects in opposite-sex pairs (Kendler et al., unpublished observations). In cousins, we replicated one aspect of these analyses; our strongest effect of cohabitation was also found in male–male pairs.
Consistently across both sibling and cousin analyses, social environmental influences impacted on resemblance for DA most strongly in male–male pairs. These results are congruent with three other lines of evidence. First, in our twin-sibling structural modeling (Kendler et al., unpublished observations), we detected evidence for shared environmental effects on DA only for male–male pairs. Second, drug use in males may be more sensitive to the effect of peer groups than in females (Borsari & Carey, 2001; Graziano et al. 2012). In particular, in a Swedish high-school student survey, Svensson (2003) found males to be both more frequently exposed to and more sensitive to the effects of deviant peer groups. Third, in a national epidemiological analysis of Sweden, DA risk in men was more sensitive to levels of neighborhood-level deprivation than in women (Sundquist et al., unpublished observations).
Our results confirm those prior twin studies of DA that have shown an etiologic impact of shared environmental factors (Tsuang et al. 1996; Kendler et al. 2000; Lynskey et al. 2002). However, twin studies have also consistently shown substantial heritability for DA, with estimates ranging from 31% to 74% (Tsuang et al. 1996; van den Bree et al. 1998; Lynskey et al. 2002; Kendler et al. 2003). Our findings are congruent with a range of prior studies showing that the familial aggregation of DA is the result of both genetic and environmental risk factors (e.g. Kendler et al. 2000, 2012; Lynskey et al. 2002).
Limitations
These results should be interpreted in the context of four potentially important methodological limitations. First, this study was confined to one Scandinavian country and only further research can determine whether the findings would generalize to other cultural and ethnic groups. Second, subjects with DA were ascertained from medical, legal and pharmacy records. Contrary to standard epidemiological surveys, this approach has the advantage of ascertaining all cases in the population independent of subject cooperation. Furthermore, our diagnoses are based on objective records independent of subject recall. However, this method is very likely to lead to both false-negative and false-positive diagnoses. An epidemiological study of DA conducted in neighboring Norway reported rates of DA and drug dependence (Kraus et al. 2003; Hibell et al. 2007), assessed using DSM- III-R criteria (APA, 1987), of 3.4%, identical to that found by our registry-based methods (Kringlen et al. 2001). Substantial under-ascertainment of DA is unlikely.
Third, in our age difference analyses, our sample size of cousin pairs was lower because we could not obtain five control pairs for all of them. In particular, the proportion of male probands in these analyses was reduced. However, we repeated our analyses with only three control pairs and the pattern of findings did not change significantly.
Fourth, could our results arise from systematic police practices? If an individual was arrested for DA, would police provide closer surveillance to their cousin, leading to an increased likelihood of arrest and conviction? Might police keep under closer surveillance the younger versus the older cousin of a convicted drug abuser? To address this possible bias, we repeated all major analyses presented here, removing cases of DA identified only through the crime register. The overall results were similar but in fact showed slightly stronger familial–environmental effects. Thus, our findings on the impact of familial environment on DA in cousins could not plausibly result from biases in police practices.
Conclusions
Using a complete population sample of first-cousin pairs and nationwide ascertainment of DA from legal, medical and pharmacy records, we were able to replicate with considerable (but not perfect) consistency prior results with sibling pairs using three analytic designs. In both sibling and cousin pairs, resemblance for DA was found to be positively correlated with closeness in age. Controlling for background factors, risk for DA was greater if an individual had an affected older versus younger sibling and an affected older versus younger cousin. In both sibling and cousin pairs, resemblance for DA was greater in individuals who grew up in closer versus more distant geographical proximity to one another. Finally, in our age difference and geographical proximity analyses, these effects were consistently strongest in male–male sibling and cousin pairs. In our analyses of older → younger versus younger → older transmission, in both siblings and cousins, effects were stronger in male–male and male–female than in female–female or female–male relative pairs. Along with a range of prior studies, these findings together provide compelling evidence that shared environmental factors are of etiological importance for DA and contribute meaningfully to its strong tendency to run in families.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant R01 DA030005 and the Swedish Research Council and by a grant to Dr K. Sundquist from the Swedish Council for Information on Alcohol and Other Drugs (CAN).
Footnotes
Declaration of Interest
None.
References
- Allen M, Donohue WA, Griffin A, Ryan D, Turner MM. Comparing the influence of parents and peers on the choice to use drugs. Criminal Justice and Behavior. 2003;30:163–186. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. Third Edition. American Psychiatric Association; Washington, DC: 1987. Revised. [Google Scholar]
- Borsari B, Carey KB. Peer influences on college drinking: a review of the research. Journal of Substance Abuse. 2001;13:391–424. doi: 10.1016/s0899-3289(01)00098-0. [DOI] [PubMed] [Google Scholar]
- Brook JS, Brook DW, Whiteman M. Older sibling correlates of younger sibling drug use in the context of parent-child relations. Genetic, Social, and General Psychology Monographs. 1999;125:451–468. [PubMed] [Google Scholar]
- Cadoret RJ, Troughton E, O’Gorman TW, Heywood E. An adoption study of genetic and environmental factors in drug abuse. Archives of General Psychiatry. 1986;43:1131–1136. doi: 10.1001/archpsyc.1986.01800120017004. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Archives of General Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. An adoption study of drug abuse/dependency in females. Comprehensive Psychiatry. 1996;37:88–94. doi: 10.1016/s0010-440x(96)90567-2. [DOI] [PubMed] [Google Scholar]
- Clayton RR, Lacy WB. Interpersonal influences on male drug use and drug use intentions. International Journal of Addictions. 1982;17:655–666. doi: 10.3109/10826088209053009. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Hops H. The role of parents and older siblings in predicting adolescent substance use: modeling development via structural equation latent growth methodology. Journal of Family Psychology. 1996;10:158–172. [Google Scholar]
- Gfroerer J. Correlation between drug use by teenagers and drug use by older family members. American Journal of Drug and Alcohol Abuse. 1987;13:95–108. doi: 10.3109/00952998709001502. [DOI] [PubMed] [Google Scholar]
- Graziano F, Bina M, Giannotta F, Ciairano S. Drinking motives and alcoholic beverage preferences among Italian adolescents. Journal of Adolescence. 2012;35:823–831. doi: 10.1016/j.adolescence.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Harris JR. The Nurture Assumption: Why Children Turn Out the Way They Do. Touchstone/Simon & Schuster; New York, NY: 2002. [Google Scholar]
- Hawkins JD, Herrenkohl T, Farrington DP, Brewer D, Catalano RF, Harachi TW. A review of predictors of youth violence. In: Loeber R, Farrington DP, editors. Serious and Violent Juvenile Offenders: Risk Factors and Successful Interventions. Sage Publications, Inc.; London: 1998. pp. 106–146. [Google Scholar]
- Hibell B, Guttormsson U, Ahlstrom S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. The Swedish Council for Information on Alcohol and Other Drugs (CAN); Sweden: 2007. [Google Scholar]
- Kandel DB. On processes of peer influences in adolescent drug use: a developmental perspective. Advances in Alcohol and Substance Abuse. 1985;4:139–163. doi: 10.1300/J251v04n03_07. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Within-family environmental transmission of drug abuse: a Swedish national study. Archives of General Psychiatry. 2013;70:235–242. doi: 10.1001/jamapsychiatry.2013.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA, Sundquist J. Genetic and familial-environmental influences on risk for drug abuse: a national Swedish adoption study. Archives of General Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus L, Augustin R, Frischer M, Kummler P, Uhl A, Wiessing L. Estimating prevalence of problem drug use at national level in countries of the European Union and Norway. Addiction. 2003;98:471–485. doi: 10.1046/j.1360-0443.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. American Journal of Psychiatry. 2001;158:1091–1098. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Needle R, McCubbin H, Wilson M, Reineck R, Lazar A, Mederer H. Interpersonal influences in adolescent drug use – the role of older siblings, parents, and peers. International Journal of Addictions. 1986;21:739–766. doi: 10.3109/10826088609027390. [DOI] [PubMed] [Google Scholar]
- Petraitis J, Flay BR, Miller TQ, Torpy EJ, Greiner B. Illicit substance use among adolescents: a matrix of prospective predictors. Substance Use and Misuse. 1998;33:2561–2604. doi: 10.3109/10826089809059341. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS Software Version 9.2. SAS Institute, Inc.; Cary, NC: 2008. [Google Scholar]
- Svensson R. Gender differences in adolescent drug use: the impact of parental monitoring and peer deviance. Youth and Society. 2003;34:300–329. [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. American Journal of Medical Genetics. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug and Alcohol Dependence. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Windle M. Parental, sibling, and peer influences on adolescent substance use and alcohol problems. Applied Developmental Science. 2000;4:98–110. [Google Scholar]
- Yi Z, Coale A, Choe MK, Zhiwu L, Li L. Leaving the parental home: census-based estimates for China, Japan, South Korea, United States, France, and Sweden. Population Studies. 1994;48:65–80. [Google Scholar]


