Abstract
There is increasing evidence showing that inflammation is an important pathogenic mediator of the development of obesity-induced insulin resistance. It is now generally accepted that tissue-resident immune cells play a major role in the regulation of this obesity-induced inflammation. The roles that adipose tissue (AT)-resident immune cells play have been particularly extensively studied. AT contains most types of immune cells and obesity increases their numbers and activation levels, particularly in AT macrophages (ATMs). Other pro-inflammatory cells found in AT include neutrophils, Th1 CD4 T cells, CD8 T cells, B cells, DCs, and mast cells. However, AT also contains anti-inflammatory cells that counter the pro-inflammatory immune cells that are responsible for the obesity-induced inflammation in this tissue. These anti-inflammatory cells include regulatory CD4 T cells (Tregs), Th2 CD4 T cells, and eosinophils. Hence, AT inflammation is shaped by the regulation of pro- and anti-inflammatory immune cell homeostasis, and obesity skews this balance towards a more pro-inflammatory status. Recent genetic studies revealed several molecules that participate in the development of obesity-induced inflammation and insulin resistance. In this review, the cellular and molecular players that participate in the regulation of obesity-induced inflammation and insulin resistance are discussed, with particular attention being placed on the roles of the cellular players in these pathogeneses.
Keywords: Adipose tissue, Obesity-induced Inflammation, Adipose tissue immune cells, Insulin resistance
1. Introduction
Insulin resistance is the condition where the body does not respond appropriately to circulating insulin [1, 2]. It associates commonly with obesity, hypertension, and cardiovascular disease and typically precedes the onset of Type 2 Diabetes (T2D). Insulin resistance occurs in several tissues, including the liver, muscle and adipose tissue (AT). The liver helps to maintain fasting glucose levels through gluconeogenesis and glycogenolysis. However, when the liver is insulin-resistant, the suppression of hepatic glucose production is impaired and thus gluconeogenesis and glycogenolysis continue at inappropriately high levels despite normal or high circulating glucose levels. AT and muscle are similarly affected by insulin resistance, although the problem here relates more to the impaired ability of insulin to promote glucose disposal. To compensate for the insulin resistance in these tissues, pancreatic β-cells produce more insulin. However, there is a limit to how much can be produced, and when this has been reached, the β-cells fail. T2D occurs when an inappropriately low level of insulin is produced in response to a given concentration of glucose.
At the cellular level, the molecular mechanism that underlies the development of insulin resistance is impairment in the insulin signaling pathway in insulin-responsive cells (adipocytes, myocytes, hepatocytes and β-cells). Normally, when insulin binds to the insulin receptor on these cells, the insulin receptor is autophosphorylated at its Tyr residues and its tyrosine kinase is activated [3, 4]. The insulin receptor then phosphorylates tyrosine residues on the insulin receptor substrates (IRSs), which then serve as docking proteins for SH2-containing enzymes such as p85 subunit of PI-3 kinase or SHP2. This leads to linear signaling cascades that result in Akt activation. The activation of Akt induces the translocation of Glut4 and glycogen synthesis and thus plays an important role in metabolic signaling. Hence, disruption of this insulin signaling cascade can induce insulin resistance and is associated with the development of T2D. It is widely agreed in the field that obesity is a major cause of impaired insulin signaling and therefore the development of insulin resistance. However, it is not fully understood how obesity causes the development of insulin resistance. Many molecular mechanisms are proposed, including ER stress, oxidative stress, dysregulation of lipid homeostasis (including FFA homeostasis), mitochondrial dysfunction, hypoxia and others [5]. However, there are now many lines of evidence that suggest that obesity-induced inflammation may also be a key cause of insulin resistance. Recent studies in this field have also identified many cellular and molecular players that participate in the development of obesity-induced inflammation and insulin resistance.
2. Obesity-induced Inflammation
Inflammation is a series of cellular and molecular responses that serve to defend the body from infections or other insults. These responses also help the tissues that were damaged by the insults (and the immune responses that were induced by the insults) to recover. Inflammation has mainly been studied in infectious models but its dysregulation has also been implicated as a major cause of many diseases such as rheumatoid arthritis, atherosclerosis, asthma and other autoimmune diseases [6–8]. The main cellular players in inflammation are immune cells, which can be roughly divided into pro- and anti-inflammatory immune cells whose relative balance and functions tightly control the inflammation processes [9]. For example, when cells are infected by foreign bacteria or viruses, the danger signals produced by these infected cells are detected by surveilling immune cells, particularly neutrophils. The neutrophils then migrate into the local sites of infection, where they produce a series of chemokines that recruit other immune cells. Macrophages are recruited first, after which lymphocytes are recruited. The infiltrating immune cells then kill and remove the infected cells, after which the inflammation resolves and tissue healing starts. Finally, B cells mediate the transition from innate immunity to adaptive immunity. When inflammation is induced without infection, it is called sterile inflammation. In this case, the cells are mainly killed by necrosis and produce DAMPs such as HMGB1 and dsDNA that bind to PRRs such as TLRs, thereby initiating inflammation. Thus, in general, inflammation is often characterized by increased cytokine levels in the circulation and local sites of inflammation along with increases in the infiltrating immune cell numbers in the local sites. Acute inflammation is often defined as the presence of neutrophils at the sites of inflammation while chronic inflammation is generally defined as the infiltration of macrophages into the inflamed tissue.
While obesity-induced inflammation resembles the inflammation in classical immunity in many ways, it also differs from the latter in that it is a low-grade inflammation that produces much lower levels of circulating cytokines. It is also considered to be a chronic inflammation because it requires relatively long diet treatments (> 8 weeks in animal models) before it becomes clearly discernible in the AT, which of all the various insulin-responsive tissues has the most severe obesity-induced inflammation (as characterized by the increased cytokine/chemokine expression and immune cell infiltration). Indeed, obesity-induced inflammation particularly resembles the inflammation observed in atherosclerosis, which is one of the complications of metabolic syndrome along with insulin resistance and lipid dysregulation [10]. Thus, obesity-induced inflammation may be a different kind of inflammation, namely one that is the result of overnutrition and stress pathways that drive abnormal metabolic homeostasis (e.g., high levels of lipid, FFA, glucose or ROS).
The role of inflammation in the development of obesity-induced insulin resistance was first suggested by the early studies on TNFα in the 1990s. These studies showed that when adipocytes were treated with TNFα, their insulin signaling was impaired, mainly because of changes in the transcription of insulin signaling molecules, in particular the insulin receptor, IRS-1, and Glut4 [11, 12]. Animal studies also revealed that interventions that increased TNFα levels induced insulin resistance, and that the blocking of TNFα functions by genetic and pharmacological interventions improved obesity-induced insulin resistance [13–17]. Thus, these were the first studies to show that an inflammatory mechanism, namely the pro-inflammatory cytokine TNFα, can regulate obesity-induced insulin resistance in vitro and in vivo. While most of these studies focused on TNFα rather than on inflammation per se, they provided the novel concept that an immune pathway may regulate the development of obesity-induced insulin resistance.
Then, in the early 2000s, a series of epidemiological studies revealed that circulating inflammatory markers associate strongly with T2D and are risk factors for the development of future T2D [18–29]. These clinical but still observational studies were then supported by preclinical studies showing that obesity activates inflammation via the IKKβ/NFκB pathway and that inhibition of this pathway by genetic deletion of IKKβ or pharmacological inhibitors of this pathway (a high dose of salicylates or aspirin) improves obesity-induced insulin resistance [30, 31]. Clinical studies then showed that when inflammation in insulin-resistant or T2D patients was suppressed by a high dose of aspirin or salsalate (a dimer of salicylate), the glycemic control of the patients improved, along with concomitant inhibition of NFκB activity in their PBMCs [32–35]. Numerous preclinical and clinical studies now strongly support the notion that obesity-induced inflammation plays an important role in the development of insulin resistance and T2D [36, 37].
The next question was, “Which tissues/cells mediate the regulation of obesity-induced inflammation?” Two seminal papers by the Chen and Ferrante groups tested this question directly [38, 39]. They showed that obesity increases AT macrophage (ATM) numbers and that ATMs, not adipocytes, produce the majority of cytokines in response to obesity. This made it clear that AT-infiltrated macrophages play a key role in the regulation of obesity-induced inflammation. Subsequently, many other types of immune cells were found in AT, most of which participate in the development of obesity-induced inflammation in AT as well. Hence, it is now generally accepted that tissue-resident immune cells play a major role in the regulation of obesity-induced inflammation and insulin resistance, like they do in classical immunity inflammation [40]. This notion is also strongly supported by studies examining the effects of genetic modulation of specific inflammatory mediators in immune cells [5, 41, 42].
3. Cellular Players in Obesity-induced AT Inflammation
Obesity is defined as the expansion of fat, and obesity, especially in abdominal fat depots, is a risk factor for the induction of metabolic diseases. Therefore, to understand the molecular mechanisms that underlie the development of obesity-induced insulin resistance, the biology of AT has been studied extensively. In terms of glucose homeostasis, liver, AT and muscle are the major players; while liver maintains glucose levels between meals by producing glucose via glycogenolysis and gluconeogenesis, AT and muscle take up glucose after a meal. However, the AT only takes up a relatively small proportion of the glucose after a meal, although the insulin signaling and insulin-sensitive Glut4 regulation in AT have been studied extensively. Thus, AT may not regulate glucose homeostasis directly via its glucose uptake ability. Instead, AT may regulate glucose homeostasis indirectly by regulating lipid homeostasis [43]. AT is the main site of lipid storage and many studies show that the modulation of the lipid pathways in AT can regulate systemic lipid homeostasis. In many cases, these modulations are accompanied by disruption of systemic glucose homeostasis. One extreme case of this is lipodystrophy, which is characterized by a near complete loss of fat that causes significant hyperlipidemia and induces insulin resistance [44]. When the fat is restored with transplantation, the metabolic dysregulation is completely reversed.
Another important function of AT in obesity is to act as an endocrine organ that regulates the production of various hormones and cytokines [45]. The hormones and cytokines that are produced by AT include leptin, adiponectin, resistin, and cytokines such as TNF-α and IL-6. Obesity regulates the production of these hormones/cytokines by AT. Indeed, AT expresses very high levels of many inflammatory mediators in obesity and is therefore considered to be the main inflammatory organ that mediates obesity-induced inflammation. While AT is composed of many different cell types, including adipocytes, pre-adipocytes (adipocyte progenitors), endothelial cells, and immune cells, recent studies show clearly that the regulation of inflammatory mediators is mainly mediated by the stromal vascular cell (SVC) fraction that contains the immune cells. Hence, adipokines can be divided into two groups: “true adipokines” that are produced by adipocytes (e.g., leptin, adiponectin, and most resistins), and “pseudo-adipokines” that are produced by non-adipocytes (most inflammatory mediators, including TNF-α and IL-10).
Several studies show that obesity changes the number and functions of the various types of immune cells in the AT SVC fraction. However, these studies should be examined closely for the way cell numbers and functions are expressed, especially when immune cell numbers are determined by flow cytometric analyses. The current literature uses three ways to present obesity-induced changes in immune cell numbers. In the first way, immune cell numbers are expressed as percentages of total SVCs or AT immune cells. However, ATMs are the main component of AT immune cells (40–60% of all AT immune cells) and obesity dramatically increases ATM numbers. Therefore, when immune cell numbers are normalized according to total SVCs or AT immune cells, they can appear to be decreased by obesity, even though their absolute numbers are increased. The second way of expressing immune cell numbers is as total number per fat pad. In this case, the changes in immune cell numbers are compounded by the obesity-induced increase in the size of the fat. As a result, if there is an actual increase in cell numbers, the changes can appear to be very dramatic; by contrast, if the immune cell numbers actually decrease, this decrease may be minimal or indiscernible when expressed in this fashion. In the third way, immune cell numbers can be normalized by fat weight (i.e., they are expressed as immune cell numbers per weight of fat) to compensate for the obesity-induced increases in ATM numbers and fat sizes. Thus, reports of obesity-induced changes in immune cell numbers should be examined carefully, especially when rare cell populations are being analyzed.
3.1 Myeloid Cells
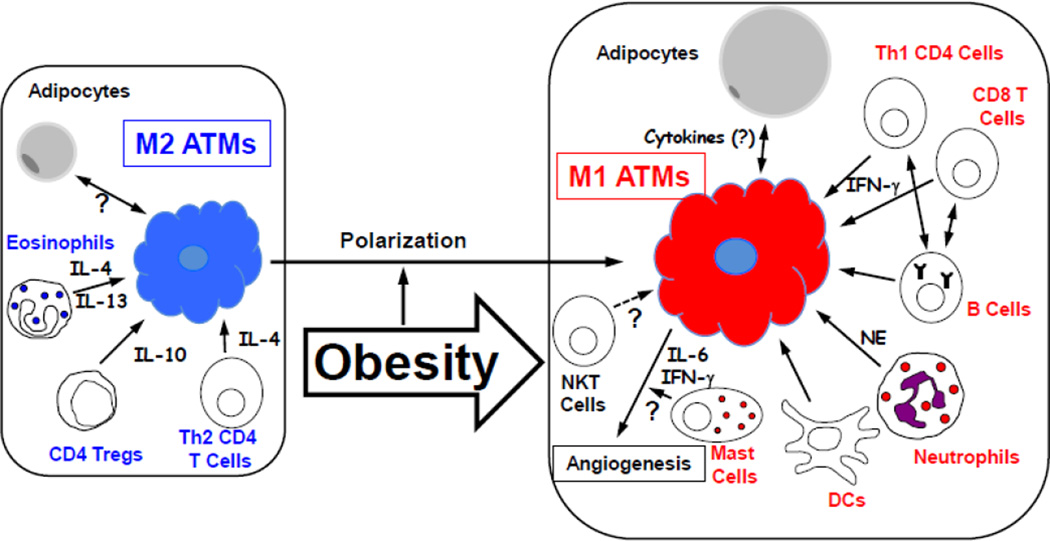
Leukocytes are grossly categorized as lymphocytes and myeloid cells [46]. Myeloid cells include macrophages, DCs, mast cells, neutrophils, basophils and eosinophils, while lymphocytes include T, B, NK and NKT cells. Although myeloid cells are believed to be key players in innate immunity, they also play important roles in adaptive immunity. For example, DCs are important for the antigen presentation that is essential for the development of adaptive immunity. Furthermore, many of the cytokines that are produced by myeloid cells help to develop and activate T and B cells, which are also critical components of adaptive immunity. The AT immune cell that was identified first was the AT macrophage (ATM), and it is presently the most well-studied AT immune cell type. However, most of the other immune cell types are also found in AT, and their roles in the development of obesity-induced inflammation and insulin resistance have been extensively investigated as well. Table 1 and Fig. 1 summarize the roles of AT immune cells in the development of obesity-induced inflammation and insulin resistance.
Table 1.
Cellular players that participate in the development of obesity-induced AT inflammation and insulin resistance.
| Cells | Targeting method: Gene or mice |
Insulin resistance |
Body weight |
Adiposity | ATM | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Number | M1 | M2 | ||||||
| Dendritic cells | T KO:Flt3 | ↓ | ↓ | ↓ | ↓ | ↓ | ND | [63] |
| BMT(H):CD11c-DTR | ↓ | ↔ | ↔ | ↓ | ↓ | ND | [62] | |
| Neutrophils | T KO:Elastase | ↓ | ↓ | ↑ | ND | ↓ | ↑ | [72] |
| Eosinophils | T KO:ΔdblGATA | ↑ | ↑ | ↑ | ↔ | ND | ↓ | [76] |
| T KO: IL-4/IL-13 | ND | ND | ND | ND | ND | ↓ | [76] | |
| Mast celsl | T KO:KitW-sh/W-sh | ↓ | ↓ | ↓ | ↓ | ND | ND | [79] |
| Th2 CD4 T Cells | T KO:Rag-1 | ↑ | ↑ | ↑ | ND | ND | ND | [83] |
| CD4 Tregs | Tg:Fxop3-DTR | ↑ | ↔ | ↔ | ND | ND | ND | [87] |
| CD8 T Cells | T KO:CD8a | ↓ | ↔ | ↔ | ND | ↓ | ↔ | [86] |
| NKT cells | T KO:CD1d | ↑ | ↑ | ↑ | ↔ | ↑ | ND | [91] |
| T KO:CD1d | ↓ | ↓ | ↓ | ND | ND | ND | [92] | |
| T KO:CD1d | ↔ | ↔ | ↔ | ↔ | ND | ND | [94] | |
| T KO:CD1d | ↔ | ↔ | ND | ND | ND | ND | [96] | |
| T KO:Jα18 | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | [91] | |
| T KO:Jα18 | ↓ | ↔ | ↓ | ↓ | ND | ND | [92] | |
| T KO:Jα18 | ↔ | ↔ | ↔ | ↔ | ND | ND | [93] | |
| T KO:β2 microglobulin | ↓ | ↔ | ↔ | ↓ | ND | ND | [97] | |
| B cells | KO T:µMT | ↓ | ↔ | ↓ | ND | ↓ | ↑ | [66] |
| KO T:µMT | ↓ | ↔ | ↓ | ↓ | ↓ | ↑ | [67] | |
T KO, Total body knockout mice; BMT(H), Deletion of target genes in the hematopoietic compartment by bone marrow transplantation (Donor: T KO or transgenic mice, Recipients: wild type controls); ND, No determine.
Fig. 1. The roles of AT immune cells in the development of obesity-induced inflammation.
This diagram is based on the current published findings that are discussed in this review. The blue fonts represent cells that suppress inflammation, while the red fonts represent cells that induce inflammation. Since there are many contradictory reports regarding the role of NKT cells, NKT cells are not categorized in this diagram.
3.1.1. Macrophages
A key function of macrophages is to protect the body from infection by removing infected cells via phagocytosis and by killing infected cells via cytokines [47]. While the phagocytosis function of these cells is rarely considered in obesity-induced inflammation, it could play a role as this is a key function in the wound repair and tissue remodeling that occurs after the resolution of inflammation. Macrophages also promote wound repair and tissue remodeling by producing extracellular matrix (ECM) and proteinases to modify ECMs. Apart from these functions, macrophages are also believed to play important roles in the regulation of chronic inflammation, mainly because of their strong capacity to produce cytokines. In most chronic diseases, macrophages produce chemokines that recruit other immune cells into the local inflammatory sites; they also produce pro-inflammatory cytokines that polarize and activate other immune cells. However, in some cases, macrophages also exhibit anti-inflammatory characteristics. An example of these macrophages are the tumor-associated macrophages (TAMs) [48]. Hence, macrophages can play various roles in the initiation, progression and resolution of inflammation.
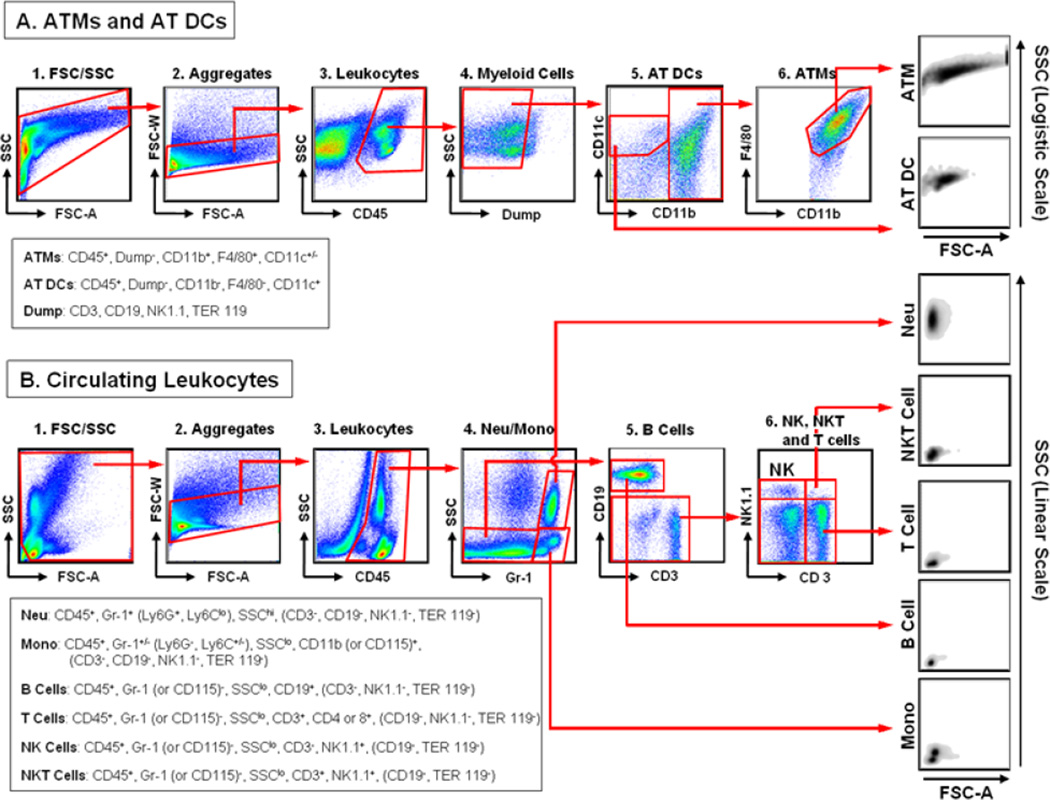
Macrophages are typically defined as CD11b+ and F4/80+ in flow cytometric analyses and do not express CD3, CD19 and NK1.1. Other markers used to identify macrophages by flow cytometry include CD14, CD16 and CD68. In addition, macrophages are bigger and more granular than lymphocytes and DCs and therefore do not typically make a distinctive cluster in FSC (size)/SSC (granularity) gating, unlike other immune cells. However, most of the macrophage cell-surface markers are also expressed by many different cell types (e.g., CD11b is also expressed by monocytes, granulocytes, NK cells, T cells and B cells). Therefore, the best flow cytometric results can be often obtained by using negative selection (e.g., CD3, CD19 and NK1.1 for macrophages) and positive selection (e.g., CD45, CD11b and F4/80 for macrophages). Examples of gating strategies for the flow cytometric analyses of mouse AT and blood immune cells were shown in Fig. 2. Also Tables 2 and 3 summarized the cell surface markers that are commonly used for determining cell types by flow cytometric analyses.
Fig. 2. Examples of flow cytometric analyses of mouse AT and circulating immune cells.
A. Gating strategies for ATMs and AT DCs. After SVCs were isolated from AT by using a collagenase method, they were stained with antibodies against cell-specific markers and subjected to flow cytometric analysis with LSR II. The data were analyzed by FlowJo software with the following gating strategy. Since ATMs have high granularity (SSC), we used the logistic scale for the SSC gating. After the appropriate cells were selected by FSC-A (size)/SSC (granularity) gating (1), the aggregated cells were removed by FSC-A/FSC-W gating (2). The total leukocytes were then identified by positivity for CD45 (3). Thereafter, the lymphocytes and RBCs were excluded by removing the CD3+ (T cells), CD19+ (B cells), NK1.1+ (NK and NKT cells) and TER119+ (RBC) cells (4). The AT DCs were selected as CD11b− and CD11c+ (5). Finally, ATMs were determined by identifying the CD11b+ and F4/80+ cells (6). B. Gating strategies for circulating immune cells. Whole blood was incubated with FcBlock to block the non-specific binding of Abs and then stained with antibodies against cell-specific surface markers. After staining, the RBCs were removed by lysing with FACS Lysing buffer (BD Biosciences). The cells were then analyzed with LSR II. The data were analyzed by FlowJo software with the following gating strategy. After the appropriate cells were selected by FSC-A (size)/SSC (granularity) gating (1), the aggregated cells were removed by FSC-A/FSC-W gating (2). The total leukocytes were then selected by positivity for CD45 (3). The neutrophils were selected as Gr-1+ and SSChi, while monocytes were selected as Gr-1+ and SSClo (4). It should be noted that this gating strategy selects only the Ly6C+ (Gr-1+) monocyte subpopulation and that the total monocyte population containing both the Ly6C+ and Ly6C− monocyte subpopulations should be selected on the basis of their CD11b and/or CD115 positivity. Thereafter, the lymphocyte population was selected as Gr-1− and SSClo (4). B cells were then selected as CD19+ from the lymphocyte population (5). Finally, NK, NKT and T cells from the CD19− non-B cell population were further determined on the basis of their CD3 and/or NK1.1 expression levels (6). Cell surface markers that were used to define specific cell types by these gating strategies were listed in the boxes. Potential negative selection markers that can be used together were also listed with the parentheses at the end of positive selection markers. It should be also noted that the examples of the gating strategies shown here are only for the mouse immune cell analyses and that they are only for the general guidance.
Table 2.
Cell surface markers that can be used to determine mouse immune cell types by the flow cytometric analysis.
| Cell Types | Pan/Commonly Used Markers |
Other Pan Markers | Subset/Activation Markers |
Negative Selection Markers |
|---|---|---|---|---|
| T Cells | CD3, CD4 and/or CD8 | CD25, CD69, CD44, CD62L, Foxop3 (intracellular) | CD19, CD115, F4/80 NK1.1 | |
| B Cells | CD19 | CD22, CD45R/B220, IgM | CD25, CD69, CD86, CD279/PD-1 | CD3, CD115, F4/80 NK1.1 |
| NKT Cells | CD3, NK1.1 | CD16, CD1d-α-gal-cer Tetramer | CD19, CD115, F4/80 | |
| Macrophages | CD11b, F4/80 | CD14, CD16, CD64, CD68, CD115, MerTK | CD11c for ATMs, CD206, CD209, CD301, MHC II | CD3, CD19, Ly6G NK1.1 |
| DCs | CD11c, CD83 | CD86, CD205/DEC-205, | CD4, CD8, CD11b, CD45R/B 220, CD80, CD103, CD209/DC-Sign, MHC II | CD11b for ATDCs, CD3, CD19, Ly6G, NK1.1 |
| Neutrophils | GR-1 or Ly6G | Ly6B/7/4 | CD11b, CD14, CD16, CD64 | CD3, CD19, CD115, NK1.1 |
All of cells are typically gated first with CD45+ (leukocytes) and TER119− (red blood cells) (see Fig. 2). It should be noted that markers in this table are only for the analyses of mouse immune cells. It should be also noted that negative selection markers listed in this table are only for the general guidance.
Table 3.
Cell surface antigens that are commonly used for the flow cytometric analyses of mouse immune cells.
| Antigen Name | Other Name | Cells |
|---|---|---|
| CD3 | T, NKT | |
| CD4 | T, DC, Mono | |
| CD8 | T, DC | |
| CD11b | Mac-1, Itgam | Mac, Mono, Neu, NK, T, B |
| CD11c | Itgax | DC, Mono, Mac, NK, T, B, Grn |
| CD14 | LPS receptor | Mac, Mono, Grn, DC |
| CD16 | FcγRIII | Mac, Neu, Mast, NK, NKT, B |
| CD19 | B4 | B, Follicular DCs |
| CD64 | FcγRI | Mono, Mac, DC, Grn |
| CD68 | Mac, Mono, DC (low) | |
| CD83 | DC, T, B | |
| CD115 | CSF-1 receptor | Mono, Mac |
| CD205 | DEC-205 | DC |
| CD206 | Mrc-1, Macrophage man nose receptor C Type 1 | Mac, Mono, DC |
| CD209 | DC-Sign | DC |
| CD301 | MGL-1/2 | Mac, DC |
| B220 | CD45R | B, DC, Mono, T, B, NK |
| F4/80 | EMR-1 | Mac, Mono (low), Neu (low) |
| MHC II | DC, B, Mac, mono, T | |
| MK1.1 | CD161 | NK, NKT |
| Gr-1 | Ly6C/G | Neu, Mono, Mac, T, B, DC |
| Ly6B | 7/4 | Neu, Mono |
| Ly6C | Mono, Mac, Neu, T, B, DC | |
| Ly6G | Neu |
It should be noted that this table is only for the general guidance. While some of markers are highly specific for the cell types (e.g., CD3 and CD19), some of markers are less specific (e.g., CD11c for ATMs). Hence, each investigator should carefully analyze the expression of markers for the target cells for individual experiment. Markers with bold fonts represent the cell types that are commonly defined by these markers. T, T cells; B, B cells; NKT, NKT cells; DC, Dendritic cells (DCs); Mac, macrophages; Mono, monocytes; Neu, Neutrophils; NK, NK cells; Grn, granulocytes; low, low expression levels that can be differentiated from the cells expressing high levels by flow cytometric analyses.
Since macrophages are the key players in most cases of chronic inflammation, it was a reasonable hypothesis that obesity-induced inflammation is regulated by macrophages. Indeed, the Ferrante and Chen groups showed that ATMs produce the majority of cytokines (or “pseudoadipokines”) in obesity and that obesity increases ATM numbers [38, 39]. Subsequent studies in animals and humans confirmed that obesity increases ATM numbers [5, 9, 49]. They also showed that loss of weight decreases ATM numbers and that decreased ATM numbers often accompany improvements in insulin resistance (such as that due to TZD treatment). Moreover, in mouse models, overexpression of MCP-1 in AT increases ATM recruitment and mildly exacerbates obesity-induced insulin resistance [50, 51], which supports the notion that ATMs regulate the development of systemic insulin resistance in obesity.
Probably the most significant but also immunologically unusual feature of the obesity-induced changes in ATM is that obesity dramatically increases CD11c+ ATM numbers [52]. CD11c is considered to be a classical cell-surface marker of DCs, and thus DCs are generally defined as CD11b− and CD11c+ while macrophages are defined as CD11b+ and CD11c− [53–55] (also see Fig. 2A). However, in obese AT, the CD11c+ ATMs also express CD11b and F4/80 and are distinct from the AT DCs, which express CD11c, but not CD11b (see Section 3.1.2). Interestingly, obesity only appears to increase the numbers of the triple-positive (CD11b+ F4/80+ CD11c+) ATM population: the CD11b+ F4/80+ CD11c− ATM numbers remain unchanged [56]. An in vivo labeling study then showed that the ATMs that reside in the AT do not have CD11c whereas the ATMs that are newly recruited by obesity express CD11c. Thus the obesity-induced changes in ATM numbers are mainly due to increases in the triple-positive CD11b+ F4/80+ CD11c+ ATM subpopulation. However, it is not clear yet how obesity increases this special subpopulation. It is possible that this population starts as a special subpopulation of circulating monocytes that is recruited specifically into AT by obesity, after which these cells differentiate into CD11c+ ATMs. It is also possible that the obese environment of AT locally induces the expression of CD11c in newly-recruited CD11c− ATMs.
Macrophages are a very diverse group of cells: they are functionally and morphologically different in different tissues, as indicated by the alveolar macrophages in the lung, Kupffer cells in the liver, microglia in the neural tissues, and osteoclasts in bone [47, 57]. Furthermore, even in the same tissue, there are many different subpopulations of macrophages. The best-known example of this is the splenic macrophage population. A paper from the ImmGen consortium recently confirmed that different tissues share this vast macrophage subpopulation diversity [54]. Despite this diversity, macrophages are often categorized by the binary M1 and M2 system [57]. The M1 and M2 phenotypes are defined by the in vitro system: M1 macrophages are generated by treating BMDMs with LPS and IFNγ or other pro-inflammatory cytokines while M2 macrophages are generated by treating these cells with IL-4/IL13 or IL-10. The resulting M1 macrophages are Th1-biased and considered to be pro-inflammatory macrophages and most notably express TNFα and IL-6. Most of the macrophages found in the sites of inflammation in inflammatory diseases are considered to be M1 macrophages. By contrast, M2 macrophages are Th2-biased and are thought to play more diverse roles, namely in anti-inflammatory pathways, tissue remodeling and wound healing. Tumor-associated macrophages (TAMs) are considered to be M2 macrophages.
Many studies have sought to categorize ATMs according to the M1/M2 system and the general consensus in the field at present is that obesity induces the exclusive polarization of ATMs from M2 phenotypes into M1 phenotypes. CD11c has been used as an M1 ATM marker, while CD206, CD209 and CD301 (Mgl1/2) are often used as M2 ATM markers. However, flow cytometry analyses show that ATMs simultaneously express both CD11c and MGL1 (CD301) [56]. This suggests that individual ATMs can bear both M1 and M2 characteristics simultaneously. Furthermore, M2 phenotypes, not M1 phenotypes, correlate with BMI in humans [58], and ATMs from obese human express the genetic signature of TAMs [59]. This suggests that obesity may induce the polarization of M2 macrophages, not M1 macrophages. These contradictions could suggest that an ATM subpopulation with a predominantly M1 phenotype coexists with another ATM subpopulation that predominantly bears an M2 phenotype and that it is the overall balance between these two ATM subpopulations that shapes obesity-induced inflammation. It is also possible that the M1/M2 system is just too simplified to adequately represent the heterogeneity of ATMs in obesity, and thus may not be helpful for understanding the role of these cells in obesity-induced inflammation.
Another notable feature of obese ATMs is that obese AT contains unique clusters of macrophages that surround dead adipocytes. These clusters are called crown-like structures (CLSs) and are mainly observed in AT from obese animals and humans [60]. They also express high levels of TNFα. However, since CLSs can be only determined by microscopical techniques and the CLS-specific surface markers that can be used for flow cytometric analyses have not yet been identified, the molecular characterization of CLSs remains limited at present.
All published studies strongly show that obesity increases the numbers of total and CD11c+ ATMs and that the infiltrating ATMs express high levels of pro-inflammatory cytokines such as TNFα. However, considering the diverse roles of macrophages in classical immunity, studies that determine the heterogeneity of ATMs and the functions of each subpopulation in the development of obesity-induced inflammation and insulin resistance are warranted.
3.1.2. Dendritic cells (DCs)
Dendritic cells (DCs) play an important role in the transition of innate immunity to adaptive immunity by presenting antigens via MHC II molecules to the T cell receptors (TCRs) of CD4 helper T (Th) cells [61]. Although macrophages can also present antigens, DCs are considered to be the primary and professional antigen-presenting cells (APCs) of the immune system. By contrast, macrophages are considered to be the primary cells that produce cytokines/chemokines in chronic inflammation. However, DCs can also produce a variety of cytokines that serve to mature and/or activate other immune cells. For example, the IL-12 produced by DCs induces the differentiation of naïve T cells into Th1 T cells, while DC-derived IL-15 is important for the proliferation and activation of CD8 T cells and NK cells. The primary DC cell-surface marker for flow cytometric analyses is CD11c, although CD80, CD83 and CD86 are also commonly used with other markers [55] (Tables 2 and 3). DCs are also smaller and less granular than macrophages and therefore form a distinctive cluster in FSC/SSC gating in flow cytometry (Fig. 2A). While DCs are considered to be myeloid cells, there are also subpopulations of DCs that derive from the lymphoid lineage: they can be differentiated from the myeloid lineage-derived DCs by their expression of CD4 or CD8. However, they do not express CD3.
Since the primary function of DCs is antigen presentation in adaptive immunity and obesity-induced inflammation is generally considered to be due to innate immunity, the role of DCs in obesity-induced inflammation has not yet been extensively studied. However, depletion of CD11c+ cells by using CD11c-DTR (diphtheria toxin receptor) transgenic mice improves obesity-induced insulin resistance and decreases pro-inflammatory cytokine expression in AT and muscle [62]. The depletion of CD11c+ cells also decreases hepatic steatosis and lipid metabolism-related gene expression in the liver. The original objective of this study was to specifically target CD11c+ ATMs and therefore determine the role CD11c+ ATMs play in the development of obesity-induced inflammation and insulin resistance. Since there are more CD11c+ ATMs than CD11c+ DCs in AT, the effects of CD11c+-cell deletion on local AT inflammation may be largely due to the deletion of CD11c+ ATMs. However, since CD11c is the classical marker of DCs and there are more systemic CD11c+ DCs than CD11c+ macrophages in the whole body, this study actually examined the effects of depleting all CD11c+ cells in the body, including the majority of systemic DCs and the small numbers of CD11c+ ATMs [55]. Hence, this study inadvertently suggests that DCs may actually play an important role in the regulation of obesity-induced inflammation and insulin resistance.
Two recent studies directly asked what role DCs play in the development of obesity-induced insulin resistance. They both showed that obesity increases DC numbers in AT [63, 64]. Bertola et al. also found that AT contains all three DC subpopulations, namely, myeloid, CD4+, and CD8+ DCs, and that obesity decreased the frequency of CD103 DCs [64], which are known to be important in the differentiation of regulator T (Treg) cells in small intestinal lamina propria [65]. Interestingly, the AT DCs isolated from obese animals and humans can induce the differentiation of Th17 cells (IL-17-expressing T cells) in vitro [64]. Treg differentiation and Th17-cell differentiation often counter-balance each other and it has already been shown that the Treg numbers are decreased by obesity. However, Th17 cells have not yet been identified in AT from lean or obese animals. Thus, it will be interesting to see whether CD103 DCs can regulate obesity-induced inflammation in AT by regulating the balance of Treg and Th17 cell differentiation in vivo. Stefanovic-Racicit et al. also showed that deletion of DCs in Flt3l−/− knockout mice improved obesity-induced insulin resistance along with decreasing the numbers of ATMs and liver macrophages. Moreover, the reconstitution of DCs in the Flt3l−/− knockout mice increased the number of ATMs and liver macrophages [63]. However, the deletion of Flt31 caused significant losses in body weight and adiposity, probably because of increased energy expenditure. Therefore, it is not clear whether the improvement of obesity-induced insulin resistance in this model is solely due to the depletion of DCs.
It was shown recently that B cells also participate in the development of obesity-induced insulin resistance [66, 67] (see also Section 3.2.2). This suggests that adaptive immunity may also contribute to the development of obesity-induced inflammation. Therefore, it will be interesting to determine whether DCs play a role in the development of obesity-induced inflammation by promoting adaptive immunity.
3.1.3 Neutrophils
Neutrophils are a member of the granulocytes, which also include basophils and eosinophils [68–70]. These three granulocyte types can be differentiated by hematoxylin and eosin (H&E) staining: the neutrophils are neutral pink while the basophils and eosinophils are dark blue and bright red, respectively. Neutrophils play a critical role in innate immunity, especially in microbial infections. As indicated by the term “granulocyte”, neutrophils bear large numbers of granules (intracellular vesicles) that contain anti-microbial reagents, including lysozyme, neutrophil elastase (NE), myeloperoxidase (MPO), and defensins. When neutrophils encounter foreign invading pathogens, they release these anti-microbial reagents by degranulation. They also remove the foreign pathogens by phagocytosis. Hence, neutrophils play a primary role in defending the body from infections and are the first cells to migrate into sites of infection. This rapid responsiveness is aided by the fact that neutrophils are very mobile and fully mature when they egress from the bone marrow (BM); by contrast, most other immune cells are naïve when they egress from BM and circulate in the blood. However, neutrophils can also be matured further in the local sites so that they produce large amounts of cytokines and chemokines, including TNF-α, IL-1β, IL-8 and MIP-1α. This allows them to recruit the second wave of other immune cells, in particular macrophages. The recruited cells can be further activated by the cytokines that are produced by neutrophils. In flow cytometric analyses, neutrophils are often defined by their high granularity (high SSC) and by using the Gr-1 antibody. Since the Gr-1 antibody recognizes both Ly6C and Ly6G, and Ly6G is expressed only by neutrophils but not by monocytes, Ly6G antibody is also used for neutrophil-specific gating in flow cytometry. Antibodies to CD11b are also used to recognize activated neutrophils, especially in in vitro studies.
Since the presence of neutrophils at local infected sites is often considered as evidence of inflammation, classical immunologists have frequently asked whether neutrophils can be found in obese AT. This question was answered by a study that assessed the expression of MPO in total AT [71] and by a flow cytometric study of AT immune cells [72]. Both studies showed that obesity increases neutrophil infiltration, although the AT neutrophil numbers are much smaller than the ATM numbers (>30 fold smaller). Both studies also revealed that neutrophil numbers increase within the first week of HFD treatment, which suggests that like in classical immune responses, neutrophils may be one of the first immune cells to migrate into AT in response to obesity. However, the MPO expression levels decreased after the first week of HFD treatment [71] whereas the flow cytometric analyses showed that AT neutrophil numbers were sustained after the first week [72]. This suggests that AT neutrophils may play different roles during the different stages of obesity. When Talukdar et al. further investigated the role of NE in the development of obesity-induced insulin resistance, they showed that the inhibition of NE by genetic deletion or pharmacological inhibition improves obesity-induced insulin resistance along with suppressing inflammation in AT and liver [72]. In particular, this inhibition decreased CD11c+ ATM numbers.
3.1.4 Eosinophils
Eosinophils are important for innate immunity, especially in the defense against parasite infections; they also play a central role in the development of asthma and allergy [73, 74]. Eosinophils are notable in that they play roles in Th2 immunity, unlike neutrophils. Hence, they produce many Th2 cytokines (such as IL-4, IL-10, IL-13 and TGF-β) that participate in anti-inflammatory immune responses, M2 polarization of macrophages, and differentiation of Th2 effector T cells. In flow cytometric analyses, Siglec-F is commonly used as an eosinophil-specific marker.
The studies on IL-4 in obesity suggest that eosinophils participate in the development of obesity-induced insulin resistance. The systemic deletion of Stat6, which IL-4 utilizes for signaling, increases insulin resistance, although Stat6−/− knockout mice also weigh less and are leaner than the wild-type control littermates [75]. By contrast, systemic infusion of IL-4, which presumably activates Stat6 (perhaps along with other signaling pathways), improves insulin resistance. However, the IL-4 infusion also decreases body weight and adiposity [75]. Analyses of IL-4-GFP mice (4 get mice) then revealed that ~90% of the IL-4-producing AT immune cells under normal chow conditions are eosinophils, although the number of AT eosinophils is very low (~20,000/g of fat) [76]. Furthermore, obesity decreases AT eosinophil numbers. It was then shown further that eosinophil knockout mice generated from ΔdblGATA mice or IL-4/IL-13 double knockout mice had decreased numbers of M2 (alternatively activated) ATMs. Furthermore, the eosinophil number decreases in ΔdblGATA mice exacerbate obesity-induced insulin resistance, while increases in eosinophils due to overexpression of IL-5 or helminth infections improve obesity-induced insulin resistance [76]. However, body weight and adiposity changes occurred in all of these models: the ΔdblGATA eosinophil knockout mice had increased body weight and adiposity compared to the wild-type controls, while mouse models that increased eosinophil numbers showed decreased body weight and adiposity. Hence, it is not clear yet whether the eosinophil-mediated regulation of obesity-induced insulin resistance and AT inflammation (M2 ATM numbers) is due to the direct and primary effects of eosinophils on insulin resistance or due to secondary effects of eosinophils on changes in body weight and adiposity. It should also be noted that eosinophils mainly play a role in Th2 immunity [73] and that the mouse backgrounds of most studies on this issue are Balb/c mice, which are Th2 biased. By contrast, most studies on obesity-induced insulin resistance, including diet-induced obesity models and the ob/ob and db/db mice, involve C57/B6 mice, which are Th1-biased. Therefore, it is not yet known whether the different backgrounds shape the role of eosinophils in the development of obesity-induced inflammation. Nevertheless, the eosinophil studies suggest a new possibility, namely that induction of Th2 immunity (and M2 ATMs) can improve obesity-induced insulin resistance, either directly by immune modulation of AT or indirectly by changing obesity. The latter effect is often observed when the immune system is modulated and then subjected to obesity; however, the molecular mechanisms that underlie changes in body weight and obesity have not yet been extensively explored.
3.1.5 Mast cells
Mast cells are also myeloid lineage cells and are abundant in the barriers that are exposed to the environment, such as skin and the mucosa [77, 78]. It is well established that the uncontrolled activation of mast cells associates strongly with asthma, allergy and anaphylaxis. However, recent studies suggest that mast cells also may play an important role in primary host defense against infections by parasites and bacteria. When mast cells are activated (e.g., by the aggregation of the IgE receptor Fcε RI), they secrete various arrays of inflammatory mediators including histamine, heparin, major basic protein (MBP), lipid mediators (PGE2 and LTB4), proteases (chymases and trptases), and pro- and anti-inflammatory cytokines (TNFα, IL-1β, IL-6, TGF-β, IL-4 and IL-10). Hence, mast cells play various functions in different tissues, especially in terms of activation of other immune cells. Interestingly, mast cells are often compared to basophils due to their overlapping phenotypes and functions in immunity. However, basophils and mast cells differ in their development: in particular, basophils egress from the BM as mature cells (like neutrophils) whereas mast cells egress as an immature form and are only fully activated when they migrate into the local tissue. In addition, they produce different mediators. In particular, basophils mainly produce Th2 cytokines (IL-4 and IL-13) while mast cells produce both Th1 and Th2 cytokines. The common technique for detecting mast cells is immunohistochemistry with toluidine blue staining and/or immunohistology with antibody against tryptase. In toluidine blue staining, mast cells stain a red-purple (metachromatic staining) with a blue background (orthochromatic staining). In terms of genetic studies of mast cells, KitW-sh/KitW-sh mice have been widely used. They have a mutation upstream of the c-Kit gene that mainly impairs mast cell development without inducing too many other abnormalities.
In humans and mouse models, the mast cell numbers in AT are also increased by obesity [79]. Furthermore, in mast cell-deficient KitW-sh/KitW-sh mice or mice treated with a mast cell stabilizer (disodium cromoglycate), obesity-induced insulin resistance is improved. This deletion or stabilization of mast cells also almost completely abolishes ATM numbers. However, body weight and adiposity in these mouse models are also decreased. When KitW-sh/KitW-sh mice are reconstituted with mast cells, their obesity-induced insulin resistance worsens. Interestingly, the reconstitution of these mice with mast cells from Il6 or Ifng knockout mice did not increase insulin resistance, which suggests that IL-6 and IFN-γ from mast cells play an important role in the ability of mast cells to regulate metabolism [79]. The authors of this report proposed that mast cells regulate ATMs, after which ATMs regulate angiogenesis in obesity. While there is no direct strong evidence to support this hypothesis, it is interesting because it can explain the changes in body weight and adiposity that are mediated by mast cells. Nevertheless, mast cell deletion mainly appears to improve insulin resistance by changing body weight and adiposity rather than by directly regulating obesity-induced inflammation.
3.2. Lymphocytes
Lymphocytes include T cells, B cells, NK cells and NKT cells, all of which are synthesized in the BM [46]. T and B cells recognize specific antigens with their receptors (TCR for T cells and BCR for B cells) and therefore play important roles in adaptive immunity. By contrast, NK and NKT cells are thought to play roles in innate immunity, although recent studies suggest that they are also important in adaptive immunity.
3.2.1 T cells
T cells are generated in BM, egress from BM in an immature form, and are then fully matured in the Thymus [80]. T cells are very heterogeneous and are categorized on the basis of their surface expression markers and/or functions. The first categorization of T cells is based on the TCR subtypes that are expressed on all T cell populations. Most T cells express αβTCR and are therefore called αβT cells. However, there is a small population (typically <2% of total T cells) of T cells that express γδTCRs (γδT cells). γδT cells are present at high numbers in the gut mucosa and the epidermal compartment of skin and do not require MHC molecules for their functions (i.e. they show no MHC-restriction). Although they express CD3, they do not usually express CD4, and their CD8 expression is variable. γδT cells also produce various anti- and pro-inflammatory cytokines, notably IL-17. However, the role of γδT cells in the development of obesity-induced inflammation and insulin resistance has not yet been extensively explored, although it will be interesting to examine their roles in subcutaneous AT due to its close proximity to the skin intraepithelial area and its lower levels of inflammation compared to epididymal AT.
αβT cells are divided by the CD4 and CD8 surface markers and/or by their functions (T helper cells, cytotoxic T cells, regulatory T cells etc.). Most helper cells are CD4 cells and they interact with antigens presented by MHC II molecules (MHC II-restriction) on APCs. T helper cells are themselves further divided into Th1, Th2, Th17 and regulatory T (Treg) cells [81]. These CD4 T cell subpopulations are mainly defined on the basis of intracellular staining for their signature cytokines: IFNγ for Th1 cells, IL-4 or IL-13 for Th2 cells, IL-17 for Th17 cells, and IL-10 (and Foxp3) for Treg cells. This staining is often performed after ex vivo stimulation of the cells with PMA and anisomycin. However, cell-surface markers specific for each subpopulation have not yet been fully established. It should also be noted that these categorizations may be too simplified. For example, there are T cell subpopulations that simultaneously express multiple signature cytokines (e.g., IFNγ and IL17). Furthermore, these immune responses are dependent on the background of the mice. The C67B/6 background is Th1 biased while the Balb/c or 129 backgrounds are Th2 biased. The molecular mechanisms underlying this are not fully understood. Nevertheless, these T cell categorizations are widely used in the immunology field, including in studies of obesity-induced inflammation.
CD8 T cells are often thought to be synonymous with cytotoxic T cells (CTLs) [82]. However, depending on the experimental conditions, CD4 T cells can also show cytotoxic activity. The primary role of cytotoxic lymphocytes is to kill infected or non-self cells. To identify infected/non-self cells, CD8 T cells recognize MHC I-presented non-self antigens via their antigen-specific TCRs (MHC I-restriction). While MHC II molecules are only expressed by APCs, including DCs, macrophages, neutrophils and B cells, MHC I molecules are ubiquitously expressed by all cells. Hence, when CD8 cells detect foreign antigens that are presented by MHC I molecules, they recognize that those cells are infected and kill them by using perforin and granzymes. In addition, CD8 T cells secrete large amounts of cytokines (e.g., IFN-γ) that help to induce the death of infected cells. However, these cytokines can also regulate the development and activation of other immune cells.
The roles of most of the αβT cell subpopulations, except Th17 cells, in the development of obesity-induced inflammation and insulin resistance have been studied. These studies are summarized here.
3.2.1.1 CD4 T cells
Recombination-activating genes (RAGs) play an important role in the recombination and rearrangement of TCRs in T cells and immunoglobulins in B cells. Hence, deletion of RAGs will abolish T cells (including NKT cells) and B cells. Therefore, Rag knockout mice are often used for T and B cell studies. When Rag1−/− knockout mice are placed on a HFD, they gain weight and adiposity and become insulin resistant, which suggests that T and B cells may play a role in obesity-induced insulin resistance [83]. However, these studies should be interpreted carefully because their effects on insulin resistance are partnered by effects on obesity.
Obesity also increases total CD3+ T cell numbers and IFNγ+ CD3 or CD3+ CD4+ T cell numbers in AT [83, 84]. Furthermore, deletion of IFNγ in the whole body improves obesity-induced insulin resistance without changing body weight [84]. The deletion of IFNγ also lowers obesity-induced macrophage infiltration and the AT expression of pro-inflammatory cytokines such as TNFα or RANTES. Hence, IFNγ-expressing Th1 T cells may exacerbate the development of obesity-induced inflammation and insulin resistance. However, these studies directly contradict the results with the Rag1−/− knockout mice: Rag1−/− knockout mice lack CD4 Th1 T cells (along with other cells) and would be expected to show improved obesity-induced insulin resistance rather than exacerbation [83].
However, Rag1−/− mice also lack Th2 T cells, which may explain their metabolic profile. Winer et al. showed that the reconstitution of CD4 T cells in Rag1−/− knockout mice lowered their body weight and improved insulin resistance [83]. By contrast, reconstitution with CD4 T cells from Stat6−/− knockout mice (which have impaired Th2 T-cell development, but have normal Th1 T-cell development) did not improve insulin resistance. Depletion of CD3 T cells by using a neutralizing anti-CD3 antibody also improves obesity-induced insulin resistance; a phenotyping switch of the ATMs from M1 to M2 macrophages was observed as well. However, in another study, CD3 T cell depletion using the same approach with a F(ab’)2 anti-CD3 antibody only induced a mild improvement in young (but not old) HFD-fed mice [85]. Nevertheless, these data altogether strongly suggest that CD4 Th2 T cells play a suppressive role in the development of obesity-induced insulin resistance by checking ATM inflammation. Interestingly, Winer et al. also showed that reconstitution of Rag1−/− knockout mice with CD8 T cells did not improve obesity-induced insulin resistance [83]. Furthermore, reconstitution of these mice with Foxp3− CD4 T cells or CD4 T cells from Il10 knockout mice resulted in the same degree of improvement in obesity-induced insulin resistance as reconstitution with total CD4 T cells [83]. These results directly contradict the reports that will be described below, which show strongly that CD8 T cells and CD4 Tregs play an important role in the development of obesity-induced inflammation and insulin resistance [86, 87].
Significantly, all three reports showed consistently that the TCR repertoires of AT CD4 T cells, CD8 T cells, and CD4 Tregs are very different from those populations in other tissues (e.g., spleen) [83, 86, 87]. This suggests that either the AT T cells are specialized populations or that there is clonal expansion in AT in response to an unknown antigen.
3.2.1.2 CD4 Regulatory T cells (Tregs)
Tregs play an important role in the suppression of inflammation [88]. Hence, Treg deletion causes the spontaneous development of several diseases, at least in mouse models, including Type 1 Diabetes, rheumatoid arthritis and colitis. In most cases, Tregs are defined as CD4+, CD25+ and Foxp3+, although CD8 Tregs have also been shown to exist. Foxp3 is a nuclear transcription factor that is a key regulator of the development and activation of Tregs. Since CD25 is often used as an activation marker for T and B cells, the “real” Treg population can only be determined by Foxp3 staining. However, since Foxp3 staining requires cell permeabilization and intracellular staining of Foxp3, it is not suitable for many experiments, such as those measuring gene expression profiles. Therefore, many studies have used CD4 and CD25 (IL-2Rα) to identify Tregs, even though only half or one-third of CD4+ CD25+ cells are Foxp3+. Recently, Foxp3-GFP knockin mice are often used to identify and sort Tregs, as they do not require intracellular staining of Foxp3. Tregs suppress other immune cells by producing IL-10 and TGFβ, which are classical anti-inflammatory cytokines and signature cytokines of Tregs. Interestingly, the frequencies of Tregs and Th17 cells often show an inverse relationship, as their differentiation processes are also counterbalanced. However, the in vivo presence of Th17 T cells in AT under normal chow conditions or a HFD has not yet been reported.
When Feuerer et al. examined the role of Tregs in the development of obesity-induced inflammation and insulin resistance, AT was found to contain a surprisingly high frequency of CD4 Tregs in normal chow conditions (~40% of CD4 T cells) [87]. Indeed, the frequency of this cell population in AT is higher than its frequency in other tissues, including the spleen, lymph node, lung and liver. Furthermore, the AT Tregs had very different genetic signatures compared to splenic and LN Tregs. Interestingly, obesity dramatically decreased Treg frequencies. When Tregs were depleted by using Foxp3-DTR (diphtheria toxic receptor) transgenic mice, insulin resistance developed spontaneously and the in vivo insulin signaling in insulin-responsive tissues, including AT, was also impaired. In addition, this was associated with increased pro-inflammatory gene expression in AT. By contrast, expansion of Tregs in HFD-fed mice by injecting the IL-2/anti-IL-2 antibody complex improved obesity-induced insulin resistance. An in vitro study suggested that the improvement of inflammation and insulin resistance is mediated by IL-10 produced by Tregs. These results suggested strongly that Tregs regulate obesity-induced insulin resistance by checking AT inflammation in obesity [87].
Interestingly, the AT Tregs from obese animals contain high levels of lipid. Furthermore, genetic analyses of these Tregs and other tissue Tregs revealed that only AT Tregs strongly express PPARγ and PPARγ-target genes [89]. Moreover, treatment of HFD-fed mice with thiazolidinediones (TZDs) increased Treg numbers in AT compared to those in untreated HFD controls. The deletion of PPARγ in Tregs by using Foxp3-Cre mice showed that under normal chow conditions, only the Treg numbers in AT were decreased; the Treg numbers in the spleen were not affected. Under a HFD, the Treg-specific PPARγ−/− knockout mice had the same number of Tregs in AT as the wild-type control mice and developed the same degree of obesity-induced insulin resistance. However, when the Treg-specific PPARγ−/− knockout mice were treated with TZDs, their Treg numbers in AT did not increase and obesity-induced insulin resistance did not improve, whereas the wild-type control mice showed increased AT Treg numbers and improved insulin resistance. Hence, these results together suggest strongly that TZDs may improve insulin resistance by targeting the AT Tregs.
3.2.1.3 Cytotoxic CD8+ T cells
The primary function of CD8 T cells to kill infected cells by using perforin and granzyme and by producing inflammatory cytokines. Obesity increases CD8 T cell numbers in AT and also induces IFNγ and granzyme B expression [83, 85, 86]. Nishimura et al. then showed that depletion of CD8 T cells improves obesity-induced insulin resistance; this is associated with a specific decrease in CD11c+ ATM numbers, whereas CD11c− ATM numbers are unchanged [86]. In addition, the depletion of CD8 T cells lowered the obesity-induced increases in the expression of pro-inflammatory cytokines such as IL-6 and TNFα. Furthermore, reconstitution of CD8a−/− KO mice fed on a HFD with splenic CD8 T cells exacerbated obesity-induced insulin resistance; this was associated with increases in CD11c+ ATM numbers, no changes in CD11c− ATM numbers, and increased AT IL-6 and TNFα gene expression. Since these data show strongly that CD8 T cells regulate ATM numbers and that the changes in AT CD8 T cell numbers precede the increases in ATM numbers in obesity, Nishimura et al. proposed that AT CD8 T cells induce the recruitment of ATMs in obesity, thereby mediating the development of insulin resistance. Interestingly, they also showed that in vitro co-culture of splenic CD8 T cells with AT from HFD mice increased CD8 T cell proliferation compared to the response to AT from lean controls. This suggests that in obesity, there may be clonal expansion of AT CD8 T cells. This result is important as it indicates an important mechanism by which obesity leads to the expansion of CD8 T cells (and potentially other T cells) in AT. However, that this obese AT-induced proliferation of CD8 T cells also occurs in vivo should be confirmed.
3.2.1.4 Natural Killer T (NKT) cell
NKT cells are a very specialized T cell subpopulation that expresses both CD3 and NK1.1, which is a cell marker for NK and NKT cells [90]. Hence, NKT cells (CD3+ NK1.1+) can be differentiated from CD4 and CD8 T cells, which are NK1.1− (NK1.1− CD3+). It can also be differentiated from NK cells, which are CD3− (CD3− NK1.1+). In most cases, NKT cells do not recognize peptide antigens that are presented by MHC I or MHC II molecules. Instead, they typically recognize lipid antigens that are presented by CD1d, which is a specialized antigen-presenting molecule that is expressed by many cell types, including DCs, macrophages, adipocytes and hepatocytes. However, there are also subpopulations of NKT cells that are not CD1d-restricted and instead utilize MHC molecules. α-galactosylceramide (α-GalCer) is commonly used as an artificial ligand for NKT-cell activation. NKT cells are categorized into two major subpopulations based on their TCR sequence variations. The type I NKT cells (also known as invariant NKT cells, classical NKT cells, or iNKT) have TCRs with an invariant α chain (Va14-Ja18 in mice and Va24-Ja18 in humans). The type II NKT cells (also known as variant NKT cells, non-classical NKT cells, or vNKT cells) have more diverse TCR sequences. Hence, CD1d−/− knockout mice lack both iNKT and vNKT cells whereas Jα18−/− knockout mice only lack iNKT cells. NKT cells produce variety of cytokines, including IL-4, IL-13, IFNγ and TNFα, and therefore can mediate either Th1 or Th2 responses.
Since NKT cells recognize lipid antigens, it is of particular interest to determine the role NKT cells play in the development of obesity-induced insulin resistance. NKT cells are the major component of liver T cells (30–50% of T cells) and the deletion of iNKT cells exacerbates hepatic steatosis. Therefore, early studies in obesity mainly focused on the effect of NKT cells in the liver. These studies showed consistently that obesity decreases NKT-cell numbers in the liver. Recently, CD1d−/− and Jα18−/− knockout mice were used to examine the roles of NKT cells in obesity more directly. However, these studies reported very contradictory results: the mice exhibited improved, exacerbated, or equivalent obesity-induced insulin resistance phenotypes [91–99]. In most cases, the studies used the same mouse lines and very similar experimental procedures. At this point, the reason for these significant discrepancies is not clear. Another study using β2-microglobulin (B2M) knockout mice, which lack NKT cells, revealed that they are not susceptible to obesity-induced insulin resistance, which suggests that NKT cells may play a role in the development of obesity-induced insulin resistance [97]. However, B2M is a component of MHC I, and B2M knockout mice also lack CD8 T cells. The latter study as well as other studies also showed that the injection of α-GalCer mildly worsened obesity-induced insulin resistance. However, it is also well-known that α-GalCer-activated NKT cells cause a cytokine storm that artificially induces the secondary activation of a variety of immune cells, including CD4 and CD8 T cells and NK cells [100–105]. Hence, the role of NKT cells in the development of obesity-induced inflammation and insulin resistance is not fully established yet and must be investigated further.
3.2.2 B cells
B cells are another arm of the adaptive immune system along with T cells [106, 107]. Although the name “B cells” derives from the notion that B cells develop fully in the Bone marrow (unlike the T cells, which develop in the Thymus), B cells egress from the BM as an immature form that has fully developed BCRs and expresses IgM. The B cells then migrate into the secondary lymphoid organs such as the lymph nodes or spleen, where they mature fully and differentiate into plasma cells that produce antibodies, including various IgG subtypes. The Rag−/− and µMT mouse models are often used to analyze B cell functions. Rag−/− mice lack both T and B cells whereas µMT mice do not have IgM and therefore lack mature B cells without any primary defects in other immune cells. The typical markers for flow cytometric analysis include CD19 and CD45R (B220). The major function of B cells is to produce antibodies that are specific for foreign antigens in humoral immunity. To fully activate B cells, they must interact with Th2 cells. When BCRs recognize their free antigens (B cells do not require MHC presentation for the recognition of antigens, unlike T cells), the antigens are engulfed, digested, and presented on MHC II molecules. Primed Th2 cells (activated T cells that have a specific TCR for the matching antigen) can interact via their TCRs with the MHC II on B cells, after which the Th2 cells produce cytokines that cause the full development of B cells and maximize their production of antibody. This process is called “T cell-dependent activation” and most B cell activation occurs through this process (B cells can be activated via T cell-independent activation but only at low frequencies). However, B cells can also produce cytokines in various functions. Recently, it was shown that B cells can act as suppressors. Since these suppressive B cells are similar to Tregs in terms of their suppressor role in inflammation and their secretion of the anti-inflammatory cytokine IL-10, they are also known as regulatory B cells (Bregs).
Recent studies showed that obesity increases the B cell population in AT, especially IgG-producing mature B cells [66, 67]. Moreover, the µMT mice on a HFD show improved obesity-induced insulin resistance, although their epididymal fat weight is decreased; interestingly, changes in subcutaneous fat weight are not observed [66, 67]. The depletion of B cells by a neutralizing antibody also improves obesity-induced insulin resistance. The µMT mice on a HFD also show decreases in CD11c+ ATM numbers and CD8 T cell activation along with increased AT Treg numbers and M2 ATM signature gene expression in AT. This is accompanied by decreases in pro-inflammatory cytokine levels in AT as well [66, 67]. Interestingly, IL-17 gene expression in AT is also decreased, which suggests that there may be a link between B cells and Th17 cells.
Winer et al. examined the adaptive immunity role of B cells in the development of obesity-induced insulin resistance further [66]. They reconstituted B cells from mice fed normal chow or a HFD into HFD-fed µMT mice and found that only the B cells from HFD-fed donor mice worsened insulin resistance; the B cells from normal chow-fed donor mice did not change the insulin resistance phenotypes. They tested this further by using purified Ig from mice fed normal chow or a HFD. Again, HFD-fed µMT mice injected with IgG purified from HFD-fed mice showed increased obesity-induced insulin resistance. The IgG from normal chow-fed mice had no effect. However, when Rag1−/− knockout mice were reconstituted with B cells, obesity-induced insulin resistance did not increase. This suggests that B cells require T cells to induce the development of obesity-induced insulin resistance. This notion was tested by reconstituting µMT mice with B cells from MHC I or II knockout mice: neither mutant B cell population promoted the development of insulin resistance. These results strongly suggest that B cells must interact with CD4 T cells and/or CD8 T cells in obesity before insulin resistance can develop fully. On the other hand, injection of µMT mice with F(ab’)2 fragments of IgG purified from HFD-fed mice did not exacerbate obesity-induced insulin resistance. This suggests that the Fc receptor may be involved in the insulin resistance-inducing actions of B cells. Finally, Winer et al. screened for antigens by using antibodies isolated from insulin-sensitive and -resistant human subjects. Potential candidates that were recognized by these antibodies from each group were identified. Interestingly, the antibodies from the two groups recognized almost equal numbers of candidate proteins.
These studies altogether show that B cells play a role in the development of obesity-induced inflammation and insulin resistance. Furthermore, Winer et al. showed that adaptive immunity of B cells may also play a significant role in these processes [66]. Since this study potentially changes the paradigm of obesity-induced inflammation, which was long thought to be mediated by innate immunity, studies that verify the observations of Winer et al. are needed.
4. Molecular players in obesity-induced inflammation
Many molecules that mediate the development of obesity-induced inflammation in total AT and AT immune cell compartments have been identified. To identify these molecules, the majority of studies used genetic mouse models including total body knockout mice, bone marrow transplantation (BMT) of total body knockout mice, and myeloid-specific knockout mice using LysM-Cre mice. By targeting mediators in the development of obesity-induced inflammation and insulin resistance, these studies have elucidated important molecular mechanisms that underlie the development of obesity-induced inflammation. However, all of these studies bear intrinsic limitations that should be remembered when interpreting their results. First, all three mouse model systems involve the systemic modulation of the target gene(s) rather than modulating the gene in the AT immune cell compartment only. Obviously, total body knockout mice delete target genes in all tissues/cells, and BMT replaces all immune cells in all tissues. While myeloid-specific gene deletions by using LysM-Cre mice yield the most specific models, all myeloid cells (including DCs, macrophages, neutrophils and eosinophils) are targeted by these deletions.
Another point that should be considered is that many immunological modulations affect body weight and adiposity. In most cases, body weight and adiposity decreases are associated with improvements in obesity-induced insulin resistance, and vice versa. Hence, the metabolic phenotypes in those studies should be interpreted carefully. Table 2 summarizes the effect of gene deletion on metabolic phenotypes and body weight and adiposity. Although it may be very important to determine how these immune modulations regulate body weight and adiposity, this issue has not yet been studied extensively. Since there are many excellent reviews that overview the roles of various molecular players in the development of obesity-induced inflammation and insulin resistance, we will only discuss selected pathways in this review.
4.1. The IKKβ/NFκB Pathway
NFκB plays a critical role in regulating inflammation [108, 109]. In the basal (resting) state, NFκB is sequestered in the cytoplasm by its binding to IκBα; this binding masks its nuclear localization sequence (NLS). When cells are stimulated with various stimuli, including growth factors, cytokines and foreign pathogens or molecules such as LPS, the IKK enzyme complex is activated and IKKβ phosphorylates Ser32 and 36 of IκBα. This phosphorylation induces the proteasome-dependent degradation of IκBα, which exposes the NLS of NFκB and causes NFκB to translocate into the nucleus, where it initiates the gene expression of various inflammatory mediators. Many NFκB target genes, including TNFα, IL-6 and MCP-1, are also implicated in the development of obesity-induced insulin resistance.
In obese animals, NFκB activity is increased [31]. Furthermore, when the IKKβ/NFκB pathway in animal models is inhibited by pharmacological inhibitors of IKKβ (a high dose of salicylates and aspirin) or by genetic deletion of IKKβ, the obesity-induced insulin resistance improves [30, 31]. More importantly, treatment of insulin-resistant or T2D patients with aspirin or salsalate (a dimer of salicylates) improves their glycemic control along with inhibiting NFκB activity in their PBMCs [32–35]. The deletion of IKKβ in myeloid cells by using LysM-Cre mice also protects these mice from developing obesity-induced insulin resistance [110]. These results strongly suggest that the suppression of IKKβ/NFκB-dependent inflammation improves insulin resistance and T2D in animal models as well as in humans.
The question is then, “How does obesity activate the IKKβ/NFκB pathway?” Since TLRs are considered to be major upstream molecules in the activation of the IKKβ/NFκB pathway in many immune systems [111], the role of the TLR signaling pathway in the development of obesity-induced inflammation and insulin resistance has been examined. While LPS is the best-known ligand for TLR4, FFA can also activate TLR4 [112, 113]. Furthermore, deletion of TLR4 in the whole body protects mice from the development of lipid- and diet-induced insulin resistance [112]. Interestingly, female tlr4−/− mice gained more weight (and adiposity) while exhibiting improved insulin resistance compared to the wild type controls [112]. Moreover, C3H/HeJ mice, which have a spontaneous loss-of-function mutation in TLR4, are also protected from diet-induced insulin resistance; however, in this case, the mutant mice also have decreased weight and adiposity [114]. More recent studies show that ceramide-mediated insulin resistance is also mediated by TLR4 [115]. Since these studies used total body knockout or mutant mice, it was not clear whether it was the TLR4 on hematopoietic cells that promoted the development of insulin resistance. This question was tested by transplanting BM from total body TLR4 knockout mice into irradiated wild-type mice: the deletion of TLR4 in the hematopoietic compartment protected the mice from diet-induced insulin resistance [116]. However, this study did not examine the role of TLR4 in the peripheral compartment by reconstituting irradiated tlr4−/− knockout mice with WT BM. Curiously, TLR4 deletion does not improve insulin resistance in all studies. A recent report showed that total body TLR4 knockout or deletion of TLR4 in the hematopoietic or non-hematopoietic compartments by BMT did not improve diet-induced insulin resistance; indeed, it actually exacerbated the insulin resistance somewhat [117]. The latter findings are supported by results from MyD88 knockout mice. MyD88 is a universal adaptor molecule for the TLR signaling pathway and all TLRs require MyD88 to activate the IKKβ/NFκB pathway. Hence, deletion of MyD88 effectively inhibits all TLR signaling. The MyD88−/− knockout mice exhibited exacerbated diet-induced insulin resistance without changes in body weight or adiposity [118]. Hence, the discrepancies between the latter two studies and the other studies on TLRs mean that it is not yet clear whether obesity-induced NFκB-dependent inflammation is mediated by the TLR signaling pathway.
4.2. Jun N-terminal kinases (JNKs)
The Jun N-terminal kinase/stress-activated protein kinases (JNK/SAPKs) belong to the MAP (mitogen-activated protein) kinase family and are involved in many different stress pathways [41, 119]. In the insulin resistance field, JNK is known to play a key role in the ER stress that regulates the development of obesity-induced insulin resistance. This role relates in part to its phosphorylation of IRS-1 on the Ser307 and 310 (mouse sequence) residues, which inhibits this molecule [120]. Hence, the deletion of JNKs or its scaffolding protein JIP improves obesity-induced insulin resistance [121, 122]. Since JNK in the ER pathway appears to directly inhibit the insulin signaling pathway, it directly affects insulin-responsive cells. However, it is debatable whether JNK in hematopoietic cells participates in the development of obesity-induced insulin resistance, and if it does, whether it is solely responsible for this pathogenesis. When Solinas et al. used the BMT technique with Jnk1−/− knockout out mice, they showed that deletion of Jnk1 in the peripheral (non-hematopoietic) compartment improved diet-induced insulin resistance, although the body weight and adiposity were also decreased [123]. The deletion of Jnk1 in the hematopoietic compartment also improved diet-induced insulin resistance but without changing the body weight and adiposity [123]. Therefore, this report suggests that Jnk1 in the hematopoietic compartment (presumably in myeloid cells) plays a critical role in the development of obesity-induced insulin resistance. However, when Vallerie et al. used the same mouse model and a similar BMT technique, they found that obesity-induced insulin resistance only improved when Jnk1 was deleted in the peripheral compartment; deletion of Jnk1 in the hematopoietic compartment had no effect on insulin resistance, although it did lower the expression of pro-inflammatory genes in AT [124]. Furthermore, the myeloid cell-specific deletion of Jnk1 did not induce any changes in metabolic phenotypes either, whereas adipocyte-specific deletion of Jnk1 improved obesity-induced insulin resistance [125]. Hence, the latter two studies support the role of Jnk1 in the peripheral tissues, especially in adipocytes, but not in myeloid cells. Although the discrepancies in these studies are not yet fully understood, a recent study suggests that Jnk2 in myeloid cells may play a bigger role in the development of obesity-induced insulin resistance when Jnk1 is deleted [126]. Total body Jnk2−/− knockout mice do not affect obesity-induced insulin resistance [121]. However, when both Jnk1 and Jnk2 are deleted in myeloid cells by using LysM-Cre mice, the mutant mice exhibit improved insulin resistance along with suppression of inflammation, including decreases in the total and CD11c+ ATM numbers and the pro-inflammatory gene expression in AT [126]. Body weight and adiposity were not affected in the latter mice.
4.3. Inflammasome Pathway
The inflammasome is an intracellular multimeric complex that plays an important role in innate immunity [127]. It is often found in myeloid cells and the complex includes NRLPs, NALPs, ASC, Pycard and caspase 1. The main function of the inflammasome is to induce the maturation of IL-1β and IL-18 and therefore to produce these cytokines in response to danger signals. Hence, the inflammasome is only fully activated when two signaling events occur: increased production of pro-IL-1β and pro-IL-18 through gene transcription (mainly induced via NFκB activation by TLRs) and activation of the inflammasome so that it cleaves pro-IL-1β and IL-18 via its caspase-1 (mainly induced by DAMPs or PAMPs) [128]. Interestingly, cholesterol crystals, which may mediate atherosclerosis, activate the inflammasome [129].
In vitro studies show that FFA or ceramide can activate the inflammasome in BMDMs only when the cells are stimulated with LPS [130, 131]. In addition, Wen et al. found that FFA-induced inflammasome activation requires ROS production and is partially suppressed by the activation of AMP kinase [130]. Interestingly, Wen et al. found that FFA treatment (plus LPS) impairs autophagy and that activation of AMP kinase reverses this. Therefore, the authors proposed that FFA treatment of macrophages inhibits AMP kinase and autophagy; this in turn increases ROS production by mitochondrial damage, which facilitates the FFA/LPS-induced activation of the inflammasome [130]. Although this is a very interesting hypothesis, it will have to be tested in vivo. Supporting the role of the inflammasome in obesity-induced insulin resistance, three studies showed that deletion of inflammasome components, including NLRP3, Pycard, caspase 1, or ASC, improved obesity-induced insulin resistance while suppressing inflammation (such as decreasing the pro-inflammatory gene expression in AT and the polarization of M1 ATMs) [130–132]. Furthermore, BMT of Pycard−/− mice show that it is the inflammasome in the hematopoietic compartment, not the inflammasome in stromal compartments, that plays this role in obesity-induced insulin resistance [130]. However, the details of these three studies differ, especially in terms of the effect of inflammasome component deletion on body weight and adiposity. These discrepancies are summarized in Table 2. Hence, it is not fully clear at this moment whether the inflammasome pathway in obesity regulates obesity-induced inflammation and insulin resistance directly or whether it acts by more indirect mechanisms that are secondary to changes in body weight and adiposity.
The two cytokines that are regulated by the inflammasome are IL-1β and IL-18. Although the role of IL-18 in the development of obesity-induced inflammation and insulin resistance has not been studied extensively, the role of IL-1β was recently the focus of research because of its clinical implications. Treatment of cells or obese animals with IL-1β induces insulin resistance by inhibiting the insulin signaling pathway [133, 134]. Furthermore, treatment of obese animals with a neutralizing antibody against IL-1β improves insulin resistance, although this is thought to be mainly mediated by the improvement of β-cell functions [135]. These observations quickly led to a small clinical trial with Anakinra, an IL-1R antagonist: the antagonist improved the glycemic control in T2D by improving β-cell functions [136]. Anakinra treatment also lowered the levels of systemic inflammation markers. These studies suggest strongly that inhibiting IL-1β function, and potentially inflammasome activity, improves insulin resistance and T2D in humans. However, the more recent CANTOS clinical study of Canakinumab, a humanized monoclonal antibody against IL-1β, showed that IL-1β blocking does not improve the glycemic control in T2D, although the treatment did lower inflammation [137]. The main difference between the two clinical trials is that Anakinra targets both IL-1α and IL-1β, while Canakinumab specifically neutralizes IL-1β alone. Hence, although the inflammasome and its target cytokines, IL-1β and IL-18, are important mediators in innate immunity and may also play a role in the development of obesity-induced inflammation and insulin resistance, more clinical and preclinical studies are needed to determine the importance of these molecules in the development of obesity-induced insulin resistance and T2D.
4.4. Cytokines
At the cellular level, obesity-induced insulin resistance is caused by the impairment of the insulin signaling pathway in insulin-responsive cells (i.e., adipocytes, hepatocytes, myocytes and β-cells). By contrast, obesity-induced inflammation is thought to mainly occur in the tissue-infiltrating immune cells. Therefore, the obesity-inflamed immune cells must cross-talk or produce mediators to induce the insulin resistance in insulin-responsive cells. The main suspects in this interaction are the cytokines that are produced by the obesity-inflamed immune cells. However, the IKKβ/NFκB, JNK and inflammasome pathways in immune cells can all promote the production of pro- and anti-inflammatory cytokines. In addition, the cytokines produced by obesity-inflamed immune cells also regulate the recruitment, development and activation of other tissue-resident immune cells, thus initiating the secondary wave of cytokine production. Hence, it is difficult to determine which cytokines directly induce the impairment of insulin signaling. However, by using genetically modified mouse models in conjunction with in vitro studies, many cytokines have been tested for their roles in the development of obesity-induced inflammation and insulin resistance.
TNFα is a classical pro-inflammatory cytokines and its role in insulin signaling has been studied extensively. Indeed, TNFα was studied even before the notion of obesity-induced inflammation arose. The early in vitro studies clearly show that TNFα inhibits insulin signaling by reducing the levels of insulin signaling components [11, 12]. In addition, TNFα can induce the inhibitory phosphorylation of IRSs, thereby blocking insulin signaling [138]. Many in vivo mouse models also clearly show that inhibition of TNFα function improves obesity-induced inflammation [13–17]. However, since most of these studies were performed before the role of ATMs in obesity was established, they did not show whether inhibition of TNFα function also changes AT inflammation or ATM phenotypes. Moreover, clinical trials of an anti-TNFα antibody showed that it did not improve glycemic control in T2D [139, 140], although the same treatment does improve rheumatoid arthritis.
IL-6 is another classical pro-inflammatory cytokine that is a risk factor for the development of T2D in human [25]. However, mouse model studies have yielded contradictory results regarding the role of IL-6 in the development of insulin resistance. Acute IL-6 infusion of mice induces spontaneous insulin resistance in the absence of obesity [141]. However, mice in which IL-6 is deleted develop late-onset obesity and insulin resistance [142].
On the other hand, IL-10 is a classical anti-inflammatory cytokine. It is expressed by Tregs at high levels but is also expressed by macrophages and other cells. Since IL-10 is anti-inflammatory, it is thought to counter pro-inflammatory cytokine functions and therefore promote the improvement of obesity-induced insulin resistance. Indeed, overexpression of IL-10 increased M2 ATM numbers [143], and co-infusion of IL-10 and IL-6 reverses IL-6-mediated insulin resistance [141]. The Treg studies also support this [87, 89]. However, the hematopoietic deletion of IL-10 by BMT from Il10−/− knockout mice into wild-type mice did not change the diet-induced insulin resistance or inflammation [144]. This raises doubts over the role IL-10 plays in these pathogeneses.
As indicated by these selected examples of in vivo cytokine studies, cytokines do not follow the anti-inflammation vs. pro-inflammation paradigm very well. For example, pro-inflammatory cytokines (e.g., IL-6) can improve insulin resistance [142]. This discordance between the inflammatory functions of cytokines and their metabolic functions may be due to the temporal and local regulation of inflammation. For example, while certain cytokines are needed to initiate obesity-induced inflammation and insulin resistance during the early phase of obesity development, they may not be required in the later phases of obesity. Another possibility is that it may require several cytokines to coordinately regulate the development of disease: a single cytokine is not sufficient for mediating the complex obesity-induced inflammation and insulin resistance.
5. Limitations and Future Studies
There has been great progress in terms of our understanding of how obesity-induced inflammation is regulated and how modulations of this pathway can regulate the development of obesity-induced insulin resistance. However, there are several limitations that we may need to consider. First, there are many contradictory reports and it is not clear yet what causes these discrepancies (a good example of this is the literature on NKT cells). However, it should be also noted that the field of obesity-induced inflammation is relatively new and that many of the studies have been done only limited time. Hence, as the field grows and these conflicted results are further tested, we may be able to find causes for these discrepancies. One intriguing idea for this is that the immunophenotypes observed in different labs may vary because of microbiome differences. In the immunology field, the possibility that the microbiome can regulate systemic immunity is gaining more support [145]. It is also clear that the animals in different institutes have very different microbiomes. Furthermore, the relationship between microbiome and obesity is also well established [146]. However, how the microbiome regulates obesity-induced insulin resistance (without changing obesity) via gut immunity (inflammation) has not been examined extensively. This is a very exciting and important area per se. In any case, it will be interesting to determine whether diverse microbiomes may be responsible for the different results of different labs.
Another limitation is the tissue specificity of immune modulations. Although this review focused on the cellular and molecular players in AT inflammation, most of the studies described here actually examined the systemic roles of these players. Probably the only two examples of AT-specific mouse models are the AT-specific MCP-1 transgenic mice [50, 51] and the Treg-specific PPARγ knockout mice [89]. With the technology that is currently available, it may not be possible to specifically target AT. Thus, when interpreting the studies described here, the effects of immune modulation on systemic inflammation should also be considered.
However, probably the biggest limitation of the current studies is that most of them are in mouse models. The immune systems of mice and humans are obviously very different. For example, while neutrophils are the most abundant circulating cell type (~two-thirds) followed by lymphocytes (~one-third) in humans, the order is reversed in mice: lymphocytes are the most abundant cell type in the circulation (~two-thirds), followed by neutrophils (~ one-third). Furthermore, the backgrounds of experimental mouse model systems also regulate immune responses. For example, Balb/c is more biased to Th-2, while C57/B6, the most commonly used mouse model in obesity, is more Th1-biased. This explains why PPARγ deletion in myeloid cells in the Balb/c background improved obesity-induced insulin resistance and suppressed inflammation [147] when the same mutation on the C57/B6 background did not improve either obesity-induced insulin resistance or inflammation [148]. It is not clear whether this is an isolated case that is specifically limited to PPARγ or reflects a more universal phenomenon. Hence a systematic study that compares these two mouse backgrounds in terms of the development of obesity-induced inflammation and insulin resistance may be needed. However, humans do not generally display this skewing towards a Th1 or Th2 background phenotype. Nevertheless, it should be also noted that many of the immunological findings from mouse studies have been confirmed by human studies. The best example is that like obese animal models, obese human subjects have more ATMs than lean subjects. Furthermore, the salicylate-induced improvement of glycemic control in humans was accurately predicted by preclinical studies, and the outcomes are fairly comparable in many phenotypes. However, the biggest limitation, and also the most challenging task in obesity-induced inflammation research, is how to translate the preclinical study findings into clinical studies that examine the effect of inflammation-regulating treatments on T2D in humans.
Table 4.
Molecular players that participate in the development of obesity-induced inflammation and insulin resistance.
| Gene | Targeting Method |
Insulin Resistance |
Body Weight |
Adiposity | ATM | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Number | M1 | M2 | ||||||
| IKK-β/NFκB signaling pathway | ||||||||
| IKK-β | T KO | ↓ | ↔ | ↔ | ND | ND | ND | [31] |
| Δmye | ↓ | ↓ | ND | ND | ND | ND | [110] | |
| TLR2 | T KO | ↑ | ↑ | ↑ | ND | ND | ND | [149] |
| TLR4 | T KO | ↓ | ↓ | ↓ | ↓ | ND | ND | [114] |
| T KO | ↔(↓) | ↓ | ↓ | ↔ | ↔ | ↑ | [117] | |
| BMT(H):T KO | ↓ | ↔ | ↔ | ↓ | ND | ND | [116] | |
| BMT(H):T KO | ↔ | ↔ | ↔ | ↔ | ND | ↑ | [117] | |
| BMT(P):T KO | ↔ | ↔ | ↔ | ND | ND | ↔ | [117] | |
| MyD88 | T KO | ↑ | ↔ | ↔ | ND | ND | ND | [118] |
| JNKs | ||||||||
| Jnk1 | T KO | ↓ | ↓ | ↓ | ND | ND | ND | [121] |
| Tg:aP2 | ↓ | ↔ | ↔ | ND | ND | ND | [150] | |
| BMT(H):T KO | ↓ | ↔ | ↔ | ND | ND | ND | [123] | |
| BMT(H):T KO | ↔ | ↔ | ↔ | ↔ | ND | ND | [124] | |
| BMT(P):T KO | ↓ | ↓ | ↓ | ND | ND | ND | [123] | |
| BMT(P):T KO | ↓ | ↔ | ↔ | ↔ | ND | ND | [124] | |
| Δmye | ↔ | ↔ | ↔ | ND | ND | ND | [125] | |
| JNK2 | T KO | ↔ | ↔ | ND | ND | ND | ND | [121] |
| Jnk1/Jnk2 | Δmye | ↓ | ↔ | ↔ | ↓ | ↓ | ↓ | [126] |
| Inflammasome | ||||||||
| Nlrp3 | T KO | ↓ | ↔ | ↓ | ND | ↓ | ↔ | [131] |
| T KO | ↓ | ↔ | ↔ | ND | ND | ND | [130] | |
| T KO | ↓ | ↓ | ND | ND | ND | [132] | ||
| ASC | T KO | ↓ | ↓ | ↓ | ↔ | ND | ND | [130] |
| BMT(H):T KO | ↓ | ND | ND | ND | ND | ND | [130] | |
| ASC | T KO | ↓ | ↓ | ↓ | ↔ | ND | ND | [132] |
| Casp1 | T KO | ↓ | ↓ | ↓ | ↓ | ND | ND | [132] |
| Pycad | T KO | ↓ | ND | ND | ND | ND | ND | [130] |
| BMT(H):T KO | ↓ | ND | ND | ND | ND | ND | [130] | |
| IL-1β | T KO | ↓ | ND | ND | ND | ND | ND | [130] |
| Cytokine/Chemoattractants | ||||||||
| TNF-α | I (Zucker rat) | ↓ | ↔ | ↓ | ND | ND | ND | [13,14] |
| T KO | ↓ | ↔ | ↓ | ND | ND | ND | [15,16] | |
| BMT(H):T KO | ↓ | ↔ | ND | ND | ND | ND | [17] | |
| TNFαR | T KO | ↓ | ↔ | ↓ | ND | ND | ND | [14] |
| IFN-γ | T KO | ↓ | ↔ | ↔ | ↓ | ↓ | ND | [84] |
| IL-6 | T KO | ↑ | ↑ | ↑ | ND | ND | ND | [142] |
| Il-15 | T KO | ↑ | ↑ | ND | ND | ND | [151] | |
| IL-4 | I | ↓ | ↓ | ↓ | ND | ND | ND | [152] |
| IL-4/IL-13 | T KO | ND | ND | ND | ND | ND | ↓ | [76] |
| IL-1R1 | T KO | ↓ | ↔ | ↑ | ↔ | ND | ND | [153] |
| IL-10 | Tg | ND | ND | ND | ND | ↔ | ↑ | [143] |
| Inj | ↓ | ND | ↓ | ND | ND | ND | [141] | |
| BMT(H):T KO | ↔ | ↔ | ↔ | ↔ | ND | ND | [154] | |
| Osteopontin | T KO | ↓ | ↔ | ↔ | ↓ | ND | ND | [155] |
| MCP-1 | T KO | ↓ | ↔ | ↓ | ↓ | ND | ND | [50] |
| Tg:aP2 | ↑ | ↔ | ↔ | ↑ | ↑ | ND | [50,51] | |
| CCR2 | T KO | ↓ | ↓ | ↓ | ↓ | ND | ND | [156] |
| CX3CR1 | T KO | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | [144] |
| CXCL5 | I | ↓ | ↔ | ND | ND | ND | ND | [157] |
| CXCR2 | T KO | ↓ | ↔ | ND | ND | ND | ND | [157] |
| PPARs | ||||||||
| PPAR-γ | Δmye (Balb/c) | ↑ | ↑ | ↑ | ↓ | ND | ↓ | [147] |
| Δmye (C57BL/6) | ↔ | ↔ | ↔ | ND | [148] | |||
| PPAR-δ | Δmye | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | [158] |
| Others | ||||||||
| AIM | KO:Het | ↓ | ↑ | ↑ | ND | ↑ | ↔ | [159] |
| RP105/MD-1 complex | KO:Het | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | [160] |
| MGL1 | KO:Het | ↓ | ↓ | ↓ | ND | ↓ | ND | [161] |
| CD36 | BMT(H):T KO | ↔ | ↔ | ↔ | ND | ↓ | ↑ | [162] |
| BLT-1 | T KO | ↓ | ↔ | ↑ | ↓ | ↓ | ↑ | [163] |
| SOCS1 | Δmye | ↑ | ↔ | ↔ | ND | ↑ | ND | [164] |
| IR | Δmye | ↓ | ↔ | ↔ | ↓ | ND | ND | [165] |
| ABCA1 | Δmye | ↑ | ND | ND | ↑ | ND | ND | [166] |
| Sorbs1 | T KO | ↓ | ↔ | ↔ | ↓ | ND | ND | [167] |
| FABP4/FABP5 | BMT(H):T KO | ↓ | ↔ | ↔ | ↔ | ND | ND | [168] |
| KLF4 | Δmye | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | [169] |
| α4 integrin | T KO | ↓ | ↔ | ND | ↓ | ND | ND | [170] |
| GPR120 | T KO | ↑ | ↔ | ND | ↔ | ↔ | ↔ | [171] |
T KO, Total body knockout; Δmye, Myeloid-specific knockout mice generated by using LysM-Cre mice; Tg:aP2, Adipocyte-specific transgenic mice generated by using aP2 promoter; BMT(H), Deletion of target genes in the hematopoietic compartment by bone marrow transplantation (Donor: T KO or transgenic mice, Recipients: wild type controls); BMT(P), Deletion of target genes in the parenchymal (non-hematopoietic) compartment by bone marrow transplantation (Donors: wild type controls, Recipients: T KO or transgenic mice); Inj, Injection with target molecule; I, inhibitor; ND, No determine.
Highlights.
We discuss obesity-induced adipose tissue inflammation.
We discuss the cellular and molecular players of obesity-induced inflammation.
We discuss obesity-induced inflammation in the context of classical immunity.
Abbreviations
- ABCA
ATP-binding cassette transporter
- AIM
Apoptosis inhibitor of macrophage
- APC
antigen-presenting cell
- ASC
apoptotic speck protein containing a caspase recruitment domain
- AT
adipose tissue
- ATM
AT macrophage
- BLT
leukotriene B4 receptor
- BM
bone marrow
- BMT
bone marrow transplantation
- Bregs
regulatory B cells
- Casp1
Caspase 1
- CCL5
Chemokine (C-C motif) ligand 5
- CCR2
C-C chemokine receptor type 2
- CLSs
crown-like structures
- CTLs
cytotoxic T cells
- CX3CR1
CX3C chemokine receptor 1
- CXCL5
C-X-C motif chemokine 5
- CXCR2
C-X-C motif receptor 2
- DAMPs
Damage-associated molecular pattern molecules
- DC
Dendritic cells
- dsDNA
double stranded DNA
- ECM
extracellular matrix
- FABP
fatty-acid-binding protein
- FFA
Free fatty acids
- GFP
green fluorescent protein
- GLUT4
Glucose transporter type 4
- GPR120
G-protein coupled receptor 120
- HFD
high fat diet
- HMGB1
high-mobility group box 1
- IKKb
inhibitor of κB kinase-β
- IR
insulin receptor
- JNKs
Jun N-terminal kinases
- IRS
Insulin receptor substrate
- KLF4
Krueppel-like factor 4
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- MGL1
macrophage galactose-type lectin 1
- MHC
Major histocompatibility complex
- MPO
myeloperoxidase
- NE
neutrophil elastase
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NKT
Natural Killer T cell
- Nlrp3
NOD-like receptor family, pyrin domain containing 3
- NLS
nuclear localization sequence
- PBMC
peripheral blood mononuclear cell
- PKC
Protein kinase C
- PPAR
peroxisome proliferator-activated receptors
- PRRs
Pattern recognition receptors
- RAGs
Recombination activating genes
- RANTES
Regulated on Activation Normal T cell Expressed and Secreted
- ROS
Reactive oxygen species
- SOCS1
Suppressor of cytokine signaling 1
- Sorbs1
Sorbin and SH3 domain-containing protein 1
- SVC
stromal vascular cell
- T2D
Type 2 Diabetes
- TAMs
tumor-associated macrophages
- TCRs
T cell receptors
- TLR
Toll-like receptors
- TNF
Tumor necrosis factors
- Treg
regulator T cells
- TZDs
thiazolidinediones
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 3.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 4.Pilch PF, Lee J. Insulin Receptor Family. In: Lennarz W, Lane MD, editors. Encyclopedia of Biological Chemistry. vol. 2. San Diego: Elsevier Science; 2004. pp. 436–440. [Google Scholar]
- 5.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 6.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.De Caterina R, Zampolli A. From asthma to atherosclerosis--5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350:4–7. doi: 10.1056/NEJMp038190. [DOI] [PubMed] [Google Scholar]
- 9.Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and Type 2 Diabetes. Arch Parma Res. 2013;36:208–222. doi: 10.1007/s12272-013-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 11.Stephens JM, Pekala PH. Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis. J Biol Chem. 1992;267:13580–13584. [PubMed] [Google Scholar]
- 12.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AT, Ree D, Kolls JK, Fuselier J, Coy DH, Bryer-Ash M. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology. 1998;139:4928–4935. doi: 10.1210/endo.139.12.6336. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 15.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 16.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller DE. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 17.De Taeye BM, Novitskaya T, McGuinness OP, Gleaves L, Medda M, Covington JW, Vaughan DE. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab. 2007;293:E713–E725. doi: 10.1152/ajpendo.00194.2007. [DOI] [PubMed] [Google Scholar]
- 18.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 20.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of US adults. Am J Epidemiol. 2002;155:57–64. doi: 10.1093/aje/155.1.57. [DOI] [PubMed] [Google Scholar]
- 22.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O'Reilly DS, Packard CJ, Sattar N. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 23.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23:650–655. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 25.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 27.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 28.Barzilay J, Freedland E. Inflammation and its association with glucose disorders and cardiovascular disease. Treat Endocrinol. 2003;2:85–94. doi: 10.2165/00024677-200302020-00002. [DOI] [PubMed] [Google Scholar]
- 29.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 30.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 32.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 41.Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 42.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010;107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease, Nature reviews. Immunology. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 47.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 48.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 49.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 52.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 54.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Best AJ, Knell J, Goldrath A, Brown B, Jojic V, Koller D, Cohen N, Brennan P, Brenner M, Regev A, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S, Jianu R, Laidlaw D, Collins J, Narayan K, Sylvia K, Kang J, Gazit R, Garrison BS, Rossi DJ, Kim F, Rao TN, Wagers A, Shinton SA, Hardy RR, Monach P, Bezman NA, Sun JC, Kim CC, Lanier LL, Heng T, Kreslavsky T, Painter M, Ericson J, Davis S, Mathis D, Benoist C. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 58.Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C, Galitzky J, Lafontan M, Karpe F, Frayn KN, Bouloumie A. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 59.Mayi TH, Daoudi M, Derudas B, Gross B, Bories G, Wouters K, Brozek J, Caiazzo R, Raverdi V, Pigeyre M, Allavena P, Mantovani A, Pattou F, Staels B, Chinetti-Gbaguidi G. Human adipose tissue macrophages display activation of cancer-related pathways. J Biol Chem. 2012;287:21904–21913. doi: 10.1074/jbc.M111.315200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, Sipula IJ, Dedousis N, Scott DK, Morel PA, Thomson AW, O'Doherty RM. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61:2330–2339. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumie A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova Y, Markham D, Strissel KJ, Watkins A, Zhu M, Allen J, Bouchard J, Toraldo D, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1215840110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 69.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 70.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 71.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. 2010;88:250–256. doi: 10.1038/icb.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 75.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koch U, Radtke F. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol. 2011;27:539–562. doi: 10.1146/annurev-cellbio-092910-154008. [DOI] [PubMed] [Google Scholar]
- 81.Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat Rev Immunol. 2012;12:799–804. doi: 10.1038/nri3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 87.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Libero G, Mori L. Recognition of lipid antigens by T cells. Nat Rev Immunol. 2005;5:485–496. doi: 10.1038/nri1631. [DOI] [PubMed] [Google Scholar]
- 91.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, Williams B, Wasserman DH, Van Kaer L. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012;109:E1143–E1152. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI, Medzhitov R. Impact of CD1d deficiency on metabolism. PLoS One. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O'Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS One. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem. 2012;287:24378–24386. doi: 10.1074/jbc.M112.371807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoe K, Tsutsui H. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 98.Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, Boon L, Nieuwenhuis EE, Elewaut D, Prakken B, Kersten S, Boes M, Kalkhoven E. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, Lee SK, Alfadda AA, Kim SS, Choi SH, Lee DS, Park SH, Seong RH, Choi CS, Kim JB. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol. 2013;33:328–339. doi: 10.1128/MCB.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82:315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 101.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 102.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 103.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol. 2000;165:4305–4311. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 105.Sidobre S, Naidenko OV, Sim BC, Gascoigne NR, Garcia KC, Kronenberg M. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169:1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 106.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 108.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 109.Ghosh G, Wang VY, Huang DB, Fusco A. NF-kappaB regulation: lessons from structures. Immunol Rev. 2012;246:36–58. doi: 10.1111/j.1600-065X.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 111.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 114.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 115.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61:2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One. 2010;5:e12537. doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 120.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 122.Jaeschke A, Czech MP, Davis RJ. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Gene Dev. 2004;18:1976–1980. doi: 10.1101/gad.1216504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 124.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 128.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulange A, Capeau J, Caron M. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162–2173. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- 135.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine. 2008;44:141–148. doi: 10.1016/j.cyto.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 137.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 138.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 140.Paquot N, Castillo MJ, Lefebvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocr Metab. 2000;85:1316–1319. doi: 10.1210/jcem.85.3.6417. [DOI] [PubMed] [Google Scholar]
- 141.Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, Friedline RH, Kurt-Jones E, Finberg R, Fischer MA, Granger EL, Norbury CC, Hauschka SD, Philbrick WM, Lee CG, Elias JA, Kim JK. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 143.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kowalski GM, Nicholls HT, Risis S, Watson NK, Kanellakis P, Bruce CR, Bobik A, Lancaster GI, Febbraio MA. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 2011;54:888–899. doi: 10.1007/s00125-010-2020-5. [DOI] [PubMed] [Google Scholar]
- 145.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marathe C, Bradley MN, Hong C, Chao L, Wilpitz D, Salazar J, Tontonoz P. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARgamma−/−, PPARdelta−/−, PPARgammadelta−/−, or LXRalphabeta−/− bone marrow. J Lipid Res. 2009;50:214–224. doi: 10.1194/jlr.M800189-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, Hirabara SM, Castoldi A, Vieira P, Camara NO, Curi R, Carvalheira JB, Saad MJ. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 150.Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL, Lin A, Lam KS. Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes. 2011;60:486–495. doi: 10.2337/db10-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barra NG, Reid S, MacKenzie R, Werstuck G, Trigatti BL, Richards C, Holloway AC, Ashkar AA. Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity (Silver Spring) 2010;18:1601–1607. doi: 10.1038/oby.2009.445. [DOI] [PubMed] [Google Scholar]
- 152.Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int J Obes (Lond) 2012;36:993–998. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- 153.McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KH, Roche HM. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60:1688–1698. doi: 10.2337/db10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kowalski GM, Nicholls HT, Risis S, Watson NK, Kanellakis P, Bruce CR, Bobik A, Lancaster GI, Febbraio MA. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 2011;54:888–899. doi: 10.1007/s00125-010-2020-5. [DOI] [PubMed] [Google Scholar]
- 155.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J, Annicotte JS, Schmidt J, Mataki C, Yamamoto H, Sanches R, Guma A, Stich V, Vitkova M, Jardin-Watelet B, Renard E, Strieter R, Tuthill A, Hotamisligil GS, Vidal-Puig A, Zorzano A, Langin D, Fajas L. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9:339–349. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kurokawa J, Nagano H, Ohara O, Kubota N, Kadowaki T, Arai S, Miyazaki T. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011;108:12072–12077. doi: 10.1073/pnas.1101841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Watanabe Y, Nakamura T, Ishikawa S, Fujisaka S, Usui I, Tsuneyama K, Ichihara Y, Wada T, Hirata Y, Suganami T, Izaki H, Akira S, Miyake K, Kanayama HO, Shimabukuro M, Sata M, Sasaoka T, Ogawa Y, Tobe K, Takatsu K, Nagai Y. The radioprotective 105/MD-1 complex contributes to diet-induced obesity and adipose tissue inflammation. Diabetes. 2012;61:1199–1209. doi: 10.2337/db11-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nicholls HT, Kowalski G, Kennedy DJ, Risis S, Zaffino LA, Watson N, Kanellakis P, Watt MJ, Bobik A, Bonen A, Febbraio M, Lancaster GI, Febbraio MA. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes. 2011;60:1100–1110. doi: 10.2337/db10-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187:1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sachithanandan N, Graham KL, Galic S, Honeyman JE, Fynch SL, Hewitt KA, Steinberg GR, Kay TW. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60:2023–2031. doi: 10.2337/db11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Bluher M, Kolanus W, Kahn CR, Bruning JC. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 2010;6:e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lesniewski LA, Hosch SE, Neels JG, de Luca C, Pashmforoush M, Lumeng CN, Chiang SH, Scadeng M, Saltiel AR, Olefsky JM. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med. 2007;13:455–462. doi: 10.1038/nm1550. [DOI] [PubMed] [Google Scholar]
- 168.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118:2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feral CC, Neels JG, Kummer C, Slepak M, Olefsky JM, Ginsberg MH. Blockade of alpha4 integrin signaling ameliorates the metabolic consequences of high-fat diet-induced obesity. Diabetes. 2008;57:1842–1851. doi: 10.2337/db07-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]