Abstract
Although allergic asthma is a heterogeneous disease, allergen-specific T helper 2 (Th2) cells producing the key cytokines involved in type 2 inflammation, interleukin-4 (IL-4), IL-5 and IL-13, are thought to play a major role in asthma pathogenesis. This model is challenged by the recent discovery of group 2 innate lymphoid cells (ILC2) that represent a critical innate source of type 2 cytokines. These ILC2 are activated by epithelial cell-derived cytokines, including IL-25 and IL-33, which have been implicated in the initiation of asthma. In this review, we will discuss recent studies supporting a significant role for ILC2 in lung inflammation, with special attention to allergen-induced asthma.
Keywords: asthma, innate immunity, lung immunology disease, T cells
Introduction
Asthma is a chronic inflammation of the airways caused by a combination of genetic predisposition and environmental factors, affecting some 235 to 300 million people worldwide.1–3 Symptoms vary between individuals, but are often characterized by episodes of coughing, wheezing and shortness of breath. The term ‘asthma’ encompasses a group of clinical symptoms and for a long time it was widely believed that asthma represented an allergic, eosinophilic and T helper type 2 (Th2) -mediated disease. However, mechanistic studies show that it is a heterogeneous condition with multiple subtypes that require distinct treatment modalities (reviewed in ref. 3). To date, our knowledge on the pathophysiology of many asthma phenotypes is incomplete, but ongoing research efforts are expected to result in more targeted and personalized therapeutic approaches. In this review we will focus on type 2 immunity in allergic asthma, which is the most common and best studied form of asthma.
T helper 2 differentiation in allergic asthma
A hallmark of allergic asthma is airway hyper-responsiveness (AHR), which can be triggered by inhalation of allergens such as house dust mite (HDM), animal dander, pollen or fungal spores.1–3 This is typically associated with eosinophilic inflammation in the airways and increased numbers of eosinophils in the circulation that correlate with AHR in the clinic.4,5 Persistent inflammation eventually leads to airway remodelling due to repair processes, most notably subepithelial fibrosis, smooth muscle hyperplasia, mucous cell metaplasia and increased angiogenesis.6 Airway epithelial cells make up the frontline of defence that separates the host and the environment and is therefore essential in the control of inflammatory responses to allergens that induce asthma (see ref. 7 for a very recent review on the role of the epithelium in asthma pathogenesis). Epithelial cells express a wide variety of pattern recognition receptors that recognize pathogen-associated or damage-associated molecular patterns. For example, HDM allergens such as Der p II and Der p VII activate toll-like receptor 4 (TLR4) signalling that in turn promotes nuclear translocation of nuclear factor-κB (NF-κB), which controls a wide range of inflammatory genes.7 Using irradiated chimeric mice, it has been shown that HDM-induced asthma requires the presence of TLR4 on radioresistant lung structural cells and not on haematopoietic cells such as dendritic cells (DCs).8 Triggering of TLR4 on epithelial cells by HDM induces the production of various cytokines including thymic stromal lymphopoietin (TSLP), granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-1α (IL-1α), IL-1β, IL-25 and IL-33. Recent in vivo experiments as well as air–liquid interface cultures of bronchial epithelial cells demonstrated that TLR signals induce the release of IL-1α, which then initiates an autocrine feedback loop to trigger production of other cytokines, including GM-CSF and IL-33.9 A common effect of these cytokines is the activation of DCs towards a phenotype that promotes Th2 immunity.7 Additionally, DCs can be directly activated as they continuously sample the airway lumen by forming dendritic extensions.
Activated DCs are able to initiate sensitization in concert with the epithelium via antigen presentation to naive T cells in the draining lymph nodes. It appears that activated DCs have intrinsic capacities to drive Th1 or Th2 responses. When DCs recognize bacterial or viral products via TLRs, they produce IL-12 and induce Th1 polarization. In contrast, DCs may also sample inhaled allergens and initiate adaptive Th2 responses in asthma.7 Furthermore, DCs stimulated with agents such as fungal products, parasitic nematodes or cholera toxin induce Th2 responses. Central to the initiation of Th2 differentiation is IL-4, which induces the Th2 master regulator GATA binding protein 3 (GATA3) through signal transducer and activator of transcription 6 (STAT6).10,11 The transcription factor GATA3, which is necessary and sufficient to instruct Th2 differentiation, acts in cooperation with various other nuclear proteins to induce the production of IL-4, IL-5 and IL-13 and to suppress Th1 development. A suggested mechanism for this activity is that GATA3 causes chromatin remodelling in the Th2 cytokine locus, in which the genes encoding IL-4, IL-5 and IL-13 are clustered. Intriguingly, the induction of GATA3 by the IL-4/STAT6 axis in differentiating Th2 cells raises the paradox that IL-4 is required for the generation of the cell type that is its major producer. Although IL-4 has long been thought to control Th2 cell development, the initial events resulting in IL-4 release in vivo and the initial source of IL-4 under physiological conditions remain to be identified. Although innate immune cells might provide a source of IL-4, Th2 responses can be generated (i) when only T cells can make IL-4, and (ii) in mice lacking a functional IL-4 receptor signalling pathway, arguing against a requisite role for an external source of IL-4.12 Inflammatory DCs are essential for the induction of Th2 immunity and features of asthma, whereas basophils, which have the capacity to produce IL-4, are not required and do not take up inhaled antigen to present it to T cells.13 Although remarkably little is known about the initial pathways that induce IL-4 or GATA3 in activated T cells in vivo, elegant experiments by Amsen et al.14,15 provide evidence that DCs use the Notch signalling pathway in T cells to instruct their differentiation. Dendritic cells expressing the Notch ligand Jagged induce Th2 differentiation independently of IL-4, whereas DCs expressing the Delta-like ligand induce the alternative Th1 cell fate. Notch signalling in T cells leads to activation of the nuclear effector recombination signal binding protein for immunoglobulin kappa J region (RBPJκ), which binds to regulatory elements inducing GATA3 and IL-4 gene expression. Interestingly, intranasal administration of the Notch-inhibitor γ-secretase inhibitor reduced allergic airway inflammation in mice.16
Once an individual has been sensitized to a specific allergen, re-exposure to the allergen activates primed Th2 cells, which are thought to play a central role in orchestrating an allergic immune response (Fig. 1). Interleukin-4 can stimulate B cells to produce antigen-specific IgE, which then binds to the high-affinity FcεRI on mast cells, enabling them to be fully activated and to release histamine, leukotrienes and prostaglandins.17,18 Interleukin-5 especially influences eosinophil survival, activation, differentiation and recruitment from the bone marrow into the tissues. It has been shown that when activated, eosinophils also release leukotrienes similar to mast cells, which act as potent bronchoconstrictors.17 The combination of leukotrienes and histamine enhances AHR and airflow obstruction. Interleukin-13 impacts airway epithelial and smooth muscle cells, where it mediates AHR, mucus hypersecretion and subepithelial fibrosis. In addition, IL-13 induces production of several matrix metalloproteinases, demonstrating its importance for airway remodelling in typical asthma.19
Figure 1.
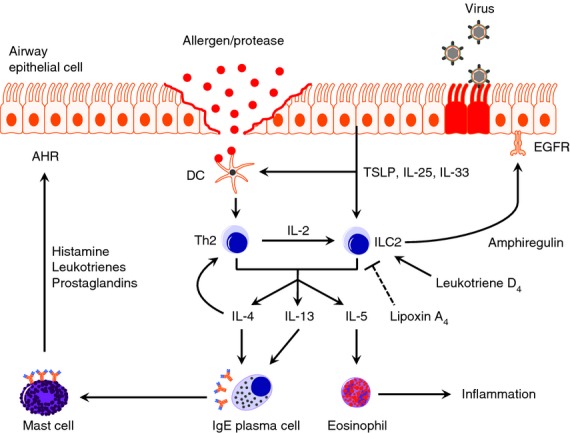
The central role of group 2 innate lymphoid cells (ILC2s) in type 2 immunity. Allergens or viral infection trigger the epithelium to express the stress signals thymic stromal lymphopoietin TSLP, interleukin-25 (IL-25) and IL-33. In response to these cytokines, ILC2s undergo proliferation and produce large amounts of IL-4, IL-5 and IL-13. Additionally, dendritic cells (DCs) are also stimulated towards a T helper type 2 (Th2) -inducing phenotype. IL-4 and IL-13 cooperate to activate B cells that produce antigen-specific IgE, which binds to the high affinity FcεRI on mast cells. Upon secondary antigen exposure, primed mast cells degranulate and release histamine, leukotrienes and prostaglandins that are responsible for airway hyper-responsiveness. Furthermore, IL-5 is a chemoattractant for eosinophils, which are well-equipped to cause inflammation. When Th2 cells come into play, ILC2s are stimulated further by the IL-2 they produce, resulting in a positive feedback loop. The ILC2s may also produce amphiregulin and can therefore play a role in restoring epithelial integrity after viral infection. An alternative pathway to activating ILC2s is possible via leukotriene D4 and a recent study has shown an inhibitory effect of lipoxin XA4 on ILC2s (see text for further information).
Although Th2 lymphocytes are regarded as the central cell type that orchestrates and amplifies allergic inflammatory events, this pathway however, fails to explain why asthmatics experience increased frequency and severity of exacerbations during viral infections of the airway, such as respiratory syncytial virus, rhinovirus or influenza, which typically evoke a type 1 response.
Identification of group 2 innate lymphoid cells
Recently, alternative ways to induce a type 2 response have been discovered and could be the reason therapies aimed at T-cell depletion have shown limited success in asthma patients.20 In fact, the first observation of a non-B/non-T-cell population in Rag2−/− mice that had the capacity to produce IL-5 and IL-13, but not IL-4, was made by Fort et al.21 They found that intraperitoneal injection of IL-25 induced type 2 cytokines and a Th2-like response, characterized by increased serum IgE, IgG1 and IgA, blood eosinophilia and pathological changes in the lungs and digestive tract. Subsequently, a previously unrecognized cell population staining positive for intracellular IL-5 was identified in Rag2−/− mice, but not in Rag2−/− mice lacking the common γ (γc) chain, when IL-25 was given intranasally.22 A few years later these cells were found to provide an important source of type 2 cytokines critically involved in Nippostrongylus brasiliensis expulsion.23 Moreover, administration of IL-33 in Rag2−/− mice efficiently induced AHR, goblet cell hyperplasia and eosinophilic infiltration in the lungs via IL-4, IL-5 and IL-13.24
In 2010 four independent groups almost simultaneously described Th2 cytokine producing non-B/non-T cells in detail. Moro et al.25 characterized a lineage-negative cell population that expresses Sca-1, CD117 (c-Kit), CD25 (IL-2Rα), CD127 (IL-7Rα) and T1/ST2 (IL-33R) in fat-associated lymphoid clusters and coined them natural helper cells. These natural helper cells were able to produce large amounts of typical Th2 cytokines like IL-5 and IL-13 in response to IL-2 and mediated protection against parasitic worms.25 Neill et al.26 reported nuocytes in the mesenteric lymph nodes that expanded in vivo in response to IL-25 and IL-33 and were an early source of IL-13 before T-cell induction during helminth infection. Mice deficient in IL-25 and IL-33 manifested a severely impaired ability to expel N. brasiliensis, which could effectively be rescued by adoptive transfer of isolated nuocytes.26 Similar cells were described by Price et al.27 who also found them in the spleen and liver and named them innate helper type 2 cells. Although most molecular surface markers are shared between natural helper cells, nuocytes and innate helper type 2 cells, subtle differences may exist.28,29 However, now it is generally agreed that they can be categorized under group 2 innate lymphoid cells (ILC2s).29,30 In addition, Saenz et al.31 observed that IL-25 also promotes the accumulation of a lineage-negative Sca-1+ CD117int multipotent progenitor cell population in gut-associated lymphoid tissue that induces Th2 responses. As these multipotent progenitor cells have the capacity to differentiate into monocyte/macrophage and granulocyte lineages, they appear to be distinct from ILC2s.
Group 2 innate lymphoid cells and allergic lung inflammation
Quickly after the identification of ILC2s in the intestine, a similar population of type 2 cytokine-producing cells was described in the respiratory tract in the context of influenza infection in mice and allergic rhinitis in humans.32–34 Furthermore, it was shown that local or systemic administration of IL-25 or IL-33 induced proliferation of ILC2s as well as production of IL-5 and IL-13 effector cytokines by these cells (Fig. 1).35–40 In vivo transfer experiments demonstrated that IL-13 produced by ILC2s was sufficient to mediate IL-33-induced airway inflammation.27,38,39 These findings are relevant for asthma in humans as well, because polymorphisms in the IL17RB gene encoding one of the chains of the IL-25R have been associated with asthma.41 Also, IL-33 levels correlate with asthma severity42,43 and both the IL33 gene and the IL1RL1 gene (encoding the IL-33R chain T1ST2) have been associated with asthma susceptibility in humans in large-scale genome-wide association studies.44–46 In these studies, the IL1RL1 locus was also associated with atopic dermatitis and allergic rhinitis.
Nevertheless, provoking type 2 immunity by intranasal administration of IL-25 or IL-33 generates an acute response and does not reflect the complex process of allergic sensitization and response in a physiological situation. Therefore, several other mouse models have been employed to study the role of ILC2s in allergic airway inflammation, including asthma induced by the ovalbumin (OVA) protein, fungal allergens derived from Alternaria alternata, glycolipid antigens from Sphingomonas bacteria that can stimulate natural killer T cells and the protease papain (see ref. 47 for a recent review). Papain has proteolytic functions by cleaving tight junctions between epithelial cells, thereby gaining access to underlying dendritic cells and possibly promoting the production of endogenous danger signals by the epithelium. It induces asthma symptoms in mice mediated by ILC2s, independent of B or T lymphocytes and is known to cause occupational asthma.36,48
Taken together, these findings showed that ILC2s have a critical role in particular mouse models for asthma in which allergic lung inflammation was studied in Rag-deficient mice, in the absence of functional B and T lymphocytes. However, the contribution of ILC2s in allergy in the context of an intact adaptive immune system is less well studied. We have shown in wild-type mice that in HDM-induced allergic asthma, the ILC2 population in lung and bronchoalveolar lavage fluid increased significantly in size and that ILC2s were a major source of IL-5 or IL-13. Also in OVA-induced asthma, the contribution of ILC2s and Th2 cells to the total production of IL-5 or IL-13 appeared in the same range.37 The ILC2s may therefore be critical for the induction of allergic airway inflammation in the lung, even in models where T cells were previously thought to be the main producers of IL-5 and IL-13. In contrast, upon induction of asthma through ovalbumin or HDM only a minor proportion of IL-4+ cells were ILC2s. Nevertheless, it has been reported that ILC2s have the capacity to produce IL-4 in response to TSLP or leukotriene D4.49–51
Much less in known about ILC2s in lung inflammation in humans. Cells with an ILC2 phenotype were described in healthy human lung parenchymal tissue and bronchoalveolar lavage fluid from lung transplant recipients.33 Although ILC2s were found to be enriched in nasal polyps from patients with chronic rhinosinusitis and in lesional skin from patients with atopic dermatitis, the involvement of ILC2s in the pathogenesis of asthma in humans remains unknown.34,52
Group 2 innate lymphoid cells and pulmonary infection
In 2011 Chang et al.32 provided evidence for a critical role of ILC2s in the development of AHR induced by influenza in mice. They demonstrated that H3N1 influenza virus infection acutely induced AHR, independently of Th2 cells and adaptive immunity. The AHR response required IL-13 and IL-33 and was associated with airway neutrophils and macrophages, but not with eosinophils. By gating on lineage-negative ST2+ c-Kit+ Sca-1+ CD25+ CD90.2+ lymphocytes, ILC2 numbers were shown to increase in the lung and peak on day 5–6 when production of IL-5 and IL-13 and AHR was also strongest. Depleting ILC2s, using a monoclonal antibody against Thy-1/CD90.2 in Rag2−/− mice, abolished the H3N1-induced AHR response. Conversely, AHR was fully reconstituted when purified ILC2s were adoptively transferred back into the recipient mice. The relationship between influenza virus infection and increased ILC2 numbers in the lung was confirmed by Monticelli et al., who employed a different strain of influenza virus, H1N1 PR8.33 Strikingly, in this study IL-33R blockade or depletion of ILC2s with anti-Thy-1/CD90.2 antibodies in Rag1−/− mice during influenza infection resulted in decreased lung function, lower blood oxygen saturation levels and loss of epithelial integrity, which suggests a previously unknown restorative role of ILC2s. These effects were effectively countered upon adoptive transfer of lung ILC2s, but appeared independent of IL-13. The authors further investigated the role of ILC2s in the maintenance of epithelial integrity by performing genome-wide transcriptional profiling of lung-resident ILC2s. From this analysis multiple genes emerged that were differentially expressed and were associated with wound repair.33 It was then suggested that amphiregulin, a member of the epithelial growth factor family, may be a key cytokine produced by ILC2s which mediates restoration of lung function (Fig. 1). Indeed, direct delivery of amphiregulin resulted in a significantly improved outcome for influenza virus-infected Rag1−/− mice depleted of ILC2s.33
The finding that ILC2s are recruited and activated during viral infection to allow local repair may be relevant to explain the phenomenon that asthma exacerbations can be triggered by viral respiratory tract infections. Although it can be assumed that ILC2 activation is self-limited, it is conceivable that repeated infection may result in sustained activation of ILC2s and type 2 immunity. In this context, it may be of note that upon infection with H3N2 X31 influenza virus in mice ILC2 numbers in the lung were still significantly increased at day 25 after infection (B.W.S. Li and R.W. Hendriks, unpublished results), although at day 10 the influenza virus is efficiently cleared in this model.53 Further experiments are required to investigate whether increased presence of ILC2s contributes to asthma exacerbation by making patients with asthma more sensitive to allergen stimulation.
Development of group 2 innate lymphoid cells
The ILC2s belong to a novel family of developmentally related ILCs. A feature that these lymphocytes have in common is the absence of Rag-dependent rearranged antigen receptors and the lack of classic lineage markers on their cell surface. ILCs were recently classified into three groups based on their signature cytokines and the transcription factors that regulate their development and function.29,54 Group 1 ILCs (ILC1s) are characterized by the production of interferon-γ (IFN-γ) and highly express the transcription factor T-bet. The well-known natural killer (NK) cell is a prototypical member of this group, but other ILC1 subsets that are phenotypically and developmentally distinct from NK cells have recently been recognized.55 Group 3 ILCs (ILC3s) produce IL-17 and/or IL-22 and are dependent on transcription factor retinoic acid receptor-related orphan receptor-γ (RORγt). A prominent member of this heterogeneous group of cells is the lymphoid tissue inducer cell, which plays a critical role in the formation of secondary lymphoid organs during embryogenesis.56 Several other ILC3 subsets have recently been discovered, including RORγt+ NKp46+ cells secreting IL-22 and RORγt+ NKp46− cells producing both IFN-γ and IL-17.57–60 Whether ILC1s and ILC3s are distinct and stable cell populations or whether they are different forms of the same plastic cell type remains to be elucidated.29 Especially, since ILC3s may switch from IL-22 to IFN-γ production, whereby RORγt expression is progressively lost and the transcription factor T-bet is essential for IFN-γ expression.61–63
It is thought that ILCs arise from common lymphoid progenitors in the bone marrow, which are Lin– IL-7Ra+ Flt3+. ILC2s require inhibitor of DNA binding 2 (Id2) for their development, which functions as an inhibitor of transcriptional activity of basic helix-loop-helix E proteins, such as E12, E47, HEB and E2-2. Deficiency studies indicate that lack of Id2 results in an absence of NK cells, RORγt+ ILCs and ILC2s.25,64–66
RORγt+-deficient mice have normal ILC2 numbers25,26, but the structurally related transcription factor RORα was shown to be important for ILC2 development in the bone marrow.67,68 Development of ILC2 from common lymphoid progenitors in vitro following culture with IL-7 and IL-33 was very inefficient, although not completely abolished. Although RORα-deficient mice appear to be able to develop low numbers of cells with an ILC2 phenotype, these are unable to proliferate in response to IL-25 stimulation.67
Interestingly, Notch signalling, which is known to be a master regulator of T-cell lineage commitment in the thymus,69 is also of particular importance in ILC2 development in vitro to block B-cell potential in addition to repressing differentiation of several other lineages.67,69 Moreover, ILC2 development also requires T-cell factor 1 (Tcf1) encoded by the Tcf7 gene, a transcription factor that is implicated in T-cell lineage specification; Tcf7−/− mice lack ILC2s and are unable to mount ILC2-mediated type 2 immune responses.70 Although the physiological role of Notch in ILC2 development in vivo remains to be determined, these findings indicate that ILC2s may be closely related to T cells. This is also reported by the recent identification of GATA3 as a critical early regulator of ILC2 development.71,72 Conditional deletion of the Gata3 gene in established Th2 cells showed that Gata3 is critical for the expression of IL-5 and IL-13, but not of IL-4.73 Likewise, Gata3 deletion in ILC2s abolished the expression of IL-5 and IL-13 in the mouse74 and Mjosberg et al.49 showed that Gata3 is crucial for function of human ILC2s. Using an inducible Gata3 ablation strategy it was shown by the group of Diefenbach that intestinal ILC2 development and homeostasis required Gata3 expression.71 Analysis of chimeric mice, as well as mice over-expressing Gata3 at the common lymphoid progenitor stage, demonstrated an essential and dose-dependent role for Gata3 in ILC2 development.72 Finally, we found that enforced expression of Gata3 in mice was sufficient to enhance Th2 and ILC2 activity, leading to increased susceptibility to eosinophilic inflammation after mild exposure to HDM (R.W. Hendriks, unpublished results). Collectively, these results identify Gata3 as a critical early regulator of ILC2 development, thereby extending the paradigm of Gata3-dependent control of type 2 immunity to include both innate and adaptive lymphocytes.
Concluding remarks
Group 2 innate lymphoid cells are gaining increasing recognition and the evidence gathered so far implicates them in the pathogenesis of allergic asthma and provides clues to their contribution to fighting viral infections and restoration of the airway epithelium. Interestingly, ILC2s were recently also found in the skin, where they carry out a dual role as an immune regulator and a pro-inflammatory effector cell and were shown to functionally interact with mast cells.50,52 Stimulated dermal ILC2s promote an eosinophil influx and mast cell activation and lead to spontaneous dermatitis in areas routinely exposed to body fluids. It remains unknown whether ILC2s also interact with mast cells in the context of allergic airway inflammation. In response to allergens or viruses, ILC2s are activated by a number of cytokines produced by epithelial cells and DCs, of which IL-25, IL-33 and TSLP are most studied. However, ILC2s in the lung and bone marrow also express the cysteinyl leukotriene receptor 1 (CysLT1R) and challenge with leukotriene D4, which can bind CysLT1R, was recently shown to increase the proportions of IL-5+ ILC2s in the lung.51 On the other hand, ILC2s in peripheral blood of humans express the pro-resolving G protein-coupled receptors, N-formyl peptide receptor ALX/FPR2 and chemokine receptor-like 1 (CMKLR1). Their ligands, such as lipoxin A4 (LXA4), have anti-inflammatory functions by inhibiting neutrophil activation and regulation of epithelial cytokine release. Importantly, LXA4 was found to decrease the release of IL-13 by ILC2s in nanomolar concentrations.75 These findings therefore highlight a potential therapeutic strategy to control asthma in patients who do not respond to corticosteroids.
Nevertheless, a large number of questions still need to be answered, especially regarding the interactions between ILC2s and the adaptive immune system. Knowledge on interaction between ILC2 and Th2 cells may be important for controlling allergic asthma. Although it has been reported that ILC2s can express MHC class II,31,40 interaction between ILC2s and Th2 lymphocytes is largely unexplored. Interestingly, very recent functional analyses revealed that RORγt-expressing ILCs can process and present antigen in the context of MHC class II and thereby limit commensal bacteria-specific CD4 T-cell responses.76 Despite the obvious central role of type 2 immunity in allergic asthma, the efficacy of humanized antibodies directed against individual cytokines IL-4, IL-5 and IL-13 appears disappointing.77 Because of the function of GATA3 as a master regulator in both Th2 cells and ILC2s, inhibiting its function and thereby targeting all Th2 cytokines may be an attractive treatment modality for asthma in humans. In this context, the observed capacity of the glucocorticoid fluticasone to inhibit GATA3 translocation from the cytosol to the nucleus would be an excellent starting point for drug discovery strategies.78
Acknowledgments
These studies were partly supported by Lung Foundation Netherlands Grant 3.2.12.067.
Disclosures
The authors declare no conflict of interests.
References
- 1.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 2.Baiz N, Annesi-Maesano I. Is the asthma epidemic still ascending? Clin Chest Med. 2012;33:419–29. doi: 10.1016/j.ccm.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 4.Liang Z, Zhao H, Lv Y, et al. Moderate accuracy of peripheral eosinophil count for predicting eosinophilic phenotype in steroid-naive non-atopic adult asthmatics. Intern Med. 2012;51:717–22. doi: 10.2169/internalmedicine.51.6834. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz N, Grossman A, Levy Y, Schwarz Y. Correlation between eosinophil count and methacholine challenge test in asymptomatic subjects. J Asthma. 2012;49:336–41. doi: 10.3109/02770903.2012.672613. [DOI] [PubMed] [Google Scholar]
- 6.Aceves SS, Broide DH. Airway fibrosis and angiogenesis due to eosinophil trafficking in chronic asthma. Curr Mol Med. 2008;8:350–8. doi: 10.2174/156652408785161023. [DOI] [PubMed] [Google Scholar]
- 7.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 8.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–17. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz J, Thiel A, Kuhn R, Rajewsky K, Muller W, Assenmacher M, Radbruch A. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J Exp Med. 1994;179:1349–53. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells – not basophils – are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different Notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 15.Amsen D, Antov A, Jankovic D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JH, Kim BS, Uhm TG, Lee SH, Lee GR, Park CS, Chung IY. γ-Secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am J Respir Crit Care Med. 2009;179:875–82. doi: 10.1164/rccm.200806-893OC. [DOI] [PubMed] [Google Scholar]
- 17.Hendeles L, Asmus M, Chesrown S. Evaluation of cytokine modulators for asthma. Paediatr Respir Rev. 2004;5(Suppl A):S107–12. doi: 10.1016/s1526-0542(04)90020-6. [DOI] [PubMed] [Google Scholar]
- 18.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 19.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just TH2 cells. Nat Rev Immunol. 2010;10:838–48. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 22.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 23.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo Y, Yoshimoto T, Yasuda K, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 25.Moro K, Yamada T, Tanabe M, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 26.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 29.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 30.Walker JA, McKenzie A. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol. 2013;25:148–55. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 35.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage – CD25+ CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 38.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–8. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 39.Kim HY, Chang YJ, Subramanian S, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–27. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilhelm C, Hirota K, Stieglitz B, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung JS, Park BL, Cheong HS, et al. Association of IL-17RB gene polymorphism with asthma. Chest. 2009;135:1173–80. doi: 10.1378/chest.08-1595. [DOI] [PubMed] [Google Scholar]
- 42.Prefontaine D, Lajoie-Kadoch S, Foley S, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 43.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 44.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–92. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirota T, Takahashi A, Kubo M, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet. 2011;43:893–6. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein Wolterink RG, Hendriks RW. Type 2 innate lymphocytes in allergic airway inflammation. Curr Allergy Asthma Rep. 2013;13:271–80. doi: 10.1007/s11882-013-0346-z. [DOI] [PubMed] [Google Scholar]
- 48.Novey HS, Marchioli LE, Sokol WN, Wells ID. Papain-induced asthma – physiological and immunological features. J Allergy Clin Immunol. 1979;63:98–103. doi: 10.1016/0091-6749(79)90198-2. [DOI] [PubMed] [Google Scholar]
- 49.Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–73. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates T2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.GeurtsvanKessel CH, Willart MA, Bergen IM, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–49. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells – how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 55.Bernink JH, Peters CP, Munneke M, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–9. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 56.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+ CD3– LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 57.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 59.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 60.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–5. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vonarbourg C, Mortha A, Bui VL, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity. 2010;33:736–51. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klose CS, Kiss EA, Schwierzeck V, et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 2013;494:261–5. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 63.Sciume G, Hirahara K, Takahashi H, et al. Distinct requirements for T-bet in gut innate lymphoid cells. J Exp Med. 2012;209:2331–8. doi: 10.1084/jem.20122097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–84. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 65.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–6. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 66.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3– NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–80. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong SH, Walker JA, Jolin HE, et al. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–36. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor α is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–74. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Radtke F, Macdonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–37. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 70.Yang Q, Monticelli LA, Saenz SA, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoyler T, Klose CS, Souabni A, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–48. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein Wolterink RG, Serafini N, van Nimwegen M, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2013;110:10240–5. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nat Immunol. 2004;5:1157–65. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 74.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–7. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011;128:495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 78.Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, Barnes PJ. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009;6:e1000076. doi: 10.1371/journal.pmed.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]


