Abstract Abstract
Temperate reefs, built by multilayers of encrusting algae accumulated during hundreds to thousands of years, represent one of the most important habitats of the Mediterranean Sea. These bioconstructions are known as “coralligenous” and their spatial complexity allows the formation of heterogeneous microhabitats offering opportunities for a large number of small cryptic species hardly ever considered.
Although sponges are the dominant animal taxon in the coralligenous rims with both insinuating and perforating species, this group is until now poorly known. Aim of this work is to develop a reference baseline about the taxonomic knowledge of sponges and, considering their high level of phenotypic plasticity, evaluate the importance of coralligenous accretions as a pocket for biodiversity conservation.
Collecting samples in four sites along the coast of the Ligurian Sea, we recorded 133 sponge taxa (115 of them identified at species level and 18 at genus level). One species, Eurypon gracilis is new for science; three species, Paratimea oxeata, Clathria (Microciona) haplotoxa and Eurypon denisae are new records for the Italian sponge fauna, eleven species are new findings for the Ligurian Sea. Moreover, seventeen species have not been recorded before from the coralligenous community. The obtained data, together with an extensive review of the existing literature, increase to 273 the number of sponge species associated with the coralligenous concretions and confirm that this habitat is an extraordinary reservoir of biodiversity still largely unexplored, not only taxonomically, but also as to peculiar adaptations and life histories.
Keywords: Porifera, cryptic species, bioconstructions, Ligurian Sea
Introduction
The term “coralligenous” refers to a secondary hard substrate, formed by the concretion of algal thalli and, to a lesser extent, by animal skeletons. Two main types of coralligenous concretions can be distinguished: banks, which are built over more or less horizontal substrata, and rims, which develop in the outer parts of marine caves and on vertical cliffs (Ballesteros 2006). Coralligenous communities represent the temperate reefs of the Mediterranean Sea and along with the meadows of Posidonia oceanica (Boudouresque, 2004) are biodiversity hot spots in the basin. The holes and crevices of the coralligenous build-ups support a complex community dominated by suspension feeders (sponges, hydrozoans, serpulid polychates, molluscs, bryozoans and tunicates).
Laubier (1966) first emphasized the high biodiversity of the coralligenous and listed 544 invertebrate species from this assemblage in Banyuls. Later, Hong (1980), in an exhaustive survey of the coralligenous of Marseille, listed a total of 682 species, whilst other authors (Ros et al. 1984) reported 497 species of invertebrates from the algal concretions of the Medes Islands. Recently, Romdhane (2003) reported 35 algal species and 93 animal species from a coralligenous formation along a vertical cliff in the gulf of Tunis. However, the number of species living in the coralligenous assemblages is still undefined, because of the richness of the fauna (Laubier 1966), the habitat complexity (Pérès and Picard 1964, Ros et al. 1985), the wide depth range of the conglomerates (Ballesteros 2006), the sporadic presence of cryptic species and the scarcity of reference studies. A rapid, non-destructive protocol for biodiversity assessment and monitoring of coralligenous, based on photographic sampling, was recently proposed by Kipson et al. (2011).
Sponges, with 142 recorded species, are one of the most diverse group of sessile animals of the coralligenous assemblage (Ballesteros 2006). Some species, mainly belonging to the family Clionaidae, are active bioeroders representing the principal driving force in the turn-over of bioconstructions, both in temperate and tropical areas (Cerrano et al. 2001, Calcinai et al. 2000, 2005, 2007c)
In the present paper, the species diversity of the coralligenous sponge fauna was studied in four sites of the Ligurian Sea, focusing on the relatively poorly known cryptic species boring or insinuating into the calcareous concretions. A new species for science and ten poorly known species, rarely recorded in the Mediterranean Sea, are treated exhaustively.
Materials and methods
Samples were collected between 30 and 40 m depth by SCUBA diving from 6 stations along the Ligurian coast where coralligenous is more developed (Fig. 1). Stations (from West to East) are: Santo Stefano Shoals, station 1; Gallinara Island, station 2 (Falconara) and station 3 (Sciusciaù); Portofino Promontory, Punta del Faro, station 4 and 5 (northern and southern side of the point); Punta Manara, station 6. Four blocks of coralligenous concretion, with an average volume of 20 l, were collected from each station.
Figure 1.
The four studied localities along the Ligurian Coast: Santo Stefano Shoal (station 1), Gallinara Island (station 2–3), Punta del Faro (Portofino Promontory) (station 4–5) and Punta Manara (station 6).
All the sponge species settled on the surface of these blocks were sampled and identified.
Two of the four blocks from each station were cut into slices about 2 cm thick and observed by a stereomicroscope to detect the cryptic, generally small, endolithic sponges.
The spicule complement of each sponge specimen was analysed according to Rützler (1978). From 30 measurements for each spicule type, size range, mean and standard deviation (in brackets) were calculated. Dissociated spicules were transferred onto stubs and sputtered with gold for SEM analyses and observed with a Philips XL 20 scanning electron microscope. Whenever possible, skeletal architecture was examined in light and scanning electron microscope (SEM) on hand-cut sections of the ectosome and choanosome. Unfortunately, due to small size and cavity dwelling habit, for most specimens it was impossible to study the skeleton.
We followed the classification given by Hooper and van Soest (2002) and the updated nomenclature reported in the World Porifera Database (van Soest et al. 2013). The geographic distribution of sponges in the Mediterranean Sea was compared with that reported by Pansini and Longo (2003, 2008), considering nine biogeographic areas for the Italian seas.
Results
During this survey we have recorded 133 sponge taxa (115 of them identified at species level and 18 at genus level). One species is new for science, 17 are new findings for the coralligenous conglomerate, 11 of which for the Ligurian Sea and 3 for the Italian sponge fauna (Table 1). In the following taxonomic part we provide the description of the new species and of ten poorly known ones.
Table 1.
List of Demospongiae and Homoscleromorpha species living outside and inside the coralligenous blocks (SSS: Santo Stefano Shoals, station 1; GI: Gallinara Island, station 2-3; PF: Punta del Faro, station 4-5; PM: Punta Manara, station 6; * new finding for the coralligenous concretion; ** new finding for the Ligurian Sea; *** new finding for the Italian sponge fauna).
| Species \ Sites | SSS | GI | PF | PM | Epilithic | Endolithic |
|---|---|---|---|---|---|---|
| Oscarella lobularis (Schmidt, 1862) | + | + | + | |||
| Plakina trilopha Schulze, 1880 | + | + | + | |||
| Plakinastrella copiosa Schulze, 1880 | + | + | ||||
| Plakortis simplex Schulze, 1880 | + | + | + | |||
| Samus anonymus Gray, 1867 | + | + | + | |||
| Stelletta grubii Schmidt, 1862 | + | + | ||||
| Stelletta lactea Carter, 1871 * | + | + | ||||
| Stelletta stellata Topsent, 1893 * | + | + | ||||
| Jaspis incrustans Topsent, 1890 ** | + | + | + | + | ||
| Jaspis johnstoni (Schmidt, 1862) | + | + | + | + | + | + |
| Penares euastrum (Schmidt, 1868) | + | + | + | + | + | |
| Dercitus (Stoeba) plicatus (Schmidt, 1868) | + | + | + | + | + | + |
| Pachastrissa sp. | + | + | ||||
| Erylus discophorus (Schmidt, 1862) | + | + | + | + | ||
| Geodia conchilega Schmidt, 1862 | + | + | + | + | + | |
| Geodia cydonium Schmidt, 1862 | + | + | + | + | ||
| Pachastrella monilifera Schmidt, 1868 | + | + | + | |||
| Poecillastra compressa (Bowerbank, 1866) | + | + | + | + | ||
| Triptolemma simplex (Sarà, 1959) | + | + | + | + | + | |
| Cliona burtoni Topsent, 1932 *, ** | + | + | ||||
| Cliona celata Grant, 1826 | + | + | + | + | + | |
| Cliona janitrix Topsent, 1932 | + | + | + | + | + | + |
| Cliona schmidtii (Ridley, 1881) | + | + | + | |||
| Cliona viridis Schmidt, 1862 | + | + | + | + | + | |
| Cliona sp. | + | + | + | |||
| Dotona pulchella mediterranea Rossell & Uriz, 2002 | + | + | ||||
| Spiroxya corallophila (Calcinai et al., 2002) | + | + | ||||
| Spiroxya heteroclita Topsent, 1896 | + | + | + | + | + | |
| Spiroxya sarai Melone, 1965 | + | + | + | |||
| Delectona ciconiae Bavestrello, Calcinai & Sarà, 1996 | + | + | ||||
| Delectona sp. | + | + | + | |||
| Paratimea oxeata Pulitzer-Finali, 1978 *, **, *** | + | + | ||||
| Polymastia sp. | + | + | + | |||
| Diplastrella bistellata (Schmidt, 1862) | + | + | + | + | + | |
| Aaptos aaptos (Schmidt, 1864) | + | + | + | + | ||
| Prosuberites longispinus Topsent, 1893 | + | + | ||||
| Pseudosuberites sulphureus (Bowerbank, 1866) | + | + | + | |||
| Suberites carnosus (Johnston, 1842) | + | + | ||||
| Suberites domuncula (Olivi, 1792) | + | + | ||||
| Suberites sp. | + | + | + | |||
| Terpios gelatinosa (Bowerbank, 1866) | + | + | + | |||
| Timea stellata (Bowerbank, 1866) | + | + | + | + | + | |
| Timea unistellata (Topsent, 1892) | + | + | + | + | ||
| Chondrosia reniformis Nardo, 1847 | + | + | + | + | ||
| Acarnus souriei Levi, 1952 *, ** | + | + | ||||
| Acarnus sp. | + | + | ||||
| Clathria (Microciona) armata (Bowerbank, 1866) *, ** | + | + | ||||
| Clathria (Microciona) atrasanguinea (Bowerbank, 1862) | + | + | + | |||
| Clathria (Microciona) gradalis Topsent, 1925 | + | + | ||||
| Clathria (Microciona) haplotoxa (Topsent, 1928) *, **, *** | + | + | ||||
| Clathria (Microciona) toxistyla (Sarà, 1959) | + | + | ||||
| Clathria (Microciona) toxivaria (Sarà, 1959) | + | + | ||||
| Clathria (Microciona) sp. | + | + | + | |||
| Antho (Antho) involvens (Schmidt, 1864) | + | + | ||||
| Eurypon cf. cinctum Sarà, 1960 | + | + | + | |||
| Eurypon clavatum (Bowerbank, 1866) | + | + | + | + | + | |
| Eurypon coronula (Bowerbank, 1874) ** | + | + | ||||
| Eurypon denisae Vacelet, 1969 *, ** | + | + | ||||
| Eurypon gracilis sp. n. Bertolino, Calcinai & Pansini | + | + | + | |||
| Eurypon major Sarà & Siribelli, 1960 | + | + | + | + | + | |
| Eurypon topsenti Pulitzer-Finali, 1983 | + | + | + | |||
| Eurypon vesciculare Sarà & Siribelli, 1960 | + | + | + | + | + | |
| Eurypon sp. | + | + | + | + | + | |
| Raspaciona aculeata (Johnston, 1842) | + | + | ||||
| Raspaciona sp. | + | + | ||||
| Forcepia (Leptolabis) brunnea (Topsent, 1904) ** | + | + | + | |||
| Lissodendoryx (Lissodendoryx) isodictyalis (Carter, 1882) | + | + | ||||
| Lissodendoryx (Anomodoryx) cavernosa (Topsent, 1892) | + | + | + | + | + | |
| Crambe crambe (Schmidt, 1862) | + | + | + | + | ||
| Crella (Crella) elegans (Schmidt, 1862) | + | + | ||||
| Crella (Crella) mollior Topsent, 1925 | + | + | ||||
| Crella (Grayella) pulvinar (Schmidt, 1868) | + | + | + | + | + | |
| Hemimycale columella (Bowerbank, 1864) | + | + | ||||
| Hymedesmia (Hymedesmia) baculifera Topsent, 1901 * | + | + | + | |||
| Hymedesmia (Hymedesmia) rissoi Topsent, 1936 | + | + | + | + | ||
| Hymedesmia sp. | + | + | + | |||
| Hymedesmia (Stylopus) coriacea (Fristedt, 1866) | + | + | + | + | ||
| Phorbas fictitius Bowerbank, 1866 | + | + | + | + | ||
| Phorbas mercator (Schmidt, 1868) * | + | + | ||||
| Phorbas lieberkuhni (Burton, 1930) | + | + | ||||
| Phorbas tenacior (Topsent, 1925) | + | + | + | + | + | |
| Phorbas sp. | + | + | + | |||
| Plocamionida ambigua (Bowerbank, 1866) * | + | + | + | + | + | |
| Tedania (Tedania) anhelans (Lieberkühn, 1859) | + | + | ||||
| Mycale (Aegogropila) tunicata (Schmidt, 1862) * | + | + | ||||
| Mycale (Paresperella) serrulata Sarà & Siribelli, 1960 **, *** | + | + | ||||
| Merlia normani Kirkpatrick, 1908 * | + | + | ||||
| Axinella damicornis (Esper, 1794) | + | + | + | + | + | |
| Axinella polypoides Schmidt, 1862 | + | + | ||||
| Axinella verrucosa (Esper, 1794) | + | + | + | |||
| Phakellia sp. | + | + | ||||
| Bubaris carcisis Vacelet, 1969 | + | + | + | + | ||
| Bubaris vermiculata (Bowerbank, 1866) | + | + | ||||
| Hymerhabdia oxytrunca Topsent, 1904 | + | + | ||||
| Hymerhabdia typica Topsent, 1892 * | + | + | ||||
| Hymerhabdia sp. | + | + | ||||
| Halicnemia geniculata Sarà, 1958 *, ** | + | + | ||||
| Halicnemia patera Bowerbank, 1864 | + | + | ||||
| Acanthella acuta Schmidt, 1862 | + | + | + | + | + | |
| Dictyonella incisa (Schmidt, 1880) | + | + | + | + | + | |
| Dictyonella marsilii (Topsent, 1893) | + | + | ||||
| Dictyonella pelligera (Schmidt, 1862) | + | + | + | + | ||
| Dictyonella sp. | + | + | ||||
| Halichondria (Halichondria) contorta Sarà, 1961 | + | + | + | |||
| Halichondria (Halichondria) cf. convolvens Sarà, 1960 | + | + | ||||
| Halichondria (Halichondria) genitrix Schmidt, 1862 | + | + | + | |||
| Halichondria (Halichondria) panicea Pallas, 1766 | + | + | + | |||
| Halichondria sp. | + | + | + | |||
| Agelas oroides Schmidt, 1864 | + | + | + | + | ||
| Dendroxea lenis (Topsent, 1892) | + | + | + | + | ||
| Haliclona (Gellius) angulata (Bowerbank, 1866) | + | + | + | + | ||
| Haliclona (Gellius) marismedi (Pulitzer-Finali, 1978) *, ** | + | + | + | + | ||
| Haliclona (Halichoclona) fulva (Topsent, 1893) | + | + | + | + | + | |
| Haliclona (Halichoclona) parietalis (Topsent, 1893) | + | + | + | |||
| Haliclona (Haliclona) sp. | + | + | + | |||
| Haliclona (Reniera) cinerea Grant, 1826 | + | + | ||||
| Haliclona (Reniera) citrina (Topsent, 1892) | + | + | + | |||
| Haliclona (Reniera) sp. | + | + | + | + | ||
| Haliclona (Soestella) arenata Griessinger, 1971 | + | + | ||||
| Haliclona (Soestella) mucosa (Griessinger, 1971) | + | + | ||||
| Haliclona sp. | + | + | ||||
| Siphonodictyon insidiosum (Johnson, 1899) | + | + | + | + | + | + |
| Petrosia (Petrosia) clavata (Esper, 1794) | + | + | + | + | ||
| Petrosia (Petrosia) ficiformis (Poiret, 1798) | + | + | + | + | + | |
| Ircinia variabilis (Schmidt, 1862) | + | + | + | + | + | + |
| Sarcotragus spinosulus Schmidt, 1862 | + | + | + | + | + | + |
| Cacospongia mollior Schmidt, 1862 | + | + | ||||
| Spongia (Spongia) officinalis Linnaeus, 1759 | + | + | ||||
| Spongia (Spongia) virgultosa (Schmidt, 1868) | + | + | + | + | + | + |
| Dysidea avara (Schmidt, 1862) | + | + | + | + | ||
| Dysidea sp. | + | + | ||||
| Pleraplysilla spinifera (Schulze, 1879) | + | + | + | + | ||
| Aplysina cavernicola Vacelet, 1959 | + | + | ||||
| Total number of species | 61 | 70 | 71 | 61 | 103 | 63 |
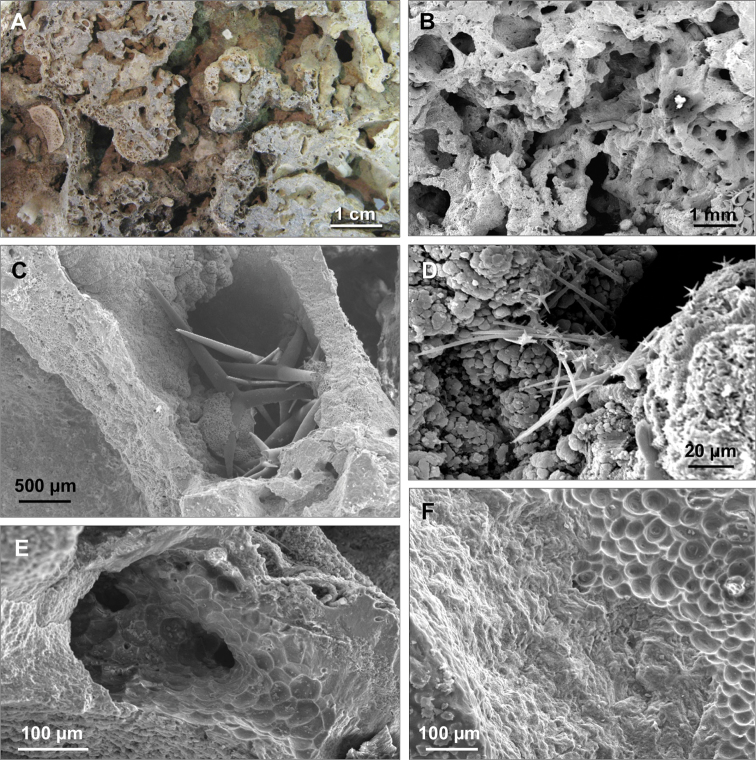
On the surfaces of the blocks 103 massive or encrusting species were recorded; inside the crevices of the conglomerate 63 species were observed and 33 shared both positions. Thirty species are exclusively endolithic demonstrating the abundance of cryptic sponges thriving inside the porous matrix of the coralligenous substrate (Table 1) (Fig. 2).
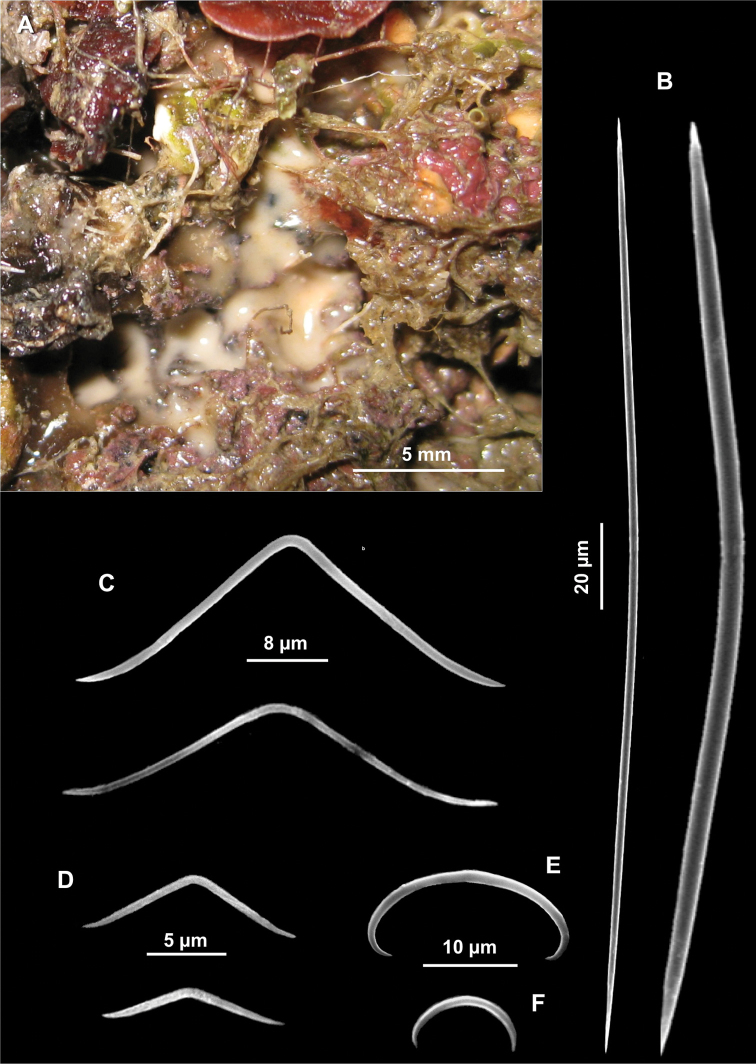
Figure 2.
Porosity of the coralligenous concretion. A Holes and cavities of the coralligenous concretion B Magnification of the holes C Magnification of a natural hole occupied by spicules of Pachastrella monilifera D Spicules of Jaspis johnstoni in a natural cavity in the coralligenous concretion E Cavity excavated by a boring sponge with excavation marks (pits) on the wall F Border between the area excavated by a boring sponge (right) and the not excavated area (left).
Among the 63 species recorded inside the conglomerate, 53 were insinuating and 10 boring (Table 1). From the first group six species: Geodia cydonium (Jameson, 1811) (Fig. 3A), Poecillastra compressa (Bowerbank, 1866) (Fig. 3D), Stelletta grubii Schmidt, 1862, Paratimea oxeata Pulitzer-Finali, 1978 (Fig. 3E), Hymedesmia (Hymedesmia) baculifera (Topsent, 1901) and Mycale (Paresperella) serrulata (Sarà & Siribelli, 1960) were hitherto recorded encrusting or massive; four species: Erylus discophorus (Schmidt, 1862), Penares euastrum (Schmidt, 1868), Geodia conchilega Schmidt, 1862 (Fig. 3B) and Pachastrella monilifera Schmidt, 1868 (Fig. 3C) were generally recorded as massive but also described as insinuating by Pulitzer-Finali (1970, 1983) and Calcinai et al. (2007b).
Figure 3.
Insinuating sponges. A Geodia cydonium B Geodia conchilega C Pachastrella monilifer a D Poecillastra compressa E Paratimea oxeata F Spongia virgultosa.
Species descriptions
Class Demospongiae: Order Hadromerida: Family Clionaidae: Genus Cliona
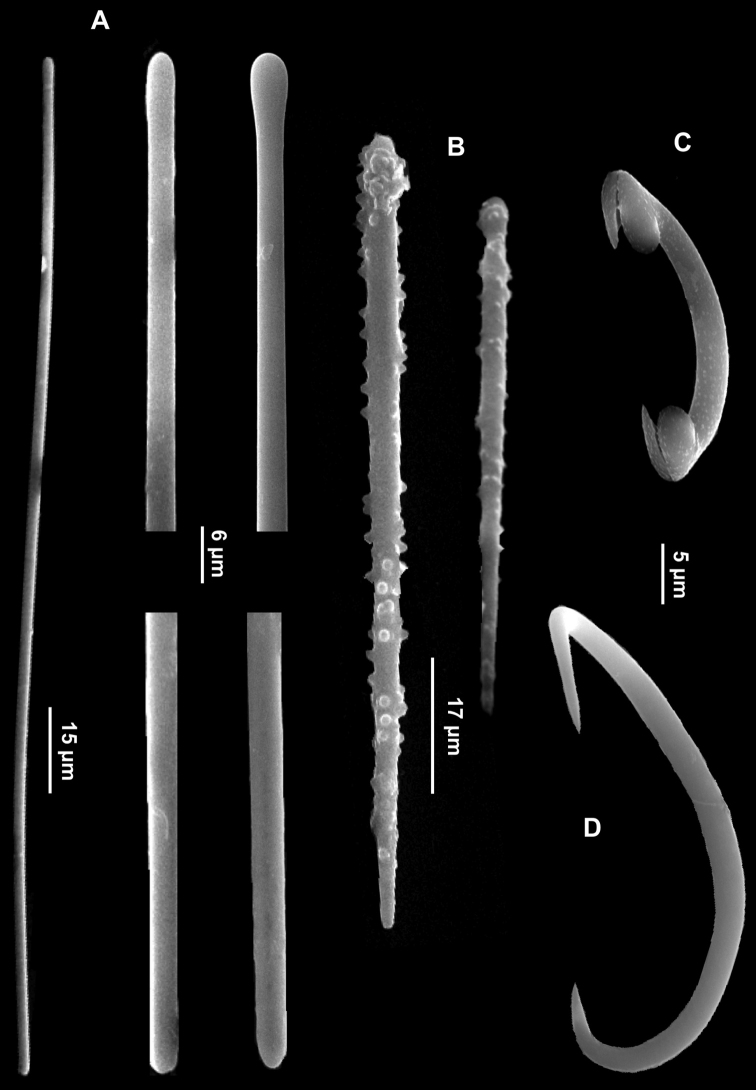
Cliona burtoni
Topsent, 1932
http://species-id.net/wiki/Cliona_burtoni
Figure 4.
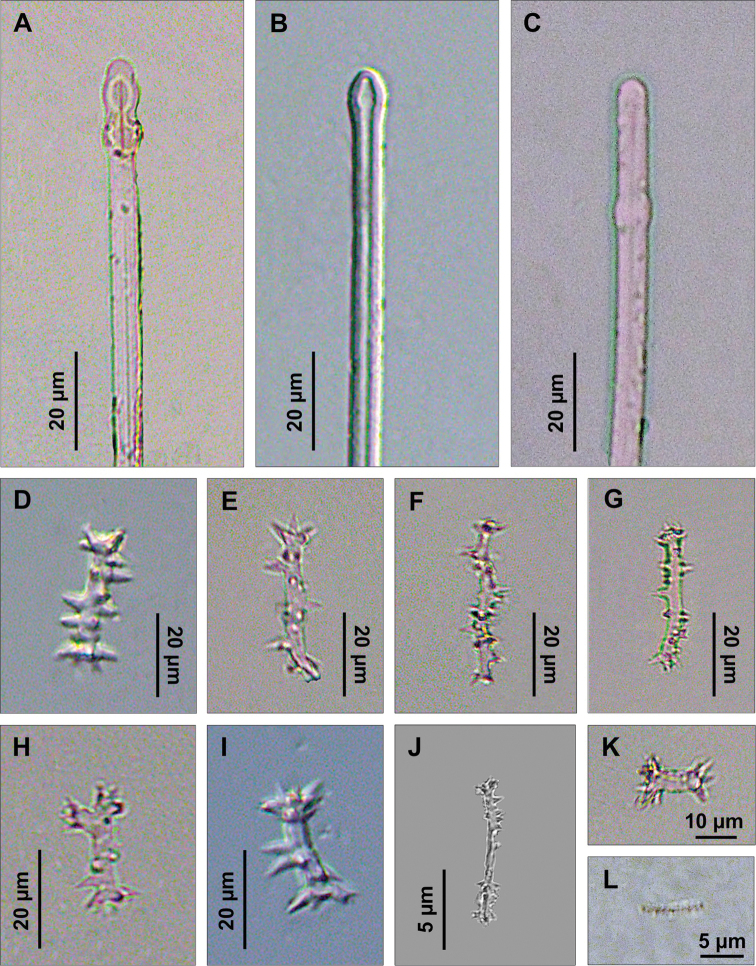
Cliona burtoni. A–C Tylostyle heads D–L Spirasters of various shape and thickness.
Cliona burtoni Topsent, 1932: 577.
Material examined.
Specimen IG-S-BL1-F5B-spB; dry state, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected 17-06-2009. The specimen was entirely used for spicule preparations.
Description.
Boring sponge in alpha growth form, occupying a surface of 1 cm2 in a section of conglomerate. Colour beige in dry state.
Skeleton. Not observed.
Spicules. Macroscleres: tylostyles to subtylostyles straight or slightly curved, 132 (225) 287 × 5 (6) 7.5 μm. Heads with a rounded or oval tyle, sometimes in terminal position but more often shifted along the shaft (Figs 4A, B, C). Microscleres: spirasters of various shape and thickness, straight or curved, 10 (26.5) 45 × 1.25 (10) 17.5 μm. The most abundant have scattered conical spines (Figs 4D, E, F, G, H, I, J, K) and numerous are amphiaster-like (Figs 4H, I, K). The smaller ones are microspinated (Fig. 4J, L).
Distribution and discussion.
This is a Mediterranean endemic species (Pansini and Longo 2008) originally described from Corsica (Strait of Bonifacio), where it is known to bore into calcareous rocks and mollusc shells (Topsent 1932). This is a new record for the Ligurian Sea (Gallinara Island) and the coralligenous assemblage and the first finding after the original description.
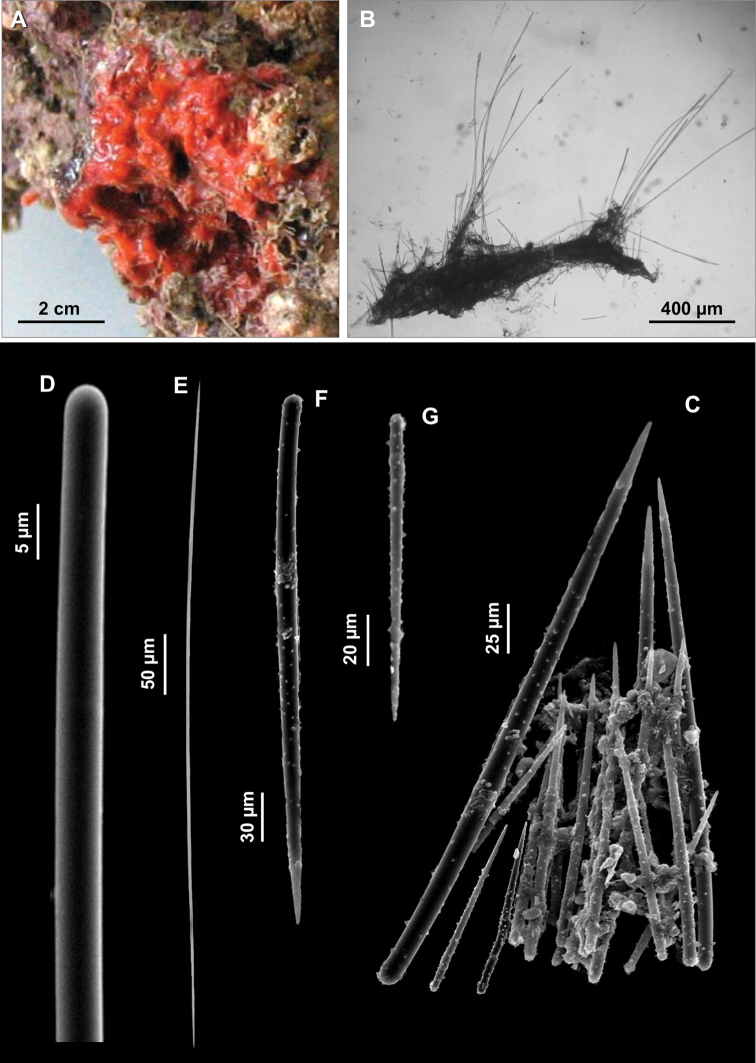
Family Hemiasterellidae: Genus Paratimea
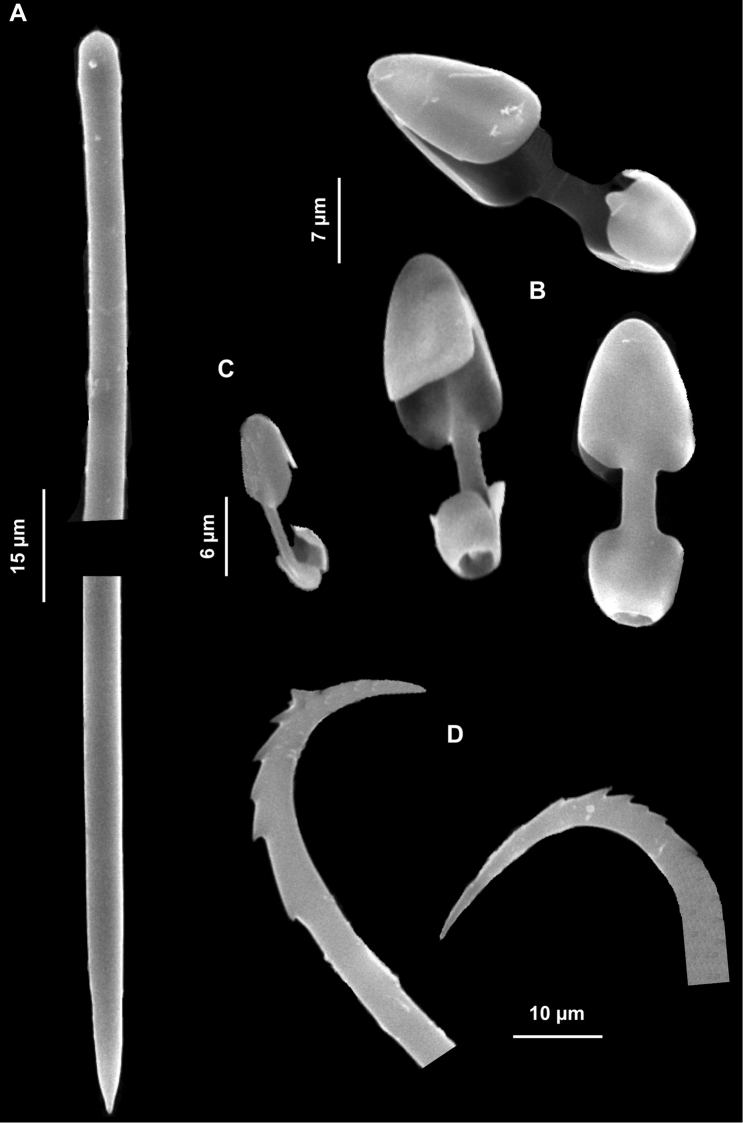
Paratimea oxeata
Pulitzer-Finali, 1978
http://species-id.net/wiki/Paratimea_oxeata
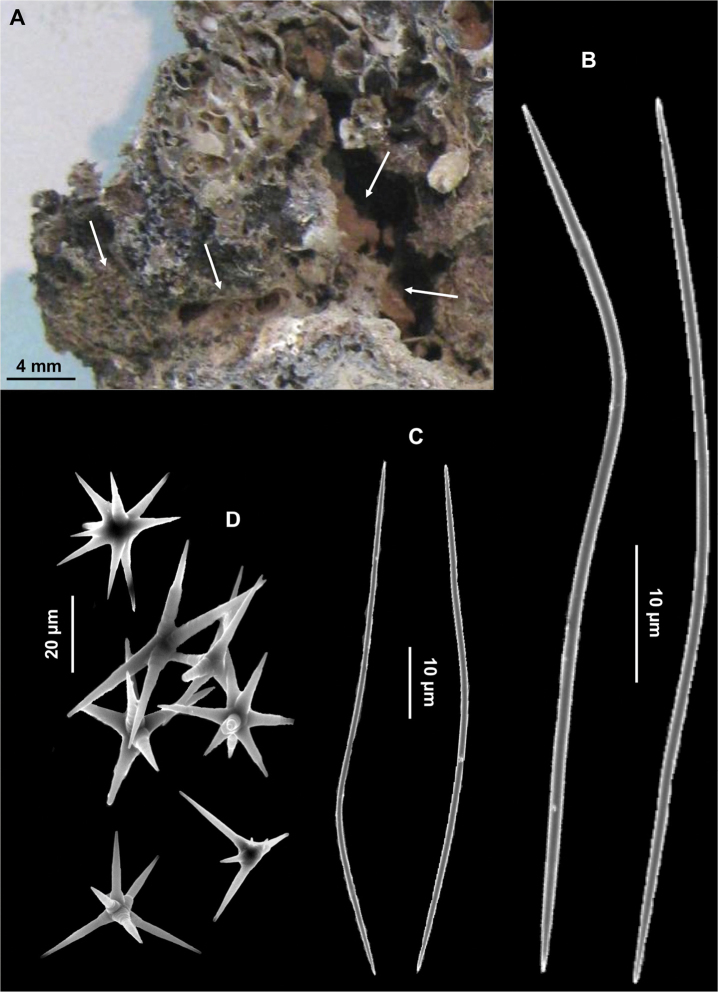
Figure 5.
Paratimea oxeata. A Specimen in the coralligenous accretions (arrows) B Large oxeas C Small oxeas D Oxyasters.
Paratimea oxeata Pulitzer-Finali, 1978: 39.
Material examined.
Specimen SSS-BL1-F3A-spH; alcohol and dry state; Santo Stefano Shoals (station 1), 43°49'N, 7°54'E, depth 35 m, collected 14-02-2008. The specimen was entirely used for spicule preparations.
Description.
Very small (0.5 cm2) insinuating sponge (Fig. 5A) detected inside a cavity of a slice of a coralligenous block. Grey coloured in dry state.
Skeleton. Not observed.
Spicules. Macroscleres: oxeas in two size categories: I) large oxeas curved, bent or flexuous, with hastate tips (Fig. 5B), 810 (961.25) 1200 × 15 (18) 25 μm; II) small oxeas curved or flexuous (Fig. 5C), 300 (546.6) 700 × 2.5 (4.75) 5 μm. Microscleres: oxyasters with more or less marked centrum with 9-12 conical rays, 25 (41.5) 60 μm in diameter. In some cases the number of rays is reduced (Fig. 5D).
Distribution and discussion.
The species wasdescribed from Naples (Pulitzer-Finali 1978) where it occurred on rocky bottoms at 60-100 meter depth. This is a new record for the coralligenous assemblage and for the Ligurian Sea and it is probably endemic for the Mediterranean Sea (Pansini and Longo 2008). This is its first finding after the original description.
Order Poecilosclerida: Suborder Microcionina: Family Microcionidae: Genus Clathria: Subgenus Microciona
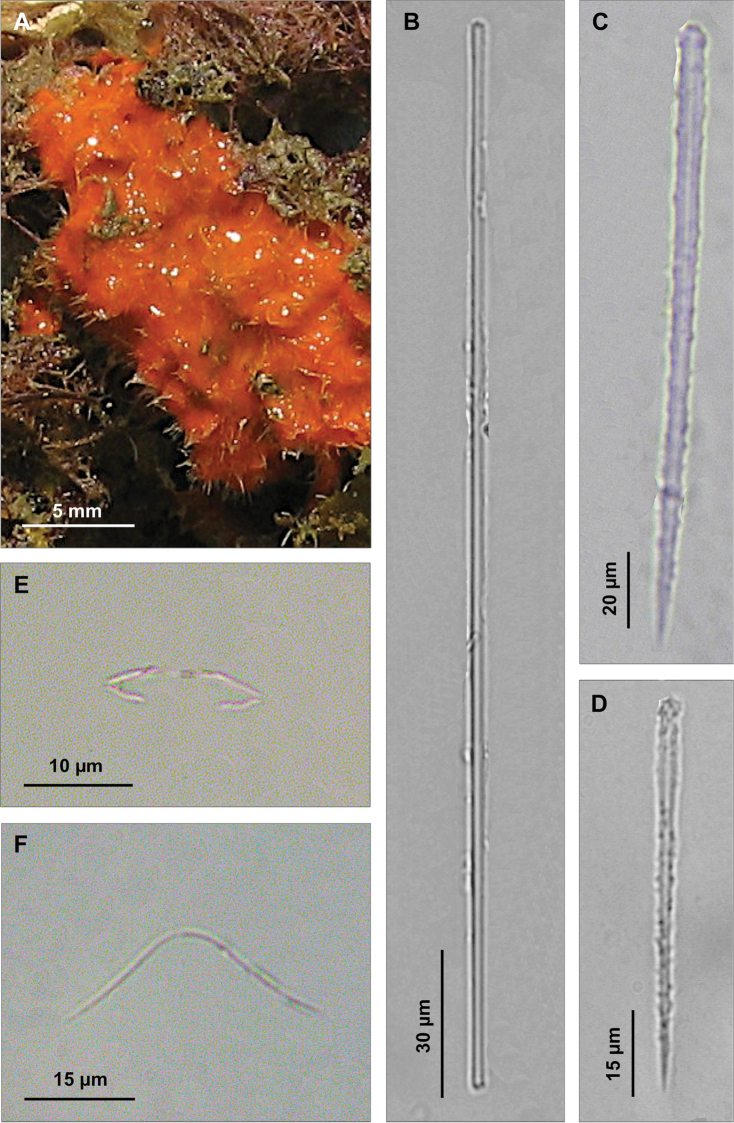
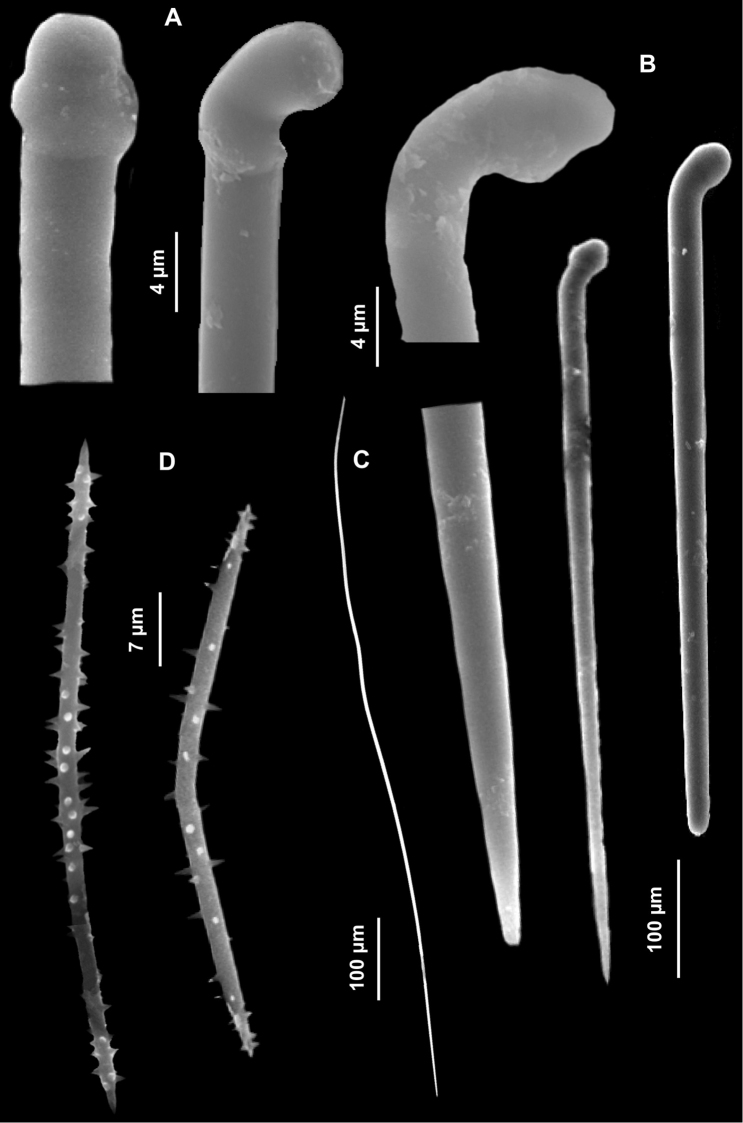
Clathria (Microciona) armata
(Bowerbank, 1862)
http://species-id.net/wiki/Clathria_armata
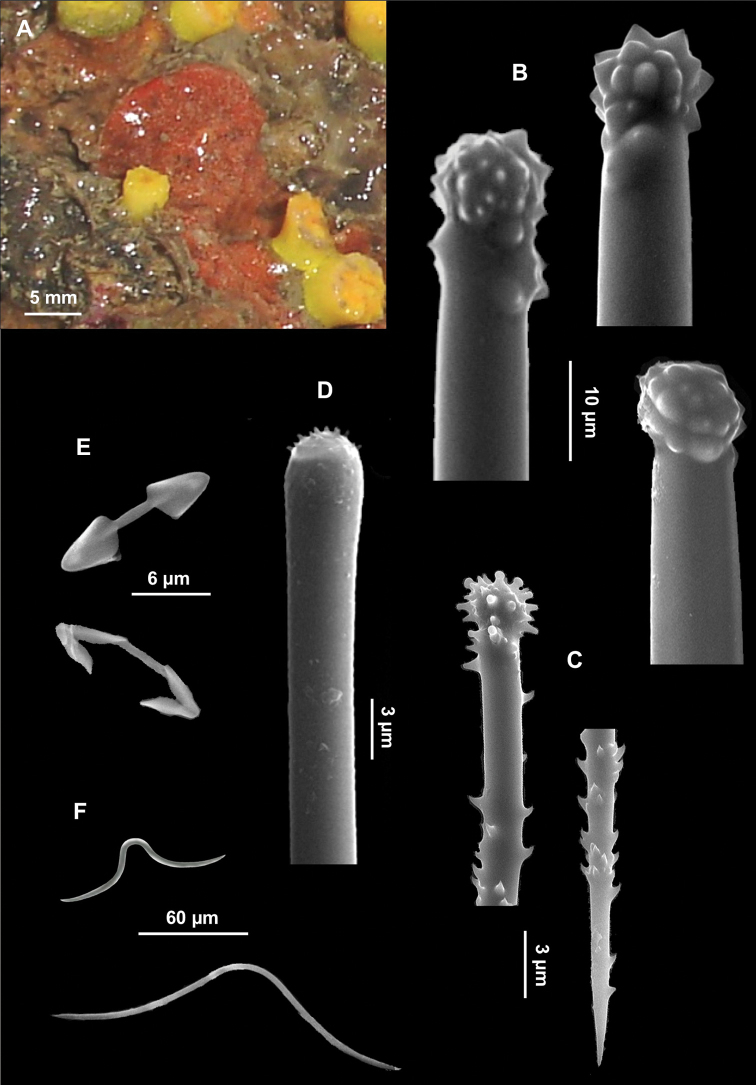
Figure 6.
Clathria (Microciona) armata. A Specimen on the surface of the coralligenous block B Large acanthostyle heads C Small acanthostyle D Subtylostyle with spined head E Palmate isochelae F Toxas of variable size, with smooth extremities.
Microciona armata Bowerbank, 1862; 1866: 129.
Material examined.
Specimen IG-F-BL4-sp2-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected 31-7-2009.
Description. Thickly encrusting sponge (3-5 mm thick) covering a surface of 1.5 cm2 on a coralligenous block (Fig. 6A). Surface irregular, smooth. Consistency soft. The red-orange colour of the living specimen slightly fades when alcohol preserved.
Skeleton. Not observed.
Spicules. Macroscleres: acanthostyles in two size categories: I) large acanthostyles slightly curved, with obtuse spines concentrated on the head (Fig. 6B), 220 (484.5) 830 × 3.75 (8.5) 12 μm; II) small acanthostyles, with scattered spines, but more concentrated on the head (Fig. 6C), 100 (110) 122.5 × 3.75 (5) 6 μm; subtylostyles straight, often with slightly spined head (Fig. 6D), 440 (503.7) 550 × 2.5 (2.9) 3.8 μm. Microscleres: palmate isochelae (Fig. 6E), 10 (12.5) 13.5 μm long. Toxas of variable size, with more or less wide central curvature and slightly reflexed smooth points (Fig. 6F), 80 (114.5) 210 μm long.
Distribution and discussion.
This species has been recorded on rocky walls and on mollusc shells from 10 to 180 m depth (Bowerbank 1866, Arndt 1934, Pulitzer-Finali 1983, van Soest and Stone 1986). It is widely distributed in the Mediterranean Sea (Northern Adriatic Sea, Alboran Sea and Ionian Sea (Pansini and Longo 2003, 2008) and along the Atlantic coast of Europe: Arctic, Sweden, Ireland, United Kingdom, France (van Soest et al. 2013).
This specimen, like that described by van Soest and Stone (1986), differs from the type material in the toxa dimensions. Actually Bowerbank measured small toxas 50 µm long and large toxas 130 µm long dividing them in two size categories. Van Soest and Stone (1986) confirm the large variability of spicule size. The species is a new finding for the coralligenous community and the Ligurian Sea.
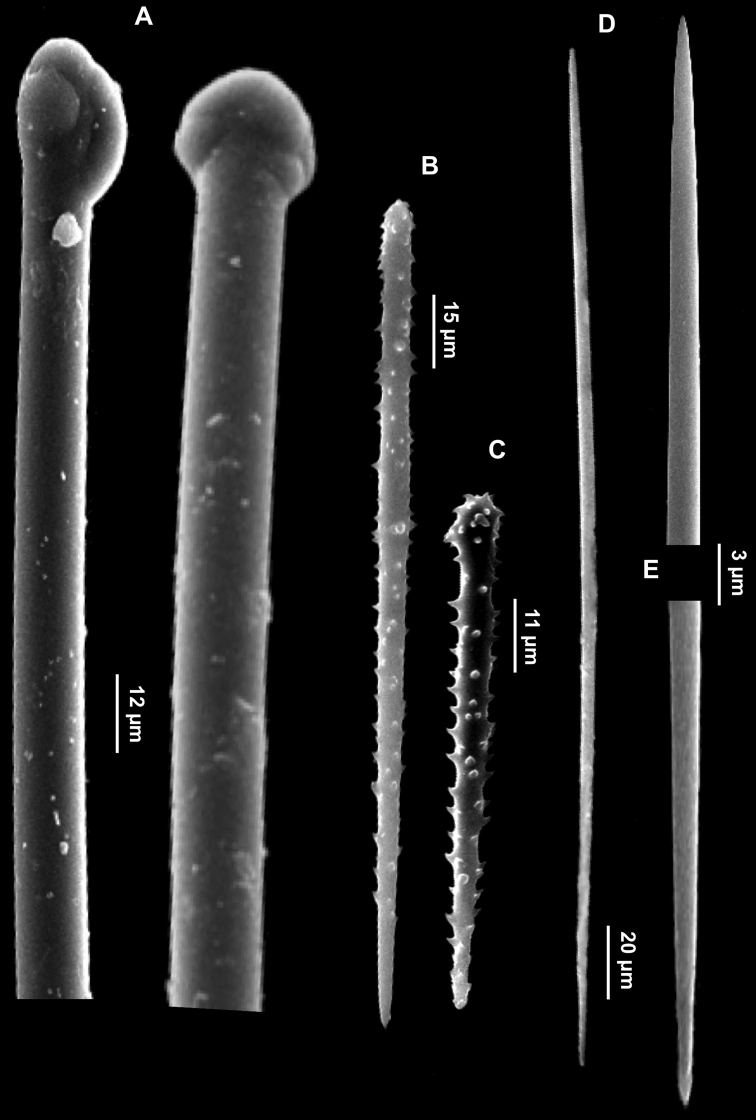
Clathria (Microciona) haplotoxa
(Topsent, 1928)
http://species-id.net/wiki/Clathria_haplotoxa
Figure 7.
Clathria (Microciona) haplotoxa. A Specimen on the surface of a coralligenous block B Strongyle C Large acanthostyle D Small acanthostyle E Isochela F Toxa.
Leptoclathria haplotoxa Topsent, 1928: 298.
Material examined.
Specimen IG-F-BL3-sp5-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected 17-06-2009. The specimen was entirely used for spicule preparations.
Description.
Encrusting sponge on the surface of a coralligenous block, 2 cm in diameter. Surface hispid. Colour brick red (Fig. 7A).
Skeleton. Not observed.
Spicules. Macroscleres: strongyles straight, smooth, 112.5 (178) 215 × 2.5 μm (Fig. 7B); acanthostyles straight with a characteristic constriction under the head, in two size categories: I) large acanthostyles (Fig. 7C), 150 (175.5) 210 μm and II) small acanthostyles (Fig. 7D), 55 (74.5) 102.5 × 2.5 (3.5) 5 μm. Microscleres: palmate isochelae with straight shaft (Fig. 7E), 12.5 (13.8) 15 μm long; toxas thin, smooth, with wide central curvature and slightly reflexed points, 30 (32.5) 37.5 μm long (Fig. 7F).
Distribution and discussion. Described from Porto Santo Bay (Madeira) the species extends south to the Sahelian Upwelling (Lévi 1956). In the Mediterranean Sea it was only recorded from Tunisia (Ben Mustapha et al. 2003). It is a new finding for the Italian sponge fauna and for the coralligenous community.
Family Raspailiidae: Subfamily Raspailiinae: Genus Eurypon
Eurypon denisae
Vacelet, 1969
http://species-id.net/wiki/Eurypon_denisae
Figure 8.
Eurypon denisae. A Tylostyles with variable head B Large acanthostyles C Small acanthostyles D Anisoxeas E Magnifications of the extremities of an anisoxea.
Eurypon denisae Vacelet, 1969: 188.
Material examined.
Specimen IG-S-BL3 sp10-fot.; alcohol preserved, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected 31-07-2009.
Description.
Encrustingsponge covering a surface of 3 cm2 on a coralligenous block. Surface hispid. Colour in life white.
Skeleton. Ectosomal skeleton absent. Choanosomal skeleton consisting of basal acanthostyles with heads embedded in a spongin layer and bundles of very long tylostyles protruding through the sponge surface which appears hispid.
Spicules. Long tylostyles, slightly curved or straight, with rather irregular heads, 1066 (1774) 2236 × 5 (8.5) 12.5 μm (Fig. 8A); anisoxeas straight or faintly curved, 200 (220) 250 × 5 (5.5) 7 μm (Figs 8D-E); acanthostyles in two size categories: I) large, straight acanthostyles, often with inconspicuous heads, uniformly but faintly spined, 107.7 (134.5) 170 × 7.5 (9) 12 μm (Fig. 8B); II) small, straight acanthostyles with stouter and longer spines, 60 (68) 77.5 × 7.5 (8) 10 μm (Fig. 8C).
Distribution and discussion.
The species was originally described by Vacelet (1969) from a coral bottom in the bathyal zone (300–350 m depth) of the Gulf of Lions. This second finding is a new record for the Italian seas and the coralligenous community.
Eurypon gracilis
Bertolino, Calcinai & Pansini sp. n.
http://zoobank.org/E2792BEE-BEC2-41E5-BB7E-E32969E50A1C
http://species-id.net/wiki/Eurypon_gracilis
Figure 9.
Eurypon gracilis sp. n. A Holotype B Skeleton C Portion of the skeleton with large and small echinating acanthostyles D Long style E Oxea F Large acanthostyle with scattered small spines G Small acanthostyle.
Material examined.
Type specimen: Holotype MSNG 57017. Specimen PdF-S-BL4-sp18-sciaf., on a coralligenous concretion, depth 40 m, Stat. 4, 27-07-2009. leg. M. Bertolino, alcohol preserved.
Type locality.
Italy, Ligurian Sea, Portofino Promontory (Punta del Faro) 44°17'54.20"N, 9°13'06.93"E.
Other examined material.
Specimen IG-F-BL1-sp4-fot.; specimen IG-F-BL1-sp15-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected 17-06-2009; specimen IG-S-BL3-sp6-fot.; alcohol preserved, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected 17-06-2009; specimen PM-BL1-sp9-sciaf.; alcohol preserved, Punta Manara (station 6) 44°15'05.61"N, 9°24'09.33"E, depth 35 m, collected 13-06-2009.
Description.
All the specimens were encrusting on the surface of coralligenous blocks, covering surfaces up to 2 cm2. The sponge surface is corrugated, hispid. The colour in life is brick red (Fig. 9A).
Skeleton. The skeleton consists of a basal layer of spongin in which the spicules are vertically positioned, perpendicular to the substrate. Both the categories of acanthostyles are close one another (Fig. 9C) with the heads embedded in the basal spongin layer. Styles and oxeas–with the same vertical arrangement–are grouped in bundles which are faintly echinated, in their lower part, by the smaller acanthostyles (Fig. 9B). Oxeas are positioned in the basal part of the bundles. The styles protrude trough the sponge surface making it hispid.
Spicules. Long styles to tylostyles, curved or flexuous (Fig. 9D), 788 (1101) 1280 × 5 (6.8) 10 µm; oxeas thin, almost straight or with a slight curvature (Fig. 9E), 365 (483) 650 × 2.5 µm; acanthostyles without head and uniformly spined, in two sizes categories: I) large acanthostyles, straight or slightly curved with rather small spines (Fig. 9F), 200 (253) 320 × 5 (6) 7.7 µm; II) small acanthostyles straight, with spines more robust than in the previous category (Fig. 9G), 90 (119.5) 160 × 2.5 (3.8) 5 µm.
Etymology.
The species is named after the slenderness of all the spicule types.
Distribution.
So far known only from the Ligurian Sea.
Ecology.
It lives at 30–40 m depth on coralligenous concretion, characterized by the presence of a Paramuricea clavata facies.
Discussion.
This species, characterized by a microcionid skeleton with a basal layer of spongin, extra-axial spicules and echinating achantostyles embedded in spongin fibres, clearly belongs to the genus Eurypon.
Only five, out of the numerous species of the genus Eurypon found in the temperate Western Atlantic have oxeas or tornotes as structural megascleres together with styles or tylostyles. All of them (Eurypon cinctum Sarà, 1960, Eurypon denisae Vacelet, 1969, Eurypon obtusum Vacelet, 1969, Eurypon major Sarà & Siribelli, 1960 and Eurypon lacazei (Topsent, 1891) occur in the Mediterranean Sea. Eurypon cinctum showing a lilac colour, achantostyles with discrete heads and different size in the other megascleres is not close to the new species. Eurypon denisae is also different according to the description given above. Eurypon obtusum is grey in colour and has smaller oxeas and acanthostyles than those of the present species, but the maximum length of its tylostyles is unknown. Eurypon lacazei remarkably differs from the present species for the green colour and spicule shape and size. The closest species to the new one is Eurypon major but its tylostyles are longer and stouter (1445–2210 × 10–17 µm) and differ in the shape of the heads, while the acanthostyles, in a single size category, have well formed heads. Only two other species from the temperate Atlantic: Eurypon lictor (Topsent, 1904) and Eurypon (Acantheurypon) mucronale (Topsent, 1928) present oxeas. However, they are both deep species (recorded deeper than 1500 m from the Azores) and they differ also in the spicule characters from Eurypon gracilis sp. n. There are two other species of Eurypon with oxeas reported in the literature: Eurypon calypsoi Lévi, 1958 from the Red Sea which is blue in colour and Eurypon fulvum Lévi, 1969 from South Africa which is yellow. Both have a single size category of acanthostyles and differ in the spicule morphology. Eurypon gracilis therefore has to be considered as new for science.
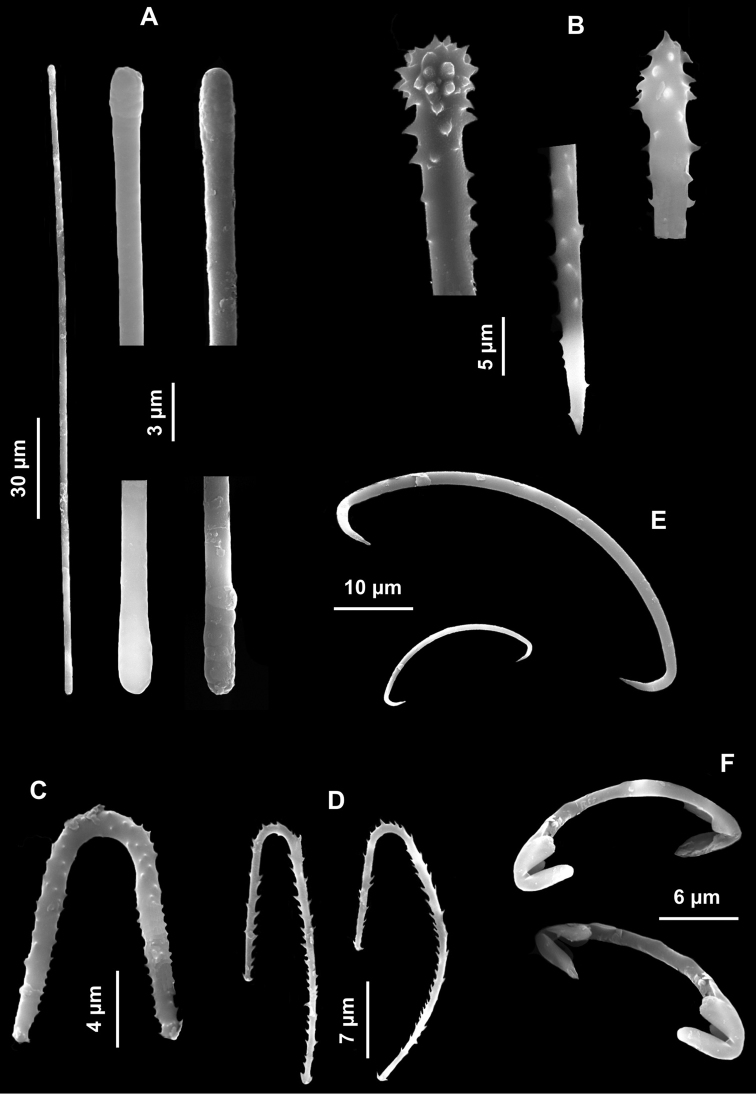
Suborder Myxillina: Family Coelosphaeridae: Genus Forcepia: Subgenus Leptolabis
Forcepia (Leptolabis) brunnea
(Topsent, 1904)
http://species-id.net/wiki/Forcepia_brunnea
Figure 10.
Forcepia (Leptolabis) brunnea. A Anisotylotes B Acanthostyles C Symmetric forceps D Asymmetric forceps E Large and small sigmas F Isochelae.
Leptolabis forcipula var. brunnea Topsent, 1904: 182.
Leptolabis brunnea Topsent, 1928: 278.
Material examined.
Specimen PdF-NE-BL2A-sp15-sciaf.; alcohol preserved, Portofino Promontory (Punta del Faro, station 4) 44°17'55.61"N, 9°13'07.95"E, 40 m depth, collected on 27-08-2009; specimen IG-S-BL3-sp13-sciaf.; alcohol preserved, Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45" E, depth 30 m, collected on 17-06-2009; specimen PdF-BL8-sp50-sciaf.; alcohol preserved, Portofino Promontory (Punta del Faro, station 4) 44°17'55.61"N, 9°13'07.95"E, 30 m depth, collected on 25-01-2013.
Description.
Thin, smallencrusting sponges (up to 0.5 cm2) on the surface of coralligenous blocks. Colour in life yellow-orange.
Skeleton. Basal acanthostyles erect on the substrate in a hymedesmioid arrangement. Other spicule types not detectable from the skeleton.
Spicules. Megascleres: anisotylotes straight or faintly curved, with slightly different extremities and a few malformations along the shaft (Fig. 10A), 127.5 (157.7) 280.5 × 1.25 (2.3) 2.5 μm; acanthostyles straight, conical with discrete but not swollen heads. Spines evenly distributed, slightly stouter on the spicule head (Fig. 10B), 61.2 (92.2) 142.8 × 5.2 (7.5) 10.4 μm. Microscleres: acanthose symmetric forceps with straight legs, ending in small, button-like swellings with toothed margin (Fig. 10C). They measure 12.5 (15.8) 17.5 × 2.5 μm in length, the distance between the legs being 5.2 (7.2) 7.5 μm. Acanthose asymmetric forceps, very thin, have unequal legs (Fig. 10D), the longer of which is straight or curved inward, 20.4 (22.3) 25 × 1.5 μm. Sigmas in two size categories: the larger ones, “C" shaped (Fig. 10E) or more rarely “S" shaped, 40.8 (64.3) 80 × 2.5 μm are very abundant, the smaller, 17.5–25.5 μm are rare. Palmate isochelae (Fig. 10F), 18 (20) 20.8 μm long.
Distribution and discussion.
Topsent (1904) describes three species of Leptolabis from the Azores: Leptolabis forcipula var. brunnea, Leptolabis arcuata and Leptolabis assimilis. The same author in 1928 states that the former three species actually belong to a single species: Leptolabis brunnea which showsa high variability in the large forceps shape.
Leptolabis brunnea was afterwards recorded from the Far-Oer Islands, the Azores, Spain (NW coast, Strait of Gibaltar, Castellón, Girona), France (Marseille, Monaco), Italy (Gulf of Naples), between 4 and 1360 m depth. It lives in caves, detritic bottoms, coralligenous concretions and epibiotic on other organisms (Topsent 1904, 1928, Sarà 1960, Pouliquen 1972, Carballo 1994, Cristobo 1996). This is the second finding for the Italian seas and a new finding for the Ligurian Sea.
Family Hymedesmiidae: Genus Hymedesmia: Subgenus Hymedesmia
Hymedesmia (Hymedesmia) rissoi
Topsent, 1936
http://species-id.net/wiki/Hymedesmia_rissoi
Figure 11.
Hymedesmia (Hymedesmia) rissoi. A Tornote, sometimes modified into subtylotes and strongyles B Acanthostyles C Arcuate isochelae D Thin sigmas.
Hymedesmia gracilisigma var. rissoi Topsent, 1936: 35.
Material examined.
Specimen IG-F-BL3-F18b-spA; Specimen IG-F-BL4-sp9-sciaf.; specimen IG-F-BL4 sp11-fot.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected on 17-06-2009; specimenSSS-BL1-sp11-sciaf.; Santo Stefano Shoals, (station 1), 43°49'N, 7°54'E, depth 35 m, collected on 14-02-2008.
Description.
Small (0.5 cm2), slimy, coriaceous encrusting sponge, grey in colour after alcohol preservation, recorded both on the surface and inside the coralligenous blocks.
Skeleton. Not observed.
Spicules. Megascleres: straight or slightly sinuous anisotornotes, sometimes modified in anisotylotes or strongyles (Fig. 11A), 140 (175) 177.5 × 2.5 (2.7) 3.75 μm; acanthostyles in a single size category, 67.5 (84) 105 × 2.5 (3.5) 3.75 μm, devoid of conspicuous heads. The extremities may be pointed or blunt (Figs 11B, C). Microscleres: arcuate isochelae (Fig. 11D), 25 (25.6) 27.5 μm long; thin sigmas “C” (Fig. 11E) and “S” shaped, 32.5 (35) 37.5 × 1.25 μm.
Distribution and discussion.
In the original description Topsent (1936) distinguished in this species two size classes of acanthostyles similar in shape: the larger were 185–265 μm in length and the smaller 75–115 μm. Subtylotes straight or sometimes slightly sinuous, 225–275 × 3.5–4.5 μm, arcuate isochelae 23–25 μm long and sigmas 40–50 μm long and less than 1 μm thick. The specimens here described match with Topsent’s description apart from the presence of a single size class of acanthostyles. However, other authors (Sarà and Siribelli 1962), recorded a single class of acanthostyles as well. This is a Mediterranean endemic species (Ligurian Sea and Central Tyrrhenian Sea). It was found on Cladocora caespitosa, at 15–40 m depth (Topsent 1936) and on coralligenous bottom, at 40–70 m depth (Sarà and Siribelli 1962).
Suborder Mycalina: Family Mycalidae: Genus Mycale: Subgenus Paresperella
Mycale (Paresperella) serrulata
Sarà & Siribelli, 1960
http://species-id.net/wiki/Mycale_serrulata
Figure 12.
Mycale (Paresperella) serrulata. A–B Mycalostyles B Large anisochelae C Small anisochelae D Magnifications of the serrated edge of a sigma.
Mycale (Paresperella) serrulata Sarà & Siribelli, 1960: 51.
Material examined.
Specimen IG-F-BL3-F4B-spA; specimen IG-F-BL3-F17B-spA alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected on 31-07-2009. The specimen was entirely used for spicule preparations.
Description. Small, encrusting and insinuating sponge, beige in the dry state, occupying a small cavity (1 cm3) in a coralligenous block.
Skeleton. Not observed.
Spicules. Megascleres: mycalostyles straight or flexuous, with acerate tip (Fig. 12A), 310 (325) 340 × 3.75 (5) 7.5 µm. Microscleres: anisochelae in two size categories. I) The larger ones, 25 (29.5) 35 µm, have the bigger tooth palmate and the smaller often characterized by a conspicuous point and slightly diverging outwords alae; a hole is detectable at the smaller extremity (Fig. 12B). II) The smaller ones measure, 12.5 (13.7) 15 µm (Fig. 12C). Sigmas “C” shaped, 64 (78) 100 × 2.5 (2.7) 5 µm, with the convex edge serrated (Fig. 12D).
Distribution and discussion.
Mycale (Paresperella) serrulata Sarà & Siribelli, 1960, was originally described from a detritic bottom of the Gulf of Naples at 30-40 m depth. Voultsiadou and Vafidis (2004) recorded the species encrusting on Fasciospongia cavernosa at 90 m depth in the Aegean Sea. Mycale (Paresperella) serrulata is a Mediterranean endemic species. Pansini and Longo (2008) recorded it for the first time for the Ligurian Sea and the coralligenous community.
Order Halichondrida: Family Eteroxyidae: Genus Halicnemia
Halicnemia geniculata
Sarà, 1958
http://species-id.net/wiki/Halicnemia_geniculata
Figure 13.
Halicnemia geniculata. A Magnifications of the tylostyle heads B Rabdhotylostyles C Oxeas, long, sinuous and thin D Acanthoxeas.
Halicnemia geniculata Sarà, 1958: 237.
Material examined.
Specimen IG-F-BL4-sp1-sciaf.; alcohol preserved, Gallinara Island (station 2, Falconara) 44°01'22"N, 8°13'34"E, depth 35 m, collected on 17-06-2009. The specimen was entirely used for spicule preparations.
Description.
Small and thin, yellow-ochre encrustation (1 cm2) on a coralligenous block.
Skeleton. Not observed.
Spicules. Long tylostyles, 405 (1351.7) 1976 × 1.5 (2.7) 4 μm, generally straight, with terminal or subterminal swellings variable in shape; irregular and polytylote forms are to be found (Fig. 13A). Rabdhotylostyles with heads as above, 147(242)705 × 1.5 (2.7) 4 μm (Fig. 13B); oxeas long, sinuous and thin, 460 (757) 1118 × 1.5 (2.5) 5 mm (Fig. 13C); acanthoxeas slightly curved or bent, uniformly spined, 42.5 (51.8) 62.5 × 1.5 (1.8) 2 μm (Fig. 13D).
Distribution and discussion.
This species, originally described from a superficial cave of the Gulf of Naples (Sarà 1958) was recorded at 60–70 m depth in the same area (Sarà and Siribelli 1962) and in caves close to Marseille (Pouliquen 1972). It is a Mediterranean endemic species (Pansini and Longo 2008) and a new finding for the Ligurian Sea and the coralligenous community.
Order Haplosclerida: Suborder Haplosclerina: Family Chalinidae: Genus Haliclona: Subgenus Gellius
Haliclona (Gellius) marismedi
(Pulitzer-Finali, 1978)
http://species-id.net/wiki/Haliclona_marismedi
Figure 14.
Haliclona (Gellius) marismedi. A Specimen on the surface of the coralligenous block and insinuating into it B Oxeas C Large toxas D Small toxas E Large sigma F Small sigma.
Gellius marismedi , Pulitzer-Finali, 1978: 81.
Material examined.
Specimen PM-BL1-sp7-sciaf.; specimen PM-BL1-sp8-sciaf.; specimen PM-BL2b-sp6-sciaf.; specimen PM-BL2b-sp6a-sciaf.; Punta Manara (station 6) 44°15'05.61"N, 9°24'09.33"E, depth 35 m, collected 13-07-2009; specimen IG-S-BL1-sp2-sciaf.; Gallinara Island (station 3, Sciusciaù) 44°01'34"N, 8°13'45"E, depth 30 m, collected on17-06-2009.
Description. Small (1-1.5 cm2) encrusting and insinuating sponge, beige or brown, detected on the surface and inside a coralligenous block. Surface smooth, consistency soft (Fig. 14A).
Skeleton. The choanosome consists of multispicular primary lines connected by unispicular secondary tracts, creating a confused reticulation.
Spicules. Oxeas gently curved with hastate extremities detectable only in the larger spicules (Fig. 14B), 220 (245) 275 × 2.5 (4.5) 6.25 μm; toxas with more or less angulate central curvature and slightly reflexed points in two size categories: I) 27.5 (45.5) 57.5 μm (Fig. 14C) and II) 10 (11.5) 12.5 μm (Fig. 14D); two types of thin sigmas, “C” shaped, I) 22.5 (23.7) 25 μm and II) 10 (13.6) 17.5 μm (Figs 14E, F).
Distribution and discussion. Pulitzer-Finali (1978) described the species from a specimen epibiothic on Hyrtios collectrix (Schulze, 1880) found on dead, sanded Posidonia beds, at 50 m depth in the Bay of Naples. The same author considered conspecific with Gellius marismedi the specimen from Banyuls-sur-Mer (rocky walls in shaded areas at 2–17 m depth and horizontal substrates at 20–40 m depth) attributed to Gelliodes luridus (Lundbeck, 1902) by Boury-Esnault (1971).
This is a new finding for the Ligurian Sea and the coralligenous community and the third record after the original description.
Discussion
According to the latest available revision of coralligenous biodiversity (Ballesteros 2006), 142 species of sponges have been recorded associated with this community. Adding to this list the species recorded on the coralligenous of Apulia (Sarà 1968, 1969), Liguria (Pansini and Pronzato 1973; Calcinai et al. 2007a; Calcinai et al. in prep.; Bertolino et al. 2008) and the Aegean Sea (Kefalas et al. 2003; Kefalas and Castritsi-Catharios 2012) those found associated to red coral (Melone 1965; Templado et al. 1986; Corriero et al. 1988; 1997; Maldonado 1992; Bavestrello et al. 1996; Calcinai et al. 2007b) and the data of the present study, the total number of sponge species hitherto associated to the coralligenous community increases to 273 (Table 2).
Table 2.
List of sponge species (Demospongiae and Homoscleromorpha) hitherto recorded associated to the coralligenous community.
| Oscarellidae | |
| 1. | Oscarella lobularis (Schmidt, 1862) |
| Plakinidae | |
| 2. | Corticium candelabrum Schmidt, 1862 |
| 3. | Placinolopha moncharmonti (Sarà, 1960) |
| 4. | Plakina monolopha Schulze, 1880 |
| 5. | Plakina dilopha Schulze, 1880 |
| 6. | Plakina trilopha Schulze, 1880 |
| 7. | Plakinastrella copiosa Schulze, 1880 |
| 8. | Plakinastrella mixta Maldonado, 1992 |
| 9. | Plakortis simplex Schulze, 1880 |
| Tetillidae | |
| 10. | Craniella cranium (Müller, 1776) |
| Samidae | |
| 11. | Samus anonymus Gray, 1867 |
| Ancorinidae | |
| 12. | Stelletta dorsigera Schmidt, 1862 |
| 13. | Stelletta grubii Schmidt, 1862 |
| 14. | Stelletta lactea Carter, 1871 |
| 15. | Stelletta stellata Topsent, 1893 |
| 16. | Jaspis incrustans (Topsent, 1890) |
| 17. | Jaspis johnstonii (Schmidt, 1862) |
| 18. | Stryphnus mucronatus (Schmidt, 1868) |
| 19. | Stryphnus ponderosus (Bowerbank, 1866) |
| 20. | Penares candidata (Schmidt, 1868) |
| 21. | Penares euastrum (Schmidt, 1868) |
| 22. | Penares helleri (Schmidt, 1864) |
| 23. | Holoxea furtiva Topent, 1892 |
| 24. | Dercitus (Dercitus) bucklandi (Bowerbank, 1858) |
| 25. | Dercitus (Stoeba) plicata (Schmidt, 1868) |
| Calthropellidae | |
| 26. | Calthropella (Calthropella) pathologica (Schmidt, 1868) |
| 27. | Calthropella (Corticellopsis) stelligera (Schmidt, 1868) |
| Geodiidae | |
| 28. | Erylus discophorus (Schmidt, 1862) |
| 29. | Erylus papulifer Pulitzer-Finali, 1983 |
| 30. | Caminus vulcani Schmidt, 1862 |
| 31. | Pachymatisma johnstonia (Bowerbank in Johnston, 1842) |
| 32. | Geodia anceps (Vosmaer, 1894) |
| 33. | Geodia conchilega Schmidt, 1862 |
| 34. | Geodia cydonium Jamenson, 1811 |
| 35. | Caminella intuta (Topsent, 1892) |
| Pachastrellidae | |
| 36. | Pachastrella monilifera Schmidt, 1868 |
| 37. | Poecillastra compressa (Bowerbank, 1866) |
| 38. | Nethea amygdaloides (Carter, 1876) |
| 39. | Thenea muricata (Bowerbank, 1858) |
| 40. | Triptolemma simplex (Sarà, 1959) |
| 41. | Vulcanella (Vulcanella) gracilis (Sollas, 1888) |
| 42. | Annulastrella verrucolosa (Pulitzer-Finali, 1983) |
| Clionaidae | |
| 43. | Cliona burtoni Topsent, 1932 |
| 44. | Cliona carteri (Ridley, 1881) |
| 45. | Cliona celata Grant, 1826 |
| 46. | Cliona lobata Hancock, 1849 |
| 47. | Cliona janitrix Topsent, 1932 |
| 48. | Cliona rhodensis Rützler & Bromley, 1981 |
| 49. | Cliona schmidtii (Ridley, 1881) |
| 50. | Cliona thoosina Topsent, 1888 |
| 51. | Cliona vermifera Hancock, 1867 |
| 52. | Cliona viridis Schmidt, 1862 |
| 53. | Dotona pulchella mediterranea Rosell & Uriz, 2002 |
| 54. | Pione vastifica (Hancock, 1849) |
| 55. | Spiroxya corallophila (Calcinai, Cerrano & Bavestrello, 2002) |
| 56. | Spiroxya heteroclita Topsent, 1896 |
| 57. | Spiroxya levispira (Topsent, 1898) |
| 58. | Spiroxya sarai (Melone, 1965) |
| Thoosidae | |
| 59. | Alectona millari Carter, 1879 |
| 60. | Delectona ciconiae Bavestrello, Calcinai & Sarà, 1996 |
| 61. | Delectona madreporica Bavestrello et al., 1997 |
| 62. | Thoosa armata Topsent, 1888 |
| 63. | Thoosa mollis Volz, 1939 |
| Hemiasterellidae | |
| 64. | Paratimea constellata (Topsent, 1893) |
| 65. | Paratimea oxeata Pulitzer-Finali, 1978 |
| Stelligeridae | |
| 66. | Stelligera rigida (Montagu, 1818) |
| Polymastiidae | |
| 67. | Polymastia inflata Cabioch, 1968 |
| 68. | Polymastia mamillaris (Müller, 1806) |
| 69. | Polymastia polytylota Vacelet, 1969 |
| 70. | Quasillina brevis (Bowerbank, 1861) |
| 71. | Pseudotrachya hystrix (Topsent, 1890) |
| Spirastrellidae | |
| 72. | Diplastrella bistellata (Schmidt, 1862) |
| 73. | Spirastrella cunctatrix Schmidt, 1868 |
| Suberitidae | |
| 74. | Aaptos aaptos (Schmidt, 1864) |
| 75. | Prosuberites longispina Topsent, 1893 |
| 76. | Protosuberites ectyoninus (Topsent, 1900) |
| 77. | Protosuberites epiphytum (Lamarck, 1815) |
| 78. | Protosuberites rugosus (Topsent, 1893) |
| 79. | Pseudosuberites hyalinus (Ridley & Dendy, 1867) |
| 80. | Pseudosuberites sulphureus (Bowerbank, 1866) |
| 81. | Suberites carnosus (Johnston, 1842) |
| 82. | Suberites carnosus incrustans Topsent, 1900 |
| 83. | Suberites domuncula (Olivi, 1792) |
| 84. | Suberites syringella (Schmidt, 1868) |
| 85. | Terpios gelatinosa (Bowerbank, 1866) |
| Tethyidae | |
| 86. | Tethya aurantium (Pallas, 1766) |
| 87. | Tethya citrina Sarà & Melone, 1965 |
| Timeidae | |
| 88. | Timea cumana Pulitzer-Finali, 1978 |
| 89. | Timea fasciata Topsent, 1934 |
| 90. | Timea irregularis Sarà & Siribelli, 1960 |
| 91. | Timea stellata (Bowerbank, 1866) |
| 92. | Timea stellifasciata Sarà & Siribelli, 1960 |
| 93. | Timea unistellata (Topsent, 1892) |
| Trachycladidae | |
| 94. | Trachycladus minax (Topsent, 1888) |
| Chondrillidae | |
| 95. | Chondrosia reniformis Nardo, 1847 |
| 96. | Chondrilla nucula Schmidt, 1862 |
| Desmanthidae | |
| 97. | Desmanthus incrustans (Topsent, 1889) |
| Acarnidae | |
| 98. | Acarnus souriei (Lévi, 1952) |
| 99. | Acarnus tortilis Topsent, 1892 |
| Microcionidae | |
| 100. | Clathria (Clathria) compressa (Schmidt, 1862) |
| 101. | Clathria (Clathria) coralloides (Olivi, 1792) |
| 102. | Clathria (Clathria) depressa Sarà & Melone, 1966 |
| 103. | Clathria (Clathria) toxivaria (Sarà, 1959) |
| 104. | Clathria (Microciona) armata (Bowerbank, 1862) |
| 105. | Clathria (Microciona) assimilis Topsent & Olivier, 1943 |
| 106. | Clathria (Microciona) gradalis Topsent, 1925 |
| 107. | Clathria (Microciona) haplotoxa (Topsent, 1928) |
| 108. | Clathria (Microciona) spinarcus (Carter & Hope, 1889) |
| 109. | Clathria (Microciona) toxistyla (Sarà, 1959) |
| 110. | Antho (Antho) inconstans (Topsent, 1925) |
| 111. | Antho (Antho) involvens (Schmidt, 1864) |
| 112. | Antho (Acarnia) coriacea (Bowerbank, 1874) |
| 113. | Antho (Acarnia) cf. novizelanica (Ridley & Duncan, 1881) |
| Raspailiidae | |
| 114. | Raspailia (Raspailia) viminalis Schmidt, 1862 |
| 115. | Aulospongus spinosus (Topsent, 1927) |
| 116. | Eurypon cinctum Sarà, 1960 |
| 117. | Eurypon clavatum (Bowerbank, 1866) |
| 118. | Eurypon coronula (Bowerbank, 1874) |
| 119. | Eurypon denisae Vacelet, 1969 |
| 120. | Eurypon gracilis Present paper |
| 121. | Eurypon lacazei (Topsent, 1891) |
| 122. | Eurypon major Sarà & Siribelli, 1960 |
| 123. | Eurypon topsenti Pulitzer-Finali, 1983 |
| 124. | Eurypon vesciculare Sarà & Siribelli, 1960 |
| 125. | Eurypon viride (Topsent, 1889) |
| 126. | Raspaciona aculeata (Johnston, 1842) |
| Rhabderemiidae | |
| 127. | Rhabderemia gallica van Soest & Hooper, 1993 |
| 128. | Rhabderemia indica Dendy, 1905 |
| 129. | Rhabderemia minutula (Carter, 1876) |
| 130. | Rhabderemia cf. topsenti van Soest & Hooper, 1993 |
| Chondropsidae | |
| 131. | Batzella inops (Topsent, 1891) |
| Coelosphaeridae | |
| 132. | Chaetodoryx insinuans (Topsent, 1936) |
| 133. | Forcepia (Leptolabis) apuliae (Sarà, 1969) |
| 134. | Forcepia (Leptolabilis) brunnea (Topsent, 1904) |
| 135. | Forcepia (Leptolabis) cf. luciensis (Topsent, 1888) |
| 136. | Forcepia (Leptolabis) megachela (Maldonado, 1992) |
| 137. | Lissodendoryx (Lissodendoryx) isodictyalis (Carter, 1882) |
| 138. | Lissodendoryx (Anomodoryx) cavernosa (Topsent, 1892) |
| Crambeidae | |
| 139. | Crambe crambe (Schmidt, 1862) |
| 140. | Crambe tuberosa Maldonado & Benito, 1991 |
| Crellidae | |
| 141. | Crella (Crella) elegans (Schmidt, 1862) |
| 142. | Crella (Crella) mollior Topsent, 1925 |
| 143. | Crella (Grayella) pulvinar (Schmidt, 1868) |
| 144. | Crella (Pytheas) fusifera Sarà, 1969 |
| 145. | Crella (Pytheas) sigmata Topsent, 1925 |
| 146. | Crella (Yvesia) rosea (Topsent, 1892) |
| Desmacididae | |
| 147. | Desmacidon adriaticum Sarà, 1969 |
| 148. | Desmacidon fruticosum (Montagu, 1818) |
| Hymedesmiidae | |
| 149. | Hemimycale columella (Bowerbank, 1864) |
| 150. | Hymedesmia (Hymedesmia) baculifera (Topsent, 1901) |
| 151. | Hymedesmia (Hymedesmia) paupertas (Bowerbank, 1866) |
| 152. | Hymedesmia (Hymedesmia) peachi Bowerbank, 1882 |
| 153. | Hymedesmia (Hymedesmia) plicata Topsent, 1928 |
| 154. | Hymedesmia (Hymedesmia) rissoi Topsent, 1936 |
| 155. | Hymedesmia (Hymedesmia) versicolor (Topsent, 1893) |
| 156. | Hymedesmia (Stylopus) coriacea (Fristedt, 1885) |
| 157. | Phorbas dives (Topsent, 1891) |
| 158. | Phorbas fibulatus (Topsent, 1893) |
| 159. | Phorbas fictitius Bowerbank, 1866 |
| 160. | Phorbas mercator (Schmidt, 1868) |
| 161. | Phorbas tenacior (Topsent, 1925) |
| 162. | Plocamionida ambigua (Bowerbank, 1866) |
| Myxillidae | |
| 163. | Myxilla (Myxilla) rosacea (Lieberkühn,1859) |
| Tedaniidae | |
| 164. | Tedania (Tedania) anhelans Lieberkühn, 1849 |
| Desmacellidae | |
| 165. | Biemna parthenopea Pulitzer-Finali, 1978 |
| 166. | Biemna variantia (Bowerbank, 1858) |
| 167. | Desmacella annexa Schmidt, 1870 |
| 168. | Desmacella inornata (Bowerbank, 1866) |
| Esperiopsidae | |
| 169. | Ulosa stuposa (Esper, 1794) |
| Hamacanthidae | |
| 170. | Hamacantha (Vomerula) falcula (Bowerbank, 1874) |
| Mycalidae | |
| 171. | Mycale (Mycale) lingua (Bowerbank, 1866) |
| 172. | Mycale (Mycale) massa (Schmidt, 1862) |
| 173. | Mycale (Aegogropila) contarenii (Lieberkühn, 1859) |
| 174. | Mycale (Aegogropila) tunicata (Schmidt, 1862) |
| 175. | Mycale (Paresperella) serrulata Sarà & Siribelli, 1960 |
| Merliidae | |
| 176. | Merlia normani Kirkpatrick, 1908 |
| Podospongiidae | |
| 177. | Podospongia lovenii Bocage, 1870 |
| Latrunculiidae | |
| 178. | Latrunculia (Biannulata) citharistae Vacelet, 1969 |
| 179. | Sceptrella biannulata (Topsent, 1892) |
| 180. | Sceptrella insignis (Topsent, 1890) |
| Axinellidae | |
| 181. | Axinella cannabina (Esper, 1794) |
| 182. | Axinella damicornis (Esper, 1794) |
| 183. | Axinella rugosa (Bowerbank, 1866) |
| 184. | Axinella polypoides Schmidt, 1862 |
| 185. | Axinella verrucosa (Esper, 1794) |
| 186. | Phakellia robusta Bowerbank, 1866 |
| 187. | Phakellia ventilabrum (Linnaeus, 1767) |
| Bubaridae | |
| 188. | Bubaris carcisis Vacelet, 1969 |
| 189. | Bubaris vermiculata (Bowerbank, 1866) |
| 190. | Cerbaris curvispiculifer (Carter, 1880) |
| 191. | Monocrepidion vermiculatum Topsent, 1898 |
| Hymerhabdiidae | |
| 192. | Hymerhabdia oxytrunca Topsent, 1904 |
| 193. | Hymerhabdia typica Topsent, 1892 |
| Heteroxyidae | |
| 194. | Halicnemia geniculata Sarà, 1958 |
| 195. | Halicnemia patera Bowerbank, 1864 |
| Dictyonellidae | |
| 196. | Acanthella acuta Schmidt, 1862 |
| 197. | Dictyonella incisa (Schmidt, 1880) |
| 198. | Dictyonella marsilii (Topsent, 1893) |
| 199. | Dictyonella obtusa (Schmidt, 1862) |
| 200. | Dictyonella pelligera (Schmidt, 1862) |
| Halichondriidae | |
| 201. | Axinyssa aurantiaca (Schmidt,1864) |
| 202. | Halichondria (Halichondria) bowerbanki Burton, 1930 |
| 203. | Halichondria (Halichondria) contorta (Sarà, 1961) |
| 204. | Halichondria (Halichondria) convolvens Sarà, 1960 |
| 205. | Halichondria (Halichondria) genitrix (Schmidt, 1870) |
| 206. | Halichondria (Halichondria) panicea (Pallas, 1766) |
| 207. | Halichondria (Halichondria) semitubulosa Lieberkühn, 1859 |
| 208. | Hymeniacidon perlevis (Montagu, 1818) |
| 209. | Hymeniacidon rugosa (Schmidt, 1868) |
| 210. | Laminospongia subtilis Pulitzer-Finali, 1983 |
| 211. | Spongosorites intricatus (Topsent, 1892) |
| 212. | Spongosorites flavens Pulitzer-Finali, 1983 |
| 213. | Topsentia glabra (Topsent, 1898) |
| 214. | Topsentia vaceleti Kefalas & Castritsi–Catharios, 2012 |
| Agelasidae | |
| 215. | Agelas oroides Schmidt, 1864 |
| Callyspongiidae | |
| 216. | Callyspongia subcornea Griessinger, 1971 |
| Chalinidae | |
| 217. | Dendroxea lenis (Topsent, 1892) |
| 218. | Haliclona (Gellius) angulata (Bowerbank, 1866) |
| 219. | Haliclona (Gellius) dubia (Babic, 1922) |
| 220. | Haliclona (Gellius) flagellifer (Ridley & Dendy, 1866) |
| 221. | Haliclona (Gellius) lacazei (Topsent, 1893) |
| 222. | Haliclona (Gellius) marismedi (Pulitzer-Finali, 1978) |
| 223. | Haliclona (Gellius) tenuisigma (Sarà & Siribelli, 1960) |
| 224. | Haliclona (Halichoclona) fulva (Topsent, 1893) |
| 225. | Haliclona (Haliclona) simulans (Johnston, 1842) |
| 226. | Haliclona (Reniera) aquaeductus (Schmidt, 1862) |
| 227. | Haliclona (Reniera) citrina (Topsent, 1892) |
| 228. | Haliclona (Reniera) cratera (Schmidt, 1862) |
| 229. | Haliclona (Reniera) mediterranea Griessinger, 1971 |
| 230. | Haliclona (Rhizoniera) rosea (Bowerbank, 1866) |
| 231. | Haliclona (Rhizoniera) sarai (Pulitzer-Finali, 1969) |
| 232. | Haliclona (Soestella) arenata Griessinger, 1971 |
| 233. | Haliclona (Soestella) implexa (Schmidt, 1868) |
| 234. | Haliclona (Soestella) mamillata (Griessinger, 1971) |
| 235. | Haliclona (Soestella) mucosa (Griessinger, 1971) |
| 236. | Haliclona (Soestella) valliculata (Griessinger, 1971) |
| 237. | Haliclona elegans (Lendenfeld, 1887) |
| Phloeodictyidae | |
| 238. | Siphonodictyon coralliirubri (Calcinai et al., 2007) |
| 239. | Siphonodictyon insidiosum (Johnson, 1899) |
| 240. | Calyx nicaeensis (Risso, 1826) |
| Petrosiidae | |
| 241. | Petrosia (Petrosia) clavata (Esper, 1794) |
| 242. | Petrosia (Petrosia) ficiformis (Poiret, 1798) |
| Irciniidae | |
| 243. | Ircinia dendroides (Schmidt, 1862) |
| 244. | Ircinia oros (Schmidt, 1864) |
| 245. | Ircinia variabilis (Pallas, 1766) |
| 246. | Sarcotragus fasciculatus (Pallas, 1766) |
| 247. | Sarcotragus foetidus Schmidt, 1862 |
| 248. | Sarcotragus pipetta (Schmidt, 1868) |
| 249. | Sarcotragus spinosulus Schmidt, 1862 |
| Thorectidae | |
| 250. | Cacospongia mollior Schmidt, 1862 |
| 251. | Cacospongia scalaris Schmidt, 1862 |
| 252. | Hyrtios collectrix (Schulze, 1880) |
| 253. | Fasciospongia cavernosa (Schmidt, 1862) |
| Spongiidae | |
| 254. | Spongia (Spongia) agaricina Pallas, 1766 |
| 255. | Spongia (Spongia) nitens (Schmidt, 1862) |
| 256. | Spongia (Spongia) officinalis Linnaeus, 1759 |
| 257. | Spongia (Spongia) virgultosa (Schmidt, 1868) |
| 258. | Spongia (Spongia) zimocca Schmidt, 1862 |
| 259. | Hippospongia communis (Lamarck, 1814) |
| Dysideidae | |
| 260. | Dysidea avara (Schmidt, 1862) |
| 261. | Dysidea fragilis (Montagu, 1818) |
| 262. | Dysidea tupha (Martens, 1824) |
| 263. | Pleraplysilla spinifera (Schulze, 1879) |
| Darwinellidae | |
| 264. | Aplysilla rosea (Barrois, 1876) |
| 265. | Aplysilla sulfurea Schulze, 1878 |
| 266. | Chelonaplysilla noevus (Carter, 1876) |
| Dictyodendrillidae | |
| 267. | Spongionella gracilis (Vosmaer, 1883) |
| 268. | Spongionella pulchella (Sowerby, 1804) |
| Halisarcidae | |
| 269. | Halisarca dujardini Johnston, 1842 |
| Aplysinidae | |
| 270. | Aplysina aerophoba Nardo, 1843 |
| 271. | Aplysina cavernicola Vacelet, 1959 |
| Ianthellidae | |
| 272. | Hexadella pruvoti Topsent, 1896 |
| 273. | Hexadella racovitzai Topsent, 1896 |
This increasing is related to the difficulty of studying the organisms inhabiting the coralligenous concretions due to the complexity of the habitat, the high diversity, and the depth where these structures are located (Kipson et al. 2011). Our study, based on the collection of blocks and their sectioning into slices, allowed the identification of species that would have been otherwise completely disregarded.
Among the insinuating species observed in the coralligenous crevices we have found several species previously recorded with a massive habitus in deeper waters. Pachastrella monilifera Schmidt, 1868 and Poecillastra compressa (Bowerbank, 1866) were the species with the highest phenotypic plasticity, since they usually appear with large, fun shaped specimens, in deep habitats (Bo et al. 2012), while in the coralligenous community they live in crevices and fissures of the concretion. Our results support the idea that environments rich in microhabitats may act as shelters essential for the dispersal of many deep water species, enlarging their distribution range (Bo et al. 2011). Therefore we can emphasize the importance of the coralligenous concretion, not only as reservoir of biodiversity, but also as an important “stepping-stone” able to facilitate the dispersal of species along vertical gradients.
As to the boring sponges, Cliona janitrix is indicated by Ballesteros (2006) and Calcinai et al. (2007b) as the key species in the bio-erosive processes involving Corallium rubrum, whereas Cliona viridis has the same rolein the coralligenous matrix (Rosell et al. 1999). According to our data Cliona celata Grant, 1826, Cliona schmidtii (Ridley, 1881), Spiroxya corallophila (Calcinai, Cerrano & Bavestrello, 2002), Spiroxya heteroclita Topsent, 1896 and Siphonodictyon insidiosum (Johnson, 1899) may also be considered important in the bio erosive processes acting upon the coralligenous structure. SEM analyses showed that three other species: Jaspis johnstoni (Schmidt, 1862), Dercitus (Stoeba) plicatus (Schmidt, 1868), Samus anonymus Gray, 1867, suspected to be excavating (Carter 1880, Thomas 1973, van Soest and Hooper 2002), actually do not bore the coralligenous substratum but only occupy cavities of the porous concretion and the chambers previously excavated by boring sponges (Figs 2E–F). Cliona viridis, Jaspis johnstoni and Dercitus (Stoeba) plicatus, able to penetrate 5 cm into the substrate, are the species reaching the greatest depth inside the concretion.
Supplementary Material
References
- Babić K. (1922) Monactinellida und Tetractinellida des Adriatischen Meeres. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere 46(2): 217-302. [Google Scholar]
- Ballesteros E. (2006) Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanography and Marine Biology: An Annual Review 44: 123-195. [Google Scholar]
- Bertolino M, Calcinai B, Cerrano C. (2008) Poriferi. In: Ponti M, Mescalchin P. (Eds) Meraviglie sommerse delle Tegnue. Guida alla scoperta degli organismi marini, Editrice La Mandragora, Imola, 85-135. [Google Scholar]
- Bo M, Bertolino M, Borghini M, Castellano M, Covazzi Harriague A, Di Camillo CG, Gasparini GP, Misic C, Povero P, Pusceddu A, Schroeder K, Bavestrello G. (2011) Characteristics of the Mesophotic Megabenthic Assemblages of the Vercelli Seamount (North Tyrrhenian Sea). PLoS ONE 6(2): e16357. doi: 10.1371/journal.pone.0016357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo M, Bertolino M, Bavestrello G, Canese S, Giusti M, Angiolillo M, Pansini M, Taviani M. (2012) Role of deep sponge grounds in the Mediterranean Sea: a case study in southern Italy. Hydrobiologia 687: 163-177. [Google Scholar]
- Boury-Esnault N. (1971) Spongiaires de la zone rocheuse littorale de Banyuls-sur-Mer. I. Ecologie et répartition. Vie et Milieu 22(1): 159-191. [Google Scholar]
- Bowerbank JS. (1866) A Monograph of the British Spongiadae. Ray Society, London, 2(i-xx): 1–388. [Google Scholar]
- Bowerbank JS. (1874) A Monograph of the British Spongiadae. Volume 3. Ray Society, London, 3(i-xvii): 1–367. [Google Scholar]
- Calcinai B, Cerrano C, Bavestrello G, Sarà M. (2000) Boring sponges (Porifera, Demospongiae) from the Indian Ocean. Italian Journal of Zoology 67: 203-219. doi: 10.1080/11250000009356314 [DOI] [Google Scholar]
- Calcinai B, Bavestrello G, Cerrano C. (2005) Excavating Sponge Species from the Indo-Pacific Ocean. Zoological Studies 44(1): 5-18. [Google Scholar]
- Calcinai B, Bavestrello G, Bertolino M. (2007a) La comunità endolitica a poriferi del concrezionato coralligeno. Biologia Marina Mediterranea 14: 152-153. [Google Scholar]
- Calcinai B, Cerrano C, Bavestrello G. (2007b) Three new species and one re-description of Aka. Journal of the Marine Biological Association of the United Kingdom 87(6): 1355-1365. doi: 10.1017/S0025315407058377 [DOI] [Google Scholar]
- Calcinai B, Azzini F, Bavestrello G, Gaggero L, Cerrano C. (2007c) Excavating rates and boring pattern of Cliona albimarginata (Porifera, Clionaidae) in different substrata. In: Custódio MR, Hajdu E, Lôbo-Hajdu G, Muricy G. (Eds) Porifera Research: Biodiversity, Innovation & Sustainability, 203–210.
- Calcinai B, Bertolino M, Bavestrello G, Montori S, Mori M, Pica D, Cerrano C. (in prep) Assessment of the diversity and biomass of the coralligenous cryptic sponges.
- Carballo JL. (1994) Taxonomía, zoogeografía y autoecología delos Poríferos del Estrecho de Gibraltar. Universidad de Sevilla, PhD Thesis: 1–331. [Google Scholar]
- Carter HJ. (1880) Report on Specimens dredged up from the Gulf of Manaar and presented to the Liverpool Free Museum by Capt. W.H. Cawne Warren. Annals and Magazine of Natural History (5) 6 (31): 35–61; 129–156. [Google Scholar]
- Cerrano C, Bavestrello G, Bianchi CN, Calcinai B, Cattaneo-Vietti R, Morri C, Sarà M. (2001) The role of sponge bioerosion in the Mediterranean coralligenous accretion. In: Faranda FM, Guglielmo L, Spezie G. (Eds) Mediterranean Ecosystems: Structures and Processes. Springer-Verlag, Milano, 235-240. doi: 10.1007/978-88-470-2105-1_30 [DOI] [Google Scholar]
- Corriero G, Abbiati M, Santangelo G. (1997) Sponges inhabiting a Mediterranean red coral population. Marine Ecology 18(2): 147-155. doi: 10.1111/j.1439-0485.1997.tb00433.x [DOI] [Google Scholar]
- Cristobo FJ. (1996) Esponjas del order Poecilosclerida (Porifera, Demospongiae) de la Ria del Ferrol (NW. de España). Universidade de Santiago de Compostela; PhD Thesis: 1–571. [Google Scholar]
- Hong JS. (1980) Étude faunistique d’un fond de concrètionnement de type coralligène soumis à un gradient de pollution en Méditerranée nord-occidentale (Golfe de Fos). Thèse de Doctorat. Université d’Aix-Marseille II. [Google Scholar]
- Hong JS. (1982) Contribution à l’étude des peuplements d’un fond coralligène dans la region marseillaise en Méditerranée nord.occidentale. Bullettin of Korea Ocean Research and Development Institut 4: 27-51. [Google Scholar]
- Hooper JNA, van Soest RWM. (Eds) (2002) Systema Porifera: a guide to the classification of Sponges. Vol. 1 Kluwer Academic/Plenum Publishers, New York, 1101 pp. doi: 10.1007/978-1-4615-0747-5 [DOI] [Google Scholar]
- Kefalas E, Tsirtsis G, Castritsi-Catharios I. (2003) Distribution and ecology of Demospongiae from the circalittoral of the islands of the Aegean Sea (Eastern Mediterranean). Hydrobiologia 499: 125-134. doi: 10.1023/A:1026343113345 [DOI] [Google Scholar]
- Kipson S, Fourt M, Teixidó N, Cebrian E, Casas E, Ballesteros E, Zabala M, Garrabou J. (2011) Rapid Biodiversity Assessment and Monitoring Method for Highly Diverse Benthic Communities: A Case Study of Mediterranean Coralligenous Outcrops. PLoS ONE 6(11): e27103. doi: 10.1371/journal.pone.0027103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubier L. (1966) Le coralligène des Albères: monographie biocènotique. Annales de l’Institut Ocèanographique de Monaco 43: 139-316. [Google Scholar]
- Lévi C. (1956) Spongiaires de la région de Dakar. Bulletin de l’Institut français d’Afrique noire (A, Sciences naturelles) 18(2): 391-405. [Google Scholar]
- Melone N. (1965) Poriferi associati a Corallium rubrum (L.) della Sardegna. Annali del Museo Civico di Storia Naturale Giacomo Doria 75: 343-358. [Google Scholar]
- Mustapha KB, Zarrouk S, Souissi A, El Abed A. (2003) Diversité Des Desmosponges Tunisiennes. Bulletin de l’Institut National des Sciences et Technologies de la Mer de Salammbô. Vol. 30. [Google Scholar]
- Pansini M, Longo C. (2003) A review of the Mediterranean Sea sponge biogeography with, in appendix, a list of the demosponges hitherto recorded from this sea. Biogeographia, Marine biogeography of the Mediterranea Sea: patterns and dynamics of biodiversity 24: 59-90. [Google Scholar]
- Pansini M, Longo C. (2008) Porifera. In: Relini G. (Ed) Checklist della flora e della fauna dei mari italiani. Ministero dell’Ambiente e tutela del territorio, Roma. Biologia Marina Mediterranea 15(1): 42-66.
- Pansini M, Pronzato R. (1973) Il coralligeno di Bogliasco ed il suo popolamento di Poriferi. Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova 41: 5-34. [Google Scholar]
- Pérès J, Picard JM. (1958) Recherches sur les peuplements benthiques de la Méditerranée nord-orientale. Annales de l’Institut Océanographique de Monaco 34: 213-291. [Google Scholar]
- Pérès J, Picard JM. (1964) Nouveau manuel de bionomie benthique de la mer Méditerranée. Recueil Travaux Station Marine Endoume 31(47): 1-131. [Google Scholar]
- Pouliquen L. (1972) Les spongiaires des grottes sous-marines de la région de Marseille: Ecologie et systématique. Téthys 3(4): 717-758. [Google Scholar]
- Pulitzer-Finali G. (1978. (1977)) Report on a Collection of Sponges from the Bay of Naples. III Hadromerida, Axinellida, Poecilosclerida, Halichondrida, Haplosclerida. Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova 45: 7-89. [Google Scholar]
- Pulitzer-Finali G. (1983) A collection of Mediterranean Demospongiae (Porifera) with, in appendix, a list of the Demospongiae hitherto recorded from the Mediterranean Sea. Annali del Museo civico di storia naturale Giacomo Doria 84: 445-621. [Google Scholar]
- Romdhane N. (2003) Enclave coralligène de l’infrallitoral de Korbous: Aire minimale qualitative et inventaires spécifiques. Diplôme Etude Approfondie; Université de Carthage, Institut National d’Agronomie de Tunis, 78 pp. [Google Scholar]
- Ros J, Olivella I, Gili JM. (1984) Els sistemes naturals de les Illes Medes. Arxius Secció Ciències. Vol. 73 Institut d'Estudis Catalans Barcelona, 828 pp. [Google Scholar]
- Ros J, Romero J, Ballesteros E, Gili JM. (1985) Diving in the blue water: the benthos. In: Margalef R. (Ed) Western Mediterranean. Pergamon, Oxford, 233-295. [Google Scholar]
- Rosell D, Uriz MJ, Martin D. (1999) Infestation by excavating sponges on the oyster (Ostrea edulis) populations of the Blanes littoral zone (North-western Mediterranean Sea). Journal of the Marine Biological Association of the United Kingdom 79: 409-413. doi: 10.1017/S0025315498000526 [DOI] [Google Scholar]
- Rützler K. (1978) Sponges in coral reefs. In: Stoddart DR, Johannes RE. (Eds) Coral Reefs: Research Methods, Monographs on Oceanographic Methodology. UNESCO, Paris, 5: 299–313. [Google Scholar]
- Sarà M. (1958) Studio sui Poriferi di una grotta di marea del Golfo di Napoli. Archivio Zoologico Italiano 43: 203-281. [Google Scholar]
- Sarà M. (1960) Poriferi del litorale dell’isola d’Ischia e loro ripartizione per ambienti. Pubblicazioni della Stazione Zoologica di Napoli 31: 421-472. [Google Scholar]
- Sarà M. (1968) Un coralligeno di piattaforma (coralligène de plateau) lungo il litorale pugliese. Archivi di Oceanografia e Limnologia 15 (Suppl.): 139–150. [Google Scholar]
- Sarà M. (1969) Specie nuove di Demospongiae provenienti dal coralligeno pugliese. Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova 37(255): 89-96. [Google Scholar]
- Sarà M, Siribelli L. (1960) La fauna di Poriferi delle ‘secche’ del Golfo di Napoli. 1. La ‘secca’ della Gaiola. Annuario dell’Istituto e Museo di Zoologia dell’Università di Napoli 12(3): 1-93. [Google Scholar]
- Sarà M, Siribelli L. (1962) La fauna di Poriferi delle ‘secche’ del golfo di Napoli. II. La secca di Benda Palummo. Annuario dell’Istituto e Museo di Zoologia dell’Università di Napoli 14(2): 1-62. [Google Scholar]
- Sarà M, Balduzzi A, Boero F, Pansini M, Pessani D, Pronzato R. (1978) Analisi di un popolamento bentonico di falesia del Promontorio di Portofino: dati preliminari. Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova 46: 119-137. [Google Scholar]
- Van Soest RWM, Stone SM. (1986) Antho brattegardi sp. n. (Porifera: Poecilosclerida), with remarks on and a key to the clathriids of Norwegian waters. Sarsia 71(1): 41-48. [Google Scholar]
- Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, Voogd NJ de, Glasby B Alvarez de, Hajdu E, Pisera AB, Manconi R, Schoenberg C, Janussen D, Tabachnick KR, Klautau M, Picton B, Kelly M, Vacelet J. (2013) World Porifera Database. http://www.marinespecies.org/porifera
- Van Soest RWM, Hooper JNA. (2002) Family Samidae Sollas, 1888. In: Hooper JNA, Van Soest RWM. (Eds) Systema Porifera: a guide to the classification of sponges. Vol. 1 Kluwer Academic/Plenum Publishers, New York, 99-101. doi: 10.1007/978-1-4615-0747-5_9 [DOI] [Google Scholar]
- Thomas PA. (1973) Marine demospongiae of Mahe Island in the Seychelles Bank. Annales du Musée royal de l’Afrique centrale. Sciences zoologiques (203): 1–96.
- Topsent E. (1904) Spongiaires des Açores. Résultats des campagnes scientifiques accomplies par le Prince Albert I. Monaco 25: 1-280. [Google Scholar]
- Topsent E. (1928) Spongiaires de l’Atlantique et de la Méditerranée provenant des croisières du Prince Albert ler de Monaco. Résultats des campagnes scientifiques accomplies par le Prince Albert I. Monaco 74: 1-376. [Google Scholar]
- Topsent E. (1932) Notes sur des clionides. Archives de Zoologie expérimentale et générale 74(28): 549-579. [Google Scholar]
- Topsent E. (1936) Eponges observées dans les parages de Monaco. (Deuxième partie). Bulletin de l’Institut océanographique, Monaco 686: 1-70. [Google Scholar]
- Vacelet J. (1969) Eponges de la Roche du Large et de l’étage bathyal de Méditerranée (Récoltes de la soucoupe plongeante Cousteau et dragages). Mémoires du Muséum national d’Histoire naturelle (A, Zoologie) 59(2): 145-219. [Google Scholar]
- Voultsiadou E. (2009) Reevaluating sponge diversity and distribution in the Mediterranean Sea. Hydrobiologia 628: 1-12. doi: 10.1007/s10750-009-9725-9 [DOI] [Google Scholar]
- Voultsiadou E, Vafidis D. (2004) Rare sponge (Porifera: Demospongiae) species from the Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 84(3): 593–598. doi: 10.1017/S0025315404009592h [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.