Abstract
Functional neuroimaging has provided new tools to study cerebral gait control in Parkinson disease (PD). First, imaging of blood flow functions has identified a supraspinal locomotor network that includes the (frontal) cortex, basal ganglia, brainstem tegmentum and the cerebellum. These studies emphasize also the cognitive and attentional dependency of gait in PD. Furthermore, gait in PD and related syndromes like progressive supranuclear palsy may be associated with dysfunction of the indirect, modulatory prefrontal–subthalamic–pedunculopontine loop of locomotor control. The direct, stereotyped locomotor loop from the primary motor cortex to the spinal cord with rhythmic cerebellar input appears preserved and may contribute to the unflexible gait pattern in parkinsonian gait. Second, neurotransmitter and proteinopathy imaging studies are beginning to unravel novel mechanisms of parkinsonian gait and postural disturbances. Dopamine displacement imaging studies have shown evidence for a mesofrontal dopaminergic shift from a depleted striatum in parkinsonian gait. This may place additional burden on other brain systems mediating attention functions to perform previously automatic motor tasks. For example, our preliminary cholinergic imaging studies suggest significant slowing of gait speed when additional forebrain cholinergic denervation occurs in PD. Cholinergic denervation of the pedunculopontine nucleus and its thalamic projections have been associated with falls and impaired postural control. Deposition of β-amyloid may represent another non-dopaminergic correlate of gait disturbance in PD. These findings illustrate the emergence of dopamine non-responsive gait problems to reflect the transition from a predominantly hypodopaminergic disorder to a multisystem neurodegenerative disorder involving non-dopaminergic locomotor network structures and pathologies.
Keywords: acetylcholine, amyloid, cerebellum, dopamine, gait, MRI, network, SPECT, Parkinson disease, pedunculopontine nucleus, PET, progressive supranuclear palsy
Introduction
Gait and postural dysfunction presents early in Parkinson disease (PD) 1, is a significant cause of disability, and responds poorly to dopaminergic replacement except in the early phase of the disease 2. Clinical characteristics of parkinsonian locomotor patterns include a slow and small stepped gait with reduced angular excursion of the joints, e.g. shoulder, knee and trunk joints 3, 4. PD affects “complex” gait activities, such as gait initiation, braking, and turning which need modulation of the stereotyped (spinal) gait pattern. Akinesia, defined as inability to initiate movement or sustain movement (e.g., “sudden freezes”) is considered by some to be the fifth cardinal feature of PD 5. Freezing of gait (FOG), usually manifested as abrupt cessation of leg movement during walking, is a common cause of falls. Sudden freezes may be related to altered cortical regulation of movement execution, together with progressive impairment of mesencephalic locomotor center function 6. The levodopa resistance of parkinsonian gait disturbances has been proposed to result from the extension of the degenerative process to non-dopaminergic structures 7. We will review first imaging studies of locomotor network functions in PD followed by neurochemical imaging studies that may underlie such network changes. We will discuss also directions for future neuroimaging research to better understand mechanisms of parkinsonian gait disturbances. Specifically, we will emphasize the need for future research based on correlation of imaging studies with improved and quantitative gait analysis assessments.
Imaging methods and human mobility functions
Positron emission tomography (PET) and single photon emission computed tomography (SPECT) are molecular imaging techniques that use radiolabeled molecules to image molecular interactions of biological processes in vivo, such as binding of neuroreceptors, metabolism by cerebral enzymes or regional cerebral blood flow (rCBF). The first in vivo human imaging studies of locomotion were performed using a radiotracer rCBF SPECT technique 8, 9. Similar to rCBF [15O]H2O PET or regional cerebral glucose metabolic [18F]fluorodeoxyglucose (FDG) PET, these techniques suffer from poor temporal resolution and radiation exposure. The desired characteristics of a neuroimaging technique to study mobility include: high spatial resolution; high temporal resolution; whole brain (cortex/subcortical structures) imaging; imaging of actual gait or mobility functions; non-invasive; portable; cheap; no side-effects, and compatible with implanted neurostimulators (Table 1).
Table 1.
Desired characteristics of imaging modalities to study mobility.
| fMRI | rCBF SPECT & FDG PET | [15O]H2O PET | Neurotrans-mitter PET or SPECT | fNIRS | MEG/EEG | |
|---|---|---|---|---|---|---|
| Spatial resolution | +++ | ++ | + | ++ | + | ++++ |
| Temporal resolution | +++ | +/− | + | +/− | +++ | ++++ |
| Subcortical definition | ++++ | +++ | +++ | +++ | − | ++ |
| Actual gait | − | ++ | − | + | ++++ | + (EEG) |
| Non-reactive | + | − | − | − | + | + |
| Portable | − | − | − | − | + | + (EEG) |
However, no imaging modality technique meets all these preferred criteria at the present time. With the exception of functional Near InfraRed Spectroscopy (fNIRS) and electroencephalography (EEG), SPECT, PET, magnetic resonance imaging (MRI) and magnetoencephalography (MEG) cameras are not portable. The use of functional MRI (fMRI) has allowed unique insights into brain network changes underlying mobility. Functional MRI is at present the only method that allows investigation of whole brain activity and different gait conditions (e.g., gait initiation, turning, obstacle avoidance) in the same experiment. However, fMRI requires that the subject does not move the head during data acquisition. To overcome this problem different paradigms have been developed to record cerebral blood oxygen level dependent (BOLD) signals as an equivalent for brain activity during motor planning, during repetitive foot and leg movements, and during imagery of the motor function (MI). The latter has been proven to be a very promising approach (see Maillet et al. for an in-depth review of this topic 10). It is well known that neuronal networks show a substantial overlap between execution and imagery of a task. That is also a reason why MI is broadly used for training motor skills in sports and for relearning motor skills during rehabilitation. Miyai et al. compared treadmill walking (fNIRS) and MI (fMRI) and showed overlapping activity in the medial primary motor cortex (M1) and SMA 11. Recently, the locomotor network was found to be very similar between actual walking (FDG-PET) and MI (fMRI) 12. Further, the network is specifically activated during imagery of active walking, but not imagery of passive transfer or observing a second person’s walking 13. Based on these and other findings MI is increasingly used to study human locomotion.
Imaging and mobility: Network correlates
The spinal pattern generators (CPG) that provide the basic stepping pattern interact with sensory feedback and supraspinal structures to ensure flexible mobility. In analogy to cat electrophysiology and supported by human imaging data (Figure 1) one can assume that prefrontal cortical areas are important for gait initiation and convey the locomotor signals via basal ganglia to brainstem locomotor regions 14–16. The subthalamic locomotor region (SLR) in the lateral hypothalamic area and the mesencephalic locomotor region (MLR), corresponding to the cuneiform and pedunculopontine nuclei in the dorsal midbrain, are disinhibited from tonic basal ganglia control for gait initiation 17. The cerebellar locomotor region (CLR), located close to the fastigial nuclei in the cerebellar midline, receives rhythmic input from the vermis and paravermal cerebellar cortex to control gait speed and variability 18. The CLR output converges with descending MLR projections in the pontine brainstem where locomotor signals are transmitted to the spinal cord CPGs. The cerebellar vermis is thought to integrate proprioceptive, vestibular, and visual afferent information into the locomotor program. It has been postulated anatomically and physiologically that the pallidum has mutual connections to the MLR 19. The frontal cortices also have connections with the cerebellum via the thalamus and pontine nuclei and with the basal ganglia via the basal ganglia-thalamocortical circuit 20.
Figure 1.
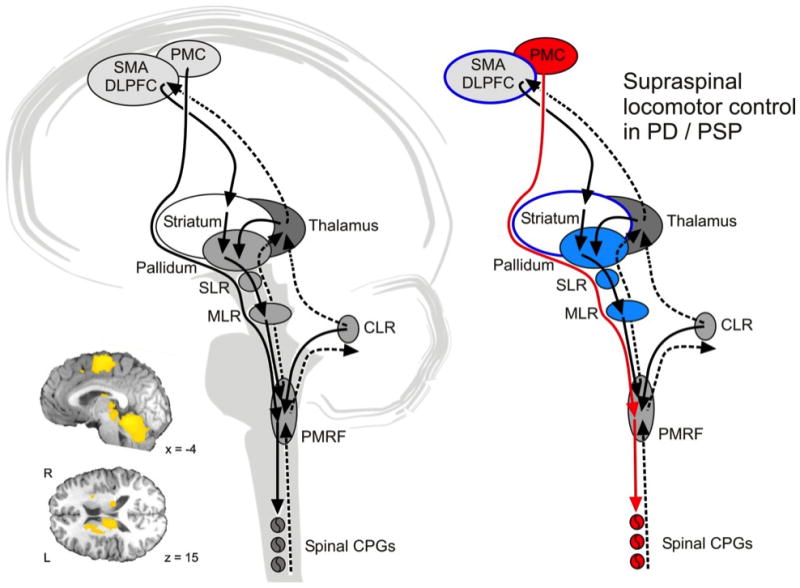
Schema on supraspinal locomotor control in humans. The left panel shows the presumed network in healthy subject as derived from animal electrophysiology and human functional imaging. The indirect pathway from frontal cortex via basal ganglia to the brainstem locomotor centers allows modulation of the gait pattern in response to external demands. The direct pathway from motor cortex to the spinal cord can bypass the brainstem centers during undisturbed locomotion. Rhythmic input from the cerebellum conveys with both pathways in the brainstem tegmentum. The insert shows BOLD signal increases during mental imagery of locomotion13. The right panel illustrates network changes in PD. The direct pathway (depicted in red) compensates for deficiencies in the indirect pathways but does not allow adequate adaptation of gait to environmental demands. Abbreviations: CLR: cerebellar locomotor region; CPG: central pattern generator; DLPFC: dorsolateral prefrontal cortex; MLR: mesencephalic locomotor region; PMRF, pontomedullary reticular formation; PMC: primary motor cortex; SLR: subthalamic locomotor region; SMA: supplementary motor area. Figure adapted from 12, 16, 24.
Imaging studies on parkinsonian gait
Using rCBF SPECT and treadmill walking, PD patients showed less activity in the left medial frontal lobe, right precuneus, and left cerebellar hemisphere whereas activity in the cerebellar midline, right insula, left temporal and cingulated gyri was increased compared to controls 21, 22. It is plausible that the increased activity in the insula may reflect increased sensory control and the increased flow in cerebellar midline may compensate for frontal deficits in parkinsonian gait. FDG-PET at rest has been recently used to assess the effect of a gait rehabilitation program in PD 23. Before therapy, patients showed hypometabolism in the right parietal lobe, temporal lobes, and left frontal lobe. Hypermetabolism was detected in the left cerebellum. After therapy, metabolism increased in the right cerebellum, right parietal lobes, and temporal lobes, showing an effect of the therapy both on physical performance and on supraspinal locomotor control. In a recent FDG-PET study on progressive supranuclear palsy (PSP) patients we found dysfunction of the indirect, modulatory prefrontal–subthalamic–pedunculopontine loop of locomotor control. The direct, stereotyped locomotor loop from the primary motor cortex to the spinal cord with rhythmic cerebellar drive showed increased activity. This may reflect a compensatory mechanism that may also contribute to the stereotyped gait pattern in PSP 24. Results of a recent fMRI study call for a similar interpretation: PD patients showed signal increases in the right dorsal premotor area, precentral, right inferior parietal lobule, and bilateral precuneus while imagining stepping over obstacles 25.
Imaging studies of freezing of gait
FOG is an extremely debilitating symptom of parkinsonian disorders, with an unknown pathophysiological mechanism. There have been several studies that tried to localize the altered cerebral activity associated with FOG (see table 2 for overview and see Bartels et al. for review 26). These findings emphasize disruption of cortical, in particular orbitofrontal and parietal, functions in patients with FOG who also tend to have to have more severe caudate nucleus dopaminergic denervation 26. The involvement of typical non-motor cortical areas may emphasize the cognitive and attentional dependency of gait in PD. However, a pitfall of these studies is that confounding effects of comorbid cognitive and mood changes in27 patients with FOG cannot be excluded.
Table 2.
Radiotracer imaging studies in PD patients with gait difficulties, including freezing of gait (FOG).
| Imaging technique | Patient populations | Findings |
|---|---|---|
| rCBF [133Xe]-SPECT5 | PD with FOG and atypical parkinsonisms | Frontal hypoperfusion only seen in patients with progressive supranuclear palsy but not in PD with FOG using imaging technique with limited spatial resolution. |
| rCBF [99mTc]-HMPAO SPECT22 | PD vs control subject walking on treadmill | Gait disturbance in PD was associated with underactivity in the medial motor area and the cerebellar hemisphere, together with overactivity in the cerebellar vermis. |
| rCBF [123I]-IMP SPECT68 | PD with and without FOG | Decreased flow in orbitofrontal cortex (Brodmann area 11) in PD with FOG |
| rCBF [123I]-IMP SPECT69 | PD subgroups with and without severe gait disturbances | Hypoperfusion of the lateral frontal and temporal association cortex and the medial frontal gyrus in PD subgroup with severe gait disturbances. |
| FDOPA and FDG PET70 | PD with and without FOG | Reduced caudate nucleus FDOPA and FDG activity and reduced right parietal cortical FDG activity in PD with FOG |
| rCBF [123I]-IMP SPECT71 | PD with and without FOG | Decreased flow in bilateral Brodmann areas 10 and 11 and left 32 in PD with FOG |
A recent resting state fMRI brain connectivity study identified reduced connectivity in right cortical fronto-parietal “executive-attention” and right occipitotemporal “visual” networks in PD with FOG suggesting a role of network connectivity disruption 28. Furthermore, atrophy of frontal and parietal gray matter occurs in PD patients with FOG 29. FOG in PD seems to reflect executive dysfunction and perception deficits corresponding to changes in frontal and parietal cortices. In accordance with these studies and despite the limitation of studying FOG with MI, a recent fMRI study showed hypoactivation of frontal and parietal cortex with hyperactivation of the MLR pointing to a possible subcortical compensation of the cortical deficit in parkinsonian FOG 6. A fMRI study of patients with PD with known FOG during a timed ‘up and go’ task using a virtual reality gait paradigm provides evidence of dysfunction across coordinated neural networks, including the caudate nucleus, globus pallidus pars interna, thalamus, and MLR 27. In addition, patients with FOG may be unable to recruit specific cortical and subcortical region during the performance of simultaneous motor and cognitive functions 30. These findings suggest that the pathophysiology of freezing involves context-dependent dysfunction across multiple levels of the locomotor system, including cortical, subcortical and brainstem regions 31, 32.
Deep brain stimulation activation studies and gait in PD
Deep brain stimulation (DBS) has become a routine treatment modality in advanced PD. The effect of subthalamic nucleus (STN) DBS on gait and balance vary in PD and the underlying mechanisms remain unclear 33. Hill and colleagues compared effects of dorsal versus ventral STN regions on gait functions in PD using [15O]H2O PET in a within-subject design 33. These authors found differential correlations with gait velocity and premotor cortex rCBF changes with ventral STN DBS whereas dorsal STN DBS produced similar changes in the anterior cerebellum. These findings suggest that effects of STN DBS on gait may be mediated by different circuits depending on the site of STN region stimulation through basal ganglia-thalamo-cortical versus cerebellar-thalamo-cortical circuits 34. These findings illustrate also complementary roles of basal ganglia and cerebellum in motor control.
Evidence that PPN degeneration occurs in PD and the important role of the PPN in gait and postural stability, coupled with the fact that stimulation of the PPN in animal models increases locomotor activity 35–37 led to interest in PPN stimulation for gait and postural dysfunction in PD 38, 39. A [15O]H2O PET activation study found that PPN DBS was associated with rCBF increases in the thalamus, cerebellum, midbrain region, and cortical areas involved in balance and motor control 40.
Neurotransmitter imaging studies and gait and postural functions in PD
The basal ganglia and the neurotransmitter dopamine have been key targets for research exploring the pathophysiology underlying movement disorders. The extent of nigrostriatal dopaminergic denervation can be quantified in PD using PET or SPECT techniques using DOPA decarboxylase, dopamine transporter (DAT), and vesicular monoamine transporter ligands. These studies have shown that striatal dopamine deficiency is most closely correlated with bradykinesia 41. Ouchi and colleagues reported on changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in PD 42. These investigators used DAT PET imaging before and after 1 hour of strenuous walking in PD and control subjects and found that uptake in the striatum (specifically the putamen) was decreased by gait to a greater extent in normal subjects, whereas a significant reduction in DAT uptake was no longer found in the already denervated putamen but occurred in the caudate nucleus and orbitofrontal cortex in PD patients. This shifted activity to predominant non-motor structures of the anterior striatum and the mesocortical dopaminergic system may represent a key element of the pathophysiology of parkinsonian gait.
Evidence is accumulating that degeneration of both major cholinergic projection systems - the brainstem PPN and the basal forebrain corticopetal complex - is a major contributor to PD gait and postural dysfunction 43, 44.
We found that PPN-thalamic cholinergic innervation is reduced more severely in PD fallers compared to non-fallers 44, 45 as confirmed by post-mortem findings 46. These results are consistent with PPN degeneration as a cause of impaired postural control in PD (Table 3). We have preliminary data that forebrain cholinergic degeneration is associated with slower gait speed in patients with PD, likely reflecting degradation of attentional capacities 47. Gait speed is not significantly slower than normal in subjects with PD with predominant nigrostriatal dopaminergic denervation. These data suggest that forebrain cholinergic denervation is a more robust marker of slowing of gait in PD than nigrostriatal denervation alone and may reflect failing cognitive processing abilities during ambulation.
Table 3.
Neurotransmitter imaging studies in PD patients with gait difficulties or history of falls.
| Imaging technique | Patient populations | Findings |
|---|---|---|
| AChE [11C]-PMP PET44, 45 | PD fallers versus non faller | Decreased cholinergic PPN-thalamic activity in PD fallers without difference in striatal dopamine loss. |
| AChE [11C]-PMP PET72 | PD and atypical parkinsonian syndromes | Greater severity of gait and balance difficulties correlated with more severe cholinergic losses in the brainstem and cerebellum. |
| AChE [11C]-PMP PET47 | PD and control subjects | Gait speed is not significantly slower than normal in subjects with PD with predominant nigrostriatal dopaminergic denervation but significantly slower in the PD subgroup with forebrain cholinopathy. |
Proteinopathy imaging and gait disturbances in PD: amyloidopathy
The Sydney Multicenter Study of PD showed that longer duration of disease is increasingly accompanied by post-mortem evidence of comorbid Alzheimer pathology, especially after 5 years 48. This time span agrees with the epidemiological studies indicating increasing dopamine unresponsiveness of (axial) motor symptoms in PD 49. We recently reported on the relationship between postural instability and gait difficulty (PIGD) feature severity and neocortical β-amyloid burden in PD patients at risk for development of dementia using [11C]-PiB PET 50. We found that increased PIGD feature severity was significantly associated with increased neocortical β-amyloid burden after controlling for effects of possible confounding variables such as the degree of striatal dopaminergic denervation, age, and the degree of cognitive capacity impairment. These results are further substantiated by our preliminary finding of an association between higher neocortical β-amyloid burden and cadence (steps per minute). In contrast, there was no association between cadence and the degree of striatal dopaminergic denervation. It is noteworthy that the inability to control cadence is associated with FOG in PD 51. These findings suggest that even low levels of comorbid neocortical amyloidopathy may significantly exacerbate gait impairments in PD.
Discussion
Neuroimaging studies of network functions provide support for a proposed model where a cortico-striatal loop of motor control involves functions of volition, cognition and attention. In contrast, a subcortical-brainstem system seems to be required for the automatic regulation and modulation of muscle tone and rhythmic limb movements 24, 52. Studies of FOG in PD emphasize disruption of cortical, in particular orbitofrontal and parietal, functions related to this complex gait disorder. Imaging studies also identify the PPN and its thalamic and cerebellar connections as key network changes important for rhythmic functions of gait and postural control 53.
Gait and balance impairments in PD result probably from an intricate interplay of multi-system degenerations and neurotransmitter deficiencies. The hypothesis of a progressive extension of the degenerative process to non-dopaminergic structures controlling locomotion has been put forward and deficiencies in other neurotransmission systems, involving acetylcholine, serotonin and norepinephrine have also been evoked 10. PET imaging studies have shown novel evidence of extra-striatal non-dopaminergic mechanism underlying gait and postural disturbances in PD: cholinergic denervation and cortical β-amyloid deposition. Therefore, the emergence of dopamine non-responsive gait and postural problems may reflect the transition from a predominantly hypodopaminergic disorder to a multisystem neurodegenerative disorder involving cholinergic and other neurotransmitter projections. It is conceivable that cortical amyloid pathology may exert a disruptive effect on intrinsic cortical functions of motor control or disrupt important subcortical (basal ganglia, thalamic and cerebellar) to cortical connections. Although serotoninergic denervation can be prominent in PD, at least in the forebrain, as shown by serotonin transporter [11C]DASB PET imaging 54, there have been no published reports of a relationship between gait and serotoninergic imaging studies. However, it is of interest to note that greater serotoninergic denervation has been associated with greater β-amyloid deposition in PD, in particular the striatum 55.
Future directions
Need for improved understanding of the role of the cerebellum in parkinsonian gait
The cerebellum receives massive real-time sensory input. Proprioceptive sensory information is utilized not only to regulate ongoing movements, but also to maintain stable standing posture 52. The cerebellar locomotor region, which is thought to regulate speed and gives rhythmical impulses to the brainstem and spinal cord 56, remains a largely unexplored area of research in parkinsonian gait. Functional MRI studies have shown dynamic changes in cerebellar thalamocortical motor circuitry with increased cerebellar recruitment during disease progression 57. This is in keeping with volumetric MRI findings of cerebellar atrophy that are only seen in older PD patients 58. Studies in the monkey have shown that beside the well-established cerebellothalamic projection, there is also a projection from the deep cerebellar nuclei to the PPN (cerebellotegmental projection) 59. The recent development of a novel vesicular acetylcholine transporter (VAChT) PET ligand, [18F]fluoroethoxybenzovesamicol (FEOBV), provides an unprecedented opportunity to quantify cholinergic terminals, particularly PPN projections to the cerebellum. Our early observation of dense cerebellar vermis VAChT expression in our preliminary PET studies (Figure 2) implicates an important cholinergic modulation of cerebellar control of posture. The use of VAChT may provide a novel tool to explore cholinergic cerebellar functions of PD mobility changes.
Figure 2.

Normal biodistribution of vesicular acetylcholine transporters using the [18F]-FEOBV ligand. More prominent uptake is seen in areas important for attention and sensorimotor locomotor functions. Abbreviations: 1o SM Cortex= primary sensorimotor cortex; PPN/LDTN= pedunculopontine nucleus-laterodorsal tegmental complex.
Need for improved understanding of the role of noradrenergic denervation in parkinsonian gait
Noradrenergic pathways have been implicated in alertness and other cortical attention functions important for gait control in PD 60. The locus ceruleus is a small nucleus located in the pontine tegmentum and is the main source of norepinephrine for the brain and spinal cord 61. There is significant degeneration of the locus ceruleus in PD 62. We have preliminary findings that cardiac post-ganglionic sympathetic denervation correlates with gait velocity in PD independently from the degree of nigrostriatal denervation 63. However, it remains uncertain whether cardiac sympathetic denervation can be taken as a proxy for central noradrenergic degeneration in PD. Future research using novel ligands that allow direct quantitative assessment of central noradrenergic activity is needed 64.
Need to correlate imaging findings to neurophysiology of gait
Advanced MRI techniques are providing optimal spatial resolution. Ultra-high-field scanners (7.0 T or greater) and quantitative MRI techniques, including diffusion tensor MRI and susceptibility-weighted imaging, hold substantial promise for an accurate quantification of tissue injury in involved brain areas. Combined with sophisticated post-processing, such as voxel-wise mapping and tractography, these techniques are contributing to the characterization of in vivo pathologic substrates of the clinical manifestations of PD 65. What is lacking so far is a good correlation of the imaging findings to parameters like gait variability, dual task cost, or frequency of falls based on quantitative gait and postural assessments. Studies that combine imaging and quantitative gait assessment will help to elucidate how the brain network interacts with gait and postural control.
Need to correlate selective stimulation protocols in deep brain stimulation to more accurately predict connectivity patterns and interaction of specific centers in the supraspinal locomotor network of parkinsonian gait
Selective stimulation of implanted DBS targets combined with rCBF imaging can provide an unique experiment of nature to better characterize the connectivity patterns of the various locomotor centers underlying parkinsonian gait. For example, the recent study by Hill and colleague provide early evidence that effects of STN DBS on gait may be mediated by different circuits through basal ganglia-thalamo-cortical versus cerebellar-thalamo-cortical circuits 33, 34. Recordings of local field potential from implanted electrodes can provide additional important physiological information about gait parameters at key locomotor areas, such as the PPN 66.
Need of standardized paradigms for functional imaging in PD
Emerging areas for future development include the development of new fMRI motor imagery paradigms, use of portable devices (fNIRS, EEG), and the application of multimodal imaging (EEG-fMRI, PET-fMRI). However, it will be crucial to define standards to find the best method for a given research question. fMRI remains the best method for depicting subcortical structures and for repetitive measurements. EEG has an excellent temporal resolution and will make it possible to investigate changes in brain activity during the gait cycle. A recent study by Handojoseno et al. successfully demonstrated the feasibility surface EEG and quantitative analysis for early detection of FOG in PD that may electrically appear 5 seconds before the clinical symptoms 67. FDG-PET is well suited for cognitively impaired patients as it does not require the same amount of cooperation as fMRI with MI of gait.
Acknowledgments
The authors thank their teams for the excellent contributions they made to the topic of this review, in particular Andreas Zwergal and Roger Albin. The authors gratefully acknowledge research support from the NIH–National Institute of Neurological Disorders and Stroke, German Research Foundation, German Federal Ministry of Education and Research, Department of Veterans Affairs and the Michael J. Fox Foundation.
Abbreviations
- BOLD
blood oxygen level dependent
- fMRI
functional magnetic resonance imaging
- fNIRS
functional Near InfraRed Spectroscopy
- FOG
freezing of gait
- MEG
magnetoencephalography
- MI
motor imagery
- PD
Parkinson disease
- PET
positron emission tomography
- PPN
pedunculopontine nucleus
- PSP
progressive supranuclear palsy
- SPECT
single photon emission computed tomography
- PIGD
postural instability and gait difficulty
Footnotes
Relevant conflicts of interest/financial disclosures: The authors have no relevant financial or conflict of interest to disclose.
Financial Disclosures
Bohnen: Research support from the NIH (P01 NS015655 and R01 NS07085), Department of Veterans Affairs and the Michael J. Fox Foundation.
Jahn: Research support from the German Research Foundation (DFG JA1087/1-1) and the German Federal Ministry of Education and Research (BMBF 01EO0901).
References
- 1.Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM. Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci. 2006;24:1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x. [DOI] [PubMed] [Google Scholar]
- 2.Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov Disord. 2008;23 (Suppl 3):S521–533. doi: 10.1002/mds.22049. [DOI] [PubMed] [Google Scholar]
- 3.Azulay JP, Van Den Brand C, Mestre D, et al. Automatic motion analysis of gait in patients with Parkinson disease: effects of levodopa and visual stimulations [French] Rev Neurol (Paris) 1996;152:128–134. [PubMed] [Google Scholar]
- 4.Kemoun G, Defebvre L. Clinical description, analysis of posture, initiation of stabilized gait] [French] Presse Med. 2001;30:452–459. [PubMed] [Google Scholar]
- 5.Factor SA. The clinical spectrum of freezing of gait in atypical parkinsonism. Mov Disord. 2008;23 (Suppl 2):S431–438. doi: 10.1002/mds.21849. [DOI] [PubMed] [Google Scholar]
- 6.Snijders AH, Leunissen I, Bakker M, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet AM, Loria Y, Saint-Hilaire MH, Lhermitte F, Agid Y. Does long-term aggravation of Parkinson’s disease result from nondopaminergic lesions? Neurology. 1987;37:1539–1542. doi: 10.1212/wnl.37.9.1539. [DOI] [PubMed] [Google Scholar]
- 8.Greenstein JJ, Gastineau EA, Siegel BH, Macsata R, Conklin JJ, Maurer AH. Cerebral hemisphere activation during human bipedal locomotion. Hum Brain Mapp. 1995;3 (Suppl 1):320. [Google Scholar]
- 9.Fukuyama H, Ouchi Y, Matsuzaki S, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228:183–186. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- 10.Maillet A, Pollak P, Debu B. Imaging gait disorders in parkinsonism: a review. J Neurol Neurosurg Psychiatry. 2012;83:986–993. doi: 10.1136/jnnp-2012-302461. [DOI] [PubMed] [Google Scholar]
- 11.Miyai I, Tanabe HC, Sase I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- 12.la Fougere C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Wutte MG, Glasauer S, Jahn K, Flanagin VL. Moving and being moved: differences in cerebral activation during recollection of whole-body motion. Behav Brain Res. 2012;227:21–29. doi: 10.1016/j.bbr.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 14.Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976;56:465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahn K, Deutschlander A, Stephan T, et al. Supraspinal locomotor control in quadrupeds and humans. Prog Brain Res. 2008;171:353–362. doi: 10.1016/S0079-6123(08)00652-3. [DOI] [PubMed] [Google Scholar]
- 17.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123:1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Matsuyama K, Mori F, Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol. 2001;87:25–40. [PubMed] [Google Scholar]
- 19.Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T. Speculation on the responsible sites and pathophysiology of freezing of gait. Parkinsonism Rel Dis. 2006;12:S55–S62. [Google Scholar]
- 21.Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease. Ann Neurol. 1999;45:329–336. doi: 10.1002/1531-8249(199903)45:3<329::aid-ana8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Hanakawa T, Katsumi Y, Fukuyama H, et al. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain. 1999;122:1271–1282. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- 23.del Olmo MF, Arias P, Furio MC, Pozo MA, Cudeiro J. Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients--a combined motor and [18F]-FDG PET study. Parkinsonism Relat Disord. 2006;12:155–164. doi: 10.1016/j.parkreldis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zwergal A, la Fougere C, Lorenzl S, et al. Functional disturbance of the locomotor network in progressive supranuclear palsy. Neurology. 2013;80:634–641. doi: 10.1212/WNL.0b013e318281cc43. [DOI] [PubMed] [Google Scholar]
- 25.Wai YY, Wang JJ, Weng YH, et al. Cortical involvement in a gait-related imagery task: comparison between Parkinson’s disease and normal aging. Parkinsonism Relat Disord. 2012;18:537–542. doi: 10.1016/j.parkreldis.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Mov Disord. 2008;23 (Suppl 2):S461–467. doi: 10.1002/mds.21912. [DOI] [PubMed] [Google Scholar]
- 27.Shine JM, Matar E, Ward PB, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain. 2013;136:1204–1215. doi: 10.1093/brain/awt049. [DOI] [PubMed] [Google Scholar]
- 28.Tessitore A, Amboni M, Esposito F, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. 2012;18:781–787. doi: 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Kostic VS, Agosta F, Pievani M, et al. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- 30.Shine JM, Matar E, Ward PB, et al. Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load. PLoS One. 2013;8:e52602. doi: 10.1371/journal.pone.0052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naismith SL, Lewis SJ. A novel paradigm for modelling freezing of gait in Parkinson’s disease. J Clin Neurosci. 2010;17:984–987. doi: 10.1016/j.jocn.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Shine JM, Naismith SL, Lewis SJ. The pathophysiological mechanisms underlying freezing of gait in Parkinson’s Disease. J Clin Neurosci. 2011;18:1154–1157. doi: 10.1016/j.jocn.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Hill KK, Campbell MC, McNeely ME, et al. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol. 2012;241C:105–112. doi: 10.1016/j.expneurol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 35.Brudzynski SM, Houghton PE, Brownlee RD, Mogenson GJ. Involvement of neuronal cell bodies of the mesencephalic locomotor region in the initiation of locomotor activity of freely behaving rats. Brain Res Bull. 1986;16:377–381. doi: 10.1016/0361-9230(86)90059-6. [DOI] [PubMed] [Google Scholar]
- 36.Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport. 2004;15:2621–2624. doi: 10.1097/00001756-200412030-00012. [DOI] [PubMed] [Google Scholar]
- 37.Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 1988;452:273–285. doi: 10.1016/0006-8993(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 38.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 39.Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 40.Ballanger B, Lozano AM, Moro E, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum Brain Mapp. 2009;30:3901–3909. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirker W, Asenbaum S, Bencsits G, et al. [123I]beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord. 2000;15:1158–1167. doi: 10.1002/1531-8257(200011)15:6<1158::aid-mds1015>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi Y, Kanno T, Okada H, et al. Presynaptic and postsynaptic dopaminergic binding densities in the nigrostriatal and mesocortical systems in early Parkinson’s disease: a double-tracer positron emission tomography study. Ann Neurol. 1999;46:723–731. doi: 10.1002/1531-8249(199911)46:5<723::aid-ana7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26:2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 44.Bohnen NI, Mueller MLTM, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson disease. J Cereb Blood Flow Metab. 2012;32:1609–1617. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohnen NI, Kotagal V, Albin RL, Koeppe RA, Frey KA, Muller MLTM. Gait speed is preserved in oligosystem compared to multisystem neurodegeneration in Parkinson disease. Neurology. 2013;80:P04165. [Google Scholar]
- 48.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol. 2008;115:409–415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 49.Lopez IC, Ruiz PJ, Del Pozo SV, Bernardos VS. Motor complications in Parkinson’s disease: ten year follow-up study. Mov Disord. 2010;25:2735–2739. doi: 10.1002/mds.23219. [DOI] [PubMed] [Google Scholar]
- 50.Muller ML, Frey KA, Petrou M, et al. β-amyloid and postural instability and gait difficulty in Parkinson’s disease at risk for dementia. Mov Disord. 2013;28:296–301. doi: 10.1002/mds.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149:187–194. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- 52.Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol. 2008;255 (Suppl 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 53.Zwergal A, la Fougere C, Lorenzl S, et al. Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology. 2011;77:101–109. doi: 10.1212/WNL.0b013e318223c79d. [DOI] [PubMed] [Google Scholar]
- 54.Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab. 2008;28:441–444. doi: 10.1038/sj.jcbfm.9600599. [DOI] [PubMed] [Google Scholar]
- 55.Kotagal V, Bohnen NI, MLTMM, Koeppe RA, Frey KA, Albin RL. Cerebral amyloid deposition correlates inversely with seotoninergic innervation in Parkinson disease. Arch Neurol. 2012;69:1628–1631. doi: 10.1001/archneurol.2012.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage. 2004;22:1722–1731. doi: 10.1016/j.neuroimage.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X. Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson’s disease. Neuroscience. 2010;166:712–719. doi: 10.1016/j.neuroscience.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camicioli R, Gee M, Bouchard TP, et al. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15:187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Hazrati LN, Parent A. Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res. 1992;585:267–271. doi: 10.1016/0006-8993(92)91216-2. [DOI] [PubMed] [Google Scholar]
- 60.Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson’s disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9:279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- 61.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 62.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 63.Muller M, Bohnen N, Bhaumik A, Albin R, Frey K, Gilman S. Striatal dopaminergic denervation and cardiac post-ganglionic sympathetic denervation correlate independently with gait velocity in Parkinson disease. Neurology. 2011;76:A265. [Google Scholar]
- 64.Gallezot JD, Weinzimmer D, Nabulsi N, et al. Evaluation of [(11)C]MRB for assessment of occupancy of norepinephrine transporters: Studies with atomoxetine in non-human primates. Neuroimage. 2011;56:268–279. doi: 10.1016/j.neuroimage.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filippi M, Kulisevsky J. Advances with MRI in Parkinson disease: from freezing to festination. Neurology. 2012;79:2222–2223. doi: 10.1212/WNL.0b013e3182768a31. [DOI] [PubMed] [Google Scholar]
- 66.Thevathasan W, Pogosyan A, Hyam JA, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain. 2012;135:148–160. doi: 10.1093/brain/awr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Handojoseno AM, Shine JM, Nguyen TN, Tran Y, Lewis SJ, Nguyen HT. The detection of Freezing of Gait in Parkinson’s disease patients using EEG signals based on Wavelet decomposition. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:69–72. doi: 10.1109/EMBC.2012.6345873. [DOI] [PubMed] [Google Scholar]
- 68.Matsui H, Udaka F, Miyoshi T, et al. Three-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson’s disease. Mov Disord. 2005;20:1272–1277. doi: 10.1002/mds.20520. [DOI] [PubMed] [Google Scholar]
- 69.Mito Y, Yoshida K, Yabe I, et al. Brain SPECT analysis by 3D-SSP and clinical features of Parkinson’s disease. Hokkaido Igaku Zasshi. 2006;81:15–23. [PubMed] [Google Scholar]
- 70.Bartels AL, de Jong BM, Giladi N, et al. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Mov Disord. 2006;21:1326–1332. doi: 10.1002/mds.20952. [DOI] [PubMed] [Google Scholar]
- 71.Imamura K, Okayasu N, Nagatsu T. Cerebral blood flow and freezing of gait in Parkinson’s disease. Acta Neurol Scand. 2012;126:210–218. doi: 10.1111/j.1600-0404.2012.01652.x. [DOI] [PubMed] [Google Scholar]
- 72.Gilman S, Koeppe RA, Nan B, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]


