Abstract
Bone marrow (BM) cavities are utilized for hematopoiesis and to maintain hematopoietic stem cells (HSCs). HSCs have the ability to self-renew as well as to differentiate into multiple different hematopoietic lineage cells. HSCs produce their daughter cells throughout the lifespan of individuals and thus, maintaining HSCs is crucial for individual life. BM cavities provide a specialized microenvironment termed “niche” to support HSCs. Niches are composed of various types of cells such as osteoblasts, endothelial cells and reticular cells. Osteoclasts are unique cells which resorb bones and are required for BM cavity formation. Loss of osteoclast function or differentiation results in inhibition of BM cavity formation, an osteopetrotic phenotype. Osteoclasts are also reportedly required for hematopoietic stem and progenitor cell (HSPC) mobilization to the periphery from BM cavities. Thus, lack of osteoclasts likely results in inhibition of HSC maintenance and HSPC mobilization. However, we found that osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization by using three independent osteoclast-less animal models. In this review, I will discuss the roles of osteoclasts in hematopoietic stem cell maintenance and mobilization.
Keywords: Osteoclasts, Hematopoietic stem and progenitor cell, Mobilization, Receptor activator of nuclear factor kappa B ligand, Osteomac, Osteopetrosis, op/op, C-Fos, Osteoprotegerin
INTRODUCTION
Bones reportedly play crucial roles in regulating bone marrow (BM) hematopoiesis by preparing BM cavities and bone marrow “niches” to support hematopoietic stem cell (HSC) maintenance[1-8]. Niches consist of osteoblasts, endothelial cells, reticular cells and osteomacs and are regulated by their products, such as Angiopoietin 1 and Cxcl12 (Figure 1)[9-19]. Functional BM cavities are required for HSPC mobilization from BM cavities to the periphery[20-24]. Thus, BM cavities play crucial roles in regulating HSC maintenance and HSPC mobilization to the periphery and loss of BM cavities is predicted to promote impaired HSPC maintenance and mobilization. However, the impact of lack of BM cavities on the hematopoietic system remains unclear.
Figure 1.
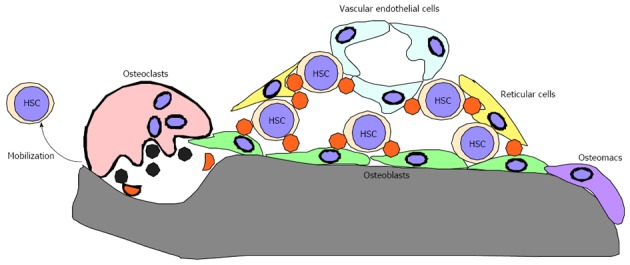
Components of bone marrow niches. Bone marrow niches are composed of cell types such as osteoblasts, reticular cells and vascular endothelial cells and factors expressed by these cells; HSC: Hematopoietic stem cell.
Osteoclasts are unique in their capacity to resorb bones: perturbation of osteoclast differentiation or function results in loss of BM cavities, a condition termed osteopetrosis[25-30]. Macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) are cytokines[31-35] that play a crucial role in inducing osteoclastogenesis[36-41]. Loss of M-CSF and RANKL, and c-Fos, a transcription factor required for osteoclastogenesis, via mutation or gene-targeting in animal models, impairs osteoclastogenesis and BM cavity formation, an osteopetrotic phenotype[25,26,28]. In contrast, loss of osteoprotegerin (OPG), a decoy RANKL receptor that inhibits osteoclastogenesis[42-45], accelerates osteoclastogenesis in mice and promotes an osteoporotic phenotype[46,47]. Osteoclast activity is reportedly upregulated following serial granulocyte colony-stimulating factor (G-CSF) injection[48], which stimulates HSPC mobilization to the periphery[49-61]. Furthermore, osteoclasts reportedly induce HSPC mobilization[62-64]. Osteoclasts were also reportedly involved in regulating the HSC niche in the bone marrow[65-70]. Thus far, however, hematopoiesis and HSPC mobilization in animals lacking M-CSF, RANKL, c-Fos or OPG remained uncharacterized before our study.
HEMATOPOIETIC STEM CELLS ARE MAINTAINED IN OSTEOPETROTIC MICE
The chemotherapeutic agent 5-fluorouracil (5-FU) kills cycling cells. Since hematopoietic stem cells (HSCs) are maintained in a quiescent state, HSCs are resistant to 5-FU induced cell death. Thus, serial 5-FU injection has been utilized to evaluate HSC cell function and maintenance in vivo[71-74]. We hypothesized that osteopetrotic mice show a reduced HSC pool and function due to lack of BM cavities and niches. Indeed, we found that op/op mice were lethally susceptible to serial 5-FU injection[75]. However, RANKL- and c-Fos-deficient mice were not as susceptible to serial 5-FU injection as op/op mice were[75]. These results suggest that osteoclasts and BM cavities are not required for HSC maintenance, while M-CSF likely functions in resistance to 5-FU-induced myelosuppression. Indeed, we found that serum M-CSF concentrations increase during 5-FU-induced myelosuppression and that various tissues from M-CSF-deficient op/op mice exhibit serious bacterial infections, suggesting that M-CSF expression antagonizes infection during myelosuppression[75].
HSPCS ARE MOBILIZED FOLLOWING SERIAL G-CSF INJECTION INTO OSTEOPETROTIC MICE
Serial G-CSF injection is utilized clinically to mobilize HSPCs for transplantation[49-51]. Similarly, serial G-CSF injection in mice induces HSPC mobilization to the periphery and is often utilized to evaluate HSPC mobilization capacity in mouse models[20,52-61]. We analyzed HSPC mobilization capacity following serial G-CSF injection in three independent osteoclast-less and thereby BM cavity-less animals, op/op mice (M-CSF-deficient mice), c-Fos and RANKL-deficient mice. HSPC mobilization to the periphery was evaluated by flow cytometry to detect the phenotypically HSPC-rich fraction; Lineage-negative, Sca1-positive and c-Kit-positive (LSK), and functional assays such as colony formation and competitive repopulation assay. Since BM cavities or osteoclasts reportedly function in HSPC mobilization[20], we speculated that osteopetrotic animals do not show HSPC mobilization into the periphery due to their loss. Interestingly, however, we found that serial G-CSF injection mobilized HSPCs at levels in all three osteopetrotic mice, compared to control mice, indicating that osteoclasts and BM cavities are not required for HSPC mobilization to the periphery[75].
HSPC MOBILIZATION IS IMPAIRED IN OSTEOPOROTIC OPG-DEFICIENT MICE
Since HSPC mobilization was detected in osteoclast-less osteopetrotic mice, we evaluated HSPC mobilization in OPG-deficient mice, which exhibit osteoporotic phenotypes due to accelerated osteoclastogenesis[75]. In contrast to osteopetrotic mice, osteoporotic OPG-deficient mice showed reduced HSPC mobilization capacity compared to wild-type mice, suggesting that osteoclasts negatively regulate HSPC mobilization following serial G-CSF injection.
SPLEEN IS NOT THE PRIMARY TISSUE TO MAINTAIN HSPCS IN AN OSTEOPETROTIC CONDITION
In osteopetrotic patients and animal models, extramedullary hematopoiesis reportedly occurs in the spleen[76,77]. Indeed, we found an increased proportion of the LSK cell fraction in op/op mouse spleen compared to control mouse spleen[75]. Thus, we reasoned that there is a pool of mobilized HSPCs in osteopetrotic mice in the spleen prior to mobilization. We then removed spleens from op/op mice and analyzed HSPC mobilization capacity of HSPCs following serial G-CSF injection (Figure 2). HSPC mobilization was significantly elevated in splenectomized op/op mice compared to splenectomized controls or non-splenectomized op/op mice, suggesting that the spleen is not the primary tissue to maintain HSPCs and that it may even antagonize HSPC mobilization in osteopetrotic mice[75]. At present, the localization of HSPCs in osteopetrotic mice is not clear, but we found that small bone lacunae are distributed in osteopetrotic bones and that c-Kit-positive hematopoietic cells are located in such lacunae[75]. These lacunae in osteopetrotic bones likely contribute to the HSPC pool in these animals.
Figure 2.
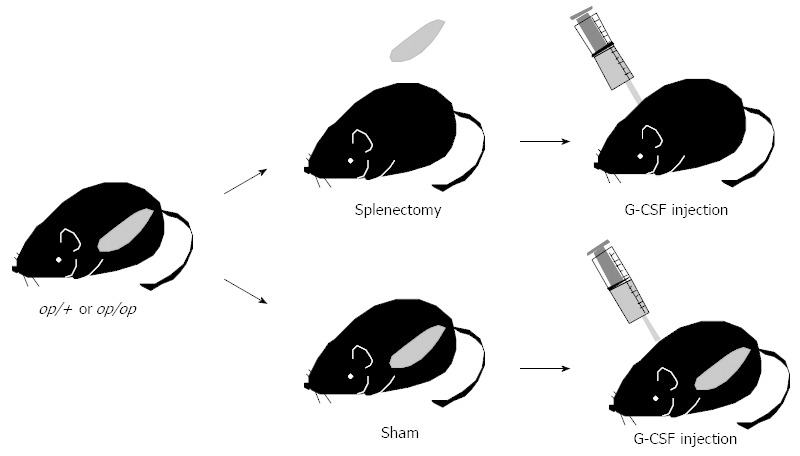
Splenectomy strategy. Splenectomy or sham surgery was performed on op/op and control mice. Seven days later, mice were injected with 250 μg/kg per day granulocyte colony-stimulating factor daily for 5 d and hematopoietic stem cell mobilization to peripheral blood was analyzed using flow cytometry and colony-forming assays. G-CSF: Granulocyte colony-stimulating factor.
F4/80-POSITIVE OSTEOMACS ARE REDUCED IN OP/OP MICE BUT ARE DETECTED NORMALLY IN C-FOS-, RANKL- AND OPG-DEFICIENT MICE
Recently, F4/80-positive bone-lining cells termed “osteomacs” have been identified[78] and demonstrated to play a crucial role in regulating hematopoiesis in vivo[18]. Osteomacs reportedly regulate osteoblast function and help retain HSPCs in BM cavities and osteomac loss is predicted to mobilize HSPCs to the periphery[18]. Since osteomacs were once thought to be identical to osteoclasts, increased mobilization seen in osteoclast-less animals was considered due to the loss of osteomacs/osteoclasts. Indeed, we observed decreased numbers of osteomacs in op/op mouse bones[75]. However, F4/80-positive osteomacs were detected normally in RANKL-deficient and c-Fos-deficient mice, indicating that osteomacs are not osteoclasts[75]. F4/80 is reportedly not expressed in osteoclasts[79], further suggesting that osteomacs are a different cell type.
PHARMACOLOGICAL INHIBITION OF OSTEOCLASTS BY BISPHOSPHONATE AND RANKL NEUTRALIZING ANTIBODY DOES NOT INHIBIT HSPC MOBILIZATION
Although we analyzed osteoclast function in regulating hematopoiesis in osteopetrotic mice, the role of osteoclasts in hematopoiesis in adult animals remained unclear. Previously, osteoclast activity was reported to increase following G-CSF injection, but treatment with pamidronate, an osteoclast-inhibiting bisphosphonate, did not inhibit HSPC mobilization to the periphery, suggesting that increased osteoclast activity is not required for HSPC mobilization following G-CSF injection[48]. In contrast, osteoclasts are reportedly required for HSPC mobilization since mobilization is induced by bleeding or LPS injection, which increases osteoclastogenesis[62]. Similarly, injection of HGF, SDF1 or RANKL also stimulated HSPC mobilization and increased osteoclast formation[62]. RANKL- or G-CSF-induced HSPC mobilization is abrogated in young female PTPe-deficient mice, which exhibit mild osteoclast dysfunction[62]. Thus, the role of osteoclasts in regulating HSPC mobilization in adults remained controversial and so we did the next experiments to resolve this controversy. We treated wild-type adult mice with an osteoclast-inhibiting agent: the bisphosphonate alendronate or a neutralizing antibody against RANKL (RANKL Ab). Both of these reagents strongly inhibit osteoclast activity and are used to treat osteoporosis patients[80-97]. Indeed, we observed increased bone mass following treatment with alendronate and RANKL Ab in wild-type adult mice[75]. Even in this osteoclast-inhibiting condition, HSPC mobilization to the periphery was normal or even highly induced compared with control mice, suggesting that osteoclast activity is neither required for nor antagonistic to HSPC mobilization[19].
ROLES OF OSTEOCLASTS AND BM CAVITIES IN HEMATOPOIESIS AND HSPC MOBILIZATION
In most mammalian and avian species, including humans and mice, hematopoiesis occurs in BM cavities and HSC daughter cells are mobilized to the periphery. To continuously supply hematopoietic cells throughout an animal’s life, HSCs must self-renew and be capable of producing multiple lineages[98]. Protection of HSCs from various stresses is crucial to maintain lifelong hematopoiesis. To maintain function, HSCs locate in a specific microenvironment in BM cavities termed the “niche”, where cells normally remain quiescent. Niches consist of various cell types, including osteoblasts, reticular cells, endothelial cells and osteoclasts, and corresponding products of these cells, such as Cxcl12 (SDF1), Angiopoietin 1 and N-Cadherin. Increased osteoblastogenesis reportedly increases the HSC pool, while degradation of Cxcl12 or surrounding extracellular matrix protein is required for HSPC mobilization to the periphery[9,10,20]. Since osteoclasts express high levels of matrix-degrading enzymes, such as matrix metalloprotenase 9 (MMP9) and Cathepsin K[99-105], and osteoclast activity increases following G-CSF injection[48], osteoclasts were predicted to be critical for HPSC mobilization through degradation of Cxcl12 and matrix protein[62]. In our study, we found that HSPC mobilization to the periphery was induced at comparable or even higher rates than that seen in controls following serial G-CSF injection of three independent osteoclast-less and therefore BM-less mice, phenotypes also seen in wild-type mice treated with two independent osteoclast inhibiting agents[75]. These findings suggest that osteoclasts and BM cavities are dispensable for HSPC maintenance and mobilization. However, it is important to note that we did not induce HSPC mobilization by bleeding or injection of LPS or cytokines, but rather treated wild-type adult mice with one bisphosphonate and one antibody. Nonetheless, our study, at least in part, demonstrates that osteoclast-less and BM cavity-less conditions or conditions in which osteoclasts are severely inhibited do not necessarily prohibit HSPC mobilization.
HEMATOPOIESIS IN OSTEOCLAST-LESS, THEREBY BM-LESS, ANIMALS
As described above, even in osteopetrotic bones, HSCs were located in bones[75], suggesting that these bones play a role in providing an HSC pool. Parathyroid hormone (PTH) reportedly has dual effects in bone and single or intermittent PTH injection leads to increased osteoblastic activity, whereas continuous PTH stimulation results in increased osteoclast activity and decreased bone mass[106-111]. Interestingly, increased osteoblastic activity due to elevated PTH signaling in constitutive active PTH receptor transgenic mice reportedly increases bone mass and the size of the HSC pool in vivo and osteoblasts are thought to serve as critical niche components[9,10]. Similarly, we found that increased bone mass due to reduced osteoclastic activity may contribute to an increased HSC pool[75]. Thus, increased bone mass, due to either increased osteoblast activity or reduced osteoclast function, likely increases the HSC pool in vivo (Figure 3). Expanding HSCs ex vivo is considered difficult since senescence is crucial for maintaining HSCs and cell cycling disrupts HSC function. Increased niche size by either increased osteoblast activity or inhibited osteoclast function likely serves as a mechanism to increase the HSC pool in vivo (Figure 3). Further studies are needed to elucidate the role of bones as a HSC niche.
Figure 3.
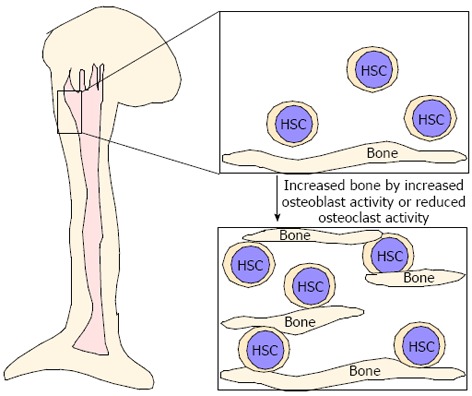
Increased bone mass is associated with an increased hematopoietic stem cell pool. Hematopoietic stem cells (HSC) are located in bone marrow cavities with surrounding niche cells. Increased bone mass due to either increased osteoblast activity or reduced osteoclast activity likely contributes to HSC expansion.
BM CAVITY FORMATION AND BONE STRENGTH
We have shown that osteoclasts and BM cavities are not required for HSPC maintenance and mobilization. So why do BM cavities develop with osteoclasts? We found that, indeed, bone mineral density was significantly higher in osteopetrotic bones than that seen in control bones since BM cavities of osteopetrotic bones were filled with bone[75]. However, osteopetrotic bone strength was low compared with control bones. Since cortical bone thickness is lower in osteopetrotic than in control bones, BM cavities likely developed along with development of cortical bones. At present, the roles of BM cavities are not well clarified and further studies are needed to elucidate their roles in bone and in hematopoiesis.
PHARAMACOLOGICAL INHIBITION OF OSTEOCLASTS
Osteoclasts emerge in the presence of M-CSF and RANKL: mutational inactivation of M-CSF or targeted disruption of RANKL results in osteoclast differentiation failure and BM cavity-less osteopetrotic phenotypes[25,28]. Recently, a neutralizing antibody against RANKL, named Denosumab, was utilized to treat osteoporosis patients and found to promote significantly reduced osteoclast activity and elevated bone mineral density compared with non-treated placebo controls[90]. Similarly, neutralizing antibody against mouse RANKL increases bone mass[94]. That antibody could be a useful tool to analyze effects of osteoclast-inhibiting conditions in mouse models. The increasing number of osteoporosis patients is now a pressing problem in developed countries and many are treated with osteoclast-inhibiting agents such as bisphosphonates. Future analysis of the effects of these drugs on the systems other than the bone system, such as hematopoiesis, is required to evaluate potential adverse affects of these agents in the future.
Footnotes
P- Reviewer Shin DM S- Editor Song XX L- Editor Roemmele A E- Editor Zhang DN
References
- 1.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 3.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- 6.Oh IH, Kwon KR. Concise review: multiple niches for hematopoietic stem cell regulations. Stem Cells. 2010;28:1243–1249. doi: 10.1002/stem.453. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P. Minireview: The stem cell next door: skeletal and hematopoietic stem cell “niches” in bone. Endocrinology. 2011;152:2957–2962. doi: 10.1210/en.2011-0217. [DOI] [PubMed] [Google Scholar]
- 8.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 10.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 11.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 18.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 19.Cain CJ, Manilay JO. Hematopoietic stem cell fate decisions are regulated by Wnt antagonists: comparisons and current controversies. Exp Hematol. 2013;41:3–16. doi: 10.1016/j.exphem.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Larsson J, Scadden D. Nervous activity in a stem cell niche. Cell. 2006;124:253–255. doi: 10.1016/j.cell.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 23.Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol. 2011;39:723–729. doi: 10.1016/j.exphem.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27:24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 26.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 27.Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 28.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 29.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 32.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 36.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood. 2000;96:4335–4343. [PubMed] [Google Scholar]
- 38.Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, Anderson DM, Suda T. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98:2544–2554. doi: 10.1182/blood.v98.8.2544. [DOI] [PubMed] [Google Scholar]
- 39.Nosaka K, Miyamoto T, Sakai T, Mitsuya H, Suda T, Matsuoka M. Mechanism of hypercalcemia in adult T-cell leukemia: overexpression of receptor activator of nuclear factor kappaB ligand on adult T-cell leukemia cells. Blood. 2002;99:634–640. doi: 10.1182/blood.v99.2.634. [DOI] [PubMed] [Google Scholar]
- 40.Miyamoto T, Suda T. Differentiation and function of osteoclasts. Keio J Med. 2003;52:1–7. doi: 10.2302/kjm.52.1. [DOI] [PubMed] [Google Scholar]
- 41.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi K, Kinosaki M, Goto M, Kobayashi F, Tsuda E, Morinaga T, Higashio K. Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem. 1998;273:5117–5123. doi: 10.1074/jbc.273.9.5117. [DOI] [PubMed] [Google Scholar]
- 46.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 48.Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Lévesque JP. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92:3465–3473. [PubMed] [Google Scholar]
- 49.Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1:1194–1198. doi: 10.1016/s0140-6736(88)92012-0. [DOI] [PubMed] [Google Scholar]
- 50.Gianni AM, Siena S, Bregni M, Tarella C, Stern AC, Pileri A, Bonadonna G. Granulocyte-macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation. Lancet. 1989;2:580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 51.Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni AM. Circulation of CD34+ hematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: enhancement by intravenous recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1989;74:1905–1914. [PubMed] [Google Scholar]
- 52.Haas R, Ho AD, Bredthauer U, Cayeux S, Egerer G, Knauf W, Hunstein W. Successful autologous transplantation of blood stem cells mobilized with recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1990;18:94–98. [PubMed] [Google Scholar]
- 53.Molineux G, Pojda Z, Hampson IN, Lord BI, Dexter TM. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990;76:2153–2158. [PubMed] [Google Scholar]
- 54.Briddell RA, Hartley CA, Smith KA, McNiece IK. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82:1720–1723. [PubMed] [Google Scholar]
- 55.Neben S, Marcus K, Mauch P. Mobilization of hematopoietic stem and progenitor cell subpopulations from the marrow to the blood of mice following cyclophosphamide and/or granulocyte colony-stimulating factor. Blood. 1993;81:1960–1967. [PubMed] [Google Scholar]
- 56.Bodine DM, Seidel NE, Gale MS, Nienhuis AW, Orlic D. Efficient retrovirus transduction of mouse pluripotent hematopoietic stem cells mobilized into the peripheral blood by treatment with granulocyte colony-stimulating factor and stem cell factor. Blood. 1994;84:1482–1491. [PubMed] [Google Scholar]
- 57.Yan XQ, Briddell R, Hartley C, Stoney G, Samal B, McNiece I. Mobilization of long-term hematopoietic reconstituting cells in mice by the combination of stem cell factor plus granulocyte colony-stimulating factor. Blood. 1994;84:795–799. [PubMed] [Google Scholar]
- 58.Yan XQ, Hartley C, McElroy P, Chang A, McCrea C, McNiece I. Peripheral blood progenitor cells mobilized by recombinant human granulocyte colony-stimulating factor plus recombinant rat stem cell factor contain long-term engrafting cells capable of cellular proliferation for more than two years as shown by serial transplantation in mice. Blood. 1995;85:2303–2307. [PubMed] [Google Scholar]
- 59.Yamamoto Y, Yasumizu R, Amou Y, Watanabe N, Nishio N, Toki J, Fukuhara S, Ikehara S. Characterization of peripheral blood stem cells in mice. Blood. 1996;88:445–454. [PubMed] [Google Scholar]
- 60.Bodine DM, Seidel NE, Orlic D. Bone marrow collected 14 days after in vivo administration of granulocyte colony-stimulating factor and stem cell factor to mice has 10-fold more repopulating ability than untreated bone marrow. Blood. 1996;88:89–97. [PubMed] [Google Scholar]
- 61.Drize N, Chertkov J, Samoilina N, Zander A. Effect of cytokine treatment (granulocyte colony-stimulating factor and stem cell factor) on hematopoiesis and the circulating pool of hematopoietic stem cells in mice. Exp Hematol. 1996;24:816–822. [PubMed] [Google Scholar]
- 62.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 63.Purton LE, Scadden DT. Osteoclasts eat stem cells out of house and home. Nat Med. 2006;12:610–611. doi: 10.1038/nm0606-610. [DOI] [PubMed] [Google Scholar]
- 64.Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 65.Shivtiel S, Kollet O, Lapid K, Schajnovitz A, Goichberg P, Kalinkovich A, Shezen E, Tesio M, Netzer N, Petit I, et al. CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med. 2008;205:2381–2395. doi: 10.1084/jem.20080072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 67.Hoggatt J, Pelus LM. Mobilization of hematopoietic stem cells from the bone marrow niche to the blood compartment. Stem Cell Res Ther. 2011;2:13. doi: 10.1186/scrt54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S, Zhai Q, Zou D, Meng H, Xie Z, Li C, Wang Y, Qi J, Cheng T, Qiu L. A pivotal role of bone remodeling in granulocyte colony stimulating factor induced hematopoietic stem/progenitor cells mobilization. J Cell Physiol. 2013;228:1002–1009. doi: 10.1002/jcp.24246. [DOI] [PubMed] [Google Scholar]
- 69.Mansour A, Wakkach A, Blin-Wakkach C. Role of osteoclasts in the hematopoietic stem cell niche formation. Cell Cycle. 2012;11:2045–2046. doi: 10.4161/cc.20534. [DOI] [PubMed] [Google Scholar]
- 70.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 72.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2342. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 74.Zhao M, Ross JT, Itkin T, Perry JM, Venkatraman A, Haug JS, Hembree MJ, Deng CX, Lapidot T, He XC, et al. FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood. 2012;120:1831–1842. doi: 10.1182/blood-2011-11-393991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okada S, Wang ZQ, Grigoriadis AE, Wagner EF, von Rüden T. Mice lacking c-fos have normal hematopoietic stem cells but exhibit altered B-cell differentiation due to an impaired bone marrow environment. Mol Cell Biol. 1994;14:382–390. doi: 10.1128/mcb.14.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lowell CA, Niwa M, Soriano P, Varmus HE. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood. 1996;87:1780–1792. [PubMed] [Google Scholar]
- 78.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 79.Quinn JM, Morfis M, Lam MH, Elliott J, Kartsogiannis V, Williams ED, Gillespie MT, Martin TJ, Sexton PM. Calcitonin receptor antibodies in the identification of osteoclasts. Bone. 1999;25:1–8. doi: 10.1016/s8756-3282(99)00094-0. [DOI] [PubMed] [Google Scholar]
- 80.Seedor JG, Quartuccio HA, Thompson DD. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res. 1991;6:339–346. doi: 10.1002/jbmr.5650060405. [DOI] [PubMed] [Google Scholar]
- 81.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 83.Zimolo Z, Wesolowski G, Rodan GA. Acid extrusion is induced by osteoclast attachment to bone. Inhibition by alendronate and calcitonin. J Clin Invest. 1995;96:2277–2283. doi: 10.1172/JCI118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 85.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, Holloway D, Peterson MC, Bekker PJ. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 86.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 87.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 88.Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24:182–195. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- 89.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 90.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 91.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, Fizazi K. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25:440–446. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 92.Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, Maasalu K, Bolognese MA, Woodson G, Bone H, et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25:2256–2265. doi: 10.1002/jbmr.149. [DOI] [PubMed] [Google Scholar]
- 93.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 94.Furuya Y, Mori K, Ninomiya T, Tomimori Y, Tanaka S, Takahashi N, Udagawa N, Uchida K, Yasuda H. Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts. J Biol Chem. 2011;286:37023–37031. doi: 10.1074/jbc.M111.246280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown JE, Coleman RE. Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol. 2012;9:110–118. doi: 10.1038/nrclinonc.2011.197. [DOI] [PubMed] [Google Scholar]
- 96.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damião R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goessl C, Katz L, Dougall WC, Kostenuik PJ, Zoog HB, Braun A, Dansey R, Wagman RB. The development of denosumab for the treatment of diseases of bone loss and cancer-induced bone destruction. Ann N Y Acad Sci. 2012;1263:29–40. doi: 10.1111/j.1749-6632.2012.06674.x. [DOI] [PubMed] [Google Scholar]
- 98.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 99.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 100.Väänänen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113(Pt 3):377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 101.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 102.Zaidi M, Blair HC, Moonga BS, Abe E, Huang CL. Osteoclastogenesis, bone resorption, and osteoclast-based therapeutics. J Bone Miner Res. 2003;18:599–609. doi: 10.1359/jbmr.2003.18.4.599. [DOI] [PubMed] [Google Scholar]
- 103.Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, Hustad CM, DaSilva C, Santora AC, Ince BA. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25:937–947. doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 104.Lewiecki EM. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol. 2011;7:631–638. doi: 10.1038/nrrheum.2011.130. [DOI] [PubMed] [Google Scholar]
- 105.Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Rodriguez Portales J, Denker A, Lombardi A, Le Bailly De Tilleghem C, Dasilva C, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res. 2012;27:2251–2258. doi: 10.1002/jbmr.1695. [DOI] [PubMed] [Google Scholar]
- 106.Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1-34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612. doi: 10.1210/endo.138.11.5505. [DOI] [PubMed] [Google Scholar]
- 107.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 108.Seeman E, Delmas PD. Reconstructing the skeleton with intermittent parathyroid hormone. Trends Endocrinol Metab. 2001;12:281–283. doi: 10.1016/s1043-2760(01)00460-x. [DOI] [PubMed] [Google Scholar]
- 109.Lotinun S, Sibonga JD, Turner RT. Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine. 2002;17:29–36. doi: 10.1385/ENDO:17:1:29. [DOI] [PubMed] [Google Scholar]
- 110.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40:1447–1452. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]


