Abstract
AIM: To investigate the relationship between increases in expression time of ABCG2 mRNA driven by cisplatin and efficacy of platinum-containing chemotherapy for gastric cancer.
METHODS: Tumor specimens and normal control tissues were collected from 78 patients with gastric cancer treated from January 2008 to December 2011. Fresh tumor tissue obtained from the surgically resected specimens was tested within 6 h. Polymerase chain reaction products were run on 2% agarose gels and analyzed under ultraviolet light after ethidium bromide staining. Increases in ABCG2 mRNA expression time were assessed after cancer cells were incubated with cisplatin, and were divided into terciles and compared in relation to clinical outcomes.
RESULTS: Among groups classified by expression time of ABCG2 mRNA, no significant differences in baseline clinical characteristics and pathological findings were detected. The median overall time was 14.2 (95%CI: 9.7-18.6), 11.4 (95%CI: 6.3-16.5) and 8.1 (95%CI: 5.4-10.8) in patients with low, intermediate and high increases in ABCG2 mRNA expression times (P < 0.05), respectively. Median survival associated with performance status and tumor node metastasis (TNM) stage showed a similar trend, with longer survival and higher risk for mortality associated with lower performance status score and TNM stage. In a multivariate analysis for survival with Cox proportional-hazards model, increased ABCG2 mRNA expression time was an independent predictor for overall survival. Overall survival was longer with increased ABCG2 mRNA expression times ≤ 0.71 than increased ABCG2 mRNA expression times > 0.71, with a hazard ratio for death of 0.855 (95%CI: 0.615-0.962, P = 0.038).
CONCLUSION: Increased ABCG2 mRNA expression time driven by cisplatin is associated with survival of gastric cancer patients, and this may help modify the therapeutic strategies.
Keywords: Gastric cancer, ABCG2 mRNA expression, Cisplatin, Overall survival
Core tip: As a prognostic marker of poor clinical outcome, the high expression of ABCG2 has become a hot research topic and focus. Nevertheless, there are few studies involving the relevance of ABCG2 expression driven by chemotherapeutic agents and its clinical significance. This is the first study examining the impact of increased ABCG2 mRNA expression time driven by cisplatin in vitro in gastric cancer and its relationship with overall survival of the patients.
INTRODUCTION
Gastric cancer is the fourth most prevalent malignant cancer worldwide, and is the second most frequent cause of cancer death[1]. Despite advances made in gastric cancer therapy, the prognosis of patients with gastric cancer remains unsatisfactory. Because of early detection in screening programs in Japan, survival is prolonged (52%), whereas survival in the United States, Europe, and China is only 20%-25% due to delayed diagnosis[2]. The 5-year survival rate for advanced or metastatic gastric cancer is nearly 5%-20%, with a median overall survival being less than 1 year[3,4]. With the development of new anticancer drugs, such as taxanes, CPT-11, oxaliplatin, gefitinib and S-1, significant improvements in the efficacy of chemotherapy against gastric cancer have been achieved[5]. However, some patients still fail on first-line chemotherapy and will relapse and eventually develop resistance to currently available treatment options due to the acquisition of multidrug resistance (MDR)[6]. Therefore, it is necessary to find markers which could accurately predict the risk of gastric cancer, and give the evidence for early prediction of the clinical outcome so as to improve the clinical management of gastric cancer patients.
Breast cancer resistance protein (BCRP/ABCG2), the second member of the ATP-binding cassette-transporter superfamily, is prominently expressed in the epithelium of small intestine and colon, liver canalicular membranes, ducts and lobules of mammary tissue and blood-brain barrier, which plays a pivotal role in the bioavailability and brain disposition of drugs[7]. A broad spectrum of anticancer drugs, sulfate and glucuronide conjugates of sterols and xenobiotics, natural compounds and toxins, fluorescent dyes, photosensitizers, and antibiotics have been identified as substrates of ABCG2[8]. As a major drug transporter, ABCG2 has been shown to play an active role in MDR in various cancers[9-11]. Increased expression of ABCG2 results in resistance to anticancer drugs, including topoisomerase inhibitors, anthracyclines, camptothecin (CPT) analogs, tyrosine kinase inhibitors (TKI), and antimetabolites[8]. The resistant phenotype is conferred through the reduction of cytoplasmic chemotherapeutic drug concentrations to levels below those required for cytotoxicity[7]. Using an immunohistochemical method, ABCG2 expression was seen in all 150 tumor samples comprising 21 types of cancer, especially in carcinomas of the digestive tract (colon, esophagus and stomach), endometrium and lung, and in melanoma, which suggested that ABCG2 represented a common mechanism of clinical drug resistance[12]. Results from clinical studies indicate that high expression of ABCG2 in tumors is a prognostic marker of poor clinical outcome[9,13,14]. However, there are a limited number of studies on the relevance of ABCG2 expression driven by chemotherapeutic agents and its clinical significance.
In light of the above information, this study was an attempt to clarify the impact of increases in ABCG2 mRNA expression times driven by cisplatin on clinical outcome in patients with gastric cancer.
MATERIALS AND METHODS
Patients and samples
Tumor specimens and normal control tissues were collected from 78 patients with newly-diagnosed histologically proven gastric cancer (GC) in Shanghai Sixth People’s Hospital, Renji Hospital and Shanghai Putuo Hospital, from January 2008 to December 2011. All of the tumor specimens were obtained before chemotherapy. None of the patients received preoperative radiotherapy or chemotherapy. The clinical stage of GC was determined on the basis of the tumor-node-metastasis (TNM) classification system recommended by the International Union Against Cancer. Demographic and clinicopathological details of the patients were collected from electronic patient records. The study was approved by the Institutional Review Board of Shanghai Sixth People’s Hospital, Renji Hospital and Shanghai Putuo Hospital. All samples were obtained after receiving patients’ written informed consent.
All patients received platinum-based first-line chemotherapy and second-line chemotherapy for GC according to the NCCN guideline of GC[6]. The platinum-based first-line were cisplatin 60-100 mg/m2 plus fluoropyrimidine (750-1000 mg/m2 iv on days 1-5 as a protracted continuous infusion, FP; n = 18), docetaxel (75 mg/m2 iv on day 1, DP; n = 12), paclitaxel (135 mg/m2 iv on day 1, PP; n = 10), capecitabine (1000 mg/m2 bid po on days 1-14, XP; n = 25) or S-1 (40 mg/m2 bid po on days 1-21, SP; n = 13). Chemotherapy was repeated every 3 wk (XP, DP, PP and FP) or every 5 wk (SP) according to the regimen. All the patients received 2 or more courses of chemotherapy or until the appearance of progressive disease. In second-line chemotherapy, patients were administered the following regimens: oral S-1 or capecitabine (n = 30); weekly paclitaxel (n = 18); irinotecan and cisplatin (n = 13); irinotecan and docetaxel (n = 9) and mitomycin C, etoposide and cisplatin (n = 8).
Cell culture with cisplatin
Fresh tumor tissues obtained from the surgically resected specimens were tested within 6 h. The tumor tissue was cut into pieces (smaller than 1 mm3) and passed through No. 100 and No. 200 stainless steel meshes respectively into a complete medium containing RPMI 1640 solution, 100 μg/mL penicillin, and 100 μg/mL streptomycin, and washed twice gently with the same solution. The viable cells were assessed using a trypsin blue exclusion method. The cell suspension was collected into sterile 96-well flat-bottomed microtiter plates (1 × 105 cells per well) with or without 0.5 μg/mL cisplatin (CDDP). The plates were then incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2 for 72 h. Microtiter wells containing tumor cells but no anticancer agents were used as control cells, in which the total number of tumor cells was equivalent to that in the test wells.
Quantitative polymerase chain reaction
Treated and untreated cancer cells were suspended at 1 × 106 cells/mL in 5 mL PBS as samples. Total RNA was then isolated using TriPure Isolation reagent (Roche Diagnostics GmbH, Germany) or RNeasy Mini Kit (Qiagen, United States) according to the manufacturer’s instructions. Quantitative polymerase chain reaction (PCR) were performed using specific hydrolysis probes targeting ABCG2 on Applied Biosystems 7300S Real Time PCR systems (ABI, United States). Total RNA was isolated from cisplatin-treated GC cells using TRIzol (Tarkara, Dalian, China) and reverse-transcription was performed to synthesize cDNA using PrimeScript Reverse Transcriptase (Tarkara, Dalian, China). Subsequent PCR amplification was performed using 2 μg of cDNA under the following conditions: 95 °C for 30 s, 35 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 30 s. SYBR® Premix Ex Taq™ (Tarkara, Dalian, China) was used. β-actin was used as an internal control. PCR products were run on 2% agarose gels and analyzed under ultraviolet light after ethidium bromide staining. The primer sequences of ABCG2 used in real-time RT-PCR were: sense 5’-TTTCCAAGCGTTCATTCA AAAA-3’, antisense 5’-TACGACTGTGACA ATGATCTGAGC-3’. β-actin: sense 5’-ACCGTG GAGAAGAGCTACGA-3’, antisense 5’-GTACTT GCGCTCAGAAGGAG-3’. Each RT-PCR amplification was repeated in triplicate. The β-actin primer was included in every plate to avoid sample variations. The PCR efficiency was examined by serially diluting the template cDNA and the melting curve data were collected to check the PCR specificity. Each cDNA sample was triplicated and the corresponding no-RT mRNA sample was included as a negative control. The β-actin primer was included in every plate to avoid sample variations. The mRNA level of each sample was normalized to that of the β-actin mRNA. Relative increases in mRNA expression times of target gene against β-actin were measured as follows: mRNAtarget = (mRNAtarget/mRNAβ-actin)cisplatin treated cells/(mRNAtarget /mRNAβ-actin) control cells. All data shown were the mean ± SD of three separate experiments.
Statistical analysis
To determine the correlation between mRNA increased expression times and survival rate after chemotherapy, the χ2 test or Fisher’s exact test was used to analyze the data with R software statistical environment (version 2.15.1; R Development Core Team, Vienna, Austria). Cumulative overall survival probability was calculated by the Kaplan-Meier method for censored failure time data, and statistical significance was calculated using the log-rank test for comparison of survival rates between the different groups. The Cox proportional-hazards model was used to calculate the hazard ratios. P < 0.05 was considered statistically significant. All P values were two-tailed and unadjusted for potential multiple comparisons.
RESULTS
Patient characteristics
The clinical and pathological characteristics of the patients are outlined in Table 1. The median age was 59 years (range, 27-81 years). All of the patients were treated with platinum-based combination chemotherapeutic regimens. The median number of chemotherapy courses was 4 (range, 2-15). The disease progression was the most common reason for the discontinuation of the chemotherapy. The median follow-up time of the 78 patients was 12 mo (range, 3-42 mo).
Table 1.
Characteristics of patients according to increased expression times of ABCG2 mRNA by terciles n (%)
| Clinical pathological parameters | Total cohort |
ABCG2 mRNA expression times |
P value | ||
| ≤ 0.71 (n = 40) | 0.71-1.8 (n = 23) | ≥ 1.8 (n =1 5) | |||
| Age (yr) | 0.123 | ||||
| Median | 59 | 58 | 61 | 59 | |
| Range | 27-81 | 27-79 | 28-81 | 30-80 | |
| Gender | 0.269 | ||||
| Female | 19 (24.4) | 10 (24.8) | 6 (28.0) | 3 (19.0) | |
| Male | 59 (75.6) | 30 (75.2) | 17 (72.0) | 12 (81.0) | |
| Performance status | 0.556 | ||||
| 0-1 | 70 (89.7) | 36 (88.8) | 20 (85.0) | 14 (91.0) | |
| 2 | 8 (10.3) | 4 (11.2) | 3 (15.0) | 1 (9.0) | |
| Histological grad | 0.106 | ||||
| Differentiated | 39 (50.0) | 19 (48.0) | 12 (51.0) | 8 (52.3) | |
| Undifferentiated | 39 (50.0) | 21 (52.0) | 11 (49.0) | 7 (47.7) | |
| Stage | 0.170 | ||||
| IIIA | 24 (30.8) | 12 (29.0) | 7 (31.0) | 5 (33.0) | |
| IIIB | 24 (30.8) | 13 (32.1) | 7 (30.0) | 4 (28.0) | |
| IV | 30 (38.5) | 15 (38.9) | 9 (39.0) | 6 (39.0) | |
| Chemotherapeutic regimen | 0.322 | ||||
| Cisplatin + fluoropyrimidine | 18 (23.1) | 9 (23.1) | 6 (26.9) | 3 (21.4) | |
| Cisplatin + docetaxel | 11 (14.1) | 6 (15.4) | 3 (14.6) | 2 (13.8) | |
| Cisplatin + paclitaxel | 10 (12.8) | 5 (12.8) | 3 (15.1) | 2 (14.3) | |
| Cisplatin + capecitabine | 19 (24.4) | 7 (32.1) | 7 (30.5) | 5 (35.8) | |
| Cisplatin + S-1 | 20 (25.6) | 13 (16.7) | 4 (12.8) | 3 (14.7) | |
ABCG2 mRNA levels were measured by quantitative PCR and increased expression times in the cancer cells incubated with cisplatin were calculated by comparing with cancer cells incubated without cisplatin. Using the method reported by Font et al[15], results from these analyses were used to categorize the patients into terciles according to their ABCG2 mRNA expression times (lowest tercile, ≤ 0.71, n = 40; intermediate tercile, 0.71-1.80, n = 23; and highest tercile, ≥ 1.80, n = 15). No differences in clinical characteristics were observed according to BRCA1 mRNA expression levels. Among groups determined according to ABCG2 mRNA expression times, no significant differences in baseline clinical characteristics and pathological findings were detected (Table 1).
ABCG2 mRNA increased expression times and clinical outcome
The median overall survival time was 14.2 mo (95%CI: 9.7-18.6), 11.4 mo (95%CI: 6.3-16.5) and 8.1 mo (95%CI: 5.4-10.8) in the patients with low, intermediate and high ABCG2 mRNA expression times (Table 2), respectively. Because survival rates were similar in patients with intermediate and high expression times, the patients in these two groups were mixed for further statistical analyses. In patients with intermediate/high ABCG2 mRNA expression times, median overall survival (OS) time was 10 mo (95%CI: 5.8-14.2), whereas in patients with low expression levels, median survival was 14.2 mo (95%CI: 9.7-18.6) (P = 0.025) (Figure 1A). There was no statistical difference in progression-free survival (PFS) between the two groups (Figure 1B).
Table 2.
Univariate analysis of median overall survival and hazard ratios for risk of mortality according to clinical parameters
| Clinical pathological parameters |
Overall survival |
Risk of mortality |
||
| Month (95%CI) | P value | HR (95%CI) | P value | |
| Performance status | 0.012 | |||
| 0-1 | 14.4 (10.8-17.0) | 1.00 (referent) | ||
| 2 | 7.6 (2.5-13.7) | 3.26 (0.96-16.46) | 0.041 | |
| Histological grade | 0.062 | |||
| Differentiated | 15.5 (8.2-22.5) | 1.00 (referent) | ||
| Undifferentiated | 10.9 (4.5-17.3) | 4.95 (0.92-18.6) | 0.122 | |
| Tumor-node-metastasis stage | 0.001 | |||
| IIIA | 19.3 (16.9-21.7) | 0.87 (0.51-9.76) | < 0.001 | |
| IIIB | 13.0 (7.8-18.2) | 0.92 (0.81-2.15) | 0.102 | |
| IV | 7.2 (4.4-10.0) | 1.00 (referent) | ||
| Chemotherapeutic regimen | 0.536 | |||
| Cisplatin + fluoropyrimidine | 10.7 (5.1-16.3) | 1.00 (referent) | ||
| Cisplatin + docetaxel | 13.2 (7.0-19.4) | 0.84 (0.76-1.79) | 0.073 | |
| Cisplatin + paclitaxel | 12.6 (6.4-18.9) | 0.91 (0.85-2.41) | 0.145 | |
| Cisplatin + capecitabine | 11.3 (5.6-18.0) | 0.98 (0.90-3.26) | 0.210 | |
| Cisplatin + S-1 | 12.0 (6.1-17.9) | 0.95 (0.89-2.84) | 0.179 | |
| ABCG2 mRNA expression times | 0.031 | |||
| ≤ 0.71 | 14.2 (9.7-18.6) | 0.71 (0.43-0.96) | < 0.001 | |
| 0.71-1.8 | 11.4 (6.3-16.5) | 0.94 (0.76-1.52) | 0.083 | |
| ≥ 1.8 | 9.0 (6.4-11.6) | 1.00 (referent) | ||
Figure 1.
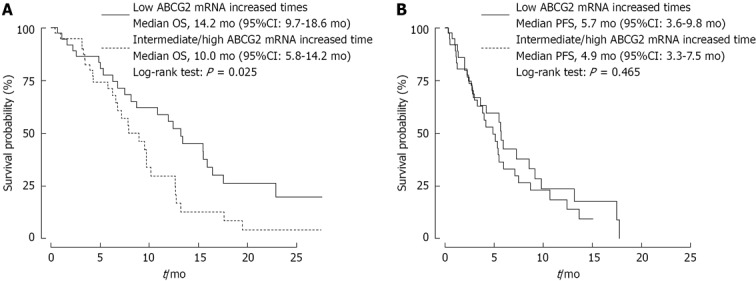
Overall survival (A) and progression-free survival (B) according to increased ABCG2 mRNA expression times (intermediate or high vs low tertiles). OS: Overall survival; PFS: Progression-free survival.
Univariate analysis was conducted to examine the impact of increases in ABCG2 mRNA expression times and other clinical pathological parameters on prognosis. In the univariate analysis, three parameters were found to be significantly associated with overall survival and risk for mortality statistically: performance status; TNM stage; and ABCG2 mRNA expression times (Table 2). Median survival associated with performance status and TNM stage showed a similar trend, with longer survival and higher risk for mortality associated with lower performance status score and TNM stage. In the multivariate analysis using the Cox proportional hazards model, only increased ABCG2 mRNA expression times and TNM stage were independent predictors for overall survival. The HR of TNM stage IIIA vs III B-IV for overall survival was 0.921 (95%CI: 0.656-0.983, P = 0.045), and the HR of ABCG2 mRNA increased expression times ≤ 0.71 vs > 0.71 was 0.855 (95%CI: 0.615-0.962, P = 0.038).
DISCUSSION
Platinum-containing regimens have now been clinically validated as effective advanced GC treatments. However, the relatively rapid acquired chemotherapeutic resistance to such therapies significantly limits their effects and remains a substantial obstacle to the clinical management of GCs. With the elucidation of molecular mechanisms of resistance, new strategies of predicting the formation of resistance have become a potential area of interest. In the current study, we found that ABCG2 mRNA expression levels were increased by incubation in sub-therapeutic concentrations of cisplatin in vitro, which could lead to the MDR phenotype associated with ABCG2. Moreover, a significant relationship between increased ABCG2 mRNA expression times and overall survival was observed in GC patients treated with platinum-containing chemotherapy. Patients with higher ABCG2 mRNA expression times had a shorter median overall survival time. In a multivariate analysis, increased ABCG2 mRNA expression times driven by cisplatin in vitro might be an independent prognostic marker of overall survival, while no baseline clinical pathological parameters, except TNM stage, had a prognostic impact on overall survival. To the best of our knowledge, there has been no study examining the role of ABCG2 mRNA expression times induced by cisplatin in vitro in GC treatment.
ABCG2 has been found to be an important molecule involved in both innate and acquired MDR by regulating drug bioavailability. Over-expression of ABCG2 shows the potential to be an independent prognostic marker of both hematopoietic and solid malignancies. In hematopoietic malignancies, the results from several follow-up studies have validated the relationship between ABCG2 expression and the prognosis and survival of acute myeloid leukemia patients[16-18]. In esophageal squamous cell carcinoma, lung cancer, digestive tract tumors and lymphoma, the presence of ABCG2-positive cells in the tumor indicated by immunohistochemical study was associated with poorer survival[12,19-21]. These studies observed the relationship between the prognosis and the presence of chemotherapeutic resistance in tumors. However, they did not take the potential of acquisition of MDR into account. In fact, sub-therapeutic concentrations of anticancer agents, including cisplatin, would result in MDR in neoplastic cells[22]. The current study might provide a helpful method to assess the potential of acquisition of MDR driven by chemotherapeutic agents.
The expression of ABCG2 in normal and cancer cells appears to be regulated at different levels including gene amplification, epigenetic modifications, transcriptional and post-transcriptional regulation[8,23]. Nuclear hormone receptor proteins, including the pregnane X receptor (PXR), constitutive androstane receptor, and farnesoid X receptor have shown the ability to regulate the expression of ABC transporters[24]. In peripheral blood mononuclear cells and small intestine, PXR-selective ligands have been shown to increase the expression of various transporters like solute carrier family 21A6 (SLC21A6), ABCC2 and ABCB and significant relationships between expression of PXR and ABCB1, ABCC2 and ABCG2 have been reported[25-27]. One recent study found that IL-1β and TNF-α induced ABCG2 and PXR expression and NF-κB activity in some breast cancer and normal cell lines, which indicated a probable relationship between ABCG2, PXR and NF-κB[25]. Pradhan et al[28] showed that the cooperative binding of ER and p65 at adjacent response elements led to a major increase in both ABCG2 mRNA and protein expressions. Wu et al[29] reported that prolactin could up-regulate ABCG2 in T-47D human breast cancer epithelial cells via activation of JAK2/STAT5, MAPK, and PI3K signaling. However, the precise mechanism of regulating the expression of ABCG2 caused by DNA damage remains unclear.
In conclusion, ABCG2 mRNA increased expression times driven by cisplatin in vitro is associated with clinical outcome, which may be a novel biological marker for optimizing the treatment of GC. However, our data should be interpreted cautiously because of the limited number of patients enrolled. This is the first report to indicate a relationship between the potential for acquisition of MDR and prognosis of patients with GC. Further prospective randomized controlled trials with a larger number of patients would be worth doing to confirm these results, and the mechanism of DNA damage caused by cisplatin also should be elucidated.
COMMENTS
Background
Gastric cancer (GC) ranks the fourth in morbidity among malignant tumors worldwide. Despite the advances made in diagnosis and treatment, the prognosis of GC patients remains poor. This is most probably attributed to the delayed diagnosis and resistance to currently available agents. Therefore, determining the markers which could predict the evidence for early clinical outcome and modify the therapeutic regimens is important.
Research frontiers
ABCG2 has been shown to represent a common mechanism of multidrug resistance in various cancers. Increasingly, it has become a hot research topic to identify the relevance of ABCG2 expression driven by chemotherapeutic agents and its clinical significance.
Innovations and breakthroughs
To date, there have been a limited number of studies regarding the potential for acquisition of MDR. In this study, the authors employed a method to assess the potential for acquisition of MDR driven by chemotherapeutic agents. Furthermore, the authors confirmed the significant correlation between the over-expression of ABCG2 and decreased overall survival rate.
Applications
By identifying the high expression of ABCG2 driven by cisplatin in vitro as being associated with overall survival, the authors evaluated the biological features and prognosis in GC, which could improve their understanding of GC, and provide a novel biological marker for optimizing the treatment of GC.
Terminology
ATP-binding cassette sub-family G member 2 is a protein that in humans is encoded by the ABCG2 gene. This protein transports various molecules across extra- and intra-cellular membranes, which may also play a role in multi-drug resistance to chemotherapeutic agents.
Peer review
The authors determined the expression of ABCG2 driven by cisplatin in vitro, and identified the high expression of this protein was associated with the prognosis of patients with GC. The result is interesting and indicates that the over-expression of ABCG2 could be used as a biomarker for prognosis in GC.
Footnotes
Supported by The Shanghai Municipal Health Bureau, No.20114296; The National Natural Science Foundation of China, No.30901738; Leading Academic Discipline Project of the State Administration of Traditional Chinese Medicine of China
P- Reviewer Imai N S- Editor Zhai HH L- Editor A E- Editor Ma S
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JS. Gastric cancer--new therapeutic options. N Engl J Med. 2006;355:76–77. doi: 10.1056/NEJMe068121. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Fan D. Multidrug resistance in gastric cancer: recent research advances and ongoing therapeutic challenges. Expert Rev Anticancer Ther. 2007;7:1369–1378. doi: 10.1586/14737140.7.10.1369. [DOI] [PubMed] [Google Scholar]
- 7.Schnepf R, Zolk O. Effect of the ATP-binding cassette transporter ABCG2 on pharmacokinetics: experimental findings and clinical implications. Expert Opin Drug Metab Toxicol. 2013;9:287–306. doi: 10.1517/17425255.2013.742063. [DOI] [PubMed] [Google Scholar]
- 8.Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3:1–27. [PMC free article] [PubMed] [Google Scholar]
- 9.Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, Nishiwaki Y, Kodama T, Suga M, Ochiai A. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 10.Niu Q, Wang W, Li Y, Ruden DM, Wang F, Li Y, Wang F, Song J, Zheng K. Low molecular weight heparin ablates lung cancer cisplatin-resistance by inducing proteasome-mediated ABCG2 protein degradation. PLoS One. 2012;7:e41035. doi: 10.1371/journal.pone.0041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diestra JE, Scheffer GL, Català I, Maliepaard M, Schellens JH, Scheper RJ, Germà-Lluch JR, Izquierdo MA. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 13.Usuda J, Ohira T, Suga Y, Oikawa T, Ichinose S, Inoue T, Ohtani K, Maehara S, Imai K, Kubota M, et al. Breast cancer resistance protein (BCRP) affected acquired resistance to gefitinib in a “never-smoked” female patient with advanced non-small cell lung cancer. Lung Cancer. 2007;58:296–299. doi: 10.1016/j.lungcan.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Galimberti S, Nagy B, Benedetti E, Pacini S, Brizzi S, Caracciolo F, Papineschi F, Ciabatti E, Guerrini F, Fazzi R, et al. Evaluation of the MDR1, ABCG2, Topoisomerases IIalpha and GSTpi gene expression in patients affected by aggressive mantle cell lymphoma treated by the R-Hyper-CVAD regimen. Leuk Lymphoma. 2007;48:1502–1509. doi: 10.1080/10428190701402895. [DOI] [PubMed] [Google Scholar]
- 15.Font A, Taron M, Gago JL, Costa C, Sánchez JJ, Carrato C, Mora M, Celiz P, Perez L, Rodríguez D, et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer. Ann Oncol. 2011;22:139–144. doi: 10.1093/annonc/mdq333. [DOI] [PubMed] [Google Scholar]
- 16.Damiani D, Tiribelli M, Michelutti A, Geromin A, Cavallin M, Fabbro D, Pianta A, Malagola M, Damante G, Russo D, et al. Fludarabine-based induction therapy does not overcome the negative effect of ABCG2 (BCRP) over-expression in adult acute myeloid leukemia patients. Leuk Res. 2010;34:942–945. doi: 10.1016/j.leukres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, Legrand O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 18.Benderra Z, Faussat AM, Sayada L, Perrot JY, Tang R, Chaoui D, Morjani H, Marzac C, Marie JP, Legrand O. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–7772. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 19.Tsunoda S, Okumura T, Ito T, Kondo K, Ortiz C, Tanaka E, Watanabe G, Itami A, Sakai Y, Shimada Y. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology. 2006;71:251–258. doi: 10.1159/000106787. [DOI] [PubMed] [Google Scholar]
- 20.Saglam A, Hayran M, Uner AH. Immunohistochemical expression of multidrug resistance proteins in mature T/NK-cell lymphomas. APMIS. 2008;116:791–800. doi: 10.1111/j.1600-0463.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim JE, Singh RR, Cho-Vega JH, Drakos E, Davuluri Y, Khokhar FA, Fayad L, Medeiros LJ, Vega F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod Pathol. 2009;22:1312–1320. doi: 10.1038/modpathol.2009.98. [DOI] [PubMed] [Google Scholar]
- 22.Furmaga WB, Tsongalis GJ, Wu AHB. Monitoring of subtherapeutic drug levels during cancer chemotherapy: a possible method to prevent acquired multidrug resistance. FASEB Journal. 2003;17:Abstract No. 384.387. [Google Scholar]
- 23.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18:1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng S, Piquette-Miller M. Regulation of transporters by nuclear hormone receptors: implications during inflammation. Mol Pharm. 2008;5:67–76. doi: 10.1021/mp700102q. [DOI] [PubMed] [Google Scholar]
- 25.Malekshah OM, Lage H, Bahrami AR, Afshari JT, Behravan J. PXR and NF-κB correlate with the inducing effects of IL-1β and TNF-α on ABCG2 expression in breast cancer cell lines. Eur J Pharm Sci. 2012;47:474–480. doi: 10.1016/j.ejps.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Mottino AD, Catania VA. Hepatic drug transporters and nuclear receptors: regulation by therapeutic agents. World J Gastroenterol. 2008;14:7068–7074. doi: 10.3748/wjg.14.7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albermann N, Schmitz-Winnenthal FH, Z’graggen K, Volk C, Hoffmann MM, Haefeli WE, Weiss J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol. 2005;70:949–958. doi: 10.1016/j.bcp.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan M, Bembinster LA, Baumgarten SC, Frasor J. Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NF{kappa}B cooperativity at adjacent response elements. J Biol Chem. 2010;285:31100–31106. doi: 10.1074/jbc.M110.155309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu AM, Dalvi P, Lu X, Yang M, Riddick DS, Matthews J, Clevenger CV, Ross DD, Harper PA, Ito S. Induction of multidrug resistance transporter ABCG2 by prolactin in human breast cancer cells. Mol Pharmacol. 2013;83:377–388. doi: 10.1124/mol.112.082362. [DOI] [PubMed] [Google Scholar]


