Abstract
Lgr5 marks adult stem cells in multiple adult organs and is a receptor for the Wnt-agonistic R-spondins (RSPOs). Intestinal, stomach and liver Lgr5+ stem cells grow in 3D cultures to form ever-expanding organoids, which resemble the tissues of origin. Wnt signalling is inactive and Lgr5 is not expressed under physiological conditions in the adult pancreas. However, we now report that the Wnt pathway is robustly activated upon injury by partial duct ligation (PDL), concomitant with the appearance of Lgr5 expression in regenerating pancreatic ducts. In vitro, duct fragments from mouse pancreas initiate Lgr5 expression in RSPO1-based cultures, and develop into budding cyst-like structures (organoids) that expand five-fold weekly for >40 weeks. Single isolated duct cells can also be cultured into pancreatic organoids, containing Lgr5 stem/progenitor cells that can be clonally expanded. Clonal pancreas organoids can be induced to differentiate into duct as well as endocrine cells upon transplantation, thus proving their bi-potentiality.
Keywords: beta cell, duct cell, pancreas, Wnt, stem cell
Introduction
As first demonstrated for intestinal crypts (Korinek et al, 1998), Wnt signalling plays a crucial role in the regulation of multiple types of adult stem cells and progenitors (Clevers and Nusse, 2012). The Wnt target gene Lgr5 marks actively dividing stem cells in Wnt-driven, continuously self-renewing tissues such as small intestine and colon (Barker et al, 2007), stomach (Barker et al, 2010) and hair follicles (Jaks et al, 2008). However, expression of Lgr5 is not observed in endodermal organs with a low rate of spontaneous self-renewal, such as liver or pancreas. In the liver, we have recently described that Wnt signalling is highly activated during the regenerative response following liver damage. Lgr5 marks an injury-induced population of liver progenitor cells capable of regenerating the tissue after injury (Huch et al, 2013).
In the adult pancreas, Wnt signalling is inactive (Pasca di Magliano et al, 2007), yet it is essential for its development during embryogenesis (Murtaugh et al, 2005; Heiser et al, 2006). The embryonic pancreas harbours multipotent progenitor cells that can give rise to all pancreatic lineages (acinar, duct and endocrine) (Zaret and Grompe, 2008). Injury to the pancreas can reactivate the formation of new pancreatic islets, called islet neogenesis, by mechanisms still not entirely understood but that resemble development of the embryonic pancreas (Bouwens, 1998; Gu et al, 2003). Lineage tracing studies have demonstrated that these ‘de novo beta cells’ can be derived from pre-existing beta cells (Dor et al, 2004), or by conversion of alpha cells, after almost 90% beta-cell ablation (Thorel et al, 2010). Also, severe damage to the pancreas, by means of partial duct ligation (PDL) or acinar ablation, can stimulate non-endocrine precursors, such as duct cells, to proliferate and differentiate towards acinar (Criscimanna et al, 2011; Furuyama et al, 2011), duct (Criscimanna et al, 2011; Furuyama et al, 2011; Kopp et al, 2011) and also endocrine lineages (including beta cells) (Xu et al, 2008; Criscimanna et al, 2011; Pan et al, 2013; Van de Casteele et al, 2013), suggesting the existence of a pancreas progenitor pool within the ductal tree of the adult pancreas.
The development of a primary culture system based on the adult, non-transformed progenitor pancreas cells would represent an essential step in the study of the relationships between pancreas progenitor cells, their descendants and the signals required to instruct them into a particular lineage fate. Also, the production of an unlimited supply of adult pancreas cells would facilitate the development of efficient cell replacement therapies. Most of the available pancreas adult stem cell-based culture protocols yield cell populations that undergo senescence over time unless the cells become transformed. It is fair to say that no robust, long-term culture system exists today that is capable of maintaining potent, clonal expansion of adult non-transformed pancreas progenitors over long periods of time under defined conditions. Recently, endoderm progenitors derived from embryonic stem cells (ESCs) (Cheng et al, 2012; Sneddon et al, 2012) or induced pluriportent stem cells (iPSCs) (Cheng et al, 2012) were serially expanded, in co-culture with pancreas mesenchyme or MEFs, respectively, and gave rise to glucose-responsive beta cells in vitro (Cheng et al, 2012) and glucose-sensing and insulin-secreting cells, when transplanted, in vivo (Sneddon et al, 2012).
We have recently described a 3D culture system that allows long-term expansion of adult small intestine, stomach and liver cells without the need of a mesenchymal niche, while preserving the characteristics of the original adult epithelium (Sato et al, 2009; Barker et al, 2010; Huch et al, 2013). A crucial component of this culture medium is the Wnt agonist RSPO1 (Kim et al, 2005; Blaydon et al, 2006), the recently reported ligand of Lgr5 and its homologues (Carmon et al, 2011; de Lau et al, 2011). Here, we describe that Wnt signalling and Lgr5 are strongly upregulated in remodelling duct-like structures upon injury by PDL. We exploit the Wnt-Lgr5-Rspo signalling axis to generate culture conditions that allow long-term expansion of adult pancreatic duct cells, which maintain the ability to differentiate towards both duct and endocrine lineages when provided the proper signals.
Results
Wnt signalling and Lgr5 expression are upregulated during pancreas regeneration following PDL
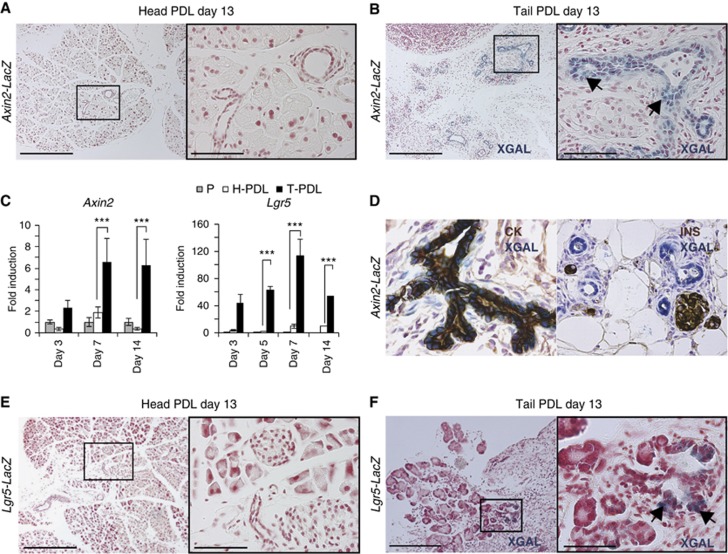
We first sought to document Wnt pathway activation in normal adult pancreas and following acute damage. We used the Axin2-LacZ allele as a general reporter for Wnt signalling (Leung et al, 2002; Lustig et al, 2002; Yu et al, 2005). In the head of a pancreas injured by PDL, where there is still healthy tissue, the reporter was inactive (Figure 1A), in agreement with the previous observations made with the TOPGAL Wnt reporter mice (DasGupta and Fuchs, 1999; Pasca di Magliano et al, 2007). However, after controlled injury by PDL (Watanabe et al, 1995; Xu et al, 2008), the Axin2LacZ reporter was highly activated along the ductal tree of the ligated part of the pancreas (Figure 1B). Axin2 activation in the pancreas was already detectable at day 3 post injury, as assessed by qPCR (Figure 1C). Co-labelling with duct (pancytokeratin, CK) and endocrine (insulin, INS) markers revealed that the Axin2 upregulation was restricted to the duct compartment (Figure 1D). Thus, pancreas injury by PDL led to activation of Wnt target genes in the proliferative duct cell compartment (Scoggins et al, 2000) during the regenerative response.
Figure 1.
Induction of Axin2 and Lgr5 expression upon damage on adult pancreas. (A, B) Axin2-LacZ induction in newly formed pancreatic ducts upon PDL. Axin2-LacZ mice (n=6) underwent PDL as explained in Materials and methods. Mice were sacrificed at the indicated time points and the non-ligated pancreatic tissue (Head-PDL) was separated from the ligated part (Tail-PDL). (A) Head-PDL and (B) Tail-PDL portion 13 days after injury. Arrows indicate XGAL-specific staining exclusively detected in the pancreatic ducts of the ligated pancreas. Scale bars 200 μm (A, B, left panels) and 50 μm (A, B, right panels). (C) qPCR analysis of Axin2 and Lgr5 mRNA in adult pancreas following PDL. Results are represented as mean±s.e.m. of at least three independent experiments. The Hprt housekeeping gene was used to normalize for differences in RNA input. Non-parametric Mann–Whitney test was used. ***P<0.0001. P, pancreas from a sham-operated mice; H-PDL, Head-PDL (non-affected area after PDL injury); T-PDL, Tail-PDL (affected area after PDL injury). (D) Representative image of a XGAL staining on an Axin2-LacZ pancreas after PDL, sections were co-stained either for pancytokeratin (CK), a duct cell marker, or for insulin (INS), an endocrine β-cell marker. XGAL staining (reflecting Axin2 expression) was detected exclusively in the pancreatic duct compartment. (E, F) Lgr5-LacZ induction in the ductal tree upon PDL. (E) Head PDL pancreas (n=6) do not show XGAL staining, indicating that Lgr5 is not expressed in non-injured pancreas. (F) Lgr5 reporter is detected (arrows) in the Tail-PDL portion of the ligated pancreas (n=6). Scale bars 200 μm (E, F, left panels) and 30 μm (E, F, right panels).
We have recently described that the Wnt target Lgr5 not only marks stem cells during physiological self-renewal (e.g., in the gut), but also marks a population of liver stem cells that is activated after liver damage (Huch et al, 2013). We utilized the Lgr5-LacZ knock-in allele (Barker, et al, 2007) to determine the expression of the Wnt target Lgr5 in the pancreas. Lgr5 is essentially undetectable in the head of a pancreas injured by PDL (non-ligated pancreas), in agreement with the absence of Wnt signalling in the tissue under homeostatic conditions (Figure 1E). However, in the tail of the pancreas upon PDL, we observed a significant Lgr5LacZ reporter activity in the duct cells of the ligated pancreas, starting at day 3 and peaking at day 7 after PDL (Figure 1C and F). No background staining was detected in wild-type mice following pancreas injury (Supplementary Figure S1). The appearance of de novo expression of Lgr5 following pancreas regeneration by PDL suggested that pancreatic Lgr5 expression may herald de novo activation of regenerative stem/progenitor cells by Wnt upon injury.
Pancreatic ducts self-renew in vitro
Given the induction of Wnt and Lgr5 after injury, and the existence of pancreas progenitors in the ductal tree (Criscimanna et al, 2011; Furuyama et al, 2011), we reasoned that adult pancreas progenitors could be expanded from the duct cell compartment under our previously defined gut and stomach organoid culture conditions (Sato et al, 2009; Barker et al, 2010). Cultures of heterogeneous populations of pancreas cells have been previously established and typically include factors such as EGF, HGF and Nicotinamide (Bonner-Weir et al, 2000; Ramiya et al, 2000; Deutsch et al, 2001; Seaberg et al, 2004; Rovira et al, 2010; Cardinale et al, 2011; Smukler et al, 2011). Most of these approaches yield cell populations that undergo senescence over time unless the cells are transformed.
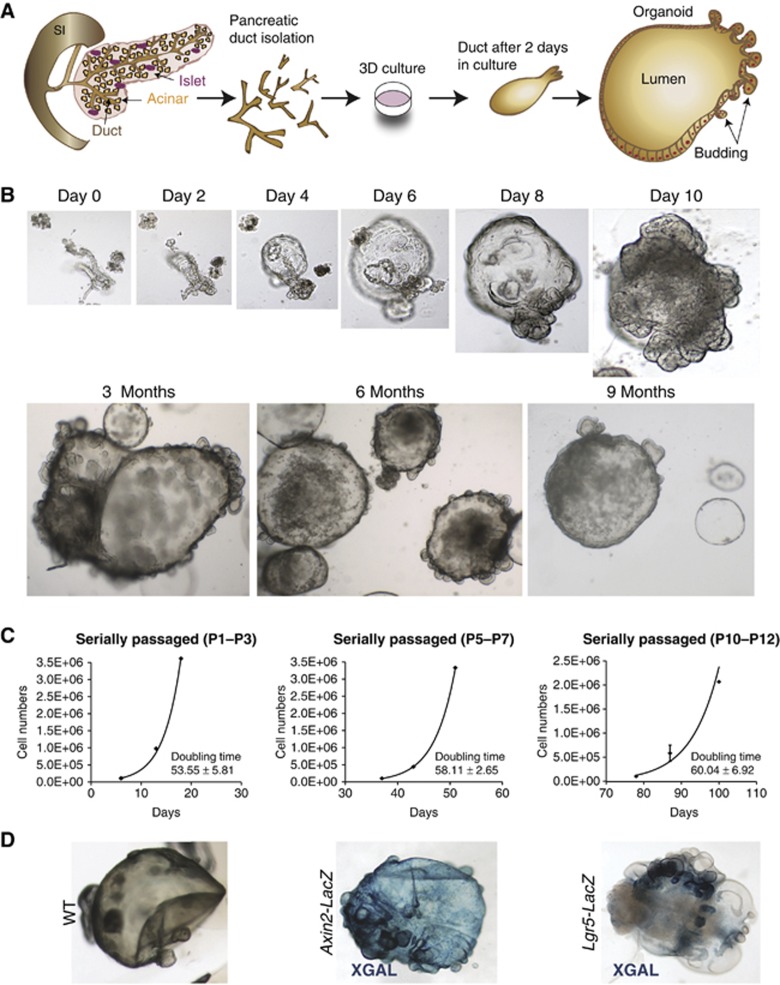
To establish pancreas cultures, isolated pancreatic duct fragments from adult healthy mice (Figure 2A) were embedded in Matrigel containing the ‘generic’ organoid culture factors EGF, RSPO1 and Noggin (Sato et al, 2009) to which FGF10 (Bhushan et al, 2001) and Nicotinamide were added. Under these conditions, small duct fragments formed closed structures within 24–48 h that expanded into budding cyst-like organoids (Figure 2B). The efficiency of cyst formation from isolated ducts and subsequent organoid formation was nearly 100%. Without EGF, RSPO1 or FGF10, the cultures deteriorated after 2–5 weeks (Supplementary Figure S2A). Noggin and Nicotinamide proved to be essential to maintain the cultures >2 months (∼passage 8) (Supplementary Figure S2A). The cultures maintained exponential growth with cell doubling times essentially unchanged during the culturing period (Figure 2C). Using these culture conditions, we have been able to expand the cultures by passaging at a 1:4–1:5 ratio weekly for over 10 months (Figure 2B). These culture conditions allowed the recovery of the cells after freezing and thawing. Of note, when transplanted into immunocompromised mice, the cultures did give rise only to ductal structures, and no tumour formation was detected in any of the mice analysed (n=5), confirming the non-transformed origin of the cultured cells (Supplementary Figure S2C and D). Also, the karyotype analysis revealed that chromosome numbers were essentially normal, even after >5 months in culture (Supplementary Figure S2B).
Figure 2.
Establishment of the pancreas organoids from adult pancreatic ducts. (A) Scheme representing the isolation method of the pancreatic ducts and the establishment of the pancreatic organoid culture. The pancreatic ducts were isolated from adult mouse pancreas after digestion, handpicked manually and embedded in matrigel. Twenty-four hours after, the pancreatic ducts closed and generated cystic structures. After several days in culture, the cystic structures started folding and budding. (B) Representative serial DIC images of a pancreatic organoid culture growing at the indicated time points. Magnifications: × 10 (days 0, 2, 4, 6, and 8) and × 4 (day 10 onwards). (C) Growth curves of pancreas cultures originated from isolated pancreatic ducts cultured as described in Materials and methods. Note that the cultures followed an exponential growth curve within each time window analysed. Graphs illustrate the number of cells counted per well at each passage from passages P1–P3 (left), P5–P7 (middle) and P10–P12 (right). The doubling time (hours) is indicated in each graph. Data represent mean±s.e.m., n=2. (D) Representative DIC images of XGAL staining in WT (left), Axin2-LacZ (middle) and Lgr5-LacZ (right) derived pancreas organoids.
Organoids generated from Axin2LacZ and Lgr5LacZ knock-in mice allowed localization of the Axin2- and Lgr5-positive cells. We observed XGAL staining in Axin2LacZ pancreas organoids throughout the cysts, whereas XGAL staining in the Lgr5LacZ-derived pancreas organoids was mainly restricted to small budding structures (Figure 2D). These results resembled the in vivo situation after pancreas injury by PDL, where only the ductal buds were Lgr5+, whereas the Axin2 reporter showed a broader expression pattern (compare Figure 1B versus Figure 1F).
Prospectively isolated single pancreatic duct cells but not endocrine or acinar cells self-renew long term in vitro
We then prospectively isolated the different pancreatic epithelial cells (duct, acinar and endocrine lineages) and cultured the different populations in our defined 3D culture system. A prospective isolation procedure that allows isolation of single cells of the different pancreatic epithelial cell types and maintenance of their viability in culture has not been established yet. The epithelial cell-surface marker EpCAM and the high concentration of Zn2+ in secretory granules of endocrine cells, that allows binding of the fluorescent chelator TSQ (6-methoxy-8-p-toluenesulfonamido-quilone), were used as a basis for cell isolation. Pancreas tissue from both WT or transgenic mice that constitutively and ubiquitously express eGFP (Okabe et al, 1997) was dissociated into single cells. After depletion of non-epithelial (EpCAM−) and haematopoietic cells (CD45+, CD31+), the cell suspension was FACS sorted in order to separate the granulated endocrine fraction (EpCAM+TSQ+) from the non-endocrine component (EpCAM+TSQ-) with high purity (>99.6%) (Figure 3A–D; Supplementary Figure S3A and B). To rule out the possibility that endocrine cells might de-granulate during the isolation procedure and thus contaminate the non-endocrine fraction, we repeated the protocol on pancreas cells obtained from mouse insulin promoter (Mip)-RFP mice and found no RFP+ cells in the non-endocrine fraction (Supplementary Figure S4A). The separated fractions were then tested for their ability to survive, proliferate and give rise to organoids under the above-defined conditions. Only the EpCAM+TSQ− exocrine cells were able to generate duct-like structures that gave rise to larger organoids (1–1.5% organoid formation efficiency) and had to be split once a week (Figure 3E). As expected, the growth pattern of the single sorted cells followed an exponential curve (Supplementary Figure S3D). The duct-derived cell cultures were maintained for >5 months (Figure 3E). The EpCAM+TSQ+ endocrine cells did not proliferate, but survived for at least 30 days in culture (Figure 3F).
Figure 3.
Isolation and in vitro expansion of single, endocrine-depleted pancreatic epithelial cells. (A) Representative fluorescence-activated cell sorting (FACS) plot illustrating the distribution of EpCAM+ and EpCAM− cells from dissociated adult mouse pancreas, following epithelial cell enrichment by magnetic beads as described in Materials and methods. (B) Representative FACS plot showing the distribution of EpCAM+ non-granulated TSQ− epithelial cells and EpCAM+ granulated TSQ+ endocrine cells. (C) EpCAM+TSQ+ and EpCAM+TSQ− sorted fractions were cytocentrifuged and immunostained (red) for Synaptophysin (SYP), Amylase (AMY) and pancytokeratin (CK); nuclei were counterstained with Hoechst 33342 (blue). Magnification: × 40. (D) Representative FACS analysis purity of sorted EpCAM+TSQ− cells indicating that this population is isolated with high purity (>99.6%). (E) EpCAM+TSQ−-sorted single cells were assessed for their growth potential in 3D expansion culture conditions: this population gave rise to organoids that could be expanded for many passages (>5 months). (F) EpCAM+TSQ+-sorted single cells were assessed for their growth potential under the same conditions: endocrine TSQ+ cells survived in culture but did not proliferate. Scale bars: 30 μm.
Acino-ductal metaplasia can happen under conditions of stress or following injury (Means et al, 2005; Blaine et al, 2010). To confirm that duct rather than acinar cells are the long-term expanded cells isolated from the EpCAM+TSQ− fraction, we traced the progeny of isolated duct (Sox9+) or acinar (Ptf1a+) cells in vitro. Transgenic mice with a Ptf1aCreER allele, that is exclusively expressed in the acinar compartment (Kopp et al, 2012; Pan et al, 2013), or mice carrying the Sox9CreER allele, that is expressed predominantly (but is not absolutely restricted to) the duct cell compartment (Furuyama, et al, 2011; Kopp et al, 2012) were crossed with Rosa26RYFP mice and subcutaneously injected with tamoxifen as described in Supplementary Figure S5A. After the washout period, the pancreas was dissociated and single Sox9YFP+ or Ptf1aYFP+ cells were FACS sorted and cultured in our defined pancreas culture medium (Supplementary Figure S5B–D). Only Sox9YFP+ cells grew into budding organoids that expanded long term in culture, even when starting from a single cell (Supplementary Figure S5D, top panel). By contrast, the cultures derived from Ptf1aYFP+ cells gave rise to smaller duct-like structures that were able to proliferate only for 3–4 passages, after which they arrested proliferation (Supplementary Figure S5D, bottom panel). In conclusion, these data indicated that the long-term expanding pancreas organoid cultures derive from duct cells.
Lgr5 cells sustain the growth of pancreas organoids that have a duct cell phenotype
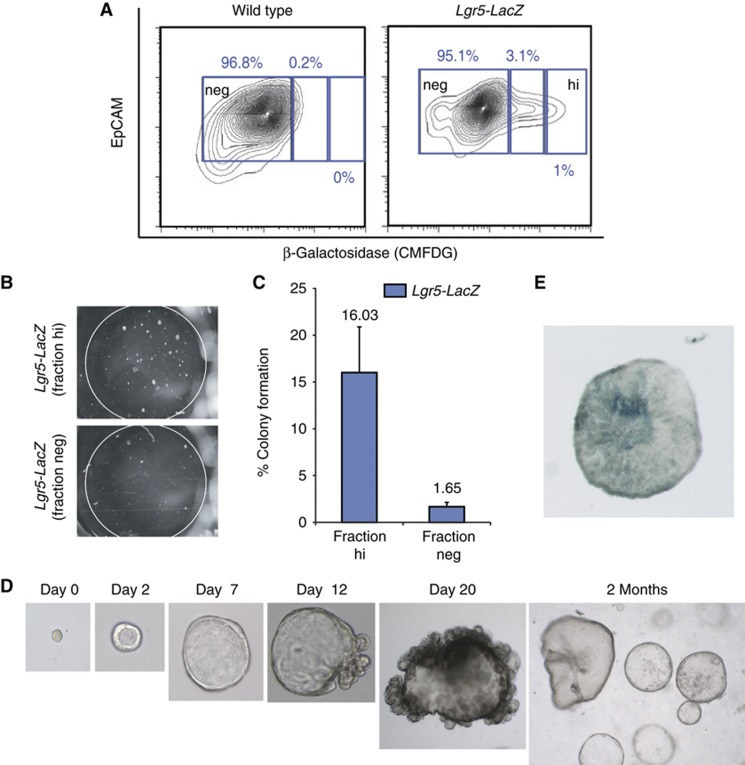
To test whether the Lgr5-expressing cells maintained the growth potential of the pancreas organoids, we sorted single Lgr5LacZ+ cells from in vitro expanded organoids derived from Lgr5-LacZ knock-in mice (Barker et al, 2007). Indeed, the isolated Lgr5+ cells grew and formed organoids (Figure 4A–E) that were subsequently expanded for >4 months in culture by splitting the cultures weekly at a 1:6–1:8 ratio. The colony formation efficiency was ∼16%, similar to the colony formation of Lgr5 cells of small intestine and stomach (Barker et al, 2010; Sato et al, 2011) (Figure 4C). Of note, 1.6% of the Lgr5neg-sorted population also grew into organoids (Figure 4C; Supplementary Figure S6A–D). These Lgr5neg-derived clones rapidly re-expressed Lgr5 (Supplementary Figure S6B and C) and expanded at a similar ratio as their Lgr5+ counterparts (Supplementary Figure S6D). This result mirrors the efficiency of colony formation of the FACS-sorted EpCAM+TSQ− exocrine cells from healthy tissue (1.65%, Lgr5neg versus 1–1.5%, exocrine cells). Overall, these results demonstrated that pancreas-derived Lgr5+ cells are capable of self-renewal and expansion in vitro, indicating that stem/progenitor cells can be activated both in organ-like structures and in secondary, single cell-derived organoids.
Figure 4.
Clonal expansion of single Lgr5 cells derived from Lgr5-LacZ pancreatic organoids. (A–E) Lgr5+ cells were sorted from Lgr5LacZ-derived pancreas organoids cultured for 20–25 days in our defined medium. Organoid formation efficiency was analysed 12 days after seeding. (A) Representative image of an FACS plot of wild-type (left) and Lgr5LacZ (right) pancreas organoids stained with Detectagene Green CMFDG (for detecting beta-galactosidase expression) and EpCAM (for selecting epithelial cells). (B) Representative image of cultures derived from 500 sorted cells from the high (Lgr5hi) or 500 sorted cells derived from the negative fraction (Lgr5neg) 12 days after seeding. (C) Graph showing the % of colony formation efficiency of the Lgr5hi and Lgr5neg fractions. (D) Representative serial DIC images showing the outgrowth of pancreatic organoids originated from a single Lgr5LacZ+cell. Magnifications: × 40 (days 1–2), × 20 (day 7), × 10 (days 12–20), × 4 (2 months). (E) Representative DIC image of XGAL staining in a 12-day-old clonal culture derived from Lgr5hi fraction. Magnification: × 10.
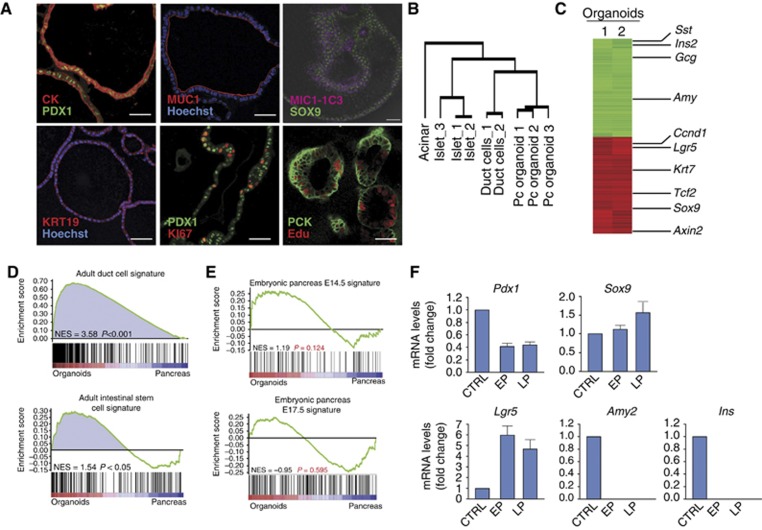
Organoids derived from single, FACS-sorted, Sox9+ duct cells or from single isolated Lgr5+ cells (FACS sorted from Lgr5LacZ cultures) allowed us to assess their lineage potential in vitro. Histologically, pancreas organoids displayed a duct-like phenotype characterized by a single-layered epithelium of cytokeratin-positive (CK) and MIC1-1C3-positive (Dorrell et al, 2008) cells (Figure 5A). Lgr5+ cells were readily detected in all organoids analysed (Figure 4E), similarly to what we had observed in the cultures derived from (non-clonal) duct fragments (Figure 2D). Ki67 and Edu staining demonstrated that only a subset of cells within the organoids proliferate (Figure 5A).
Figure 5.
Characterization of in vitro expanded organoids from single cells. Pancreatic organoids cultured in our defined medium show a ductal-like phenotype. FACS-sorted epithelial cells were isolated as shown in Figures 3 and 4, plated in matrigel and cultured for 7–9 passages before being processed for RNA and immunohistochemistry analysis. (A) Organoids were stained with anti-pancytokeratin (CK), anti-PDX1, anti-mucin 1 (MUC1), anti-SOX9, anti-cytokeratin19 (CK19) and anti-MIC1-1C3 that revealed their duct-like phenotype. Cells were proliferating, as illustrated by anti-Ki67 and Edu immunostaining. Scale bars: 35 μm. (B) Hierarchical clustering analysis of genes differentially expressed among pancreatic organoid cultures, duct, acinar and islet pancreatic lineages. Unsupervised hierarchical clustering analysis shows that the pancreas organoid arrays cluster with the ductal array while the acinar and islet profiles clustered in a separate tree. (C) Heat map of two independent clonal pancreas organoid cultures performed after subtracting pancreas tissue expression levels. Representative organoid-, duct-, endocrine- and exocrine-specific genes are listed on the right. Red, upregulated; green, downregulated. (D, E) Gene set enrichment analysis (GSEA) revealed that pancreas organoid cultures express a significant amount of genes contained in signatures of adult duct cells and intestinal stem cells (Muñoz et al, 2012) (D), while they were not enriched in gene sets representing embryonic pancreas at E14.5 or E17.5 (Juhl et al, 2008) (E). (F) Quantitative PCR showing relative fold changes of Pdx1, Sox9, Lgr5, Amy2 and Ins mRNA levels in partial duct ligated pancreas (CTRL) and cultured organoids at early (EP, passages 2–3) and late passages (LP, passages 7–9). Cyclophillin A was used to normalize for RNA input. Data represent mean±s.e.m. (n=2, independent cultures).
Then, we performed comparative gene expression profiling of 1- to 2-month-old cultures and compared it with the gene expression profile of adult duct, acinar and islet cells. The overall gene expression profile of the organoid cultures clustered with the duct cell arrays, whereas it did not cluster with the gene profiles of acinar or endocrine cells (Figure 5B). Of note, among the genes whose expression pattern did cluster between the duct pancreatic cells and the organoids we found Sox9, Krt7, Krt19 and Spp1 (full list is provided in Supplementary Dataset 1). Comparison of the gene expression profile of the pancreas organoids and the pancreatic tissue (by in silico subtraction) confirmed the segregation of the non-ductal pancreatic markers (like Sst, Ins2, Gcg and Amy) and the ductal markers (like Krt7, Tcf2 and Sox9) (Figure 5C; Supplementary Dataset 2). Of note, the Wnt target genes Lgr5, Ccnd1 and Axin2 were also specifically highly expressed in the organoids (Figure 5C; Supplementary Dataset 2). As expected, gene set enrichment analysis (GSEA) confirmed that the organoid cultures are enriched in genes specifically expressed in adult Sox9+ pancreatic duct cells (Figure 5D and Supplementary Dataset 3). Interestingly, we also observed enrichment in genes previously reported in small intestinal and pancreas stem cells, that is, Lgr5, Prom1, Sox9 and Lrig1 (Figure 5D and Supplementary Dataset 4) (Barker et al, 2007; Snippert et al, 2009, Furuyama et al, 2011; Wong et al, 2012), while we found no significant enrichment in genes expressed in the developing pancreas at E14.5 or E17.5 (Figure 5E and Supplementary Datasets 5 and 6), confirming the adult nature of our pancreas progenitor cultures. To confirm this expression pattern, we performed qPCR analysis in cultures at early and late passages (Figure 5F). While some genes could be detected in pancreas organoids over time (Pdx1, Sox9 and Lgr5), no acinar (Amy2) or endocrine (Ins) markers were observed over several passages (Figure 5F). Immunofluorescent staining confirmed that the organoids were mainly formed by cells expressing Keratin19 (KRT19), SOX9, MUCIN-1 and PDX1 (Figure 5A) while negative for the endocrine marker Synaptophysin (SYP) (Supplementary Figure S4B). Overall, these results confirmed the pancreas progenitor and duct-like nature of the pancreas organoid cultures.
Expanded organoids give rise to both pancreatic endocrine and duct cells in vivo
The embryonic pancreas harbours all necessary factors and appropriate environmental cues to support the differentiation of bona fide pancreas progenitors to mature exocrine and endocrine cells in situ or when the embryonic pancreas is transplanted under the kidney capsule of an immunodeficient mouse (Zaret and Grompe, 2008). Therefore, to assess whether the organoid cells are capable of differentiating towards fully mature endocrine lineages (e.g., insulin producing cells) we developed a whole-organ morphogenetic assay based on the re-aggregation of dissociated cells from embryonic pancreas on one hand and organoids generated from adult pancreas on the other hand (Figure 6A; Supplementary Figure S7B). This type of morphogenetic assay has successfully been used to demonstrate fate potency of both skin and thymic epithelial stem cells after expansion in vitro (Bonfanti et al, 2010). When embryonic pancreas derived from either mouse (E13.5) or rat (E14) was isolated, dissociated, re-aggregated and then transplanted under the kidney capsule of an immune-deficient mouse, the embryonic tissue fully developed into the three mature pancreas lineages: duct, acinar and endocrine cells (Supplementary Figure S7A).
Figure 6.
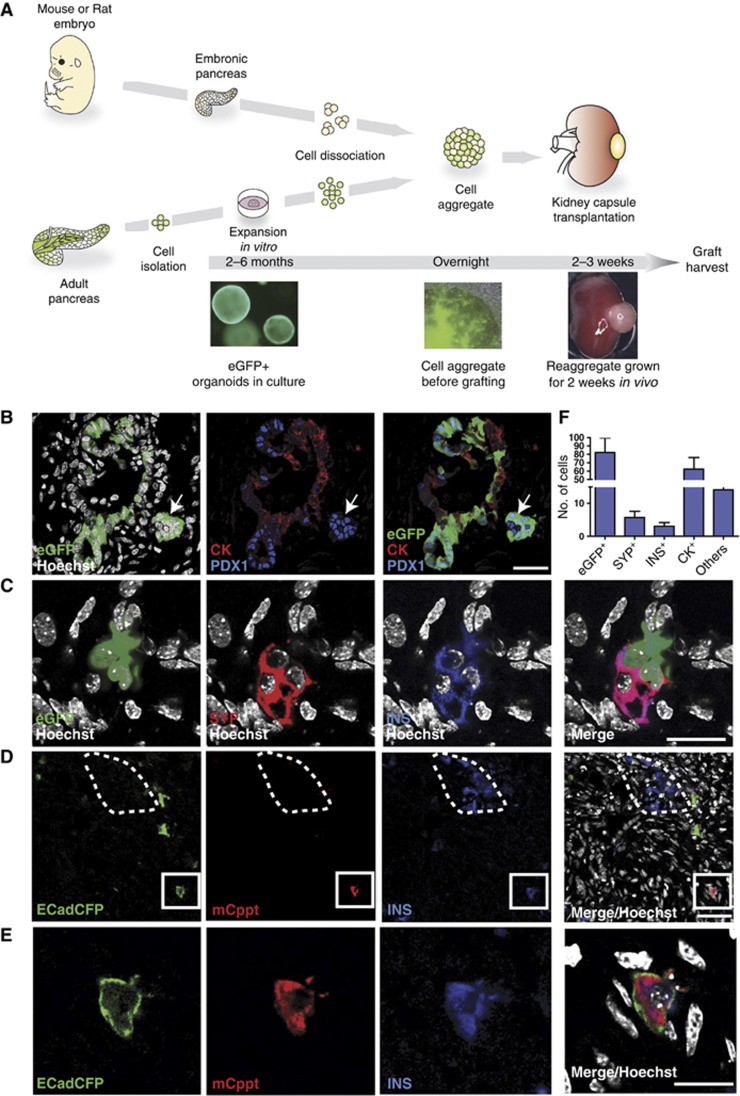
In vitro expanded organoids from single epithelial cells give rise to endocrine and duct cells when grafted in vivo in a developing pancreas. (A–F) Pancreas organoid cultures were derived from CAGeGFP+ mice or ECadCFP+ mice as described in Figure 3 and Supplementary Figure S3. The cultures were clonally expanded in vitro for 4–6 passages before dissociation into single cells. Dissociated eGFP+ or ECadCFP+ cells were re-aggregated with WT embryonic E13 mouse (B, C) or E14 rat (D, E) pancreas. The re-aggregates were kept on a filter membrane O/N and then grafted under the kidney capsule of nude mice. The grafts were harvested and analysed 2–3 weeks after. The re-aggregates consistently grew and gave rise to pancreatic tissue, as illustrated in Supplementary Figure S7A. (A) Schematic representation of the pancreatic morphogenetic assay. (B) Representative confocal microscopy image showing incorporation of eGFP+ cells (green) into pancytokeratin+ (CK, red) pancreatic duct structures; these eGFP+ cells also express low levels of PDX1 (blue). Other eGFP+ cells (white arrow) aggregated in islet-like structures near the ducts, downregulated CK and expressed high levels of PDX1 (blue). Scale bar: 35 μm. (C) Confocal microscopy demonstrates that cultured eGFP+ (green) cells differentiate into beta cells and express both synaptophysin (SYP, red) and insulin (INS, blue). Scale bar: 20 μm. (D) Confocal microscopy image illustrating mouse Insulin+ Cpeptide+ (INS+Cppt+) cells derived from ECadCFP+-grafted cells. Note that the INS+Cppt+ cells are incorporated into an embryonic rat pancreas, where rat INS+ cells are negative for mouse-specific Cppt staining (dotted line). Scale bar: 30 μm. (E) High magnification of an ECad+INS+Cppt+-grafted cell (D) that displays CFP membrane localization and cytoplasmic staining for INS and mouse Cppt. Scale bar: 20 μm. (F) Histogram showing the average quantification of differentiation of eGFP+ cells engrafted into each re-aggregate under the kidney capsule. Endocrine (SYP+), Insulin (INS+), duct cells (CK+); others: cells expressing neither duct nor endocrine markers. Average number/graft ±s.e.m. n=11 grafts from six independent cultures.
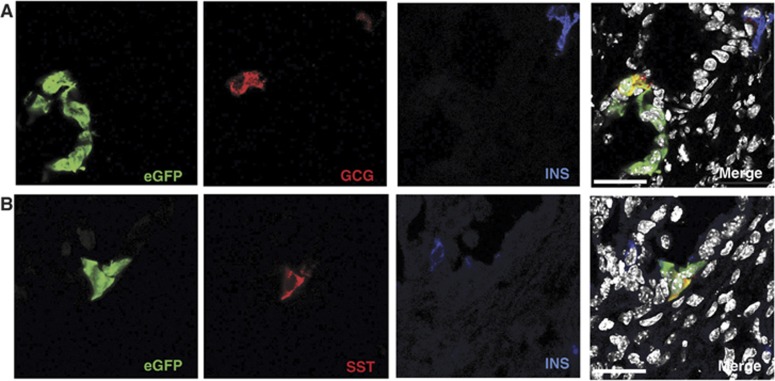
Therefore, we isolated EpCAM+ TSQ− GFP+ epithelial cells from the pancreas of CAGeGFP adult mice (Okabe et al, 1997) as described above and expanded for at least 6 weeks (Supplementary Figure S3A–C), dissociated them into a single cell suspension and re-aggregated with embryonic E13 or E14 WT mouse or rat pancreas, respectively. The re-aggregates were kept overnight on a membrane and, the day after, were grafted under the kidney capsule of nude mice. After 2 or 3 weeks, mice were sacrificed and grafts harvested (Figure 6A; Supplementary Figure S7B). The transplanted re-aggregates did consistently grow pancreatic structures organized in both exocrine and endocrine areas, several of which contained eGFP+ integrated cells (Figure 6B and C; Supplementary Figure S7C and D). Immunohistochemical analysis of the re-aggregates revealed that eGFP+ cells mainly contributed to duct cells (Figure 6B and F). Of note, some eGFP+ cells, that located outside of the ducts, downregulated cytokeratin expression and contained high level of PDX1 protein, a feature of beta cells (Figure 6B). On the basis of their expression of synaptophysin, ∼5% of integrated eGFP+ cells were of endocrine nature, 50% of which were also insulin+ (Figure 6C and F). Quantification revealed that eGFP+ cells differentiated into duct cells at a frequency of 70% (Figure 6F; Supplementary Figure S7F). It is important to remark that these percentages roughly correspond to those found in the differentiating embryonic pancreas in vivo. More importantly, we obtained the same results using a different reporter mouse that expresses CFP under the control of E-Cadherin promoter (ECadCFP) (Figure 6D and E; Supplementary Figures S7E and S8). We found that INS+ cells derived from cultivated organoids either from CAGeGFP or from ECadCFP reporter mice were functional and expressed C-peptide (Cppt) protein (Figure 6D and E; Supplementary Figure S7E). Cells with CFP membrane localization and cytoplasmic expression of both INS and mouse-specific C-peptide were readily detected throughout the grafted area, even when the organoid cells were engrafted in a rat pancreas microenvironment, where endogeneous INS+ cells were negative for the mouse/specific anti-Cppt antibody (Figure 6D and E; Supplementary Figure S8C). This last result excluded the possibility of fusion between mouse-cultivated eGFP+ or CFP+ cells and WT rat endocrine cells. The specificity of this antibody both in the ectopic rat pancreas and in the adult rat pancreas is shown in Figure 6D and Supplementary Figure S8B and C. Furthermore, the cultivated eGFP+ cells also gave rise to other endocrine lineages, such as Glucagon+ (GCG+) and Somatostatin+ (SST+) cells (Figure 7A and B). These cells were negative for INS, demonstrating that they had fully differentiated into mono-hormonal endocrine cells (Figure 7A and B).
Figure 7.
In vitro expanded organoids give rise to Glucagon (GCG) and Somatostatin (SST) mono-hormonal cells in vivo. Pancreas organoid cultures derived from sorted eGFP+ cells and expanded for at least 2 months in culture were grafted as described in Figure 6. Two weeks after transplantation, the kidney grafts were harvested and the grafted eGFP+ cells were evaluated for the expression of glucagon (GCG) and Somatostatin (SST). (A, B) Representative images illustrating that eGFP+ cells (green) differentiate towards GCG+ (A) or SST+ (B) cells in vivo. Note that both Gcg+ and SST+ cells do not express INS (insulin, blue), indicating that the organoid-derived eGFP+ cells have fully differentiated into mono-hormonal cells in vivo. Scale bars=20 μm.
Then, we assessed whether a less permissive environment would also allow the adult expanded progenitor duct cells to achieve an endocrine cell fate. We directly transplanted ∼2-month-old adult duct pancreas cultures derived from both Bl6 WT mice and EcadCFP mice into the kidney capsule of immunodeficient mice. We previously primed the 2-month-old cultures to express early endocrine markers by culturing them, 15 days prior to the transplantation, in a medium previously reported to allow ESC to acquire an endocrine fate (D’Amour et al, 2006; Kroon et al, 2008), with some modifications. We included the small molecule inhibitor ILV combined with FGF10, to induce Pdx1 expression (Bhushan et al, 2001; Chen et al, 2009), followed by DBZ treatment, to inhibit notch signalling (Milano et al, 2004) (Supplementary Figure S9A). This medium facilitated the expression of early endocrine progenitor markers (Neurogn3 and Chga) while retained the expression of the ductal marker Sox9, and suppressed Lgr5 (Supplementary Figure S9B). One month after transplantation, duct-like structures formed by Krt19+ cells were readily detectable throughout the graft (Supplementary Figure S9C). Also, albeit at much lower efficiency, Insulin+ and Cpeptide+ cells (Supplementary Figure S9C, D and E), as well as ChgA+ cells were detected (Supplementary Figure S9F).
Overall, these results conclusively demonstrate that cultured organoids derived from either sorted adult duct cells (CAGeGFP or ECadCFP mice) or from freshly isolated ducts (Bl6 WT mice) are able to acquire both duct and endocrine fates, thus demonstrating their progenitor nature and bi-potency.
Discussion
The pancreas is a glandular organ that serves two important functions: the production of the digestive enzymes and the production of the hormones responsible of glucose homeostasis. This is mirrored in the wide range of pancreas diseases that vary from pancreatic cancer to disorders related to the glucose homeostasis, such as diabetes. While pancreas cancer is the result of the accumulation of oncogenic mutations in different epithelial cell types of the pancreas, diabetes is the result of severe reduction in functional beta-cell mass. The lack of primary culture systems capable of long-term expansion of primary tissue in vitro hampers the development of therapeutic strategies for pancreas diseases. The replacement of functional pancreatic beta cells may be envisioned as a potential definitive cure for diabetes. Unfortunately, human islet transplantation is hampered by the scarcity of donors and the need for immune suppression and also by graft failure (Lysy et al, 2012). Therefore, alternative sources for cell therapy replacement hold promise as a potential treatment for diabetes.
ESCs and iPSCs can be differentiated towards beta cells in vitro (D'Amour et al, 2006; Zhang et al, 2009; Nostro et al, 2011; Cheng et al, 2012) and in vivo (Soria et al, 2000; Kroon et al, 2008; Sneddon et al, 2012), but the reproducibility of such procedures has been limited (Lysy et al, 2012). In addition, undifferentiated ESCs and iPSCs are prone to form teratomas upon transplantation in vivo, therefore any remaining undifferentiated cell must be completely removed prior to be used for transplantation. Adult pancreas progenitors able to expand long term in vitro while maintaining the potency to differentiate towards a duct or endocrine fate would potentially not encounter these limitations.
We report here that damage of adult pancreas results in the upregulation of Wnt signalling and expression of the stem-cell marker Lgr5 in the neo-formed ducts. We exploit this Wnt-driven regenerative response to define a culture medium based on the Wnt activation (RSPO1) that allows the unlimited expansion of duct fragments or even single isolated cells in a defined medium without serum. Under these conditions, pancreatic duct cells upregulate the stem-cell marker Lgr5 (receptor for RSPO1), and self-renew while maintaining their genetic stability. Importantly, when the expanded adult progenitor cells receive the appropriate differentiation signals, as for instance the signals present in a developing embryonic pancreas, they are able to integrate into both exocrine and endocrine structures that express functional markers, demonstrating that they carry the hallmarks of bi-potent progenitors.
Confirming the importance of the Wnt/Rspo signalling to facilitate the proliferation of pancreatic adult cells, Jin et al reported (while this study was under revision) that Rspo supplementation to a 3-week pancreatic culture facilitates the expansion of pancreas cells into heterogeneous cultures. The otherwise non-defined medium contains fetal bovine serum and ESC-derived conditioned medium (Jin et al, 2013).
Thus, the conditions here described, based on the induction of the Wnt-Lgr5-Rspo axis, allow the long-term in vitro expansion of pancreas progenitors. The unlimited expansion potential of the adult progenitor cells may open avenues for building patient-derived disease models, as well as the development of regenerative strategies based on the expansion of adult, genetically non-modified, pancreas cells. Future optimization of the differentiation conditions may allow the generation of high numbers of specialized and functional pancreatic cells to be used for the treatment of pancreas diseases such as diabetes.
Materials and methods
Mice lines and injury models
Generation and genotyping of the Lgr5LacZ and ECadCFP mice is already described in Barker et al (2007) and Snippert et al (2010), respectively. Axin2LacZ mice were obtained from EMMA (European Mouse Mutant Archive, Germany). C57BL/6-Tg(ACTB-EGFP)1Osb/J, Sox9CreER and Ptf1aCreER mice were previously described (Okabe et al, 1997; Furuyama et al, 2011; Pan et al, 2013) and MipRFP mice were provided by Gérard Gradwohl (IGBMC, Strasbourg, France). Wild-type Sprague–Dawley (OFA) rats and OF1 mice were obtained from Janvier. Athymic (Swiss Nu−/−) were supplied by Charles River Breeding Laboratories NSG mice (Jackson Laboratory, Bar Harbor, MA, USA). All animal experiments were performed in accordance with the institutional review committee at the Hubrecht Institute and the VUB. Animals were maintained in a 12-h light cycle providing food and water ad libitum.
To induce pancreas injury, 3- to 6-month-old mice were anaesthetized, with a mixture of fluanisone:fentanyl:midazolam injected intraperitoneally at a dosage of 3.3, 0.105 and 1.25 mg/kg, respectively. Following a median incision on the abdominal wall, the pancreas was exposed and, under a dissecting microscope, the pancreatic duct was ligated as described (Xu et al, 2008).
For Sox9 and Ptf1a lineage labelling, tamoxifen (Sigma, T5648) was prepared at the concentration of 10 mg/ml in corn oil (Sigma, C8267). A total dose of 20 mg of tamoxifen was given subcutaneously in five doses of 4 mg over a 10-day period. A washout period of 14 days preceded pancreas harvesting and dissociation into single cells.
Pancreas organoid cell culture
Pancreatic ducts were isolated from the bulk of the pancreas of mice older than 8 weeks by collagenase dissociation (Collagenase type XI 0.012% (w/v) (Sigma), dispase 0.012% (w/v) (Gibco), FBS (Gibco) 1% in DMEM media (Gibco)) at 37°C. Isolated ducts were mixed with Matrigel (BD Bioscience) and seeded and cultured as we described previously (Sato et al, 2009; Barker et al, 2010). After Matrigel formed a gel, culture medium was added. Culture media was based on AdDMEM/F12 (Invitrogen) supplemented with B27 (Invitrogen), 1.25 mM N-Acetylcysteine (Sigma), 10 nM gastrin (Sigma) and the growth factors: 50 ng/ml EGF (Peprotech), 10% RSPO1-conditioned media (kindly provided by Calvin Kuo), 100 ng/ml Noggin (Peprotech) or 10% Noggin-conditioned media (in-house prepared), 100 ng/ml FGF10 (Peprotech) and 10 mM Nicotinamide (Sigma). One week after seeding, organoids were removed from the Matrigel, mechanically dissociated into small fragments, and transferred to fresh Matrigel. Passage was performed in a 1:4–1:8 split ratio once per week for at least 9 months. To prepare frozen stocks, organoid cultures were dissociated and mixed with Recovery cell culture freezing medium (Gibco) and froze following the standard procedures. When required, the cultures were thawed using standard thawing procedures, embedded in Matrigel and cultured as described above. For the first 3 days after thawing, the culture medium was supplemented with Y-27632 (10 μM, Sigma-Aldrich).
Prospective isolation and pancreas organoid single cell (clonal) culture
For clonogenic assays, whole pancreata were harvested from adult (8–12 weeks) mice and individually digested by collagenase type XI (0.3 mg/ml, Sigma) incubation at 37°C in a shaking incubator, and then dissociated into single cells by addition of trypsin (1 mg/ml, Sigma) and DNAse (0.4 mg/ml, Roche); cell suspension was filtered through a 70-μm cell strainer. Cell pellets were incubated with anti-mouse EpCAM/APC antibody (eBiosciences) for 30′ on ice. Cells were either processed directly for FACS sorting or were enriched for epithelial cells using magnetic beads (EasySepTM APC Positive selection kit or Epithelial enrichment kit; STEMCELL Technologies Inc.). Cells were re-suspended in a solution containing propidium iodide (PI, 1 mg/ml, Sigma), and N-(6-Methoxy-8-Quinolyl)-p-Toluenesulfonamide (TSQ, 1 mg/ml, Molecular Probes) and sorted on an FACSAria (Becton Dickinson). Clean separation between EpCAM+TSQ− and EpCAM+TSQ+ cell populations was confirmed by a second FACS analysis and immunocytochemistry. According to the mouse strain, an additional gate for eGFP or YFP signal was used for sorting cells. Pulse-width gating excluded cell doublets while dead cells were excluded by addition of PI and gating on the negative cells.
For secondary clonal cultures, established cultures were dissociated into single cells and stained with the DetectaGene Green CMFDG LacZ Gene Expression Kit (Molecular Probes) according to the manufacturer’s instructions. PI staining was used to label dead cells and FSC: pulse-width gating to exclude cell doublets.
Sorted cells (EpCAM+TSQ−, EpCAM+TSQ+ or Lgr5LacZ+) were embedded in Matrigel and seeded in 96-well plates at a ratio of 1 sorted cell/well. Cells were cultured in the pancreas media described above supplemented with Y-27632 (10 μM, Sigma-Aldrich) for the first 4 days. Passage was performed in split ratios of 1:4–1:5 once per week for at least 6 months.
In vitro growth curves
Expansion ratios were calculated from both sorted cells and duct fragments as follows: pancreas organoid cultures or 20 × 103 sorted cells were grown in our defined medium for 7 days. Then, the cultures were dissociated by incubation with TrypLE Express (Gibco) until single cells. Cell numbers were counted by trypan blue exclusion at the indicated time points. From the basic formula of the exponential curve y(t)=y0 × e(growth rate × t) (y=cell numbers at final time point; y0=cell numbers at initial time point; t=time) we derived the growth rate. Then, the doubling time was calculated as doubling time=ln(2)/growth rate for each time window analysed.
Karyotyping
Organoid cultures in exponential growing phase were incubated for 1–1.5 h with 0.05 μg/ml colcemid (Gibco). Then, cultures were dissociated into single cells using TrypLE express (Gibco) and processed as described (Huch et al, 2013). Chromosomes from 100 metaphase-arrested cells were counted.
Pancreatic morphogenetic assay
Pancreatic aggregates were obtained following a previously described protocol (Bonfanti et al, 2010), modified as follows. E13 mouse embryos (OF1) or E14 rat embryos (SD) were harvested from the uteri under sterile conditions, transferred in 100 mm Petri dishes containing HBSS supplemented with 10% FCS and stored on ice. Pancreatic tissue was removed from the embryonic abdomen and transferred into a solution containing collagenase type XI (1 mg/ml, Sigma) and DNAase (0.4 mg/ml, Roche Diagnostic) for about 5 min. A known number (from 75 × 103 to 105) of GFP-labelled single cells dissociated from in vitro expanded adult organoids were mixed with an ∼10-fold excess of unlabelled embryonic pancreatic cells. Aggregates were then transferred on a 0.8-μm Isopore membrane filter (Millipore) and incubated at 37°C for 24 h in RPMI medium supplemented with 10% FCS, before being grafted under the kidney capsule of nude mice as previously described (Bonfanti et al, 2010). Two to four weeks later, the grafts were harvested and processed for cryosection and immunohistochemistry.
Pancreas organoid differentiation and kidney capsule transplantation
Pancreas organoids derived from Bl6 isolated ducts or eGFP+- or CFP+-sorted cells were expanded in vitro for at least 2 months in our defined culture medium (EM) as described above. Then, the organoids were transferred into a differentiation medium (DM) to enhance their endocrine fate. To define the differentiation medium, we adapted the protocols already described by D'Amour et al (2006) and Chen et al (2009) as follows: organoids grown in Matrigel, in our defined expansion medium (EM), were removed from the Matrigel by using BD cell recovery solution (BD Biosciences), following the manufacturer’s instructions, and transferred to suspension plates. The cells were maintained for 3 days in RPMI medium supplemented with 0.2% FBS and 100 ng/ml Activin A (Tocris BioScience). Then, the medium was changed to RPMI supplemented with 300 nM ILV (indolactam-V) (Tocris BioScience), 100 ng/ml FGF10 (Peprotech) and 2% FBS for 4–5 days. After, the medium was replaced by DMEM supplemented with 1% B27, Noggin (50 ng/ml), Retinoic Acid (2 μM) and KAAD-cyclopamine (0.25 μM) for the following 6 days. Finally, for the last 2–4 days prior to transplantation, the medium was changed to DMEM supplemented with 1% B27 and 10 μM DBZ (Tocris BioScience). During all the differentiation protocol, the cells were kept in suspension plates. After the last 2–4 days in DBZ supplemented medium, the organoids were collected and transplanted directly into the kidney capsule of nude mice using standard procedures. The grafts were allowed to grow for 1 month and then were harvested and processed for paraffin embedding and immunohistochemistry.
To determine any potential transformation of the cells, pancreas organoids derived from Bl6 mice and cultured in our defined medium for at least 2 months were also directly transplanted into the kidney capsule of nude, SCID or NSG mice. The grafts were harvested 2 weeks and 3 months later and were processed for paraffin section and H&E staining using standard techniques.
β-galactosidase (LacZ) staining, immunohistochemistry and immunoflorescence
Tissues were fixed for 2 h in ice-cold fixative (1% Formaldehyde; 0.2% Glutaraldehyde; 0.02% NP-40 in PBS0) and incubated O/N at RT with 1–2 mg/ml of X-gal (bromo-chloro-indolyl-galactopyranoside) solution as we described in Barker et al (2010). The stained tissues were transferred to tissue cassettes and paraffin blocks were prepared using standard methods. Tissue sections (4 μM) were prepared and counterstained with neutral red. For immunohistochemistry, tissues and organoids were fixed using formalin 4%, and stained using standard histology techniques as described (Barker et al, 2010). The antibodies and dilutions used are listed in Supplementary Table SI. Stained tissues were counterstained with Mayer’s Hematoxylin. Pictures were taken with a Nikon E600 camera and a Leica DFDC500 microscope (Leica). For whole-mount immunofluorescence staining, organoids were processed as described in Barker et al (2010).
Tissue sections (4 μM) or cryosections from kidney capsule grafts were processed for immunofluorescent staining using standard procedures. For the paraffin-embedded kidney capsule grafts citrate retrieval was performed. Antibodies and dilutions are listed in Supplementary Table SI. Nuclei were stained with Hoechst33342 (Molecular Probes).
Microarray
For the expression analysis of pancreas cultures, total RNA was isolated from Sox9+ duct cells (isolated as described in Supplementary Figure S5), acinar and islets cells (prepared from whole pancreas after collagenase dissociation), whole adult pancreas and pancreas organoids cultured in our defined medium, using Qiagen RNAase kit following the manufacturer’s instructions. Five hundred nanograms of total RNA were labelled with the low RNA Input Linear Amp kit (Agilent Technologies, Palo Alto, CA). Universal mouse Reference RNA (Agilent) was differentially labelled and hybridized to the tissue or cultured samples. A 4 × 44K Agilent Whole Mouse Genome dual colour Microarray (G4122F) was used. Labelling, hybridization and washing were performed according to Agilent guidelines. Microarray signal and background information were retrieved using the Feature Extraction software (V.9.5.3, Agilent Technologies). The hierarchical clustering analysis was performed in duct, acinar, islet and organoid arrays after in silico subtraction of the pancreas gene array. A cutoff of two-fold differentially expressed was used for the clustering analysis. GSEA was performed according to Subramanian et al (2005). The gene lists and gene sets used for the analysis are all provided in Supplementary Datasets 1–6. GEO accession number is GSE50103.
RT-PCR and qPCR analysis
RNA was extracted from cell cultures or freshly isolated tissue using the RNeasy Mini RNA Extraction Kit (Qiagen) or TRIzol (Invitrogen) respectively, and reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen). All targets were amplified (40 cycles) using gene-specific Taqman primers and probe sets (Applied Biosystems, London, UK). Data were analysed using the Sequence Detection Systems Software, Version 1.9.1 (Applied Biosystems). For Neurog3, cDNA was amplified in a thermal cycler (GeneAmp PCR System 9700; Applied Biosystems) as previously described (Huch et al, 2009). Primers used are listed in Supplementary Table SII.
Image analysis
Images of cultivated cells were acquired using either a Leica DMIL microscope and a DFC420C camera or a Nikon TE2000 inverted automated fluorescence microscope with motorized table and controlled by the NIS elements AR software. Immunofluorescence images were acquired using an upright Zeiss Axioplan2 fluorescence microscope with Hamamatsu C10600 ORKA-R2 camera or a confocal microscope (Leica, SP5) or a confocal microscope (Leica, SP8) or a confocal multiphoton Zeiss LSM710 NLO with the TiSa laser microscope. Images were analysed using the Leica LAS AF Lite software (Leica SP5 confocal) or Smartcapture 3 (version 3.0.8). Confocal images were processed using Improvision VolocityLE and Zeiss Zen softwares.
Data analysis
All values are represented as mean±standard error of the mean (s.e.m.). Mann–Whitney non-parametric test was used. P<0.05 was considered as statistically significant. In all cases, data from at least three independent experiments were used. All calculations were performed using the SPSS package.
Supplementary Material
Acknowledgments
We thank Maaike van den Born, Stieneke van der Brink, Ann Demarre, Gunter Leuckx and Geert Stangé for technical assistance. The Hubrecht Imaging Center for imaging assistance. This work was supported by grants to MH (EU/236954) and SFB (EU/232814) and JHvE (Ti Pharma/T3-106), PB (EMBO fellowship, EFSD/JDRF grant), HH (EU-HEALTH-F5-2009-241883, Dutch Diabetes Foundation 2007.16.001, NFSR G000609N10, NIH 1U01DK089571-01, support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 155005 (IMIDIA), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies in kind contribution). The Hubrecht Institute received financial support from the DON Foundation and Dutch Diabetes Research Foundation.
Author contributions: Experiments were conceived and designed by MH, PB, SFB, TS, HH, and HC. All experiments were performed as follows: MH, PB, TS: PDL and histology; MH, PB, SFB, TS: pancreatic duct cultures and in vitro characterization; MH: clonal culture of Lgr5 cells; microarray analysis; PB: prospective isolation and culture of purified clonal populations; morphogenetic in vivo assay; SFB: in vitro differentiation; microarray analysis; TS: development of the culture system; MH, PB, SBJ: direct transplantation. Lgr5 sortings were performed by MvdW. AG and KH helped with PCR experiments and karyotypes. MS helped with lineage labelling mice and cell cultures. JM helped in the establishment of the differentiation protocol. CJML and FR performed kidney transplants. HB helped with the immunohistochemistry analysis of the kidney grafts. JHvE, EdK and RGJV discussed the project. MH, SFB, PB and TS analysed the data. JS and VSWL help with the microarray data. MH, SFB, PB, HH and HC wrote the manuscript, the other authors commented the manuscript.
Footnotes
MH, TS and HC are inventors on a patent application related to this work.
References
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R (2001) Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 128: 5109–5117 [DOI] [PubMed] [Google Scholar]
- Blaine SA, Ray KC, Anunobi R, Gannon MA, Washington MK, Means AL (2010) Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 137: 2289–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP (2006) The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 38: 1245–1247 [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y (2010) Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466: 978–982 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ (2000) In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA 97: 7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L (1998) Transdifferentiation versus stem cell hypothesis for the regeneration of islet beta-cells in the pancreas. Microsc Res Tech 43: 332–336 [DOI] [PubMed] [Google Scholar]
- Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, Inverardi L, Dominguez-Bendala J, Ricordi C, Gerber D, Gaudio E, Alvaro D, Reid L (2011) Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes and pancreatic islets. Hepatology 54: 2159–2172 [DOI] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 108: 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D (2009) A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5: 258–265 [DOI] [PubMed] [Google Scholar]
- Cheng X, Ying L, Lu L, Galvao AM, Mills JA, Lin HC, Kotton DN, Shen SS, Nostro MC, Choi JK, Weiss MJ, French DL, Gadue P (2012) Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 10: 371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149: 1192–1205 [DOI] [PubMed] [Google Scholar]
- Criscimanna A, Speicher JA, Houshmand G, Shiota C, Prasadan K, Ji B, Logsdon CD, Gittes GK, Esni F (2011) Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology 141: 1451–1462, 1462.e1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401 [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557–4568 [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297 [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS (2001) A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128: 871–881 [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA (2004) Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46 [DOI] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M (2008) Surface markers for the murine oval cell response. Hepatology 48: 1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S (2011) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41 [DOI] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA (2003) Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev 120: 35–43 [DOI] [PubMed] [Google Scholar]
- Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M (2006) Stabilization of beta-catenin impacts pancreas growth. Development 133: 2023–2032 [DOI] [PubMed] [Google Scholar]
- Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H (2013) In vitro expansion of single Lgr5(+) liver stem cells induced by Wnt-driven regeneration. Nature 494: 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Gros A, Jose A, Gonzalez JR, Alemany R, Fillat C (2009) Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models. Neoplasia 11: 518–528 4 p following 528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jin L, Feng T, Shih HP, Zerda R, Luo A, Hsu J, Mahdavi A, Sander M, Tirrell DA, Riggs AD, Ku HT (2013) Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci USA 110: 3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl K, Sarkar SA, Wong R, Jensen J, Hutton JC (2008) Mouse pancreatic endocrine cell transcriptome defined in the embryonic Ngn3-null mouse. Diabetes 57: 2755–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K (2005) Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309: 1256–1259 [DOI] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M (2011) Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138: 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, JPt Morris, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M (2012) Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22: 737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26: 443–452 [DOI] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER (2002) Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem 277: 21657–21665 [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysy PA, Weir GC, Bonner-Weir S (2012) Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med 1: 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ Jr., Wright CV, Stoffers DA, Leach SD (2005) Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132: 3767–3776 [DOI] [PubMed] [Google Scholar]
- Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ (2004) Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 82: 341–358 [DOI] [PubMed] [Google Scholar]
- Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck AJ, Clevers H (2012) The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 31: 3079–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Law AC, Dor Y, Melton DA (2005) Beta-catenin is essential for pancreatic acinar but not islet development. Development 132: 4663–4674 [DOI] [PubMed] [Google Scholar]
- Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, Daley GQ, Elefanty AG, Stanley EG, Keller G (2011) Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138: 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y (1997) ‘Green mice' as a source of ubiquitous green cells. FEBS Lett 407: 313–319 [DOI] [PubMed] [Google Scholar]
- Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV (2013) Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140: 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T, Segara D, Dawson AC, Kench JG, Henshall SM, Sutherland RL, Dlugosz A, Rustgi AK, Hebrok M (2007) Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS One 2: e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG (2000) Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 6: 278–282 [DOI] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD (2010) Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA 107: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Scoggins CR, Meszoely IM, Wada M, Means AL, Yang L, Leach SD (2000) p53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am J Physiol Gastrointest Liver Physiol 279: G827–G836 [DOI] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D (2004) Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 22: 1115–1124 [DOI] [PubMed] [Google Scholar]
- Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, Wheeler MB, van der Kooy D (2011) The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8: 281–293 [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Borowiak M, Melton DA (2012) Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature 491: 765–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, Clevers H (2009) Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136: 2187–2194 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]
- Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F (2000) Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49: 157–162 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. (2005) Proc Natl Acad Sci USA 102: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL (2010) Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464: 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Casteele M, Leuckx G, Baeyens L, Cai Y, Yuchi Y, Coppens V, De Groef S, Eriksson M, Svensson C, Ahlgren U, Ahnfelt-Rønne J, Madsen OD, Waisman A, Dor Y, Jensen JN, Heimberg H (2013) Neurogenin 3+ cells contribute to β-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis 4: e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Abe K, Anbo Y, Katoh H (1995) Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol 58: 365–374 [DOI] [PubMed] [Google Scholar]
- Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB (2012) Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H (2008) Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207 [DOI] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W (2005) The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132: 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Grompe M (2008) Generation and regeneration of cells of the liver and pancreas. Science 322: 1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H (2009) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19: 429–438 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.