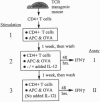
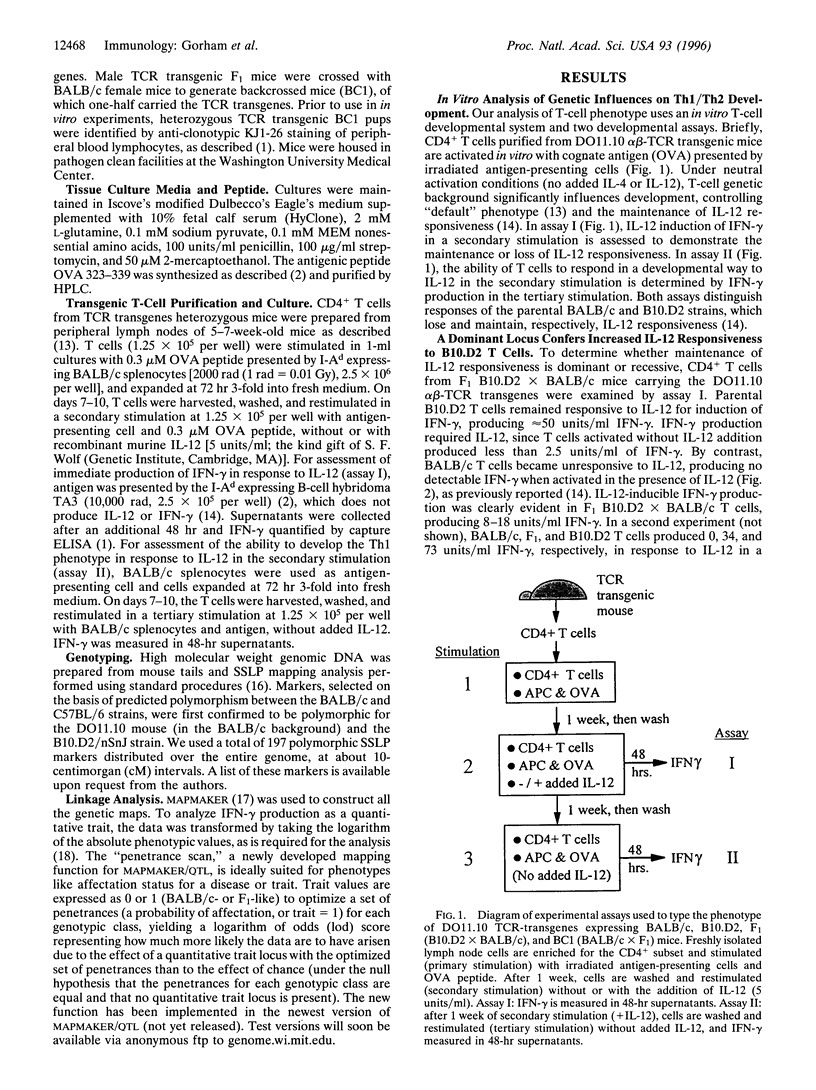
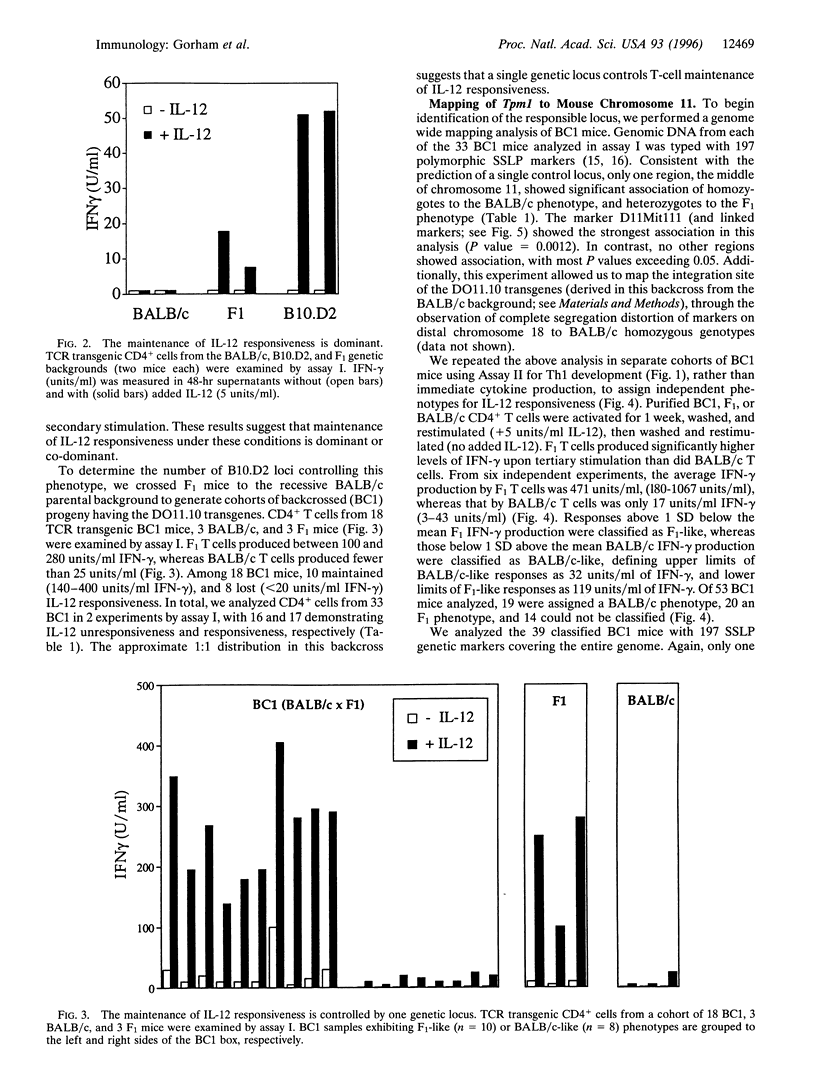
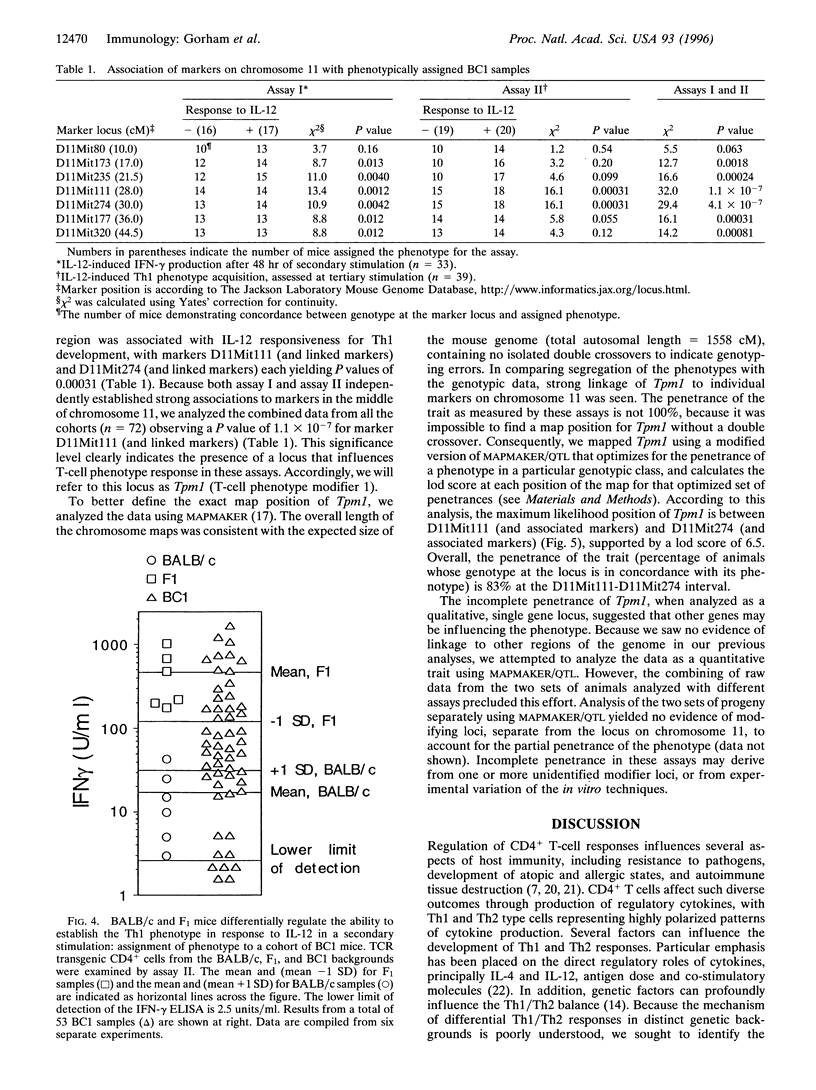
Abstract
Genetic background of the T cell can influence T helper (Th) phenotype development, with some murine strains (e.g., B10.D2) favoring Th1 development and others (e.g., BALB/c) favoring Th2 development. Recently we found that B10.D2 exhibit an intrinsically greater capacity to maintain interleukin 12 (IL-12) responsiveness under neutral conditions in vitro compared with BALB/c T cells, allowing for prolonged capacity to undergo IL-12-induced Th1 development. To begin identification of the loci controlling this genetic effect, we used a T-cell antigen receptor-transgenic system for in vitro analysis of intercrosses between BALB/c and B10.D2 mice and have identified a locus on murine chromosome 11 that controls the maintenance of IL-12 responsiveness, and therefore the subsequent Th1/Th2 response. This chromosomal region is syntenic with a locus on human chromosome 5q31.1 shown to be associated with elevated serum IgE levels, suggesting that genetic control of Th1/Th2 differentiation in mouse, and of atopy development in humans, may be expressed through similar mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D., Rosenwasser O. A., O'Neill J. K., Turk J. L. Genetic analysis of experimental allergic encephalomyelitis in mice. J Immunol. 1995 Oct 15;155(8):4046–4051. [PubMed] [Google Scholar]
- Buckwalter M. S., Lossie A. C., Scarlett L. M., Camper S. A. Localization of the human chromosome 5q genes Gabra-1, Gabrg-2, Il-4, Il-5, and Irf-1 on mouse chromosome 11. Mamm Genome. 1992;3(10):604–607. doi: 10.1007/BF00350629. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Corry D. B., Folkesson H. G., Warnock M. L., Erle D. J., Matthay M. A., Wiener-Kronish J. P., Locksley R. M. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996 Jan 1;183(1):109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. F., Miller J. C., Steen R. G., Merchant M., Damron D., Nahf R., Gross A., Joyce D. C., Wessel M., Dredge R. D. A genetic map of the mouse with 4,006 simple sequence length polymorphisms. Nat Genet. 1994 Jun;7(2 Spec No):220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler M. L., Gorham J. D., Hsieh C. S., Mackey A. J., Steen R. G., Dietrich W. F., Murphy K. M. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996 Feb 16;271(5251):984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Mutha S. S., Locksley R. M. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993 May 1;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1986;28:79–116. [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., O'Garra A., Murphy K. M. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995 Feb 1;181(2):713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Tung A. S. Regulation of IgE antibody production by serum molecules. II. Strain-specificity of the suppressive activity of serum from complete freund's adjuvant-immune low responder mouse donors. J Immunol. 1978 Jun;120(6):2060–2067. [PubMed] [Google Scholar]
- Katz J. D., Benoist C., Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995 May 26;268(5214):1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- Kruglyak L., Lander E. S. High-resolution genetic mapping of complex traits. Am J Hum Genet. 1995 May;56(5):1212–1223. [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lawrence S., Beasley R., Doull I., Begishvili B., Lampe F., Holgate S. T., Morton N. E. Genetic analysis of atopy and asthma as quantitative traits and ordered polychotomies. Ann Hum Genet. 1994 Oct;58(Pt 4):359–368. doi: 10.1111/j.1469-1809.1994.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988 Nov 1;141(9):3203–3207. [PubMed] [Google Scholar]
- Marsh D. G., Neely J. D., Breazeale D. R., Ghosh B., Freidhoff L. R., Ehrlich-Kautzky E., Schou C., Krishnaswamy G., Beaty T. H. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994 May 20;264(5162):1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Kono D. H., Clayton J., Ando D. G., Hinton D. R., Hofman F. M. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers D. A., Bleecker E. R. Approaches to mapping genes for allergy and asthma. Am J Respir Crit Care Med. 1995 Jul;152(1):411–413. doi: 10.1164/ajrccm.152.1.7599858. [DOI] [PubMed] [Google Scholar]
- Meyers D. A., Postma D. S., Panhuysen C. I., Xu J., Amelung P. J., Levitt R. C., Bleecker E. R. Evidence for a locus regulating total serum IgE levels mapping to chromosome 5. Genomics. 1994 Sep 15;23(2):464–470. doi: 10.1006/geno.1994.1524. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Mock B., Blackwell J., Hilgers J., Potter M., Nacy C. Genetic control of Leishmania major infection in congenic, recombinant inbred and F2 populations of mice. Eur J Immunogenet. 1993 Oct;20(5):335–348. doi: 10.1111/j.1744-313x.1993.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. H., Damon S., Hewitt J. D., Zamir D., Rabinowitch H. D., Lincoln S. E., Lander E. S., Tanksley S. D. Mendelian factors underlying quantitative traits in tomato: comparison across species, generations, and environments. Genetics. 1991 Jan;127(1):181–197. doi: 10.1093/genetics/127.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma D. S., Bleecker E. R., Amelung P. J., Holroyd K. J., Xu J., Panhuysen C. I., Meyers D. A., Levitt R. C. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995 Oct 5;333(14):894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- Reiner S. L., Zheng S., Wang Z. E., Stowring L., Locksley R. M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994 Feb 1;179(2):447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the development of type 2 T-helper cells in allergy. Curr Opin Immunol. 1994 Dec;6(6):838–846. doi: 10.1016/0952-7915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Scott B., Liblau R., Degermann S., Marconi L. A., Ogata L., Caton A. J., McDevitt H. O., Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994 Apr;1(1):73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Morrow T. A., Desiderio S. V. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S. J., Jacobson N. G., Dighe A. S., Gubler U., Murphy K. M. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995 Jun;2(6):665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Aitman T. J., Cornall R. J., Ghosh S., Hall J. R., Hearne C. M., Knight A. M., Love J. M., McAleer M. A., Prins J. B. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991 Jun 13;351(6327):542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- Verbeek S., Izon D., Hofhuis F., Robanus-Maandag E., te Riele H., van de Wetering M., Oosterwegel M., Wilson A., MacDonald H. R., Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995 Mar 2;374(6517):70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Wilson S. D., Billings P. R., D'Eustachio P., Fournier R. E., Geissler E., Lalley P. A., Burd P. R., Housman D. E., Taylor B. A., Dorf M. E. Clustering of cytokine genes on mouse chromosome 11. J Exp Med. 1990 Apr 1;171(4):1301–1314. doi: 10.1084/jem.171.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]