Abstract
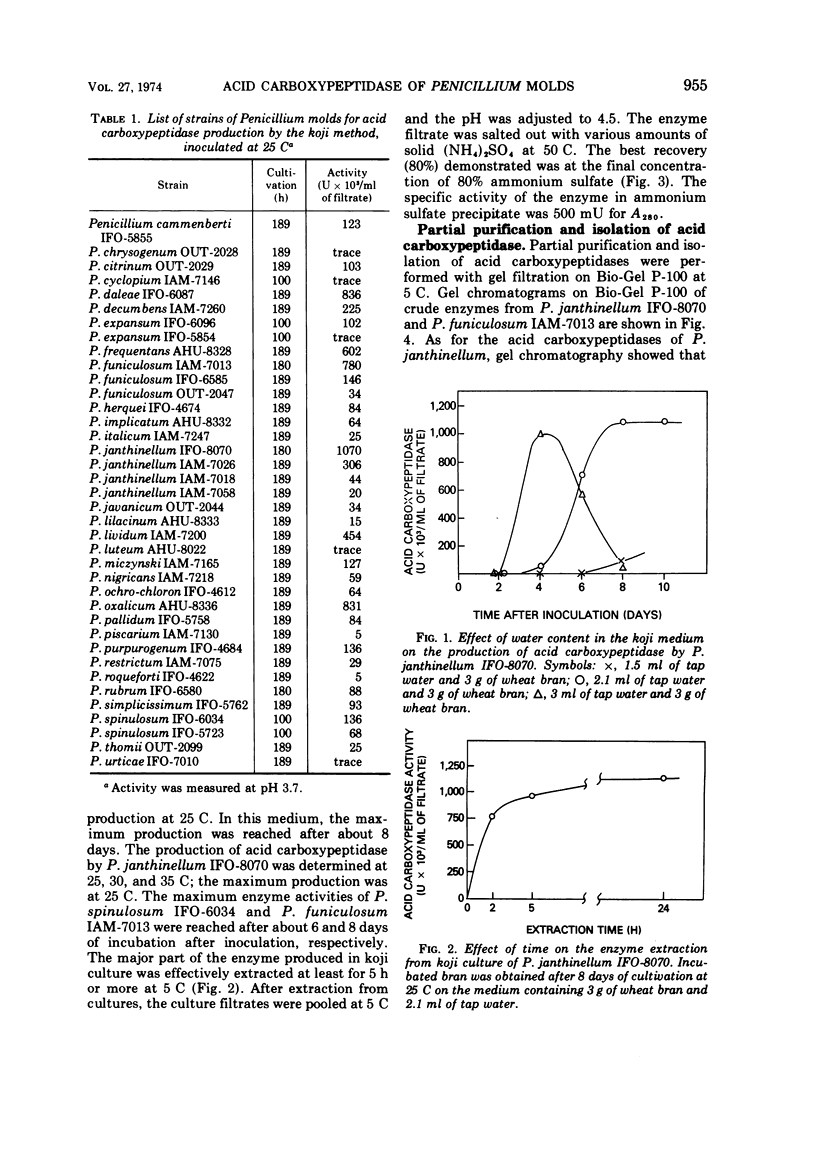
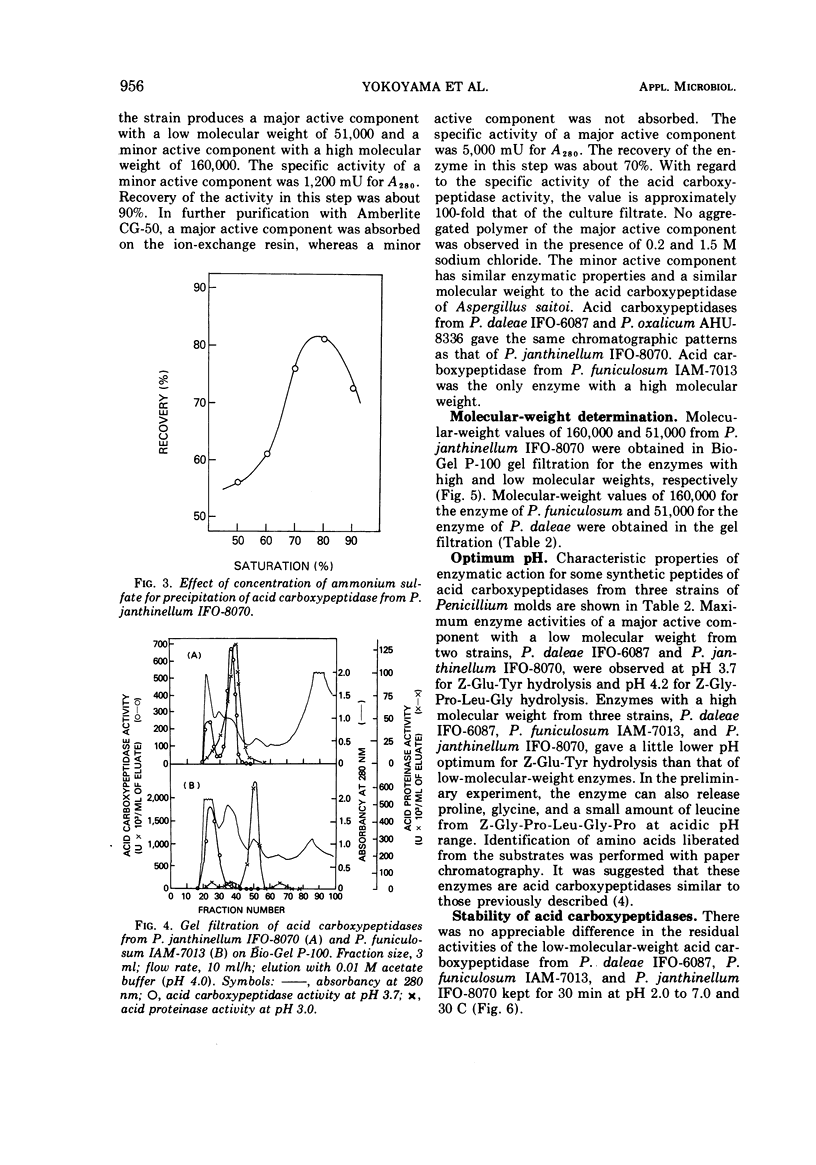
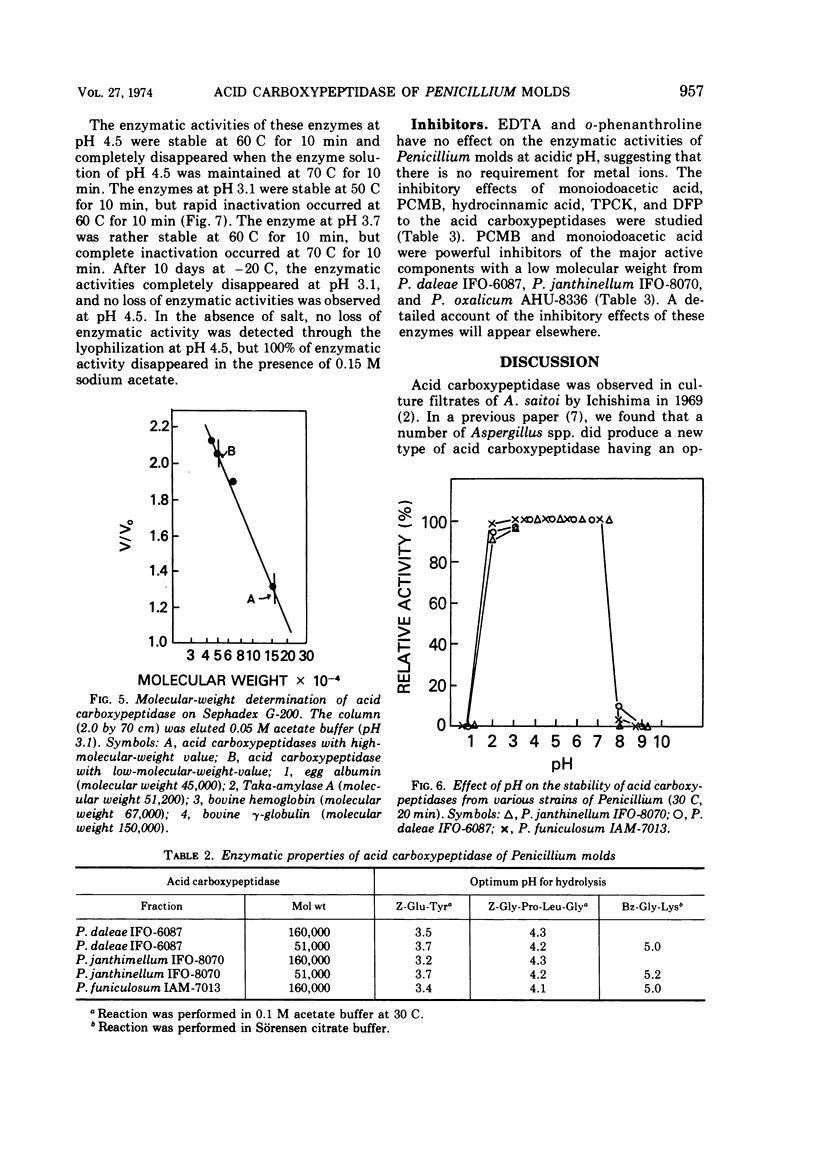
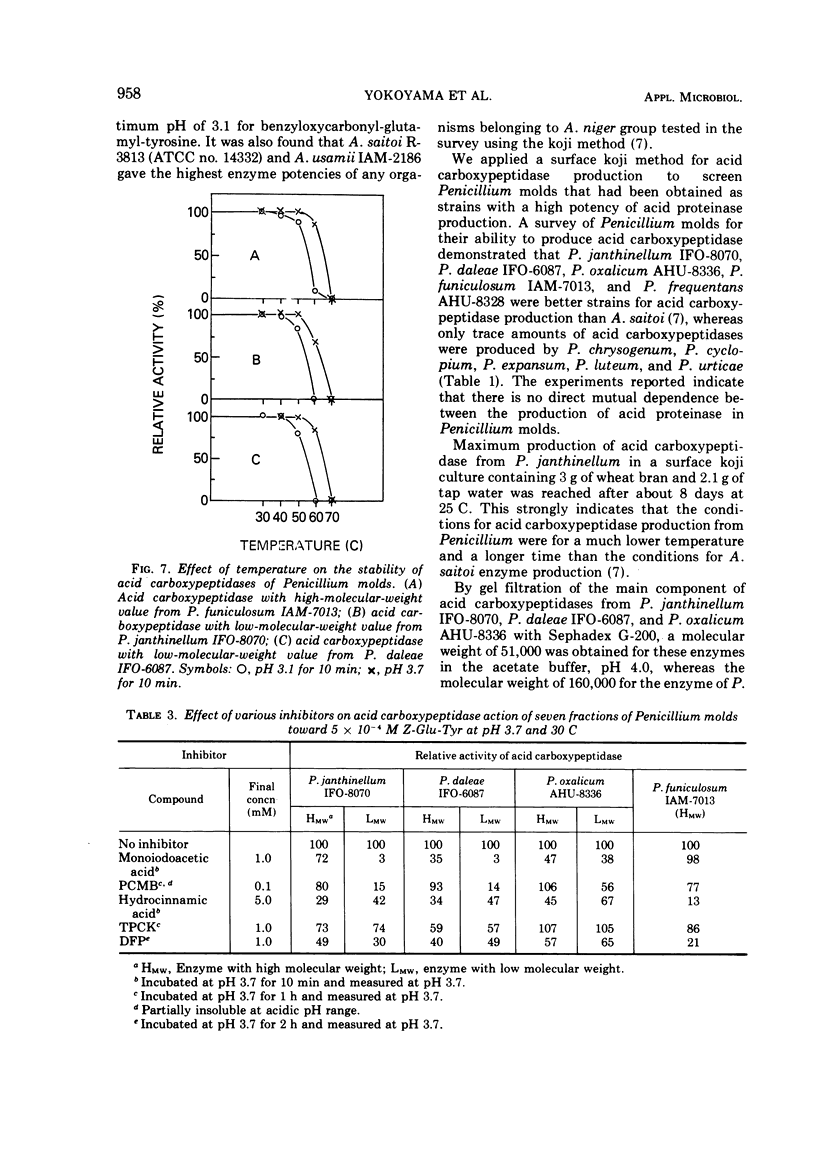
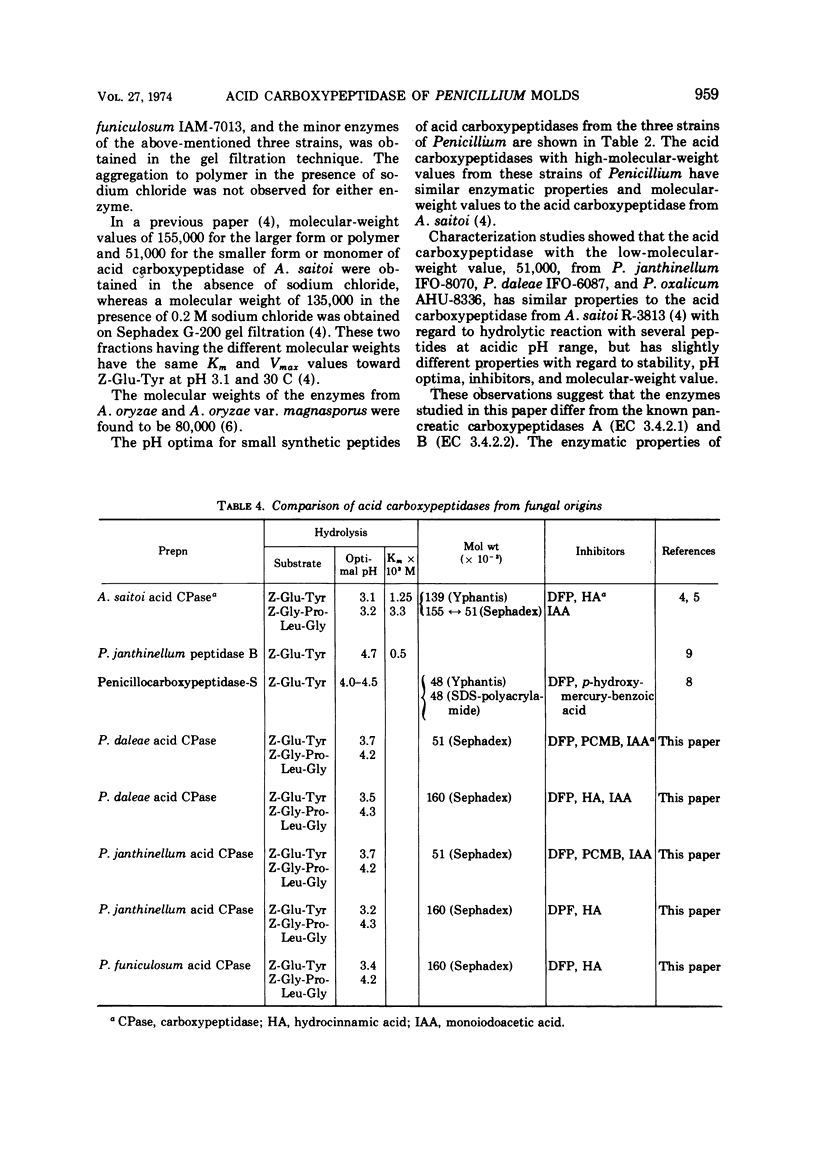
Among some 38 strains of the genus Penicillium we investigated seven wild-type strains (P. daleae IFO-6087, P. frequentans AHU-8328, P. funiculosum IAM-7013, P. janthinellum IFO-8070, IAM-7026, P. lividum IAM-7200, and P. oxalicum AHU-8336) that were found to be excellent strains for a new type of acid carboxypeptidase production in a surface koji culture at 25 C. The production of acid carboxypeptidase was determined in various culture conditions in a koji culture. The maximum yields of acid carboxypeptidase were obtained by P. janthinellum IFO-8070. Partial purification and isolation of the acid carboxypeptidase from strains of Penicillium were performed with gel filtration on Bio-Gel P-100. Characterization studies indicate that the acid carboxypeptidases from P. daleae IFO-6087, P. funiculosum IAM-7013, P. janthinellum IFO-8070, and P. oxalicum AHU-8336 have some properties similar to those of the enzyme of Aspergillus saitoi with regard to the hydrolysis of several peptides at acidic pH range but have other slightly different properties with regard to stability, pH optima, inhibitors, and molecular weights.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ichishima E., Arai T. Specificity and mode of action of acid carboxypeptidase from Aspergillus saitoi. Biochim Biophys Acta. 1973 Feb 15;293(2):444–450. doi: 10.1016/0005-2744(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Ichishima E. Purification and characterization of a new type of acid carboxypeptidase from Aspergillus. Biochim Biophys Acta. 1972 Jan 20;258(1):274–288. doi: 10.1016/0005-2744(72)90985-0. [DOI] [PubMed] [Google Scholar]
- Ichishima E., Sonoki S., Hirai K., Torii Y., Yokoyama S. Comparative study on enzymatic properties of acid carboxypeptidase of molds of the genus Aspergillus. J Biochem. 1972 Oct;72(4):1045–1048. doi: 10.1093/oxfordjournals.jbchem.a129967. [DOI] [PubMed] [Google Scholar]
- Ichishima E., Yamane A., Nitta T., Kinoshita M., Nikkuni S. Production of a new type of acid carboxypeptidase of molds of the Aspergillus niger group. Appl Microbiol. 1973 Sep;26(3):327–331. doi: 10.1128/am.26.3.327-331.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. R., Hofmann T. Penicillocarboxypeptidase-S, a nonspecific SH-dependent exopeptidase. Can J Biochem. 1972 Dec;50(12):1297–1310. doi: 10.1139/o72-175. [DOI] [PubMed] [Google Scholar]
- SHAW R. PROTEOLYTIC ENZYMES OF PENICILLIUM JANTHINELLUM. II. PROPERTIES OF PEPTIDASE B. Biochim Biophys Acta. 1964 Dec 23;92:558–566. doi: 10.1016/0926-6569(64)90015-x. [DOI] [PubMed] [Google Scholar]


