Abstract
Purpose
The search for the role(s) that HIV-1 Vpr and its HIV2/SIV paralogs Vpr and Vpx play in viral infection and pathogenesis showed that all three engage CRL4 ubiquitin ligase complexes. This association triggers ubiquitination and degradation of cellular substrates. The identity of the ubiquitin ligase substrates is only now beginning to be revealed. This review focuses on recent work that has identified one such substrate and exposed new cellular restrictions to infection.
Recent findings
Three groups have now described cellular factors that restrict HIV-1 infection in cells of the myeloid lineage. One of these factors, SAMHD1, was shown to be depleted through the CRL4 ubiquitin ligase complex in the presence of HIV-2/SIV Vpx. The other restriction can be defeated by Vpx in the absence of at least one part of the ubiquitin ligase complex that triggers SAMHD1 depletion.
Another group has shown that the previously-described up-regulation of NK-cell ligands on the surface of HIV-1-infected cells requires the actions of both the cytidine deaminase APOBEC3G and uracil-N-glycosylase 2 in association with HIV-1 Vpr.
Summary
As more cellular interaction partners are identified for HIV-1 Vpr and its paralogs from other viruses, details are emerging about Vpr function. The recent findings have highlighted the existence of two new human proteins that can act to combat HIV infection and have revealed how HIV-1 proteins act in concert to modulate the interaction between NK cells and HIV-1 infected cells.
Keywords: HIV, SIV, Vpr, Vpx, SAMHD1
Introduction
The interplay between HIV and myeloid lineage cells represents a vital role in HIV infection and pathogenesis. Dendritic cells and macrophages are important components of the immune system that bridge innate and adaptive immunity to ultimately orchestrate the defenses necessary for blocking invading microbes. Key features of HIV biology allow this virus to persist despite the host defenses.
Dendritic cells, both plasmacytoid and myeloid, are negatively affected by HIV infection. Infected individuals exhibit reduced numbers of dendritic cells in the blood [1, 2]. While the exact cause of this reduction is not known, the loss of these critical immune components impairs anti-HIV responses. Indeed, reduced dendritic cell counts are indicative of a poor prognosis in macaque models [3] and in humans [4]. The remaining dendritic cells display numerous traits that only further dampen immunity. First, these cells demonstrate an imbalance of pro- and anti- apoptotic factors that favors apoptosis [5]. Second, T-cell activation by these cells is greatly impaired [6]. Finally, interaction of these cells with HIV-1 virions results a reduced capacity to communicate with NK cells thereby limiting NK cell activity [7].
Though HIV-1 can infect dendritic cells, it does so inefficiently. This may, at least in the very early stages of infection, benefit HIV-1 as the lack of replication in these cells may allow the virus to escape detection by systems that would otherwise trigger the production of interferon (IFN) [8]. Another well established role of myeloid dendritic cells is to act as ‘trojan horses’ to facilitate transfer of virus to CD4+ T-cells. HIV-1 transfers to CD4+ T-cells along the immunological synapse that forms between the two cell types [9]. This can both aid in the infection of T-cells and at least temporarily help the virus avoid detection.
Current knowledge regarding the interaction of denderitic cells with HIV stems mainly from in vitro studies. Technical hurdles including limited availability of blood from HIV infected patients, the fact that dendritic cells constitute only a small fraction of blood cells, the loss of dendritic cells early in infection, and the lack of non-primate animal models, have restricted the analyses that could be performed. Despite the difficulties associated with in vivo work, new information is beginning to emerge. Zhang et al. were able to demonstrate, in vivo, that HIV-1 can infect plasmacytoid dendritic cells and this can lead to activation as well as functional impairment of these cells as they exhibited reduced responses to TLR7/9 stimulation [10]. This occurred in both the bone marrow and the spleen and correlated with a decline in CD4+ T-cells [10]. Studies using ex vivo tissue samples, or tissue models to mimic in vivo environments, have also provided insight as to what may be occurring in an actual human infection. Indeed models of cervico-vaginal tissue [11] and the male genital tract [12] have contributed to our understanding of HIV transmission and dissemination. Notably, models like these have led to the implication of Langerhans cells in the uptake and transmission of HIV-1 [12]. These studies allude to the importance of dendritic cells in HIV pathogensis.
The infection of macrophages with HIV-1 contributes to HIV pathology in a number of ways. HIV-1 infection of macrophages results in activation of the cells and ultimately the up-regulation of molecules which can trigger apoptosis of CD4+ and possibly CD8+ T-cells upon contact [13, 14]. Whereas infected T-cells die soon after infection with HIV, infected macrophages can persist for months and thus act as long-term virus reservoirs. Like dendritic cells, infected macrophages can transfer HIV-1 to CD4+ T-cells and may activate naive infected CD4+ T-cells resulting in enhanced transcription of proviruses [15].
Overall, HIV cripples myeloid lineage cell-mediated defenses by: directly depleting these cells, impairing their ability to communicate with other cell types, employing them to gain access to CD4+ T-cells, and establishing latent reservoirs.
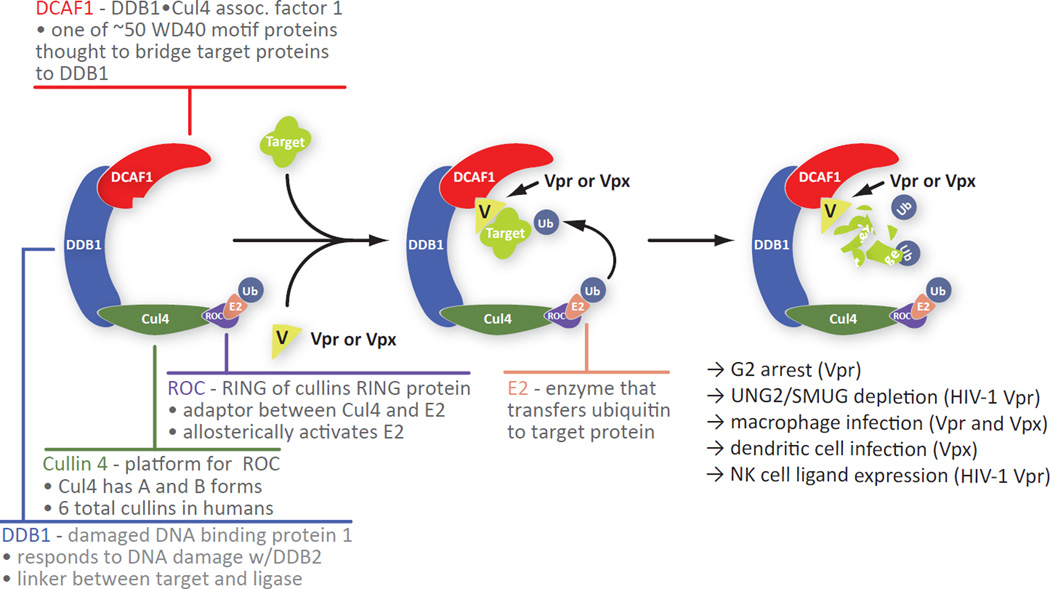
The HIV-1 genome encodes several specialized proteins that tailor the host cell environment to facilitate viral replication. Of these, the 17 kDa virion associated protein Vpr remains one of the least understood in terms of its contribution to HIV replication and pathology. Interestingly, HIV2 and some SIVs encode two Vpr-like proteins, Vpx and Vpr. While many functions have been attributed to HIV-1 Vpr, the two most widely accepted are triggering arrest at the G2 stage of the cell cycle in dividing cells and enhancing infection of terminally-differentiated macrophages. These are shared with HIV-2/SIV Vpr and Vpx, respectively. The arrest function has been linked to the association of Vpr with the CRL4 ubiquitin ligase complex through the adapter protein DCAF1 [16–22] (Figure 1). This association is required for the establishment of an intracellular state that mimics a DNA damage response [17, 23].
Figure 1.
Structure of the CRL4 ubiquitin ligase complex and a summary of its associated functions in the presence of Vpr or Vpx
Is triggering G2 cell cycle arrest the function that Vpr evolved to execute or a by-product of another role that Vpr plays? Goh et al. proposed that the G2 phase of the cell cycle, when cellular chromatin has been replicated, but before cellular structures are disassembled in preparation for cell division, provides an optimal environment for virus production [24]. More recent studies however have shown that the DNA-damage response also triggers the expression of NK-cell ligands on the surface of infected cells [25, 26]. The purpose of NK ligand expression on infected cells remains ambiguous at this time.
The role of HIV-1Vpr in macrophage infection also remains to be defined. Early studies linked HIV-1 Vpr, which has at least two nuclear import signals and one nuclear export signal [27], to translocation of the pre-integration complex into the cell nucleus [28–32]. Though Vpr does possess nuclear import signals, these are also present on other components of the pre-integration complex [33]. As such, redundancy may exist to ensure import under various conditions. Other evidence however suggests nuclear import can occur without any of the previously identified virion associated nuclear import signals [34, 35]. HIV-1 infects macrophages less efficiently than HIV-2/SIV does, but in that context Vpr can make an important difference in establishing an infection. The increased efficiency of HIV-2/SIV infection can be attributed to the action of Vpx which was shown to defeat a dominant inhibitor of retroviral replication that is found in cells of the myeloid lineage [36–38].
Recent lessons from Vpr and its HIV-2/SIV paralogs Vpr and Vpx
HIV-1 does not infect macrophages as efficiently as it does CD4+ T-cells. Nonetheless macrophages are productively infected by HIV-1 and Vpr plays a key role in this process [39]. HIV-2 and SIV, on the other hand, exhibit remarkably greater macrophage infection efficiency than HIV-1 and this has been attributed to Vpx [40]. Phylogenic analysis suggests that HIV-2 Vpr and HIV-2 Vpx arose from a gene duplication event of an HIV-1 Vpr like precursor [41]. The capacity of HIV-2 Vpx to profoundly boost macrophage infection was attributed to its ability to thwart the function of an anti-viral factor. New information regarding the interplay between HIV-1 Vpr and SIV/HIV-2 Vpx and host anti-viral proteins is rapidly emerging and providing important clues regarding the role of HIV-1 Vpr in human infections.
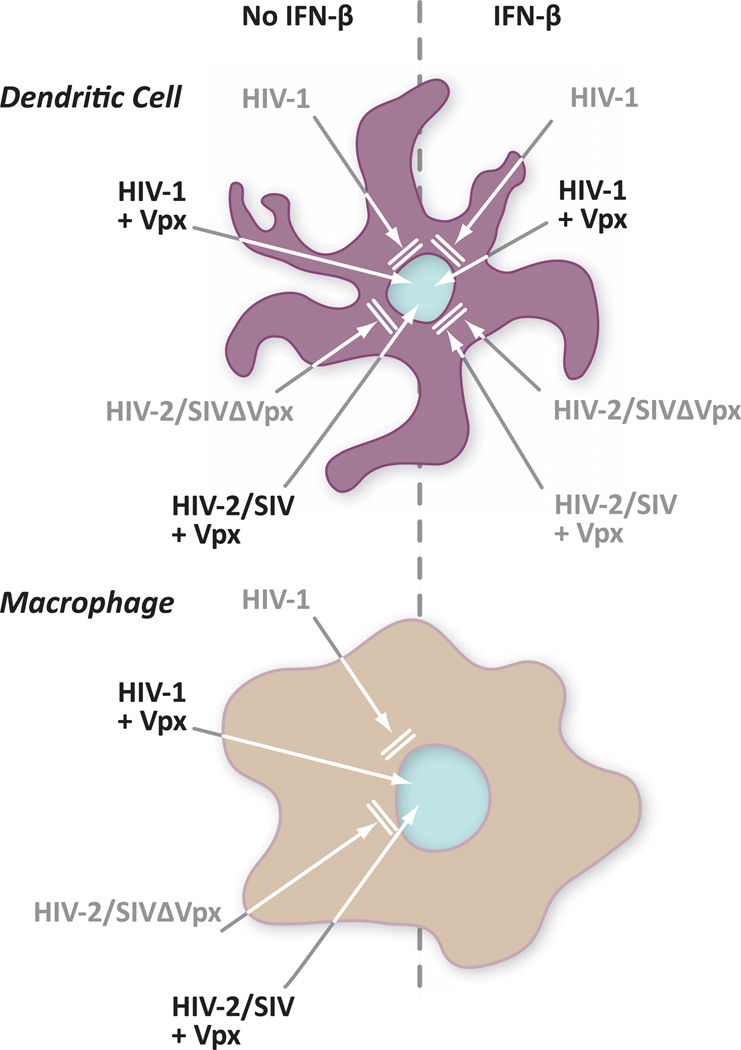
Two groups in ground-breaking, back-to-back publications described the use of co-immunoprecipitation to isolate SAMHD1 as a cellular partner for SIV/HIV-2 Vpx [42, 43]. They also demonstrated that this protein assembles with the CRL4 ubiquitin ligase complex in a Vpx dependent manner and that cells are depleted of SAMHD1 in the presence of Vpx. Importantly, depleting SAMHD1 enhanced infection of both dendritic cells and macrophages [42, 43] by helping the virus to overcome a block in reverse transcription [42]. Interestingly, while work by Laguette and colleagues shows that SAMHD1 expression correlates with viral restriction [43], Hrecka purified SAMHD1 from the HEK 293T human embryonic kidney cell line. This line is not refractory to infection by HIV-1 bearing the VSV-G protein in place of the HIV-1 env glycoprotein [42]. This observation of course suggests that SAMHD1 may be necessary but not sufficient for retroviral restriction. The pattern of virus restriction is summarized in Figure 2.
Figure 2.
Pattern of HIV-1 and HIV-2/SIV restriction in monocyte-derived dendritic cells and macrophages
SAMHD1, as the name indicates, is characterized by both a sterile alpha motif (SAM) and metal-dependent phosphohydrolase (HD) domain. Mutations in SAMHD1 are present in almost a fifth of all Aicardi-Goutières Syndrome patients. In these patients the lack of SAMHD1 function triggers symptoms that resemble viral infection. This parallels deficiencies in other enzymes that prevent accumulation of nucleic acid pools that could trigger an innate immune response. Nucleic acid pools could include those generated by viral infection. Enzymatically, SAMHD1 acts as a dGTP-regulated deoxynucleotide phosphohydrolase [44, 45].
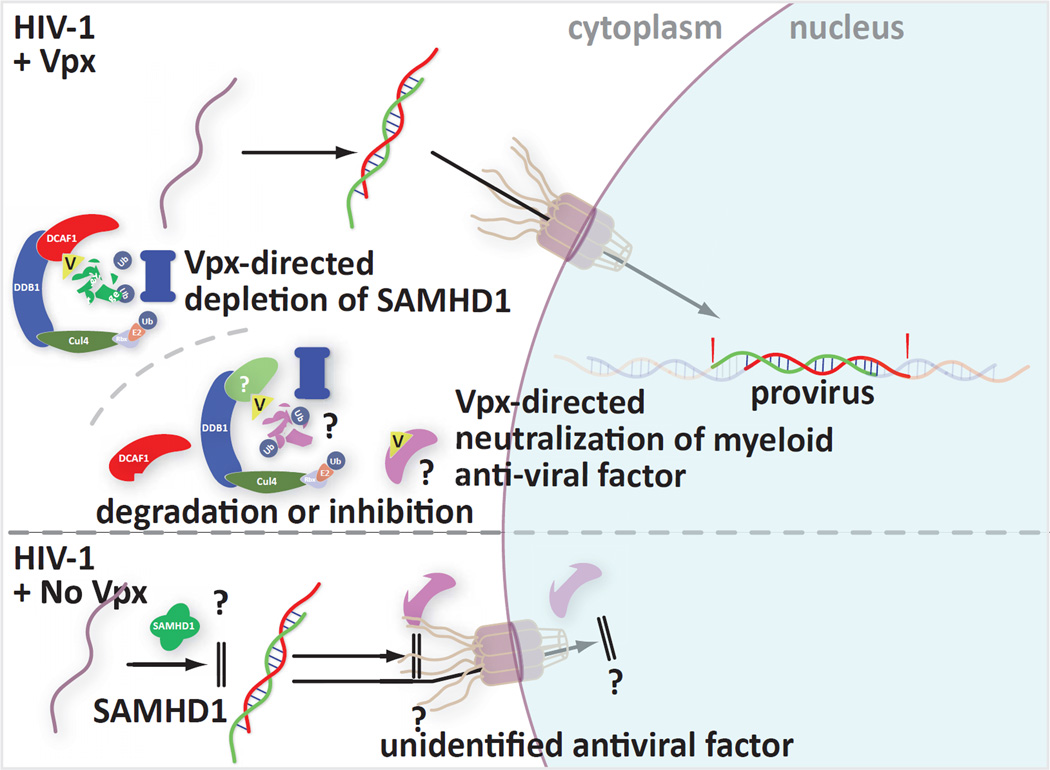
Another recent publication also focused on the impact of cellular restriction factors on retroviral infection. Pertel et al. investigated the effect of interferon-beta (IFN-β) and lipopolysacharide (LPS) on infections in monocyte-derived macrophages and dendritic cells [46]. They found that treatment with these two agents triggered mobilization of what appears to be yet another barrier to retroviral infection. While the SAMHD1 block targets the virus during reverse transcription, the LPS/IFN-β-induced restriction appears to be at or near the nuclear import of the pre-integration complex and before proviral integration. Interestingly, only HIV-1 but not HIV-2 or SIV infection benefited from Vpx action against the induced MDDC restriction. This suggests that HIV-1 may bear a unique feature in its nucleic acids or proteins that allows it to escape the antiviral factor in the presence of Vpx. Further, while Vpx relieves the block, it does not require DCAF1, the adaptor that HIV1 Vpr and Vpx use to tap ubiquitin ligase function. This leaves open the possibility that Vpx either blocks the factor directly or directs its depletion through other proteins. Thus, Pertel et al. have identified yet another HIV restriction factor that can be defeated by a viral protein. All of the dendritic cell restrictions are summarized in Figure 3.
Figure 3.
Summary of current knowledge of myeloid cell restrictions and Vpx-mediated countermeasures
Together these recent findings provide new information regarding cellular inhibitors of retroviruses but also raise a number of new questions. HIV-2 and SIV encode Vpx to thwart the as-yet-uncharacterized antiviral action of SAMHD1 that blocks replication during reverse transcription. The catalytic site of SAMHD1 had to be maintained for it to have antiviral activity, but since no enzymatic function was measured in these assays it is not apparent whether the site needed to be maintained to preserve a conformation, an intra-molecular interaction, or a function. It is also not clear whether the action of SAMHD1 impacts virus directly or indirectly.
Does HIV-1 Vpr also tag SAMHD1 for destruction albeit less efficiently? HIV-1 Vpr didn't facilitate the interaction between SAMHD1 and the CRL4DCAF1 complex but perhaps Vpr performs a similar function in a different cell-type and/or together with different co-factors. Depletion of SAMHD1 in dendritic cells improved HIV infection much more dramatically than it did in macrophages. Neither HIV-1 nor HIV-2 however is known to infect dendritic cells efficiently. Here of course the second restriction, highlighted by Pertel, may provide an explanation because while Vpx depletes SAMHD1, it also circumvents the second block. The second block acts on both HIV-1 and on HIV-2/SIV, yet Vpx aids only HIV-1 which doesn't encode Vpx. The interplay between HIV-1 and HIV-2 Vpx is most likely to occur in individuals infected with both viruses. Interestingly, a prospective study of Senegalese commercial sex workers showed that HIV-2 infection may reduce the risk of subsequent HIV-1 infection rather than enhancing it [47].
What do the studies of Vpx function reveal about HIV-1 Vpr? They highlight the fact that there are more cellular proteins that block HIV infection than were previously identified. These add to the list of antiviral defenses that so far includes Trim5α, APOBEC3G and Tetherin. These defenses may of course be constitutively present or inducible upon viral infection. We also learned from these studies that the Vpr-like proteins may have multiple targets that can be defeated by at least two mechanisms, one requiring the DCAF1 adaptor protein and the other not. Indeed, HIV-1 Vpr can direct depletion of, UNG2 and SMUG1 [48] and of an as-yet unidentified protein that is required for cell cycle progression beyond G2.
Further if Vpr cannot defeat these targets they may be good candidates for stopping HIV-1. However, given the similarities between HIV-1 Vpr and HIV-2/SIV Vpx, HIV-1 Vpr could evolve to counter the antiviral factor. Its evolution on the other hand could be constrained through overlap with another vital function. While many HIV-1 Vpr and HIV-2/SIV Vpx functions have been linked to the CRL4DCAF1 complex the Pertel work also serves as a reminder that not all Vpr action may dependent on the CRL4DCAF1 complex.
Vpr-mediated up-regulation of NK-cell ligands
The expression of HIV-1 Vpr in CD4+ T-cells, either alone or in the context of an infection, results in the up-regulation of NK cell ligands and increased NK cell mediated killing of the Vpr-expressing cells [25, 26]. Pham et al. [49] extended this work by demonstrating that this phenotype was not restricted to Vpr from common lab strains but rather also observed with Vpr from primary isolates from various HIV-1 types and clades. Previous work linked the capacity of Vpr to trigger expression of NK cell ligands with cell cycle arrest which Vpr causes by inducing an intracellular state similar to that found after DNA damage [26]. Here the authors further strengthened the correlation of NK ligand induction with G2 cell cycle arrest. Two of the Vpr alleles tested failed to up-regulate the NK cell ligand ULBP2 in CD4+ T-cells. One isolate, termed Vpr P, could not associate with the CRL4DCAF1 complex necessary to facilitate G2 arrest. Another Vpr-type, designated M/D-lo, exhibited an impaired ability to localize to the nucleus where the DNA-damage signals are likely triggered. Interestingly M/D-lo associated with the ubiquitin ligase complex but possessed an extended 12 amino acid C-terminus which was found to hinder the G2 arrest phenotype as truncation of this extension restored the ability to arrest the cell cycle in G2. The authors proposed that the extended amino acid C-terminus impairs the ability of Vpr to engage the yet-to-be-identified cellular factor responsible for cell cycle progression. The impact of Vpr-mediated NK-cell ligand up-regulation on virus biology is still unclear. As the authors indicate, up-regulation of NK cell ligands in the presence of Vpr may be a means by which HIV-1 induces NK cell tolerance by prolonged exposure to up-regulated ligands. Additionally, soluble Vpr [50] could conceivably make its way into uninfected bystander cells resulting in increased NK cell mediated killing, further contributing to T-cell depletion.
Vif, Vpr and NK ligands
Recent work by Norman et al. explored the relationship between the HIV-1 proteins Vpr and Vif, and up-regulation of the NK ligands [51] Vif, another specialized viral protein marks the cellular cytidine deaminase APOBEC3G for proteasomal degradation by linking it to the cullin5-elonginB/C complex. In the absence of effective Vif-mediated depletion of APOBEC3G, this enzyme deaminates cytidines to leave uridines in the proviral DNA. HIV-1 Vpr has long been associated with uracil-N-glycosylase 2, which executes the first step in the excision repair process to remove uridines from DNA. The authors of this work show that Vif and Vpr work together to diminish uridine in DNA, presumably because Vif lowers ABOBEC3G levels and Vpr taps UNG2 to initiate excision of uridines. The authors found that UNG2 and Vpr are both required to boosts up-regulation of NKG2D NK-cell ligands. This is ascribed to a DNA damage response triggered by the UNG2-initiated repair mechanism.
This work raises a number of new questions. It shows that HIV-1 encodes three proteins that all impact the capacity of infected cells to escape recognition by NK cells. Nef, previously shown to down-modulate cell surface proteins like CD4 and MHC-I, masks Vpr-mediated up-regulation of NK cell ligands. Vif is required to prevent APOBEC3G action, which could trigger UNG2-mediated repair and therefore activate DNA damage signals. Interestingly while recent publications show that Vpr causes depletion of UNG2 [48, 52], this work shows that depletion of UNG2 with shRNA interfered with NKG2D ligand expression albeit to a lesser degree than deletion of Vpr. Further they showed that a Vpr mutant that has been shown not to engage UNG2 or cause its depletion was impaired in its capacity to trigger NK cell ligand expression. These findings are more in line with earlier studies showing that Vpr together with UNG2 lowered viral mutation rates. The observations could also suggest that there may be different uracil-N-glycosylase populations in cells that could have diverse fates upon expression of Vpr.
Conclusion
HIV-2 is less pathogenic than HIV-1, likely because the two viruses employ different replication strategies. HIV-1 Vpr, HIV-2/SIV Vpr and Vpx are obviously critical to their respective viruses in vivo as they are all conserved and their absence or alteration produces milder diseases both in humans and simian models [53–56]. We propose that HIV-2 is better, or at least differently, adapted to its host than HIV-1. HIV-2 does not infect dendritic cells well and thus avoids triggering an interferon response. HIV-2 Vpx markedly enhances macrophage infection to gain access to a long lived host cell type. As a result, SIV/HIV-2 may have a reduced need to replicate in a more permissive host cell type such CD4+ T-cells. HIV-1 is less well adapted and thus requires better access to CD4+ T-cells to facilitate maximal replication. The observation that HIV-2 Vpx can rescue HIV-1 replication but not its own suggests that HIV-2 may have been selected for limited replication in some circumstances and enhanced replication in others. HIV-2 could have shifted, or be shifting its cell tropism to favor a better equilibrium with the host. Perhaps this is why HIV-2 is less pathogenic. A long lived host could promote viral propagation by enabling both increased viral replication over time and an increased probability of transmission to a new host. Other evidence suggests this type of strategy as well. SIV infection in natural hosts, such as sooty mangabeys, is generally not pathogenic and does not progress to AIDS despite high viral loads [57, 58]. In sharp contrast, SIV infection in non-natural hosts, like rhesus macaques, results in obvious pathology, disease progression, and the onset of AIDS. Investigation of this intriguing SIV dichotomy revealed that while both pathogenic and nonpathogenic infections initially yield a robust type I IFN response, the response is prolonged during the chronic phase of infection in pathogenic macaque models but wanes in nonpathogenic natural hosts [59, 60]. This may play a substantial role in the chronic immune activation that is thought to promote the loss of CD4+ T-cells and progression to AIDS [61, 62]. HIV-2 Nef impairs the formation of immunological synapses where as HIV-1 utilizes the synapses to enter CD4+ T-cells [9, 63]. It is conceivable that impairment of immunological synapse formation could result in less activation and therefore less type I IFN production. This coupled with factors such as Vpx that enable the virus to persist in an otherwise non-permissive cell type, could enable HIV-2 viral propagation with reduced injury to the host.
HIV-1-mediated modulation of NK-cell ligands draws attention to another interesting facet of HIV biology. Here it appears that HIV-1 depends on Vif to deplete APOBEC3G to assure that UNG, interacting with Vpr, doesn’t trigger NK-cell ligand expression at the surface of infected cells. This interplay could of course regulate not only HIV-1 function in the infected cell but also interactions between the infected cell and NK cells.
These recent findings raise a number of stimulating questions to help guide future research. These include the following:
Have HIV-1 Vpr and its HIV-2/SIV paralogs evolved entirely separate functions? All appear to have evolved the capacity to engage CRL4 ubiquitin ligases, but do they share ubiquitination targets?
Should we be looking at more than infectivity? Vpx appears to be a powerful regulator of infectivity but the studies showing that HIV-1Vpr triggers cell surface expression of NK cell ligands are a powerful reminder that Vpr could be controlling intracellular interactions.
Are we looking at the effects of Vpr on infectivity in the correct cell types?
Does HIV-1 Vpr-mediated degradation have a specific target or does it target a class of proteins? Two known HIV-1 Vpr targets: UNG2 and SMUG1 share a tryptophan-X-X-phenylalanine motif that has been shown be vital for the Vpr interaction with UNG2. Do all HIV-1 Vpr targets share this motif?
Does Vpr work in concert with additional HIV-1 proteins? From the Work of Norman et al. we see that the balance between Vif, Vpr and Nef determines whether and how much NKG2D ligand is expressed at the surface of infected cells. Are there additional interactions? For example Vpx aided HIV-1− but not HIV-2/SIV−infection in dendritic cells in the presence of IFN-β. What is unique about HIV-1 that allowed its rescue?
How do small proteins like Vpr and Vpx enhance target ubiquitination? Are they required for the ubiquitin ligase to engage the substrate or do they make the interaction more efficient?
Why does SAMHD1 exert antiviral functions in some cell types but not others? This protein is present in cells where infection is not restricted. Is restriction dependent on pathways that don’t exist in some cells?
Answering these questions will further contribute to unmasking the role that the enigmatic Vpr protein plays in HIV-1 infection and pathogenesis. Discovering the function of Vpr will of course help in the development of additional therapeutic strategies against this virus.
Key points.
The HIV-2/SIV-encoded HIV-1 Vpr paralog Vpx targets the cellular protein SAMHD1 for destruction through the CRL4 ubiquitin ligase complex.
SAMHD1 expression is required for a block in reverse transcription in cells of the myeloid lineage.
Vpx can defeat another block to HIV-1 infection in LPS- or IFN-β-treated monocyte-derived dendritic cells that stops the virus after the point of SAMHD1 action.
Vpr from various primary HIV-1 isolates triggers expression of NK-cell ligands on the surface of infected cells.
Vpr-triggered NK-cell ligand expression depends on the interplay between HIV-1 proteins Vif and Vpr and the cellular proteins APOBEC3G and UNG2.
Acknowledgements
This work is supported by NIH grant 5R01AI073178.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Derby N, Martinelli E, Robbiani M. Myeloid dendritic cells in HIV-1 infection. Curr Opin HIV AIDS. 2011 Sep;6(5):379–384. doi: 10.1097/COH.0b013e3283499d63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barratt-Boyes SM, Brown KN, Melhem N, Soloff AC, Gleason SM. Understanding and exploiting dendritic cells in human immunodeficiency virus infection using the nonhuman primate model. Immunol Res. 2006;36(1–3):265–274. doi: 10.1385/IR:36:1:265. [DOI] [PubMed] [Google Scholar]
- 3.Barratt-Boyes SM, Wijewardana V. A divergent myeloid dendritic cell response at virus set-point predicts disease outcome in SIV-infected rhesus macaques. Journal of medical primatology. 2011 Aug;40(4):206–213. doi: 10.1111/j.1600-0684.2011.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng W, Fan X, Diao Y, Cui H, Sun H, Yun K, et al. Rapid Disease Progression in HIV-1-Infected Men Who Have Sex with Men Is Negatively Correlated with Peripheral Plasmacytoid Dendritic Cell Counts at the Early Stage of Primary Infection. Journal of clinical immunology. 2011 Jun 14; doi: 10.1007/s10875-011-9556-0. [DOI] [PubMed] [Google Scholar]
- 5.Dillon SM, Friedlander LJ, Rogers LM, Meditz AL, Folkvord JM, Connick E, et al. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol. 2011 Jan;85(1):397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonkers NL, Rodriguez B, Asaad R, Lederman MM, Anthony DD. Systemic Immune Activation in HIV Infection Is Associated with Decreased MDC Responsiveness to TLR Ligand and Inability to Activate Naive CD4 T-Cells. PLoS One. 2011;6(9):e23884. doi: 10.1371/journal.pone.0023884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benlahrech A, Gotch F, Kelleher P, Patterson S. Loss of NK stimulatory capacity by plasmacytoid and monocyte-derived DC but not myeloid DC in HIV-1 infected patients. PLoS One. 2011;6(3):e17525. doi: 10.1371/journal.pone.0017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harman AN, Wilkinson J, Bye CR, Bosnjak L, Stern JL, Nicholle M, et al. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J Immunol. 2006 Nov 15;177(10):7103–7113. doi: 10.4049/jimmunol.177.10.7103. [DOI] [PubMed] [Google Scholar]
- 9.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003 May 23;300(5623):1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Jiang Q, Li G, Jeffrey J, Kovalev GI, Su L. Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2/gamma C/ mice in vivo. Blood. 2011 Jun 9;117(23):6184–6192. doi: 10.1182/blood-2011-01-331173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merbah M, Introini A, Fitzgerald W, Grivel JC, Lisco A, Vanpouille C, et al. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. Am J Reprod Immunol. 2011 Mar;65(3):268–278. doi: 10.1111/j.1600-0897.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganor Y, Bomsel M. HIV-1 transmission in the male genital tract. Am J Reprod Immunol. 2011 Mar;65(3):284–291. doi: 10.1111/j.1600-0897.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 13.Oyaizu N, McCloskey TW, Coronesi M, Chirmule N, Kalyanaraman VS, Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82(11):3392–3400. [PubMed] [Google Scholar]
- 14.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995 Sep 28;377(6547):348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 15.Herbein G, Gras G, Khan KA, Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007 Sep 14;282(37):27046–27057. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- 17.Belzile JP, Duisit G, Rougeau N, Mercier J, Finzi A, Cohen EA. HIV-1 Vpr-Mediated G2 Arrest Involves the DDB1-CUL4A(VPRBP) E3 Ubiquitin Ligase. PLoS Pathog. 2007 Jul 13;3(7):e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan L, Ehrlich E, Yu XF. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J Virol. 2007 Oct;81(19):10822–10830. doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, et al. Lentiviral Vpr usurps Cul4-DDB1VprBP E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11778–11783. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeHart JL, Zimmerman ES, Ardon O, Monteiro-Filho CM, Arganaraz ER, Planelles V. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virology journal. 2007;4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrofelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007 Mar 6;104(10):4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, et al. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell cycle (Georgetown, Tex. 2007 Jan 15;6(2):182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- 23.Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003 Jul 11;278(28):25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- 24.Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, et al. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4(1):65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 25.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010 Feb 18;115(7):1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, et al. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009 Oct;5(10):e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins Y, McEntee M, Weis K, Greene WC. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143(4):875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, et al. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994;91(15):7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, et al. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89(14):6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273(21):13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 31.Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, et al. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. Embo J. 1998;17(4):909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subbramanian RA, Kessous-Elbaz A, Lodge R, Forget J, Yao XJ, Bergeron D, et al. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J Exp Med. 1998;187(7):1103–1111. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006 Jan 5;344(1):88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Riviere L, Darlix JL, Cimarelli A. Analysis of the viral elements required in the nuclear import of HIV-1 DNA. J Virol. 2010 Jan;84(2):729–739. doi: 10.1128/JVI.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita M, Emerman M. The Cell Cycle Independence of HIV Infections Is Not Determined by Known Karyophilic Viral Elements. PLoS Pathog. 2005 Nov 11;1(3):e18. doi: 10.1371/journal.ppat.0010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008;4(5):e1000059. doi: 10.1371/journal.ppat.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, Sharkey M, et al. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008;4(5):e1000057. doi: 10.1371/journal.ppat.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell host & microbe. 2009 Jul 23;6(1):68–80. doi: 10.1016/j.chom.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher TM, 3rd, Brichacek B, Sharova N, Newman MA, Stivahtis G, Sharp PM, et al. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) Embo J. 1996;15(22):6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 41.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. Embo J. 1992;11(9):3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011 Jun 30;474(7353):658–661. doi: 10.1038/nature10195. This paper identifies SAMHD1 as a novel antiviral factor that inhibits HIV-1 monocyte-derived macrophages and links this activity to the CRL4DCAF1 ubiquitin ligase complex.
- 43. Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011 May 25; doi: 10.1038/nature10117. This paper identifies SAMHD1 as a novel antiviral factor that inhibits HIV-1 in monocyte-derived dendritic cells.
- 44.Powell RD, Holland PJ, Hollis T, Perrino FW. The Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem. 2011 Nov 7; doi: 10.1074/jbc.C111.317628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011 Nov 6; doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 46. Pertel T, Reinhard C, Luban J. Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon. Retrovirology. 2011;8:49. doi: 10.1186/1742-4690-8-49. This work revealed a LPS-/IFN-β-inducible restriction in monocyte-derived dendritic cells that has characteristics destinct from SAMHD1 restriction.
- 47.Kokkotou EG, Sankale JL, Mani I, Gueye-Ndiaye A, Schwartz D, Essex ME, et al. In vitro correlates of HIV-2-mediated HIV-1 protection. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6797–6802. doi: 10.1073/pnas.97.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005 Sep;79(17):10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pham TN, Richard J, Gerard FC, Power C, Cohen EA. Modulation of NKG2D-Mediated Cytotoxic Functions of Natural Killer Cells by Viral Protein R (Vpr) from HIV-1 Primary Isolates. J Virol. 2011 Sep 28; doi: 10.1128/JVI.05835-11. This publication extended previous work showing that Vpr can trigger cell surface expression of NK-cell ligands by demonstrating that this phenotype is conserved among Vpr from various groups and clades.
- 50.Levy DN, Refaeli Y, Weiner DB. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol. 1995;69(2):1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, et al. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nature immunology. 2011;12(10):975–983. doi: 10.1038/ni.2087. This study demonstrated the interplay among various HIV and host proteins in modulating the cell surface expression of NK-cell ligands in HIV-1 infected cells.
- 52.Fenard D, Houzet L, Bernard E, Tupin A, Brun S, Mougel M, et al. Uracil DNA Glycosylase 2 negatively regulates HIV-1 LTR transcription. Nucleic Acids Res. 2009 Oct;37(18):6008–6018. doi: 10.1093/nar/gkp673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, et al. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69(4):2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, et al. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998 Dec;4(12):1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muthumani K, Bagarazzi M, Conway D, Hwang DS, Ayyavoo V, Zhang D, et al. Inclusion of Vpr accessory gene in a plasmid vaccine cocktail markedly reduces Nef vaccine effectiveness in vivo resulting in CD4 cell loss and increased viral loads in rhesus macaques. Journal of medical primatology. 2002 Aug;31(4–5):179–185. doi: 10.1034/j.1600-0684.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- 56.Muthumani K, Hwang DS, Dayes NS, Kim JJ, Weiner DB. The HIV-1 accessory gene vpr can inhibit antigen-specific immune function. DNA and cell biology. 2002 Sep;21(9):689–695. doi: 10.1089/104454902760330237. [DOI] [PubMed] [Google Scholar]
- 57.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003 Mar;18(3):441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 58.Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, et al. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol. 2007 Feb 1;178(3):1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 59.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS pathogens. 2009 Feb;5(2):e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. Journal of virology. 2010 Aug;84(15):7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999 Apr;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 62.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006 Mar;12(3):289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 63.Arhel N, Lehmann M, Clauss K, Nienhaus GU, Piguet V, Kirchhoff F. The inability to disrupt the immunological synapse between infected human T cells and APCs distinguishes HIV-1 from most other primate lentiviruses. J Clin Invest. 2009 Oct;119(10):2965–2975. doi: 10.1172/JCI38994. [DOI] [PMC free article] [PubMed] [Google Scholar]