Abstract
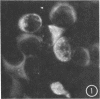
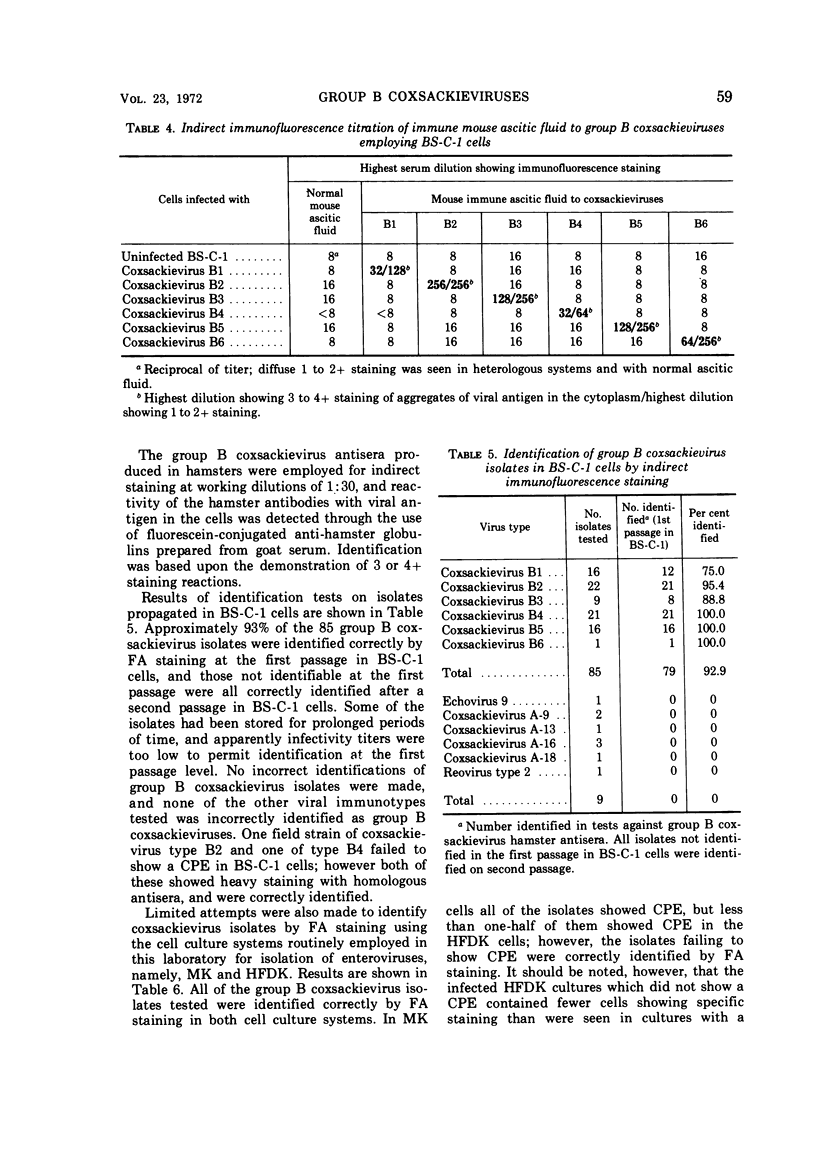
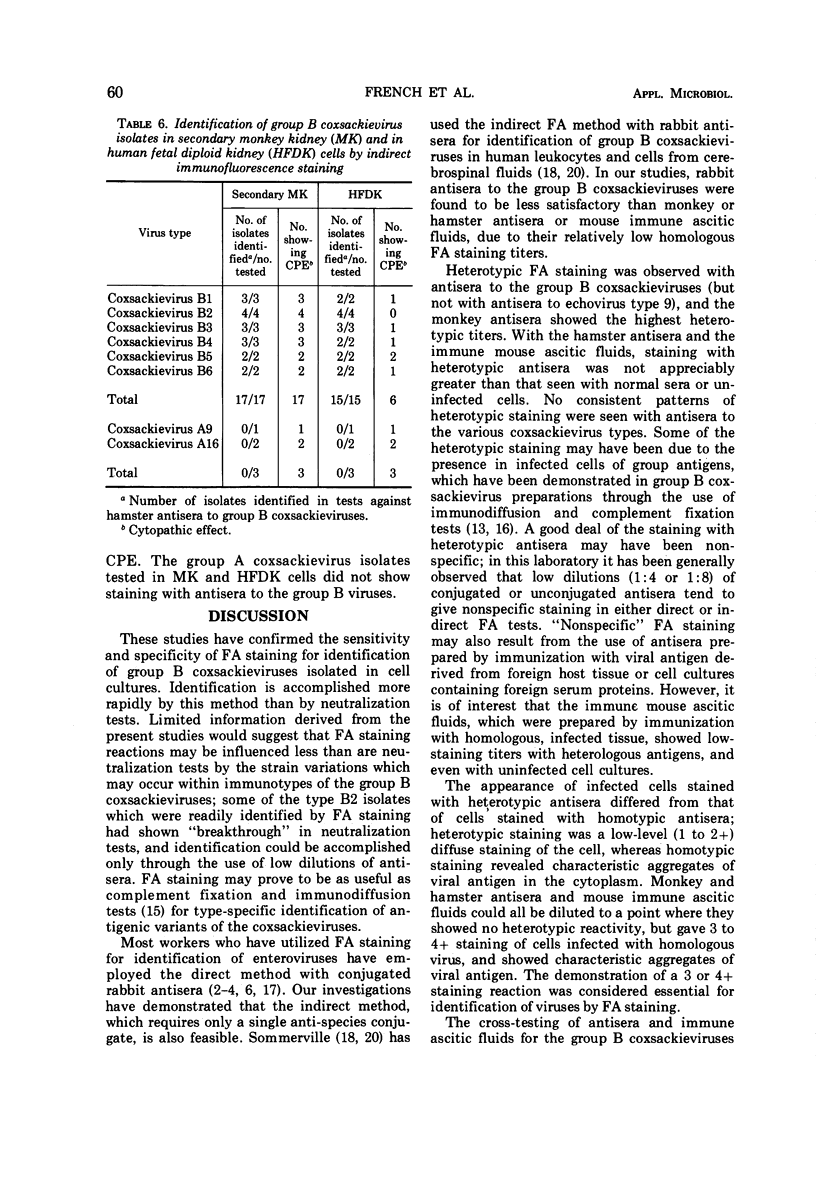
Studies were conducted on the sensitivity and specificity of indirect fluorescent-antibody (FA) staining for identification of group B coxsackieviruses. Antisera produced in four different species (monkeys, rabbits, horses, hamsters) and immune ascitic fluids prepared in mice were compared for suitability in FA staining. The horse antisera showed high titers of nonspecific staining, and the rabbit antisera showed relatively low homologous FA titers. Immune reagents from monkeys, hamsters, and mice were used for homologous and heterologous testing against cell cultures infected with the various group B coxsackieviruses. Antisera or immune ascitic fluids produced in these three species showed some heterotypic and nonspecific staining at low dilutions, with the monkey antisera showing the highest heterotypic titers. However, the immune reagents could be diluted to a point where they gave no heterotypic reactivity, but still showed characteristic homotypic staining. Heterotypic staining appeared as diffuse, low-level staining of the cells, whereas homotypic staining revealed characteristic, brightly staining aggregates of viral antigen in the cytoplasm of the infected cells. By using hamster immune sera, appropriately diluted to eliminate heterotypic staining and yet give strong homotypic staining, it was possible to identify correctly 79 (93%) of 85 field strains of group B coxsackieviruses at the first passage level in BS-C-1 cells; the remainder of the strains were identified after two passages in BS-CS-1 cells. No incorrect identifications were made. A limited number of field strains of group B coxsackieviruses were passed into rhesus monkey kidney and human fetal diploid kidney cells, and these were all correctly identified by FA staining, even the strains which failed to produce a cytopathic effect in the human fetal diploid kidney cells. Two human heart and brain tissues from which coxsackievirus type B4 had been isolated failed to show homotypic FA staining in excess of nonspecific or heterotypic staining.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates H. R., Jr Coxsackie virus B3 calcific pancarditis and hydrops fetalis. Am J Obstet Gynecol. 1970 Feb 15;106(4):629–630. doi: 10.1016/0002-9378(70)90058-x. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Chu K. C., Colcolough H. L., Sohal R. S. Immunofluorescent localization of coxsackievirus B antigen in the kidney observed at routine autopsy. Am J Med. 1969 Jul;47(1):36–42. doi: 10.1016/0002-9343(69)90239-3. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Colcolough H. L. Progressive Coxsackie viral pancarditis and nephritis. Ann Intern Med. 1969 Nov;71(5):963–970. doi: 10.7326/0003-4819-71-5-963. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Sun S. C., Colcolough H. L., Sohal R. S., DePasquale N. P. Coxsackie B viral myocarditis and valvulitis identified in routine autopsy specimens by immunofluorescent techniques. Am Heart J. 1967 Jul;74(1):13–23. doi: 10.1016/0002-8703(67)90035-x. [DOI] [PubMed] [Google Scholar]
- Chaudhary R. K., Westwood J. C. Detection of cross-reactions by immunofluorescence within the picornavirus group. Can J Microbiol. 1970 Jul;16(7):601–604. doi: 10.1139/m70-100. [DOI] [PubMed] [Google Scholar]
- HATCH M. H. IDENTIFICATION OF COXSACKIE AND ECHO VIRUS ISOLATES WITH FLUORESCENT ANTIBODY. Proc Soc Exp Biol Med. 1963 Oct;114:161–165. doi: 10.3181/00379727-114-28614. [DOI] [PubMed] [Google Scholar]
- LENNETTE E. H., WOODIE J. D., NAKAMURA K., MAGOFFIN R. L. THE DIAGNOSIS OF RABIES BY FLUORESCENT ANTIBODY METHOD (FRA) EMPLOYING IMMUNE HAMSTER SERUM. Health Lab Sci. 1965 Jan;2:24–34. [PubMed] [Google Scholar]
- LIM K. A., BENYESH-MELNICK M. Typing of viruses by combinations of antiserum pools. Application to typing of enteroviruses (Coxsackie and ECHO). J Immunol. 1960 Mar;84:309–317. [PubMed] [Google Scholar]
- Melnick J. L., Hampil B. WHO collaborative studies on enterovirus reference antisera. Third report. Bull World Health Organ. 1970;42(6):847–863. [PMC free article] [PubMed] [Google Scholar]
- RIGGS J. L., BROWN G. C. Application of direct and indirect immunofluorescence for identification of enteroviruses and titrating their antibodies. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:833–837. doi: 10.3181/00379727-110-27664. [DOI] [PubMed] [Google Scholar]
- SCHMIDT N. J., DENNIS J., FROMMHAGEN L. H., LENNETTE E. H. SEROLOGIC REACTIVITY OF CERTAIN ANTIGENS OBTAINED BY FRACTIONATION OF COXSACKIE VIRUSES IN CESIUM CHLORIDE DENSITY GRADIENTS. J Immunol. 1963 Apr;90:654–662. [PubMed] [Google Scholar]
- SCHMIDT N. J., GUENTHER R. W., LENNETTE E. H. Typing of ECHO virus isolates by immune serum pools. The "intersecting serum scheme". J Immunol. 1961 Nov;87:623–626. [PubMed] [Google Scholar]
- SCHMIDT N. J., LENNETTE E. H., DENNIS J. GEL DOUBLE DIFFUSION STUDIES WITH GROUP B AND GROUP A, TYPE 9, COXSACKIEVIRUSES. 3. ANTIGEN-ANTIBODY ABSORPTION TESTS. J Immunol. 1965 Apr;94:482–491. [PubMed] [Google Scholar]
- SHAW E. D., NEWTON A., POWELL A. W., FRIDAY C. J. Fluorescent antigen-antibody reactions in Coxsackie and ECHO enteroviruses. Virology. 1961 Oct;15:208–210. doi: 10.1016/0042-6822(61)90239-2. [DOI] [PubMed] [Google Scholar]
- SOMMERVILLE R. G., MACFARLANE P. S. THE RAPID DIAGNOSIS OF VIRUS INFECTIONS BY IMMUNOFLUORESCENT TECHNIQUES APPLIED TO BLOOD LEUCOCYTES. Lancet. 1964 Apr 25;1(7339):911–912. doi: 10.1016/s0140-6736(64)91634-4. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Antigenic variants of coxsackievirus type A24. Am J Epidemiol. 1970 Jan;91(1):99–109. doi: 10.1093/oxfordjournals.aje.a121117. [DOI] [PubMed] [Google Scholar]
- Sommerville R. G. The production of fluorescent antibody reagents for virus diagnosis in the albino mouse. I. Hyperimmune anti-species serum. Arch Gesamte Virusforsch. 1967;20(4):445–451. doi: 10.1007/BF01275225. [DOI] [PubMed] [Google Scholar]
- Zalan E., Kelen A. E., Labzoffsky N. A. Immunofluorescence studies on Coxsackie group A. I. Localization of viral antigen in infected primary human amnion cells. Arch Gesamte Virusforsch. 1965;15(5):668–680. [PubMed] [Google Scholar]